Utilization of Protein-Rich Agricultural Residues in the Biotechnological Production of L-Lactic Acid and 1,3-Propanediol for Added Value
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
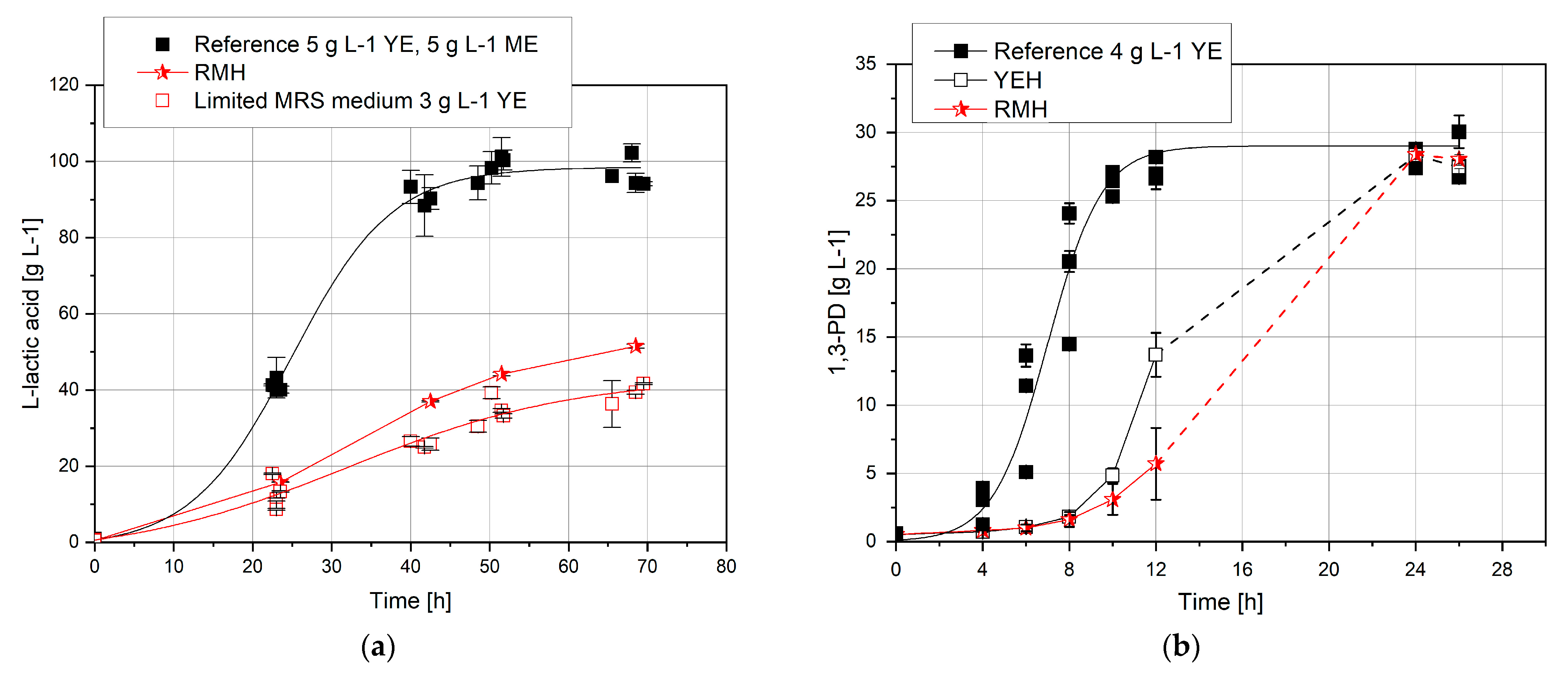
3.1. Cultivation with Complex Nutrient Source
3.2. Analysis and Replacement of Complex Nutrient Source
3.3. Nutrient Requirements
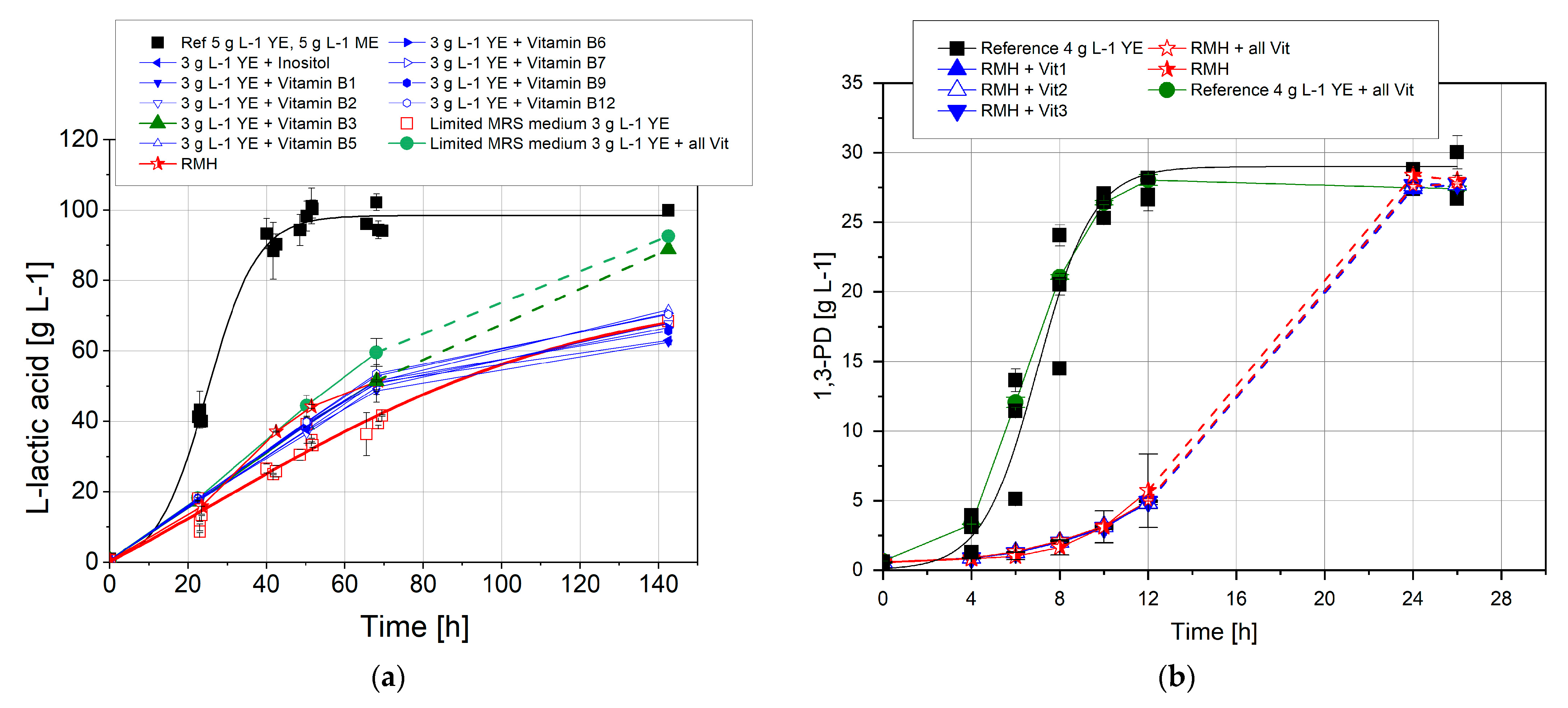
3.3.1. B Vitamin Requirements
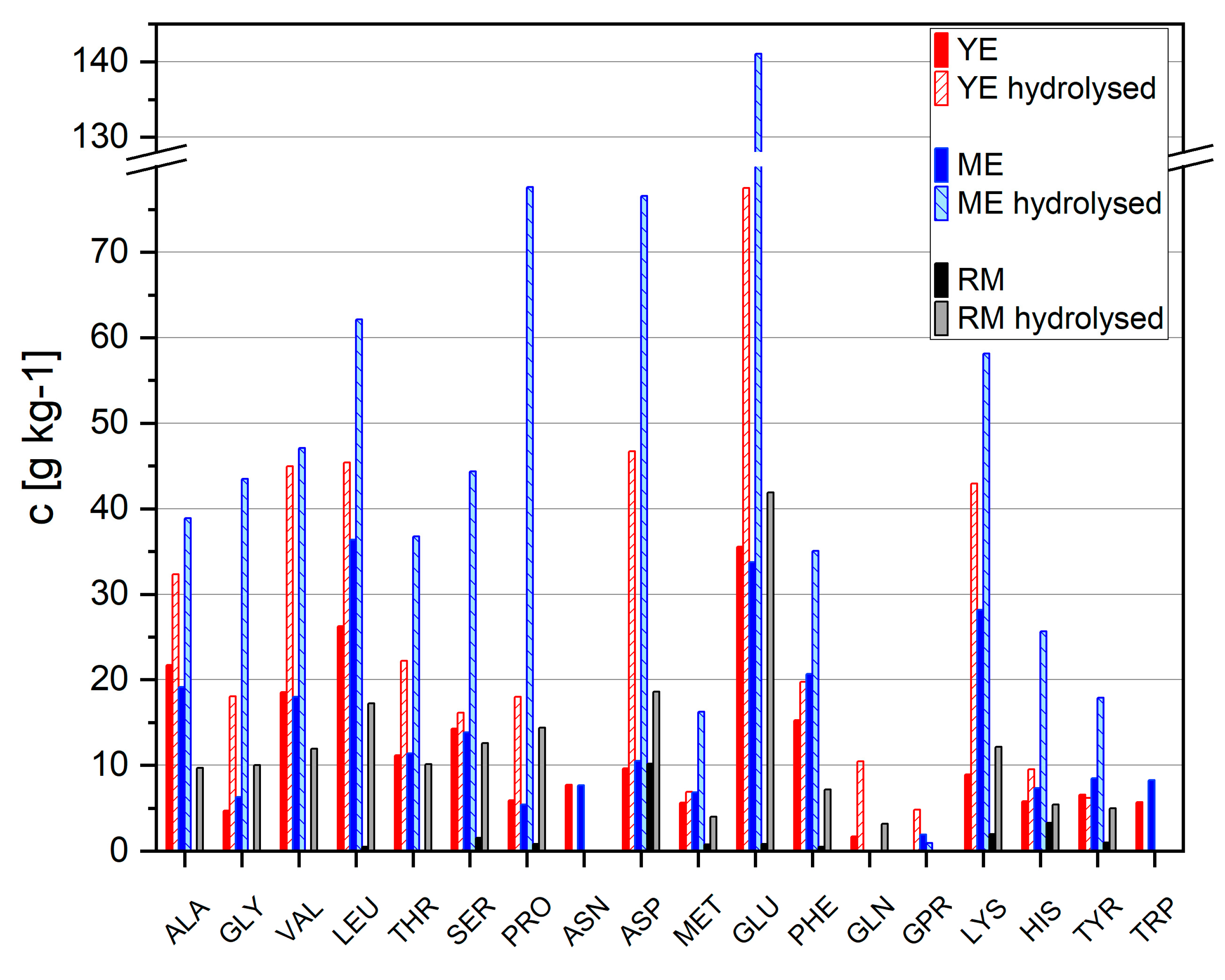
3.3.2. Amino Acids Requirements
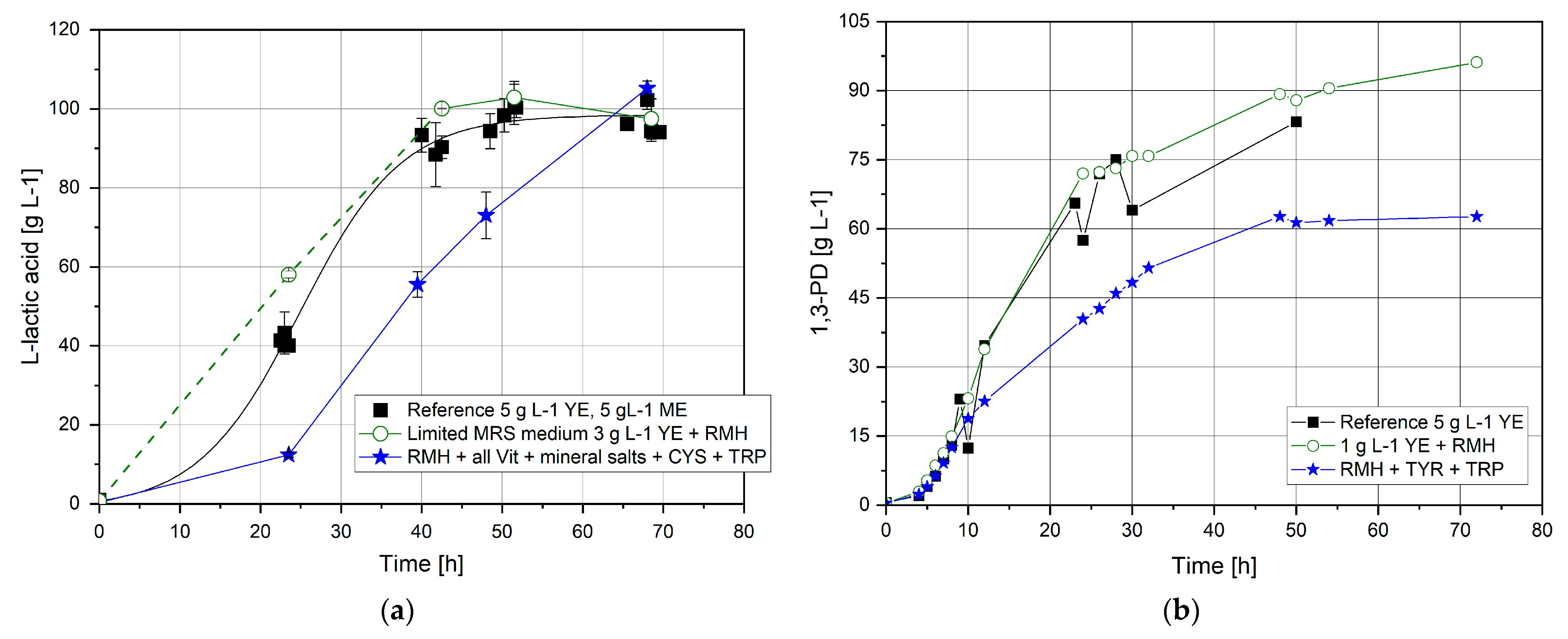
3.4. Replacement of Complex Nutrient Source
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1,3-PD | 1,3-propanediol |
| AA | amino acid |
| Arg | arginine |
| Ala | alanine |
| Asn | asparagine |
| Asp | aspartic acid |
| CSL | corn steep liquor |
| Cyc | cysteine |
| DSMZ | German Collection of Microorganisms and Cell Cultures |
| Gln | glutamine |
| Glu | glutamic acid |
| Gly | glycine |
| Gpr | glycyl-proline |
| His | histidine |
| HPLC | high-performance liquid chromatography |
| Ile | isoleucine |
| Leu | leucine |
| Lys | lysine |
| ME | meat extract |
| MES | 2-(N-morpholino)ethanesulfonic acid |
| Met | methionine |
| MRS | deMan, Rogosa, und Sharpe |
| OD | optical density |
| P | peptone |
| Phe | phenylalanine |
| PLA | poly lactic acid |
| Pro | proline |
| RM | rapeseed meal |
| RMH | rapeseed meal hydrolyzate |
| Ser | serine |
| Thr | threonine |
| TN | total nitrogen content |
| Trp | tryptophan |
| Tyr | tyrosine |
| Val | valine |
| YE | yeast extract |
| YEH | yeast extract hydrolyzate |
References
- Maddipati, P.; Atiyeh, H.K.; Bellmer, D.D.; Huhnke, R.L. Ethanol production from syngas by Clostridium strain P11 using corn steep liquor as a nutrient replacement to yeast extract. Bioresour. Technol. 2011, 102, 6494–6501. [Google Scholar] [CrossRef]
- González, M.I.; Alvarez, S.; Riera, F.; Alvarez, R. Economic evaluation of an integrated process for lactic acid production from ultrafiltered whey. J. Food Eng. 2007, 80, 553–561. [Google Scholar] [CrossRef]
- Sikder, J.; Roy, M.; Dey, P.; Pal, P. Techno-economic analysis of a membrane-integrated bioreactor system for production of lactic acid from sugarcane juice. Biochem. Eng. J. 2012, 63, 81–87. [Google Scholar] [CrossRef]
- Tejayadi, S.; Cheryan, M. Lactic-Acid from Cheese Whey Permeate—Productivity and Economics of a Continuous Membrane Bioreactor. Appl. Microbiol. Biotechnol. 1995, 43, 242–248. [Google Scholar] [CrossRef]
- Sommer, R. Yeast extracts: Production, properties and components. Food Aust. 1998, 50, 181–183. [Google Scholar]
- Yadav, A.K.; Chaudhari, A.B.; Kothari, R.M. Bioconversion of renewable resources into lactic acid: An industrial view. Crit. Rev. Biotechnol. 2011, 31, 1–19. [Google Scholar] [CrossRef]
- Brock, S.; Kuenz, A.; Prüsse, U. Impact of Hydrolysis Methods on the Utilization of Agricultural Residues as Nutrient Source for D-lactic Acid Production by Sporolactobacillus inulinus. Fermentation 2019, 5, 12. [Google Scholar] [CrossRef]
- Hujanen, M.; Linko, S.; Linko, Y.Y.; Leisola, M. Optimisation of media and cultivation conditions for L(+)-lactic acid production by Lactobacillus casei NRRL B-441. Appl. Microbiol. Biotechnol. 2001, 56, 126–130. [Google Scholar] [CrossRef]
- Pauli, T.; Fitzpatrick, J.J. Malt combing nuts as a nutrient supplement to whey permeate for producing lactic by fermentation with Lactobacillus casei. Process Biochem. 2002, 38, 1–6. [Google Scholar] [CrossRef]
- Rawoof, S.A.A.; Kumar, P.S.; Vo, D.V.N.; Devaraj, K.; Mani, Y.; Devaraj, T.; Subramanian, S. Production of optically pure lactic acid by microbial fermentation: A review. Environ. Chem. Lett. 2021, 19, 539–556. [Google Scholar] [CrossRef]
- Klotz, S.; Kuenz, A.; Prüsse, U. Nutritional requirements and the impact of yeast extract on the D-lactic acid production by Sporolactobacillus inulinus. Green Chem. 2017, 19, 4633–4641. [Google Scholar] [CrossRef]
- Georgiev, R.; Kalaydzhiev, H.; Slavov, A.; Ivanova, P.; Uzunova, G.; Chalova, V.I. Residual Waste After Protein Isolation From Ethanol-Treated Rapeseed Meal has Physico-Chemical Properties for Functional Food Systems Formulation. Waste Biomass Valori 2022, 13, 1223–1232. [Google Scholar] [CrossRef]
- Kreps, F.; Vrbiková, L.; Schmidt, S. Industrial Rapeseed and Sunflower Meal as Source of Antioxidants. Int. J. Eng. Res. Appl. 2014, 4, 45–54. [Google Scholar]
- Kalaydzhiev, H.; Ivanova, P.; Stoyanova, M.; Pavlov, A.; Rustad, T.; Silva, C.L.M.; Chalova, V.I. Valorization of Rapeseed Meal: Influence of Ethanol Antinutrients Removal on Protein Extractability, Amino Acid Composition and Fractional Profile. Waste Biomass Valori 2020, 11, 2709–2719. [Google Scholar] [CrossRef]
- Miller, C.; Fosmer, A.; Rush, B.; McMullin, T.; Beacom, D.; Suominen, P. Industrial Production of Lactic Acid. In Comprehensive Biotechnology, 2nd ed.; Elsevier: New York, NY, USA, 2011; Volume 3, pp. 179–188. [Google Scholar]
- Vijayakumar, J.; Aravindan, R.; Viruthagiri, T. Recent trends in the production, purification and application of lactic acid. Chem. Biochem. Eng. Q. 2008, 22, 245–264. [Google Scholar]
- Curk, M.C.; Peladan, F.; Hubert, J.C. Characterization of Lactobacilli Isolated in Breweries. Lait 1993, 73, 215–231. [Google Scholar] [CrossRef]
- Saxena, R.K.; Anand, P.; Saran, S.; Isar, J. Microbial production of 1,3-propanediol: Recent developments and emerging opportunities. Biotechnol. Adv. 2009, 27, 895–913. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Y.; Wei, Z.; Park, S. Recent advances in metabolic engineering of microorganisms for the production of monomeric C3 and C4 chemical compounds. Bioresour. Technol. 2023, 377, 128973. [Google Scholar] [CrossRef]
- Marr, A.C. 1,3-Propanediol, an Exemplary Bio-Renewable Organic Platform Chemical. Adv. Synth. Catal. 2024, 366, 4801. [Google Scholar] [CrossRef]
- Nimbalkar, P.R.; Dharne, M.S. A review on microbial 1,3-propanediol production: Emerging strategies, key hurdles and attainable solutions to re-establish its commercial interest. Ind. Crops Prod. 2024, 209, 117961. [Google Scholar] [CrossRef]
- Bock, R. Biokonversion von Glycerin zu 1,3-Propandiol mit Freien und Immobilisierten Mikroorganismen. Ph.D. Dissertation, TU Braunschweig, Braunschweig, Germany, 2004. [Google Scholar]
- Wilkens, E.; Ringel, A.K.; Hortig, D.; Willke, T.; Vorlop, K.D. High-level production of 1,3-propanediol from crude glycerol by Clostridium butyricum AKR102a. Appl. Microbiol. Biotechnol. 2012, 93, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, S.; Baganz, K.; Koschik, I.; Vorlop, K.D. Development of an integrated bioconversion process for the production of 1,3-propanediol from raw glycerol waters. Landbauforsch. Völkenrode FAL Agric. Res. 2005, 55, 261–267. [Google Scholar]
- Zhu, F.H.; Liu, D.H.; Chen, Z. Recent advances in biological production of 1,3-propanediol: New routes and engineering strategies. Green Chem. 2022, 24, 1390–1403. [Google Scholar] [CrossRef]
- Krull, S.; Brock, S.; Prüsse, U.; Kuenz, A. Hydrolyzed Agricultural Residues-Low-Cost Nutrient Sources for l-Lactic Acid Production. Fermentation 2020, 6, 97. [Google Scholar] [CrossRef]
- Klotz, S. Biotechnisch Hergestellte D-Milchsäure—Substitution von Hefeextrakt Durch Agrarische Rohstoffhydrolysate. Ph.D. Dissertation, TU Braunschweig, Braunschweig, Germany, 2017. [Google Scholar]
- Hofvendahl, K.; Hahn-Hägerdal, B. Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb. Technol. 2000, 26, 87–107. [Google Scholar] [CrossRef]
- Yáñez, R.; Alonso, J.L.; Parajó, J.C. D-Lactic acid production from waste cardboard. J. Chem. Technol. Biotechnol. 2005, 80, 76–84. [Google Scholar] [CrossRef]
- Slavica, A.; Trontel, A.; Jelovac, N.; Kosovec, Z.; Santek, B.; Novak, S. Production of lactate and acetate by Lactobacillus coryniformis subsp. torquens DSM 20004 in comparison with Lactobacillus amylovorus DSM 20531. J. Biotechnol. 2015, 202, 50–59. [Google Scholar]
- Wang, Y.; Tashiro, Y.; Sonomoto, K. Fermentative production of lactic acid from renewable materials: Recent achievements, prospects, and limits. J. Biosci. Bioeng. 2015, 119, 10–18. [Google Scholar] [CrossRef]
- Hujanen, M.; Linko, Y.Y. Effect of temperature and various nitrogen sources on L(+) lactic acid production by Lactobacillus casei. Appl. Microbiol. Biotechnol. 1996, 45, 307–313. [Google Scholar] [CrossRef]
- Wilkens, E. Medienoptimierung zur Kostenreduktion der Mikrobiellen Konversion von Glycerin zu 1,3-Propandiol. Ph.D. Dissertation, TU Braunschweig, Braunschweig, Germany, 2012. [Google Scholar]
- Wang, X.L.; Zhou, J.J.; Shen, J.T.; Zheng, Y.F.; Sun, Y.Q.; Xiu, Z.L. Sequential fed-batch fermentation of 1,3-propanediol from glycerol by Clostridium butyricum DL07. Appl. Microbiol. Biotechnol. 2020, 104, 9179–9191. [Google Scholar] [CrossRef]
- Wischral, D.; Zhang, J.; Cheng, C.; Lin, M.; De Souza, L.M.G.; Pessoa, F.L.P.; Pereira, N., Jr.; Yang, S.T. Production of 1,3-propanediol by Clostridium beijerinckii DSM 791 from crude glycerol and corn steep liquor: Process optimization and metabolic engineering. Bioresour. Technol. 2016, 212, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.R.; Lee, S.M.; Heo, S.Y.; Seo, J.W.; Kim, C.H. Efficient production of 1,3-propanediol from crude glycerol by repeated fed-batch fermentation strategy of a lactate and 2,3-butanediol deficient mutant of Klebsiella pneumoniae. Microb. Cell Fact. 2018, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Maina, S.; Kachrimanidou, V.; Ladakis, D.; Papanikolaou, S.; de Castro, A.M.; Koutinas, A. Evaluation of 1,3-propanediol production by two Citrobacter freundii strains using crude glycerol and soybean cake hydrolysate. Environ. Sci. Pollut. Res. Int. 2019, 26, 35523–35532. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuenz, A.; Hancock, V.; Krull, S.; Prüße, U. Utilization of Protein-Rich Agricultural Residues in the Biotechnological Production of L-Lactic Acid and 1,3-Propanediol for Added Value. Sci 2025, 7, 50. https://doi.org/10.3390/sci7020050
Kuenz A, Hancock V, Krull S, Prüße U. Utilization of Protein-Rich Agricultural Residues in the Biotechnological Production of L-Lactic Acid and 1,3-Propanediol for Added Value. Sci. 2025; 7(2):50. https://doi.org/10.3390/sci7020050
Chicago/Turabian StyleKuenz, Anja, Victoria Hancock, Susan Krull, and Ulf Prüße. 2025. "Utilization of Protein-Rich Agricultural Residues in the Biotechnological Production of L-Lactic Acid and 1,3-Propanediol for Added Value" Sci 7, no. 2: 50. https://doi.org/10.3390/sci7020050
APA StyleKuenz, A., Hancock, V., Krull, S., & Prüße, U. (2025). Utilization of Protein-Rich Agricultural Residues in the Biotechnological Production of L-Lactic Acid and 1,3-Propanediol for Added Value. Sci, 7(2), 50. https://doi.org/10.3390/sci7020050




