Validation of Easy Fabrication Methods for PDMS-Based Microfluidic (Bio)Reactors
Abstract
1. Introduction
2. Materials and Methods
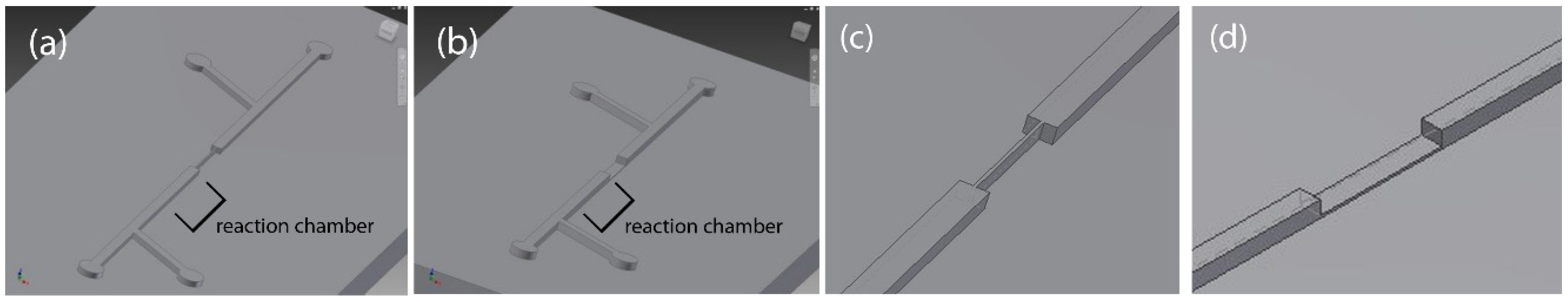
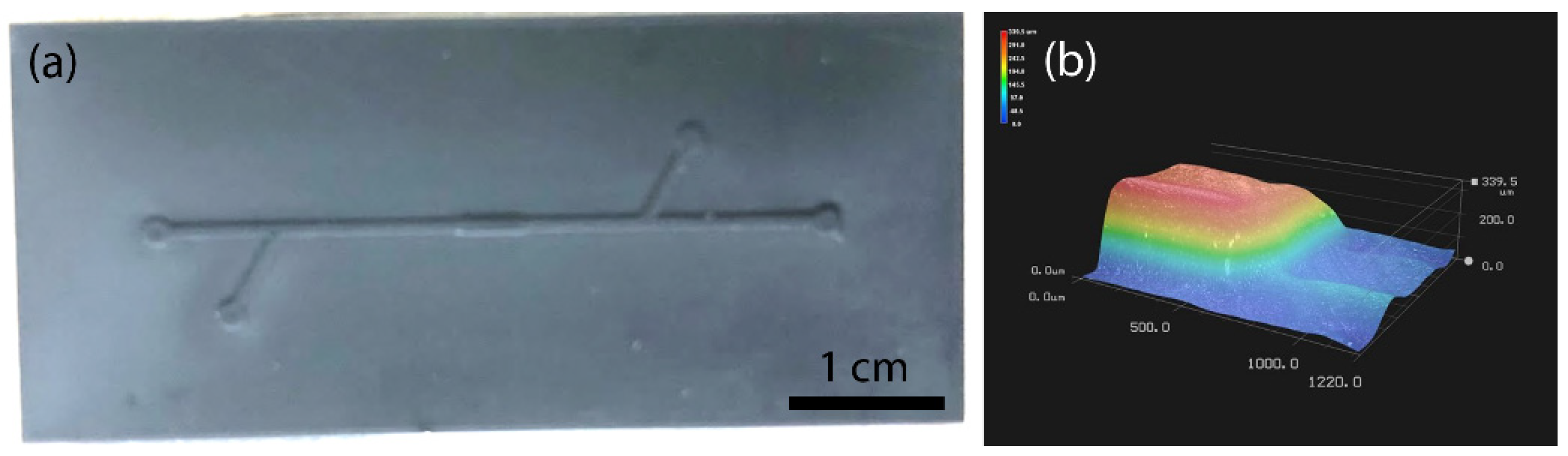
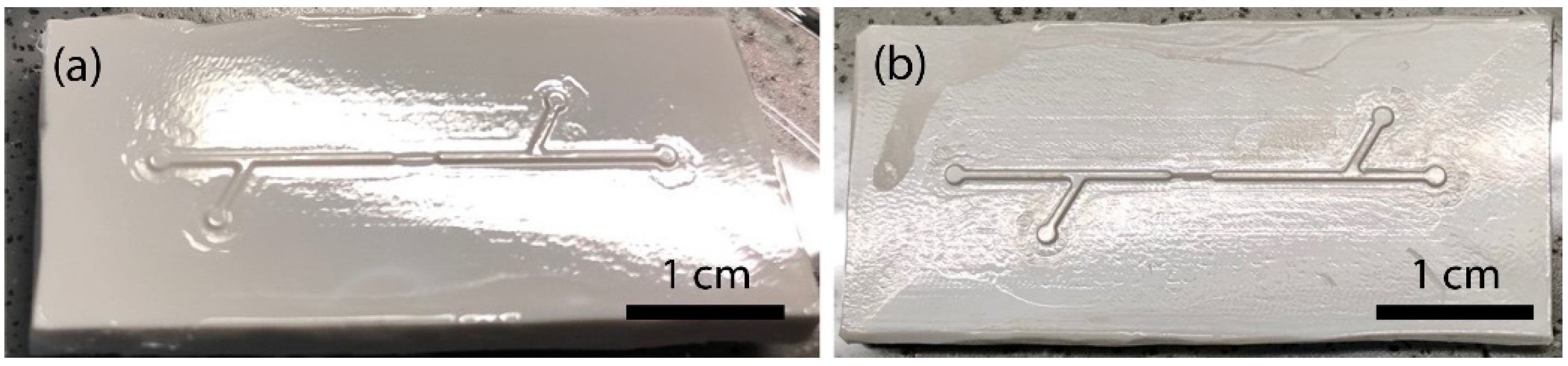
2.1. Microfluidic Particle Trapping Device Design
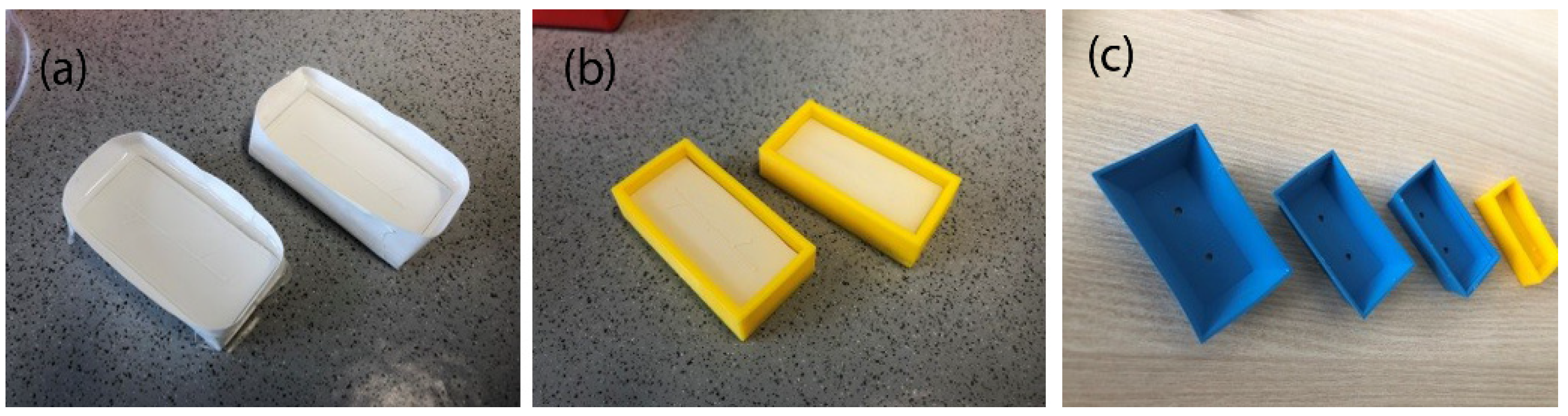
2.2. Mold Fabrication Methods
2.2.1. D Printing
2.2.2. Laser Cutting
2.2.3. Milling
2.3. PDMS Chip Preparation
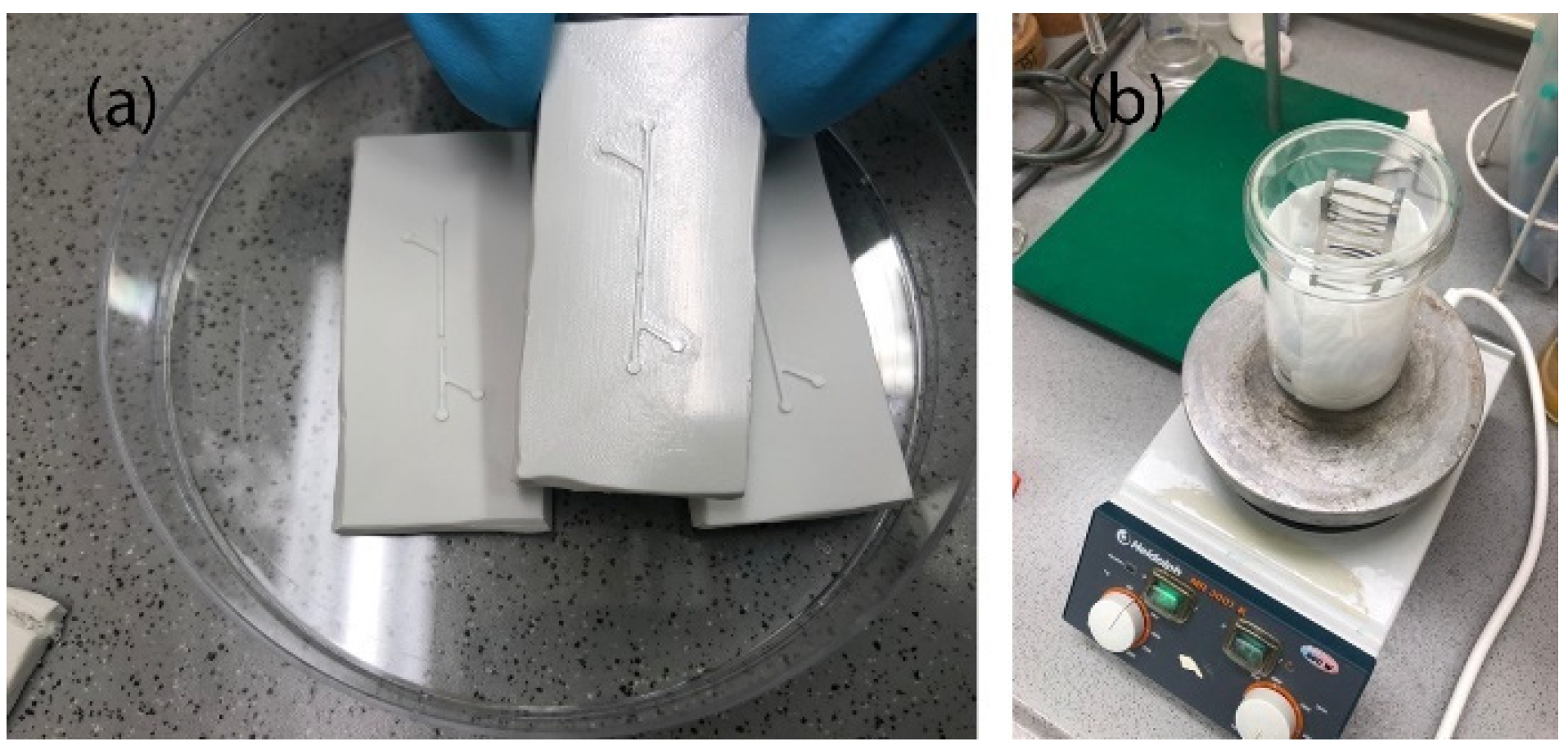
2.3.1. Preparation of Molds
2.3.2. PDMS Curing
2.3.3. Plasma Bonding
2.3.4. Tubing Connection
2.3.5. Feed
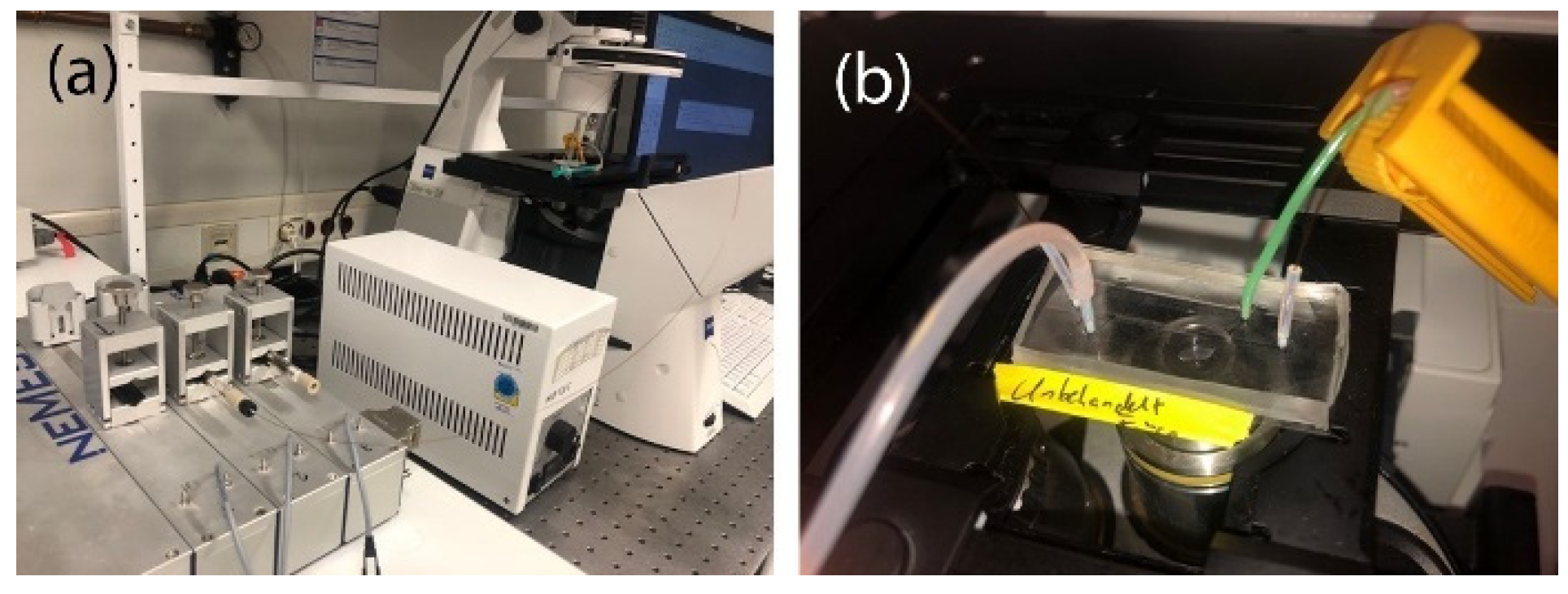
2.4. Microscopy
3. Results
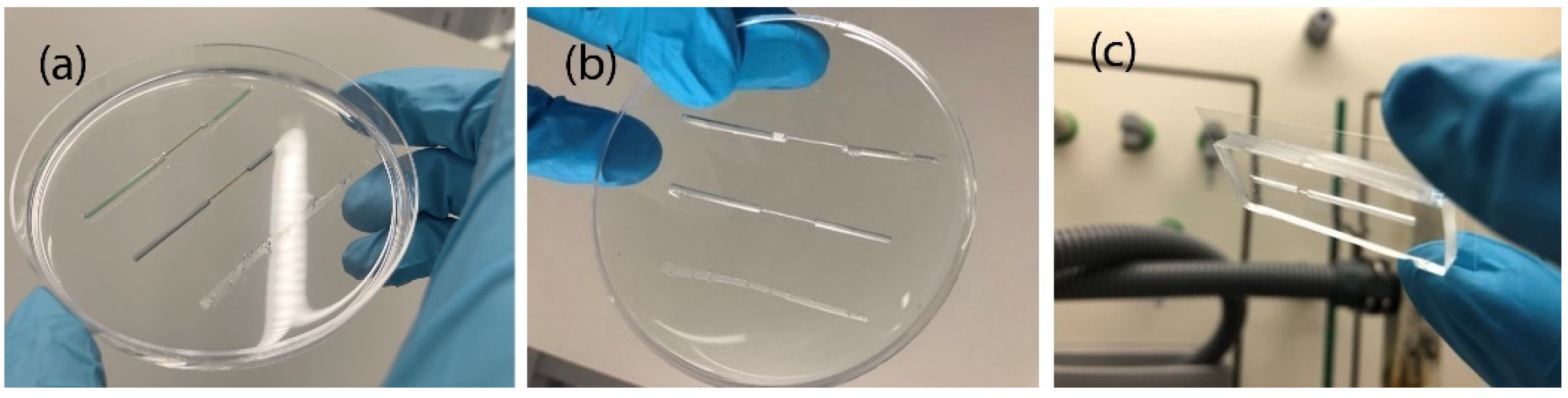
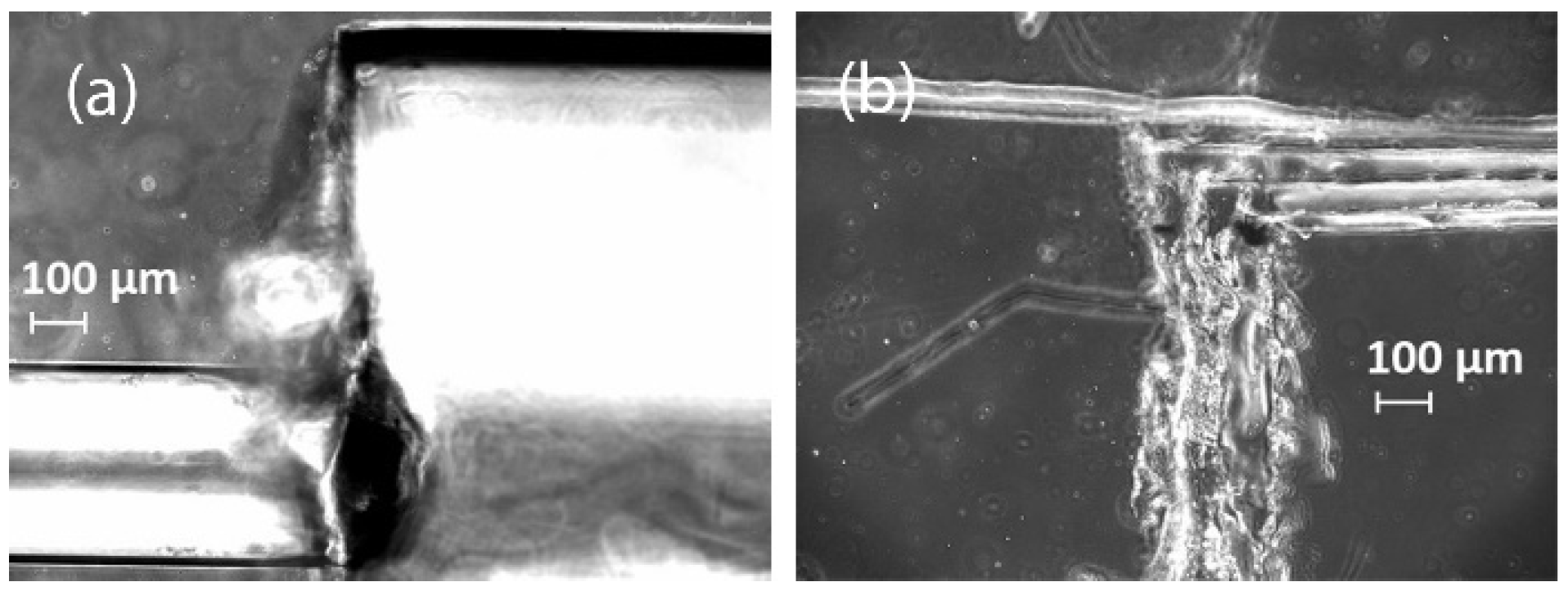
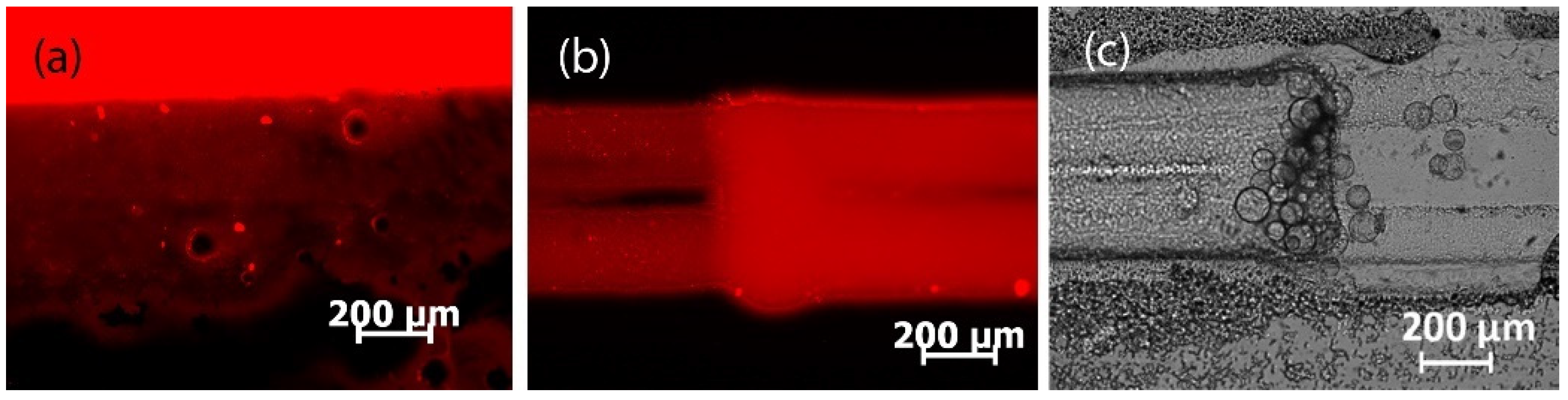
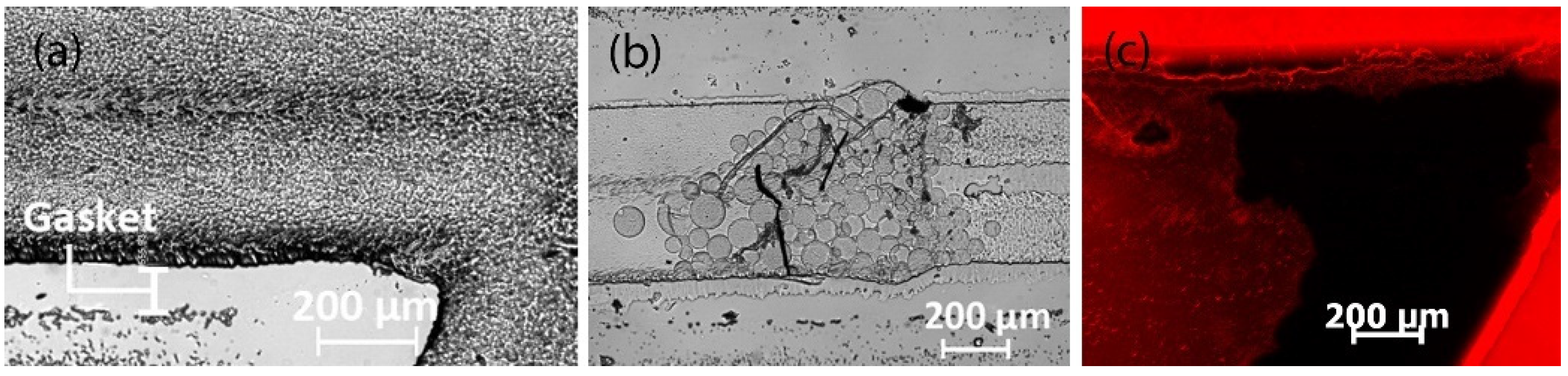
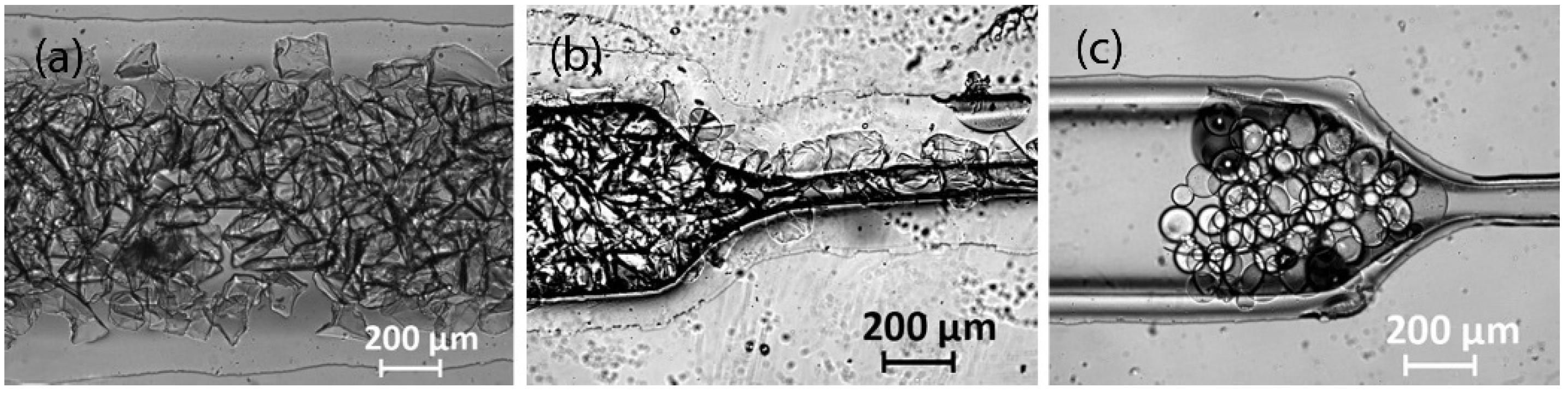
3.1. Fabrication of PDMS Chips with Capillaries and Tape as Replica Template
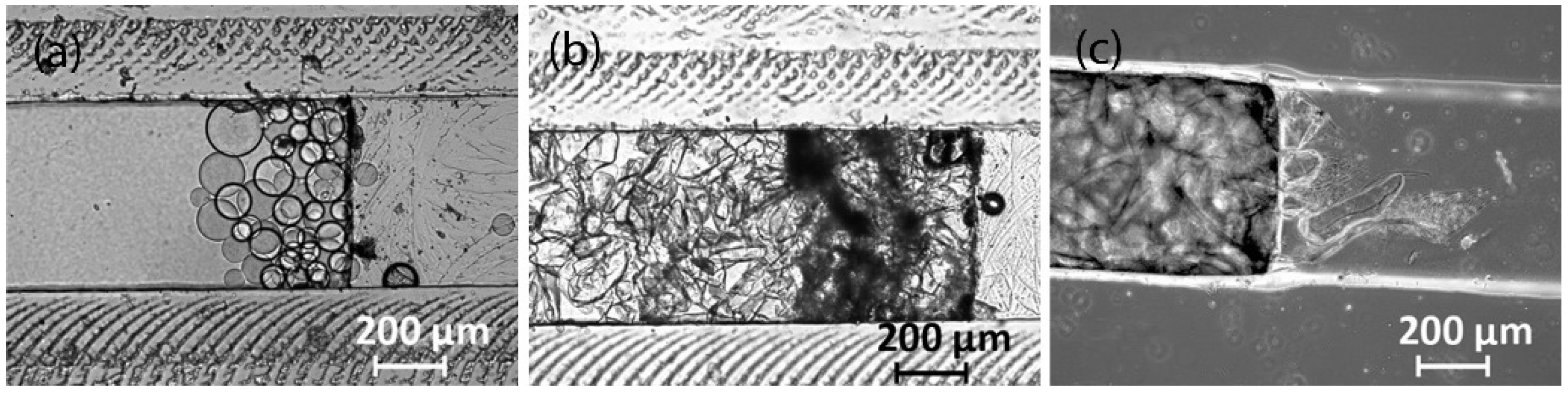
3.2. Fabrication of PDMS Chips with 3D-Printed, Laser-Engraved, and Milled Casting Molds
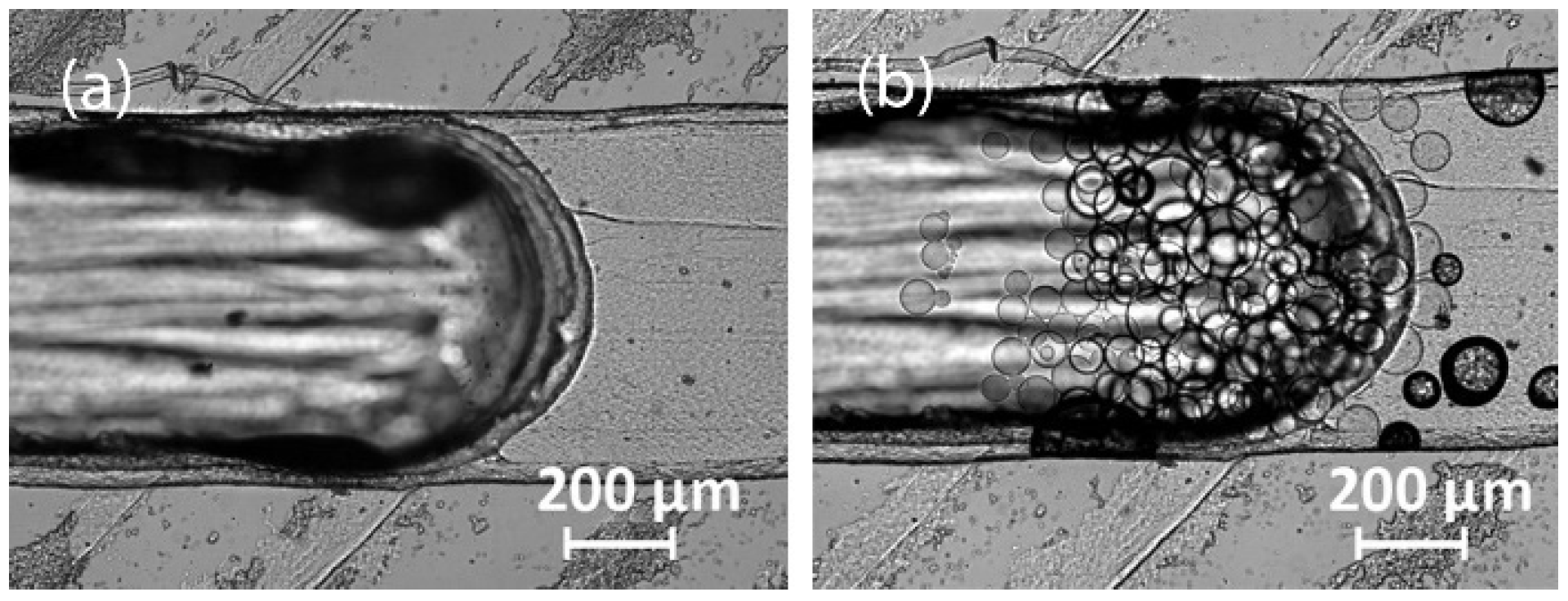
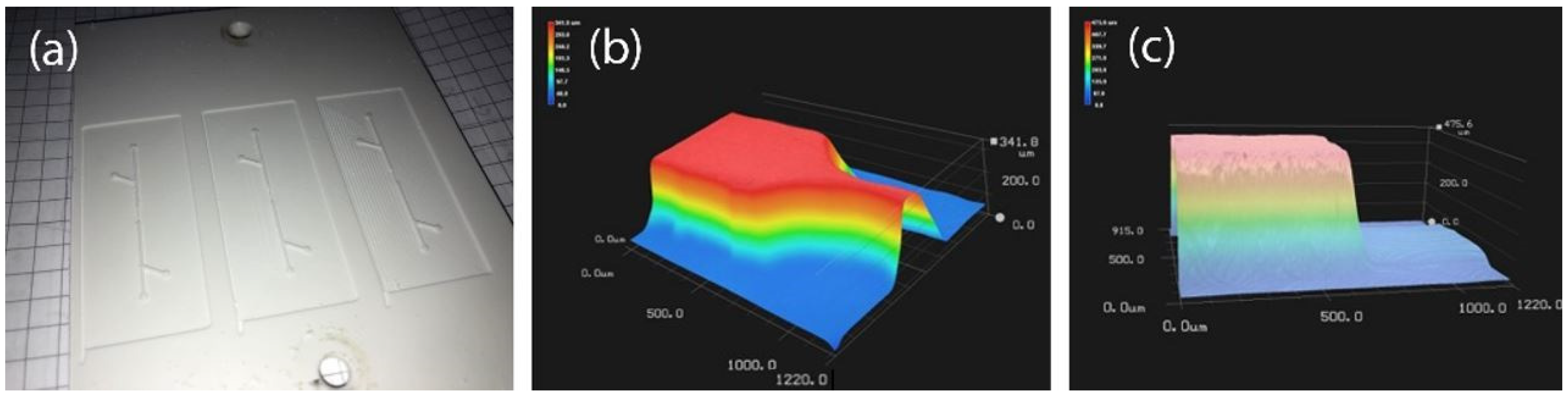
3.2.1. 3D Printing with a Fused Deposition Modeling Printer
3.2.2. 3D Printing with a Stereolithographic Printer
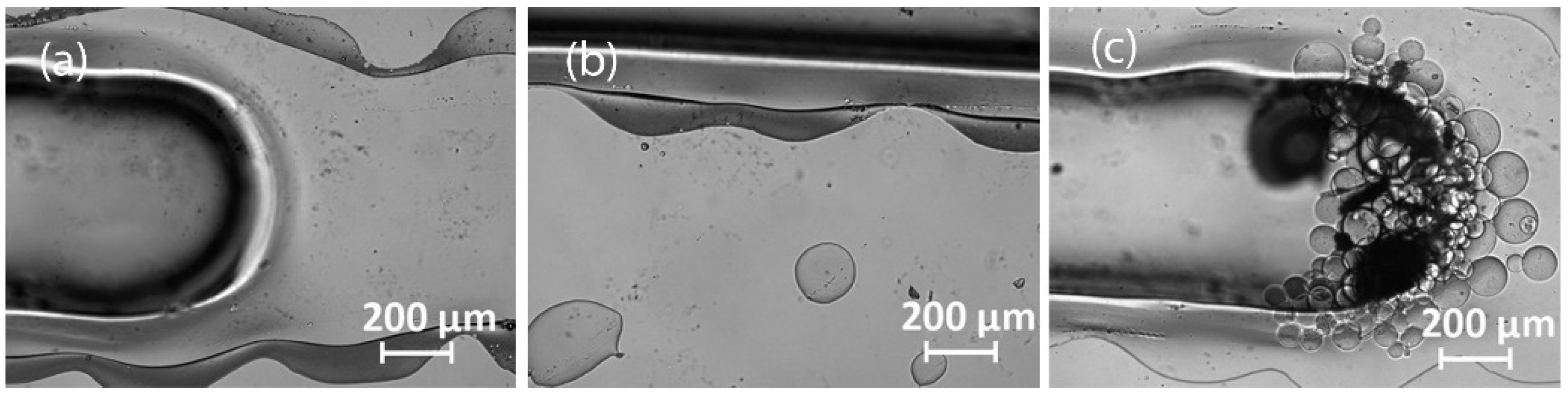
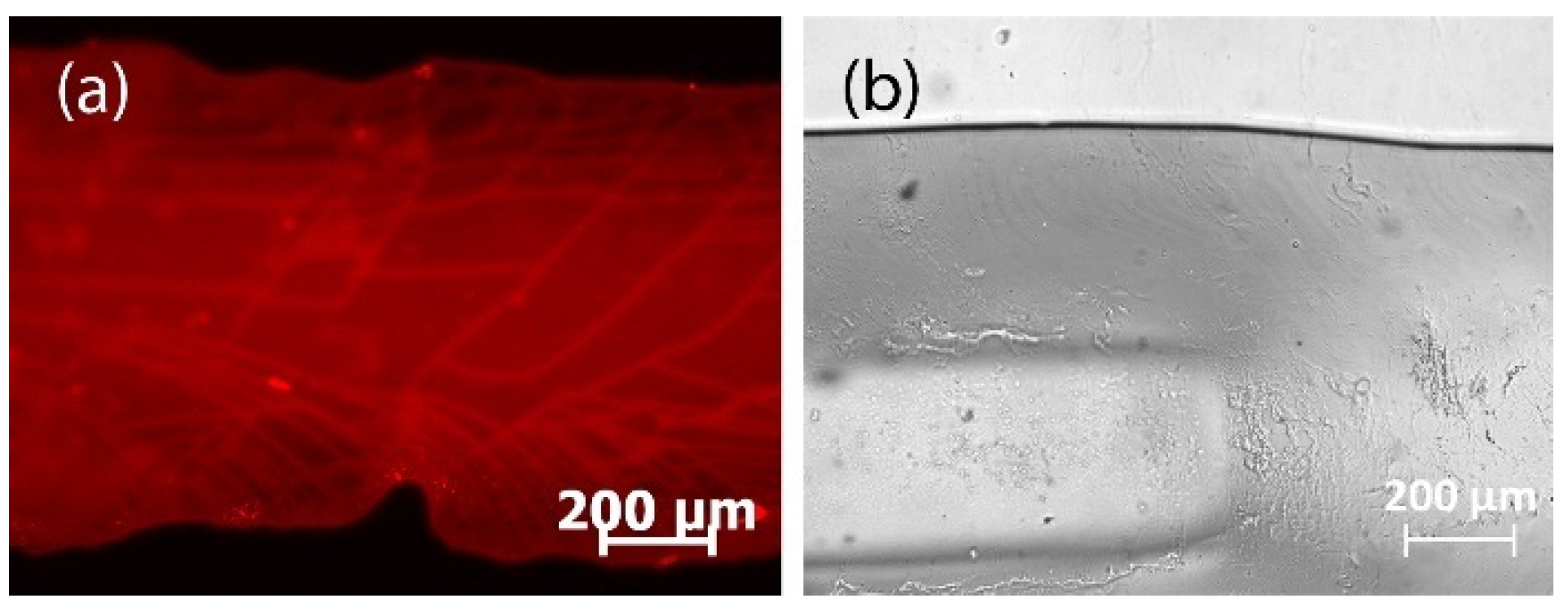
3.2.3. Laser Engraving
3.2.4. Milling
3.2.5. Acetone Polishing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmid, A.; Hollmann, F.; Park, J.B.; Bühler, B. The use of enzymes in the chemical industry in Europe. Curr. Opin. Biotechnol. 2002, 13, 359–366. [Google Scholar] [CrossRef]
- Rosenthal, K.; Lütz, S. Recent developments and challenges of biocatalytic processes in the pharmaceutical industry. Curr. Opin. Green Sustain. Chem. 2018, 11, 58–64. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Truppo, M.D. Biocatalysis in the Pharmaceutical Industry: The Need for Speed. ACS Med. Chem. Lett. 2017, 8, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.L.; Goodall, D.M.; Bruce, N.C. Enzymatic microreactors in chemical analysis and kinetic studies. Biotechnol. Adv. 2006, 24, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Manz, A.; Verpoorte, E.; Raymond, D.E.; Effenhauser, C.S.; Burggraf, N.; Widmer, H.M. µ-TAS: Miniaturized total chemical analysis systems. In Micro Total Analysis Systems; Van den Berg, A., Bergveld, P., Eds.; Springer Dordrecht: Basel, Switzerland, 1990; pp. 5–6. [Google Scholar]
- Gupta, S.; Ramesh, K.; Ahmed, S.; Kakkar, V. Lab-on-chip technology: A review on design trends and future scope in biomedical applications. Int. J. Bio-Sci. Bio-Technol. 2016, 8, 311–322. [Google Scholar] [CrossRef]
- Fritzsch, F.S.O.; Rosenthal, K.; Kampert, A.; Howitz, S.; Dusny, C.; Blank, L.M.; Schmid, A. Picoliter nDEP traps enable time-resolved contactless single bacterial cell analysis in controlled microenvironments. Lab Chip 2013, 13, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Meller, K.; Szumski, M.; Buszewski, B. Microfluidic reactors with immobilized enzymes—Characterization, dividing, perspectives. Sens. Actuators B Chem. 2017, 244, 84–106. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Q.; Shao, L.; Jia, Y.; Zhang, X. Microfluidic immobilized enzyme reactors for continuous biocatalysis. React. Chem. Eng. 2020, 5, 9–32. [Google Scholar] [CrossRef]
- Fritzsch, F.S.O.; Dusny, C.; Frick, O.; Schmid, A. Single-cell analysis in biotechnology, systems biology, and biocatalysis. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 129–155. [Google Scholar] [CrossRef]
- Deng, Y.; Guo, Y.; Xu, B. Recent development of microfluidic technology for cell trapping in single cell analysis: A review. Processes 2020, 8, 1253. [Google Scholar] [CrossRef]
- Johann, R.M. Cell trapping in microfluidic chips. Anal. Bioanal. Chem. 2006, 385, 408–412. [Google Scholar] [CrossRef]
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, J.; Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Gernaey, K.V.; Krühne, U. Connecting worlds—A view on microfluidics for a wider application. Biotechnol. Adv. 2018, 36, 1341–1366. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, K.; Falke, F.; Frick, O.; Dusny, C.; Schmid, A. An inert continuous microreactor for the isolation and analysis of a single microbial cell. Micromachines 2015, 6, 1836–1855. [Google Scholar] [CrossRef]
- Waldbaur, A.; Rapp, H.; Länge, K.; Rapp, B.E. Let there be chip—Towards rapid prototyping of microfluidic devices: One-step manufacturing processes. Anal. Methods 2011, 3, 2681–2716. [Google Scholar] [CrossRef]
- Joseph Fortenbaugh, R.; Lear, B.J. On-demand curing of polydimethylsiloxane (PDMS) using the photothermal effect of gold nanoparticles. Nanoscale 2017, 9, 8555–8559. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.D.; McCluskey, D.K.; Tan, C.K.L.; Tracey, M.C. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 2014, 24, 035017. [Google Scholar] [CrossRef]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent Compatibility of Poly(dimethylsiloxane)-Based Microfluidic Devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zheng, S.; Quach, B.Q.; Tai, Y.C. A study of the autofluorescence of parylene materials for μtAS applications. Lab Chip 2010, 10, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Loewen, A.; Allen, P.B. Simple, low-cost fabrication of acrylic based droplet microfluidics and its use to generate DNA-coated particles. Sci. Rep. 2018, 8, 8763. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, P.; Vijayan, S.; Doyle, P.S.; Hashimoto, M. Evaluation of 3D-printed molds for fabrication of non-planar microchannels. Biomicrofluidics 2021, 15, 024111. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Rath, S.N. 3D printed microfluidic devices: A review focused on four fundamental manufacturing approaches and implications on the field of healthcare. Bio-Des. Manuf. 2021, 4, 311–343. [Google Scholar] [CrossRef]
- Felton, H.; Hughes, R.; Diaz-Gaxiola, A. Negligible-cost microfluidic device fabrication using 3D-printed interconnecting channel scaffolds. PLoS ONE 2021, 16, e0245206. [Google Scholar] [CrossRef]
- Bonyár, A.; Sántha, H.; Varga, M.; Ring, B.; Vitéz, A.; Harsányi, G. Characterization of rapid PDMS casting technique utilizing molding forms fabricated by 3D rapid prototyping technology (RPT). Int. J. Mater. Form. 2014, 7, 189–196. [Google Scholar] [CrossRef]
- Bazaz, S.R.; Kashaninejad, N.; Azadi, S.; Patel, K.; Asadnia, M.; Jin, D.; Warkiani, M.E. Rapid Softlithography Using 3D-Printed Molds. Adv. Mater. Technol. 2019, 4, 1900425. [Google Scholar] [CrossRef]
- Saadat, M.; Taylor, M.; Hughes, A.; Hajiyavand, A.M. Rapid prototyping method for 3D PDMS microfluidic devices using a red femtosecond laser. Adv. Mech. Eng. 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Li, H.; Fan, Y.; Foulds, I.G. Rapid and low-cost fabrication of polystyrene-based molds for PDMS microfluidic devices using a CO2 laser. Adv. Mater. Res. 2012, 403–408, 4344–4348. [Google Scholar] [CrossRef]
- Qin, S.; Ou, G.; Wang, B.; Li, Z.; Hu, R.; Li, Y.; Yang, Y. Photolithography-free fabrication of photoresist-mold for rapid prototyping of microfluidic PDMS devices. Chin. Chem. Lett. 2022, 33, 987–989. [Google Scholar] [CrossRef]
- Srinivasan, A.; Bach, H.; Sherman, D.H.; Dordick, J.S. Bacterial P450-catalyzed polyketide hydroxylation on a microfluidic platform. Biotechnol. Bioeng. 2004, 88, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Hakala, T.A.; Bialas, F.; Toprakcioglu, Z.; Bräuer, B.; Baumann, K.N.; Levin, A.; Bernardes, G.J.L.; Becker, C.F.W.; Knowles, T.P.J. Continuous Flow Reactors from Microfluidic Compartmentalization of Enzymes within Inorganic Microparticles. ACS Appl. Mater. Interfaces 2020, 12, 32951–32960. [Google Scholar] [CrossRef] [PubMed]
- Brás, E.J.S.; Domingues, C.; Chu, V.; Fernandes, P.; Conde, J.P. Microfluidic bioreactors for enzymatic synthesis in packed-bed reactors—Multi-step reactions and upscaling. J. Biotechnol. 2020, 323, 24–32. [Google Scholar] [CrossRef]
- Lalehpour, A.; Barari, A. Post processing for Fused Deposition Modeling Parts with Acetone Vapour Bath. IFAC-PapersOnLine 2016, 49, 42–48. [Google Scholar] [CrossRef]
- Katzenberg, F. Plasma-bonding of poly(dimethylsiloxane) to glass. e-Polymers 2005, 5. [Google Scholar] [CrossRef][Green Version]
- Satyanarayana, S.; Karnik, R.N.; Majumdar, A. Stamp-and-stick room-temperature bonding technique for microdevices. J. Microelectromech. Syst. 2005, 14, 392–399. [Google Scholar] [CrossRef]
- Walsh, D.I.; Kong, D.S.; Murthy, S.K.; Carr, P.A. Enabling Microfluidics: From Clean Rooms to Makerspaces. Trends Biotechnol. 2017, 35, 383–392. [Google Scholar] [CrossRef]
- Burgoyne, F. Rapid Prototyping of Branched Microfluidics in PDMS Using Capillaries. Available online: https://blogs.rsc.org/chipsandtips/2010/05/03/rapid-prototyping-of-branched-microfluidics-in-pdms-using-capillaries/?doing_wp_cron=1544012376.7141489982604980468750 (accessed on 30 November 2021).
- Burgoyne, F. A Method for Rapid Fabrication of Microfluidic Devices. Available online: https://blogs.rsc.org/chipsandtips/2009/06/30/a-method-for-rapid-fabrication-of-microfluidic-devices/ (accessed on 30 November 2021).
- Venzac, B.; Deng, S.; Mahmoud, Z.; Lenferink, A.; Costa, A.; Bray, F.; Otto, C.; Rolando, C.; Le Gac, S. PDMS Curing Inhibition on 3D-Printed Molds: Why? Also, How to Avoid It? Anal. Chem. 2021, 93, 7180–7187. [Google Scholar] [CrossRef]
- de Almeida Monteiro Melo Ferraz, M.; Nagashima, J.B.; Venzac, B.; Le Gac, S.; Songsasen, N. 3D printed mold leachates in PDMS microfluidic devices. Sci. Rep. 2020, 10, 994. [Google Scholar] [CrossRef]
- Scott, S.M.; Ali, Z. Fabrication methods for microfluidic devices: An overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef]
- Qian, T.; Wang, Y. Micro/nano-fabrication technologies for cell biology. Med. Biol. Eng. Comput. 2010, 48, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.T.M.; Nguyen, T.D. Lithography-based methods to manufacture biomaterials at small scales. J. Sci. Adv. Mater. Devices 2017, 2, 1–14. [Google Scholar] [CrossRef]
- Shrirao, A.B.; Hussain, A.; Cho, C.H.; Perez-Castillejos, R. Adhesive-tape soft lithography for patterning mammalian cells: Application to wound-healing assays. Biotechniques 2012, 52, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yao, Z.; Hou, Z.; Song, J. Processing and Profile Control of Microhole Array for PDMS Mask with Femtosecond Laser. Micromachines 2022, 13, 340. [Google Scholar] [CrossRef] [PubMed]
- Isiksacan, Z.; Guler, M.T.; Aydogdu, B.; Bilican, I.; Elbuken, C. Rapid fabrication of microfluidic PDMS devices from reusable PDMS molds using laser ablation. J. Micromech. Microeng. 2016, 26, 035008. [Google Scholar] [CrossRef]
- Lin, L.; Chung, C.K. PDMS microfabrication and design for microfluidics and sustainable energy application: Review. Micromachines 2021, 12, 1350. [Google Scholar] [CrossRef]
- Pattanayak, P.; Singh, S.K.; Gulati, M.; Vishwas, S.; Kapoor, B.; Chellappan, D.K.; Anand, K.; Gupta, G.; Jha, N.K.; Gupta, P.K.; et al. Microfluidic chips: Recent advances, critical strategies in design, applications and future perspectives. Microfluid. Nanofluid. 2021, 25, 99. [Google Scholar] [CrossRef]
- Lv, X.; Geng, Z.; Fan, Z.; Wang, S.; Su, Y.; Fang, W.; Pei, W.; Chen, H. Route to one-step microstructure mold fabrication for PDMS microfluidic chip. AIP Adv. 2018, 8, 045207. [Google Scholar] [CrossRef]
- Gyimah, N.; Scheler, O.; Rang, T.; Pardy, T. Can 3d printing bring droplet microfluidics to every lab?—A systematic review. Micromachines 2021, 12, 339. [Google Scholar] [CrossRef]
- Comina, G.; Suska, A.; Filippini, D. PDMS lab-on-a-chip fabrication using 3D printed templates. Lab Chip 2014, 14, 424–430. [Google Scholar] [CrossRef]
- Mazlan, S.N.H.; Alkahari, M.R.; Ramli, F.R.; Maidin, N.A.; Sudin, M.N.; Zolkaply, A.R. Surface finish and mechanical properties of FDM part after blow cold vapor treatment. J. Adv. Res. Fluid Mech. Therm. Sci. 2018, 48, 148–155. [Google Scholar]
- Schoenitz, M.; Grundemann, L.; Augustin, W.; Scholl, S. Fouling in microstructured devices: A review. Chem. Commun. 2015, 51, 8213–8228. [Google Scholar] [CrossRef] [PubMed]







| Exposure Time to Acetone Vapor | Deviation of the Channel Height | Arithmetic Average Roughness Ra |
|---|---|---|
| 0 s | 0 ± 1% | 2.2 ± 0.7 µm |
| 30 s | 3 ± 6% | 0.3 ± 0.1 µm |
| 1 min | 16 ± 9% | n.d. |
| 2 min | 11 ± 7% | n.d. |
| 5 min | 23 ± 3% | n.d. |
| Laser Cutting | Fused Deposition Modeling 3D Printing | Stereolithographic 3D Printing | CNC Milling | |
|---|---|---|---|---|
| Particle retention | -- | + | + | ++ |
| Leak tightness | ++ | + * | - | ++ * |
| Machine accuracy | -- | - | - | ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogt, J.; Rosenthal, K. Validation of Easy Fabrication Methods for PDMS-Based Microfluidic (Bio)Reactors. Sci 2022, 4, 36. https://doi.org/10.3390/sci4040036
Vogt J, Rosenthal K. Validation of Easy Fabrication Methods for PDMS-Based Microfluidic (Bio)Reactors. Sci. 2022; 4(4):36. https://doi.org/10.3390/sci4040036
Chicago/Turabian StyleVogt, Josef, and Katrin Rosenthal. 2022. "Validation of Easy Fabrication Methods for PDMS-Based Microfluidic (Bio)Reactors" Sci 4, no. 4: 36. https://doi.org/10.3390/sci4040036
APA StyleVogt, J., & Rosenthal, K. (2022). Validation of Easy Fabrication Methods for PDMS-Based Microfluidic (Bio)Reactors. Sci, 4(4), 36. https://doi.org/10.3390/sci4040036









