Using Morphological Characters to Support Decision-Making in Nature-Based Solutions: A Shortcut to Promote Urban Plant Biodiversity
Abstract
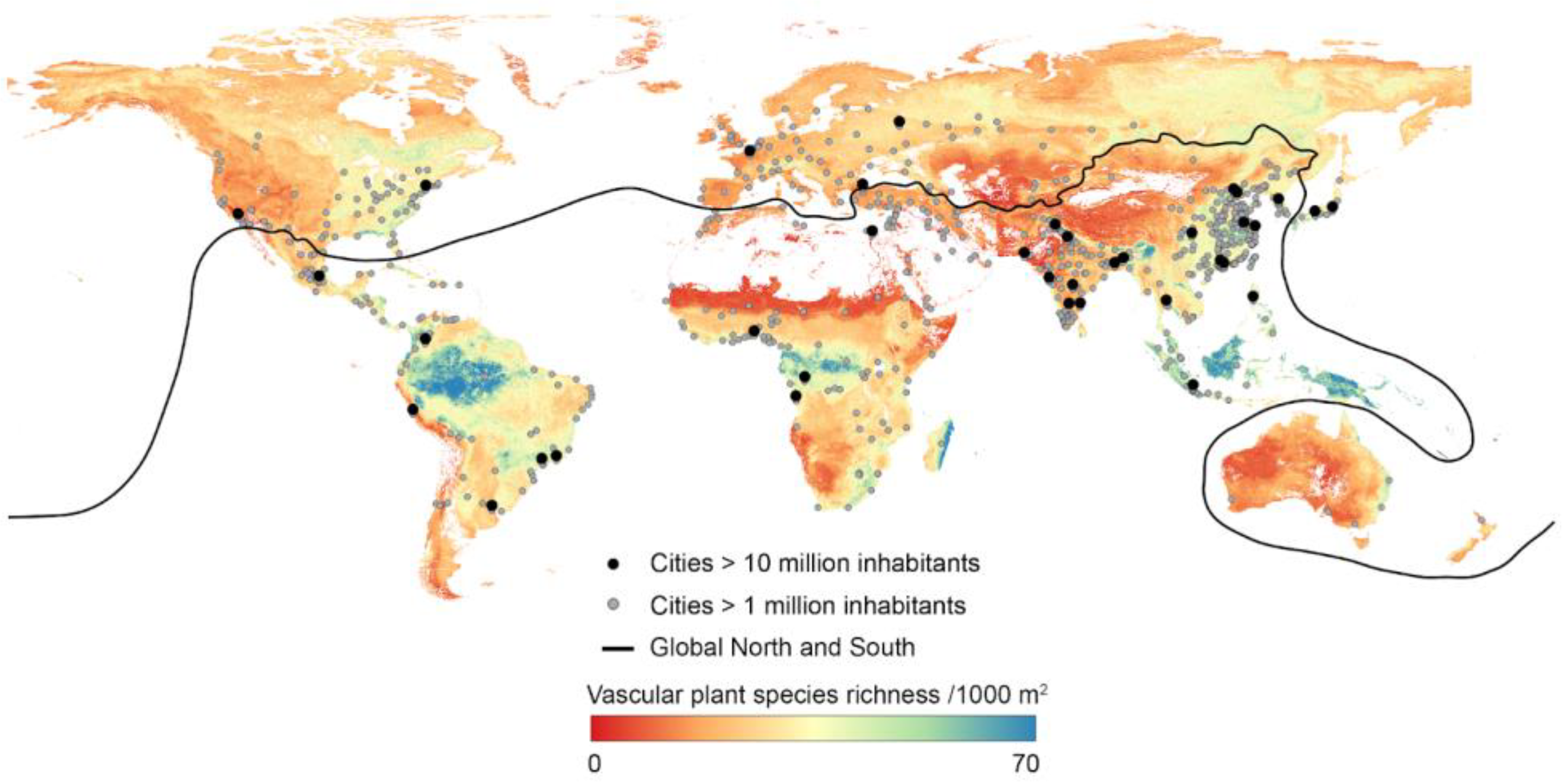
1. Introduction
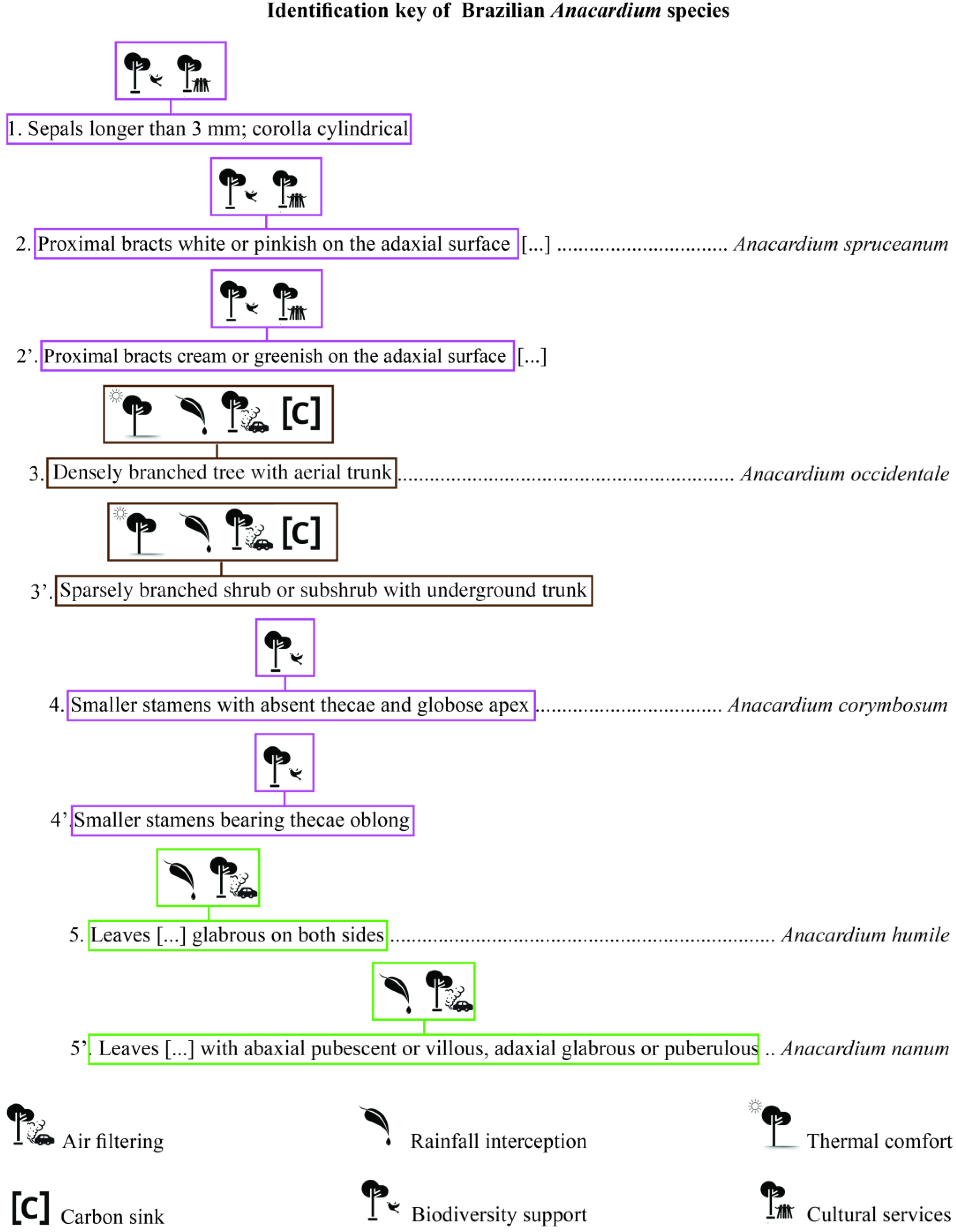
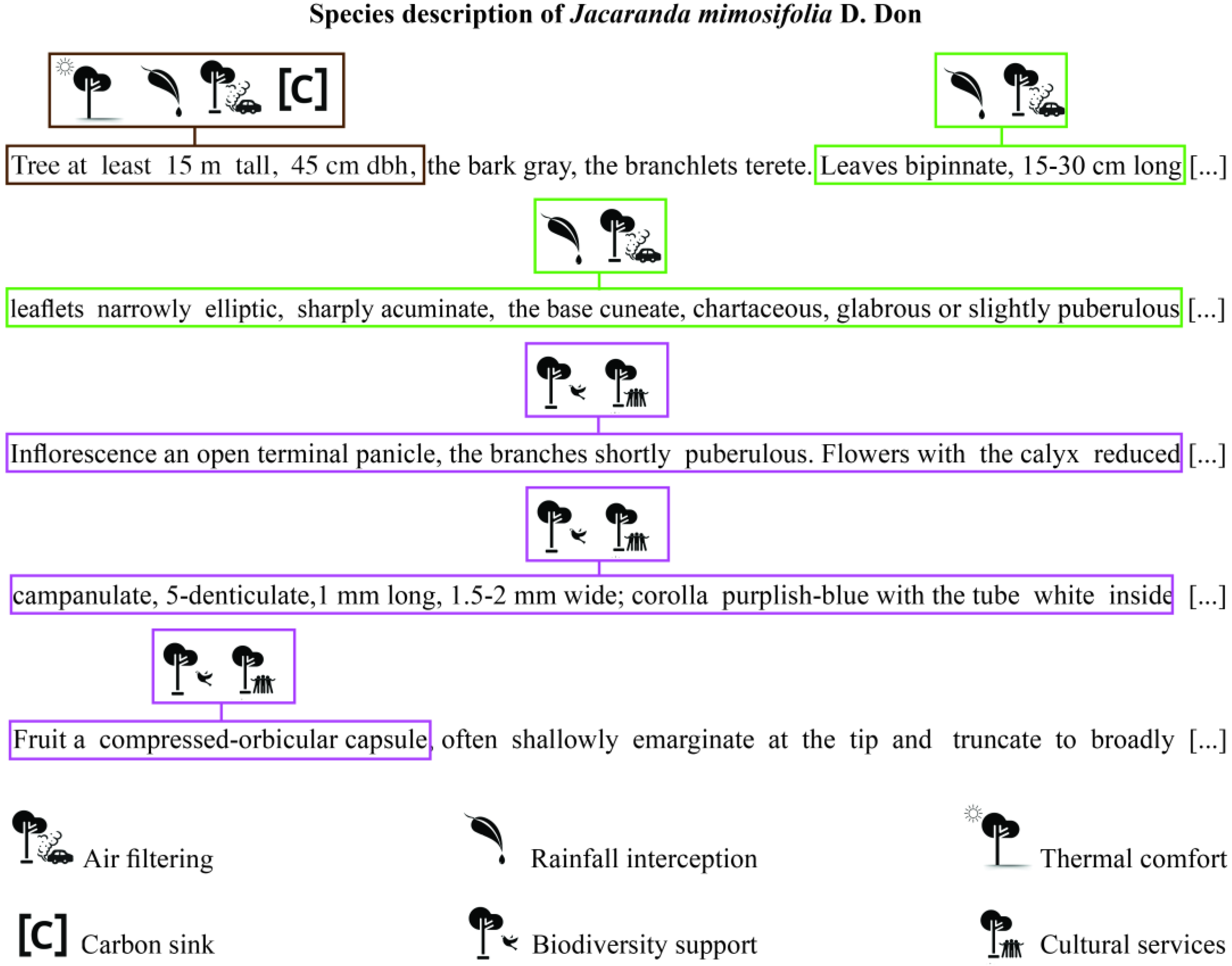
2. Hidden Treasures from Taxonomic Studies
2.1. Plant Life Form and Size
2.2. Leaf Phenology
2.3. Bark Morphology
2.4. Leaf Morphology
2.5. Reproductive Morphology and Phenology
3. Promising Ways to Use Taxonomic Datasets
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Nagar, S.; Anand, S. Climate Change and Existential Threats; Elsevier Inc.: Amsterdam, The Netherlands, 2021; ISBN 9780128229286. [Google Scholar] [CrossRef]
- Zuccaro, G.; Leone, M.F. Climate Services to Support Disaster Risk Reduction and Climate Change Adaptation in Urban Areas: The CLARITY Project and the Napoli Case Study. Front. Environ. Sci. 2021, 9, 693319. [Google Scholar] [CrossRef]
- Manoli, G.; Fatichi, S.; Schläpfer, M.; Yu, K.; Crowther, T.W.; Meili, N.; Burlando, P.; Katul, G.G.; Bou-Zeid, E. Magnitude of Urban Heat Islands Largely Explained by Climate and Population. Nature 2019, 573, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Marengo, J.A.; Alves, L.M.; Ambrizzi, T.; Young, A.; Barreto, N.J.C.; Ramos, A.M. Trends in Extreme Rainfall and Hydrogeometeorological Disasters in the Metropolitan Area of São Paulo: A Review. Ann. N. Y. Acad. Sci. 2020, 1472, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.; Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on Pollution and Health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef] [PubMed]
- United Stations. World Urbanization Prospects 2018; United Nations: New York, NY, USA, 2018; ISBN 9789211483185. [Google Scholar]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global Change and the Ecology of Cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef]
- Chang, S.E.; Gregorian, M.; Pathman, K.; Yumagulova, L.; Tse, W. Urban Growth and Long-Term Changes in Natural Hazard Risk. Environ. Plan. A 2012, 44, 989–1008. [Google Scholar] [CrossRef]
- Finewood, M.H. Green Infrastructure, Grey Epistemologies, and the Urban Political Ecology of Pittsburgh’s Water Governance. Antipode 2016, 48, 1000–1021. [Google Scholar] [CrossRef]
- Seddon, N.; Chausson, A.; Berry, P.; Girardin, C.A.J.; Smith, A.; Turner, B. Understanding the Value and Limits of Nature-Based Solutions to Climate Change and Other Global Challenges. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190120. [Google Scholar] [CrossRef]
- Locosselli, G.M.; Buckeridge, M.S. The Science of Urban Trees to Promote Well-Being. Trees Struct. Funct. 2023, 37, 1–7. [Google Scholar] [CrossRef]
- Babí Almenar, J.; Elliot, T.; Rugani, B.; Philippe, B.; Navarrete Gutierrez, T.; Sonnemann, G.; Geneletti, D. Nexus between Nature-Based Solutions, Ecosystem Services and Urban Challenges. Land Use Policy 2021, 100, 104898. [Google Scholar] [CrossRef]
- Raymond, C.M.; Frantzeskaki, N.; Kabisch, N.; Berry, P.; Breil, M.; Nita, M.R.; Geneletti, D.; Calfapietra, C. A Framework for Assessing and Implementing the Co-Benefits of Nature-Based Solutions in Urban Areas. Environ. Sci. Policy 2017, 77, 15–24. [Google Scholar] [CrossRef]
- Wild, T.; Bulkeley, H.; Naumann, S.; Vojinovic, Z.; Calfapietra, C.; Whiteoak, K. Nature-Based Solutions: State of the Art in EU-Funded Projects; Report; Publications Office of the European Union: Luxembourg, 2020; ISBN 978-92-76-17334-2. [Google Scholar] [CrossRef]
- de Bello, F.; Lavorel, S.; Díaz, S.; Harrington, R.; Cornelissen, J.H.C.; Bardgett, R.D.; Berg, M.P.; Cipriotti, P.; Feld, C.K.; Hering, D.; et al. Towards an Assessment of Multiple Ecosystem Processes and Services via Functional Traits. Biodivers. Conserv. 2010, 19, 2873–2893. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the Holy Grail: Using Plant Functional Traits to Understand Ecological Processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef]
- Brown, L.M.; Anand, M. Plant Functional Traits as Measures of Ecosystem Service Provision. Ecosphere 2022, 13, e3930. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Opportunities for Business and Industry; World Resources Institute: Washington, DC, USA, 2005; p. 31. [Google Scholar]
- Wallace, K.J. Classification of Ecosystem Services: Problems and Solutions. Biol. Conserv. 2007, 139, 235–246. [Google Scholar] [CrossRef]
- Salmond, J.A.; Tadaki, M.; Vardoulakis, S.; Arbuthnott, K.; Coutts, A.; Demuzere, M.; Dirks, K.N.; Heaviside, C.; Lim, S.; MacIntyre, H.; et al. Health and Climate Related Ecosystem Services Provided by Street Trees in the Urban Environment. Environ. Health 2016, 15, S36. [Google Scholar] [CrossRef]
- Hortal, J.; De Bello, F.; Diniz-Filho, J.A.F.; Lewinsohn, T.M.; Lobo, J.M.; Ladle, R.J. Seven Shortfalls That Beset Large-Scale Knowledge of Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 523–549. [Google Scholar] [CrossRef]
- Paquette, A.; Sousa-Silva, R.; Maure, F.; Cameron, E.; Belluau, M.; Messier, C. Praise for Diversity: A Functional Approach to Reduce Risks in Urban Forests. Urban For. Urban Green. 2021, 62, 127157. [Google Scholar] [CrossRef]
- Seddon, N.; Turner, B.; Berry, P.; Chausson, A.; Girardin, C.A.J. Grounding Nature-Based Climate Solutions in Sound Biodiversity Science. Nat. Clim. Chang. 2019, 9, 84–87. [Google Scholar] [CrossRef]
- IPPC Secretariat. Scientific Review of the Impact of Climate Change on Plant Pests; FAO on behalf of the IPPC Secretariat: Rome, Italy, 2021. [Google Scholar]
- Boyd, I.L.; Freer-Smith, P.H.; Gilligan, C.A.; Godfray, H.C.J. The Consequence of Tree Pests and Diseases for Ecosystem Services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef]
- Hantsch, L.; Bien, S.; Radatz, S.; Braun, U.; Auge, H.; Bruelheide, H. Tree Diversity and the Role of Non-Host Neighbour Tree Species in Reducing Fungal Pathogen Infestation. J. Ecol. 2014, 102, 1673–1687. [Google Scholar] [CrossRef] [PubMed]
- Prospero, S.; Cleary, M. Effects of Host Variability on the Spread of Invasive Forest Diseases. Forests 2017, 8, 80. [Google Scholar] [CrossRef]
- Roberts, M.; Gilligan, C.A.; Kleczkowski, A.; Hanley, N.; Whalley, A.E.; Healey, J.R. The Effect of Forest Management Options on Forest Resilience to Pathogens. Front. For. Glob. Chang. 2020, 3, 7. [Google Scholar] [CrossRef]
- Sjöman, H.; Östberg, J. Vulnerability of Ten Major Nordic Cities to Potential Tree Losses Caused by Longhorned Beetles. Urban Ecosyst. 2019, 22, 385–395. [Google Scholar] [CrossRef]
- Kirnbauer, M.C.; Baetz, B.W.; Kenney, W.A. Estimating the Stormwater Attenuation Benefits Derived from Planting Four Monoculture Species of Deciduous Trees on Vacant and Underutilized Urban Land Parcels. Urban For. Urban Green. 2013, 12, 401–407. [Google Scholar] [CrossRef]
- Hwang, W.H.; Wiseman, P.E.; Thomas, V.A. Enhancing the Energy Conservation Benefits of Shade Trees in Dense Residential Developments Using an Alternative Tree Placement Strategy. Landsc. Urban Plan. 2017, 158, 62–74. [Google Scholar] [CrossRef]
- Gatti, R.C.; Reich, P.B.; Gamarra, J.G.P.; Crowther, T.; Hui, C.; Morera, A.; Bastin, J.F.; de-Miguel, S.; Nabuurs, G.J.; Svenning, J.C.; et al. The Number of Tree Species on Earth. Proc. Natl. Acad. Sci. USA 2022, 119, e2115329119. [Google Scholar] [CrossRef]
- Pakeman, R.J.; Stockan, J. Using Plant Functional Traits as a Link between Land Use and Bee Foraging Abundance. Acta Oecol. 2013, 50, 32–39. [Google Scholar] [CrossRef]
- Biella, P.; Akter, A.; Ollerton, J.; Tarrant, S.; Janeček, Š.; Jersáková, J.; Klecka, J. Experimental Loss of Generalist Plants Reveals Alterations in Plant-Pollinator Interactions and a Constrained Flexibility of Foraging. Sci. Rep. 2019, 9, 7376. [Google Scholar] [CrossRef]
- He, C.; Qiu, K.; Pott, R. Reduction of Urban Traffic–Related Particulate Matter—Leaf Trait Matters. Environ. Sci. Pollut. Res. 2020, 27, 5825–5844. [Google Scholar] [CrossRef]
- Raven, P.H.; Gereau, R.E.; Phillipson, P.B.; Chatelain, C.; Jenkins, C.N.; Ulloa, C.U. The Distribution of Biodiversity Richness in the Tropics. Sci. Adv. 2020, 6, eabc6228. [Google Scholar] [CrossRef] [PubMed]
- Van Stan, J.T.; Underwood, S.J.; Friesen, J. Urban Forestry: An Underutilized Tool in Water Management. Adv. Chem. Pollut. Environ. Manag. Prot. 2018, 3, 35–61. [Google Scholar] [CrossRef]
- Zabret, K.; Šraj, M. Rainfall Interception by Urban Trees and Their Impact on Potential Surface Runoff. Clean 2019, 47, 1800327. [Google Scholar] [CrossRef]
- Davis, C.C. The Herbarium of the Future. Trends Ecol. Evol. 2023, 38, 412–423. [Google Scholar] [CrossRef]
- Hevia, V.; Martín-López, B.; Palomo, S.; García-Llorente, M.; de Bello, F.; González, J.A. Trait-Based Approaches to Analyze Links between the Drivers of Change and Ecosystem Services: Synthesizing Existing Evidence and Future Challenges. Ecol. Evol. 2017, 7, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Lavorel, S.; Díaz, S.; Cornelissen, J.H.C.; Garnier, E.; Harrison, S.P.; McIntyre, S.; Pausas, J.G.; Pérez-Harguindeguy, N.; Roumet, C.; Urcelay, C. Plant Functional Types: Are We Getting Any Closer to the Holy Grail? In Terrestrial Ecosystems in a Changing World; Springer: Berlin/Heidelberg, Germany, 2007; pp. 149–164. [Google Scholar] [CrossRef]
- Convention on Biological Diversity. Global Strategy for Plant Conservation: 2011–2020; Convention on Biological Diversity: Montreal, QC, Canada, 2012; ISBN 978-1-905164-41-7. [Google Scholar]
- Heberling, J.M.; Miller, J.T.; Noesgaard, D.; Weingart, S.B.; Schigel, D. Data Integration Enables Global Biodiversity Synthesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2018093118. [Google Scholar] [CrossRef]
- Joswig, J.S.; Kattge, J.; Kraemer, G.; Mahecha, M.D.; Rüger, N.; Schaepman, M.E.; Schrodt, F.; Schuman, M.C. Imputing Missing Data in Plant Traits: A Guide to Improve Gap-Filling. Glob. Ecol. Biogeogr. 2023, 32, 1395–1408. [Google Scholar] [CrossRef]
- Liang, D.; Huang, G. Influence of Urban Tree Traits on Their Ecosystem Services: A Literature Review. Land 2023, 12, 1699. [Google Scholar] [CrossRef]
- Borsch, T.; Berendsohn, W.; Dalcin, E.; Delmas, M.; Demissew, S.; Elliott, A.; Fritsch, P.; Fuchs, A.; Geltman, D.; Güner, A.; et al. World Flora Online: Placing Taxonomists at the Heart of a Definitive and Comprehensive Global Resource on the World’s Plants. Taxon 2020, 69, 1311–1341. [Google Scholar] [CrossRef]
- Paton, A.J.; Brummitt, N.; Govaerts, R.; Harman, K.; Allkin, B.; Lughadha, E.N.; Taxon, S.; May, N.; Wiley, P.; Paton, A.J.; et al. Towards Target 1 of the Global Strategy for Plant Conservation: A Working List of All Known Plant Species—Progress and Prospects. Taxon 2008, 57, 602–611. [Google Scholar]
- IBGE. Censo Brasileiro de 2022; IBGE: Belém, Brazil, 2022. Available online: https://censo2022.ibge.gov.br/panorama/?utm_source=ibge&utm_medium=home&utm_campaign=portal (accessed on 18 September 2024).
- Park, D.S.; Lyra, G.M.; Ellison, A.M.; Maruyama, R.K.B.; dos Reis Torquato, D.; Asprino, R.C.; Cook, B.I.; Davis, C.C. Herbarium Records Provide Reliable Phenology Estimates in the Understudied Tropics. J. Ecol. 2023, 111, 327–337. [Google Scholar] [CrossRef]
- Park, D.S.; Xie, Y.; Ellison, A.M.; Lyra, G.M.; Davis, C.C. Complex Climate-Mediated Effects of Urbanization on Plant Reproductive Phenology and Frost Risk. New Phytol. 2023, 239, 2153–2165. [Google Scholar] [CrossRef]
- Koski, M.H.; MacQueen, D.; Ashman, T.L. Floral Pigmentation Has Responded Rapidly to Global Change in Ozone and Temperature. Curr. Biol. 2020, 30, 4425–4431.e3. [Google Scholar] [CrossRef]
- Deletic, A.; Fletcher, T.D. Performance of Grass Filters Used for Stormwater Treatment—A Field and Modelling Study. J. Hydrol. 2006, 317, 261–275. [Google Scholar] [CrossRef]
- Ryan, C.M.; Pritchard, R.; McNicol, I.; Owen, M.; Fisher, J.A.; Lehmann, C. Ecosystem Services from Southern African Woodlands and Their Future under Global Change. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150312. [Google Scholar] [CrossRef]
- McPherson, E.G.; Nowak, D.J.; Rowntree, R.A. Chicago’s Urban Forest Ecosystem: Results of the Chicago Urban Forest Climate Project; Gen. Tech. Rep. NE-186; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1994; p. 201. [Google Scholar]
- Sydnor, T.D.; Subburayalu, S.K. Should We Consider Expected Environmental Benefits When Planting Larger or Smaller Tree Species? Arboric. Urban For. 2011, 37, 167–172. [Google Scholar] [CrossRef]
- Mullaney, J.; Lucke, T.; Trueman, S.J. A Review of Benefits and Challenges in Growing Street Trees in Paved Urban Environments. Landsc. Urban Plan. 2015, 134, 157–166. [Google Scholar] [CrossRef]
- Gage, E.A.; Cooper, D.J. Relationships between Landscape Pattern Metrics, Vertical Structure and Surface Urban Heat Island Formation in a Colorado Suburb. Urban Ecosyst. 2017, 20, 1229–1238. [Google Scholar] [CrossRef]
- Vaz Monteiro, M.; Doick, K.J.; Handley, P.; Peace, A. The Impact of Greenspace Size on the Extent of Local Nocturnal Air Temperature Cooling in London. Urban For. Urban Green. 2016, 16, 160–169. [Google Scholar] [CrossRef]
- Lin, T.-P.; Matzarakis, A.; Hwang, R.-L. Shading Effect on Long-Term Outdoor Thermal Comfort. Build. Environ. 2010, 45, 213–221. [Google Scholar] [CrossRef]
- Speak, A.; Montagnani, L.; Wellstein, C.; Zerbe, S. The Influence of Tree Traits on Urban Ground Surface Shade Cooling. Landsc. Urban Plan. 2020, 197, 103748. [Google Scholar] [CrossRef]
- Nur Hannah Ismail, S.; Stovin, V.; Cameron, R.W.F. Functional Urban Ground-Cover Plants: Identifying Traits That Promote Rainwater Retention and Dissipation. Urban Ecosyst. 2023, 26, 1709–1724. [Google Scholar] [CrossRef]
- Davis, A.P.; Stagge, J.H.; Jamil, E.; Kim, H. Hydraulic Performance of Grass Swales for Managing Highway Runoff. Water Res. 2012, 46, 6775–6786. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.L.; Formiga, K.T.M.; Traldi, M.A.B. Rainfall Interception Capacity of Tree Species Used in Urban Afforestation. Urban Ecosyst. 2018, 21, 697–706. [Google Scholar] [CrossRef]
- Escobedo, F.J.; Wagner, J.E.; Nowak, D.J.; De la Maza, C.L.; Rodriguez, M.; Crane, D.E. Analyzing the Cost Effectiveness of Santiago, Chile’s Policy of Using Urban Forests to Improve Air Quality. J. Environ. Manag. 2008, 86, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Redondo Bermúdez, M.d.C.; Kanai, J.M.; Astbury, J.; Fabio, V.; Jorgensen, A. Green Fences for Buenos Aires: Implementing Green Infrastructure for (More than) Air Quality. Sustainability 2022, 14, 4129. [Google Scholar] [CrossRef]
- Kanai, J.M.; Fabio, V.; Mirás, M.; Gastiarena, L. Making Green Heritage Schools Work: Nature-Based Solutions and Historical Preservation When Infrastructure Fails. Sustainability 2024, 16, 6981. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human Health Effects of Air Pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Locosselli, G.M. Soluções Baseadas Na Natureza Para Redução Da Poluição Do Ar Na Cidade. In Planejamento Urbano e Políticas Ambientais: Métodos, Instrumentos e Experiências; Gunter, W.M.R., Philipp, A., Jr., Eds.; Universidade de São Paulo: São Paulo, Brazil, 2020; pp. 86–106. [Google Scholar] [CrossRef]
- Churkina, G. The Role of Urbanization in the Global Carbon Cycle. Front. Ecol. Evol. 2016, 3, 144. [Google Scholar] [CrossRef]
- Nowak, D.J.; Greenfield, E.J.; Hoehn, R.E.; Lapoint, E. Carbon Storage and Sequestration by Trees in Urban and Community Areas of the United States. Environ. Pollut. 2013, 178, 229–236. [Google Scholar] [CrossRef]
- Hardiman, B.S.; Wang, J.A.; Hutyra, L.R.; Gately, C.K.; Getson, J.M.; Friedl, M.A. Accounting for Urban Biogenic Fluxes in Regional Carbon Budgets. Sci. Total Environ. 2017, 592, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Tigges, J.; Churkina, G.; Lakes, T. Modeling Above-Ground Carbon Storage: A Remote Sensing Approach to Derive Individual Tree Species Information in Urban Settings. Urban Ecosyst. 2017, 20, 97–111. [Google Scholar] [CrossRef]
- Brilli, L.; Carotenuto, F.; Chiesi, M.; Fiorillo, E.; Genesio, L.; Magno, R.; Morabito, M.; Nardino, M.; Zaldei, A.; Gioli, B. An Integrated Approach to Estimate How Much Urban Afforestation Can Contribute to Move towards Carbon Neutrality. Sci. Total Environ. 2022, 842, 156843. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Ren, Z.; Guo, Y.; Wang, C.; Cao, F.; Zhang, P.; Hong, S.; Ma, Z. Spatiotemporal Changes in Urban Forest Carbon Sequestration Capacity and Its Potential Drivers in an Urban Agglomeration: Implications for Urban CO2 Emission Mitigation under China’s Rapid Urbanization. Ecol. Indic. 2024, 159, 111601. [Google Scholar] [CrossRef]
- Pregitzer, C.C.; Hanna, C.; Charlop-Powers, S.; Bradford, M.A. Estimating Carbon Storage in Urban Forests of New York City. Urban Ecosyst. 2022, 25, 617–631. [Google Scholar] [CrossRef]
- Zhao, D.; Cai, J.; Xu, Y.; Liu, Y.; Yao, M. Carbon Sinks in Urban Public Green Spaces under Carbon Neutrality: A Bibliometric Analysis and Systematic Literature Review. Urban For. Urban Green. 2023, 86, 128037. [Google Scholar] [CrossRef]
- Rawat, M.; Arunachalam, K.; Arunachalam, A.; Alatalo, J.; Pandey, R. Associations of Plant Functional Diversity with Carbon Accumulation in a Temperate Forest Ecosystem in the Indian Himalayas. Ecol. Indic. 2019, 98, 861–868. [Google Scholar] [CrossRef]
- Finegan, B.; Peña-Claros, M.; de Oliveira, A.; Ascarrunz, N.; Bret-Harte, M.S.; Carreño-Rocabado, G.; Casanoves, F.; Díaz, S.; Eguiguren Velepucha, P.; Fernandez, F.; et al. Does Functional Trait Diversity Predict Above-Ground Biomass and Productivity of Tropical Forests? Testing Three Alternative Hypotheses. J. Ecol. 2015, 103, 191–201. [Google Scholar] [CrossRef]
- Ali, A.; Yan, E.-R. Functional Identity of Overstorey Tree Height and Understorey Conservative Traits Drive Aboveground Biomass in a Subtropical Forest. Ecol. Indic. 2017, 83, 158–168. [Google Scholar] [CrossRef]
- Ayma-Romay, A.I.; Bown, H.E.; Pérez-Harguindeguy, N.; Enrico, L. Trait Similarity among Dominant Highly-Competitive Species Rather than Diversity Increases Productivity in Semi-Arid Mediterranean Forests. For. Ecol. Manag. 2021, 486, 118969. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, C.; Zhang, X.; Chen, B.; Wu, J.; Tu, Y.; Miao, Y.; Luo, L. A Modified Framework for the Regional Assessment of Climate and Human Impacts on Net Primary Productivity. Ecol. Indic. 2016, 60, 184–191. [Google Scholar] [CrossRef]
- Wilkes, P.; Disney, M.; Vicari, M.B.; Calders, K.; Burt, A. Estimating Urban above Ground Biomass with Multi-Scale LiDAR. Carbon Balance Manag. 2018, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, C.E.; Stefanello, M.; dos Reis, N.C.S.; Facco, D.S.; Teleginski Ferraz, S.; Boiaski, N.T.; Herdies, D.L.; Degrazia, G.A. Winter Heat Waves Characteristics Associated with Downslope Windstorm in South Brazil. Int. J. Climatol. 2023, 43, 5951–5965. [Google Scholar] [CrossRef]
- da Silva Brito, N.D.; dos Santos Medeiros, M.J.; de Souza, E.S.; de Lima, A.L.A. Drought Response Strategies for Deciduous Species in the Semiarid Caatinga Derived from the Interdependence of Anatomical, Phenological and Bio-Hydraulic Attributes. Flora 2022, 288, 152009. [Google Scholar] [CrossRef]
- Livesley, S.J.; Baudinette, B.; Glover, D. Rainfall Interception and Stem Flow by Eucalypt Street Trees—The Impacts of Canopy Density and Bark Type. Urban For. Urban Green. 2014, 13, 192–197. [Google Scholar] [CrossRef]
- Van Stan, J.T.; Dymond, S.F.; Klamerus-Iwan, A. Bark-Water Interactions Across Ecosystem States and Fluxes. Front. For. Glob. Chang. 2021, 4, 660662. [Google Scholar] [CrossRef]
- Schelle, E.; Rawlins, B.G.; Lark, R.M.; Webster, R.; Staton, I.; McLeod, C.W. Mapping Aerial Metal Deposition in Metropolitan Areas from Tree Bark: A Case Study in Sheffield, England. Environ. Pollut. 2008, 155, 164–173. [Google Scholar] [CrossRef]
- Caldana, C.R.G.; Hanai-Yoshida, V.M.; Paulino, T.H.; Baldo, D.A.; Freitas, N.P.; Aranha, N.; Vila, M.M.D.C.; Balcão, V.M.; Oliveira Junior, J.M. Evaluation of Urban Tree Barks as Bioindicators of Environmental Pollution Using the X-Ray Fluorescence Technique. Chemosphere 2023, 312, 137257. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qin, M.; Xu, P.; Huang, D.; Jin, X.; Chen, J.; Dong, D.; Ren, Y. Atmospheric Particulate Matter Retention Capacity of Bark and Leaves of Urban Tree Species. Environ. Pollut. 2024, 342, 123109. [Google Scholar] [CrossRef]
- Baklanov, A.; Molina, L.T.; Gauss, M. Megacities, Air Quality and Climate. Atmos. Environ. 2016, 126, 235–249. [Google Scholar] [CrossRef]
- Ponette-González, A.G. Accumulator, Transporter, Substrate, and Reactor: Multidimensional Perspectives and Approaches to the Study of Bark. Front. For. Glob. Chang. 2021, 4, 716557. [Google Scholar] [CrossRef]
- Moreira, T.C.L.; Amato-Lourenço, L.F.; da Silva, G.T.; de André, C.D.; de André, P.A.; Barrozo, L.V.; Singer, J.M.; Saldiva, P.H.N.; Saiki, M.; Locosselli, G.M. The Use of Tree Barks to Monitor Traffic Related Air Pollution: A Case Study in São Paulo–Brazil. Front. Environ. Sci. 2018, 6, 72. [Google Scholar] [CrossRef]
- Zhang, X.; Lyu, J.; Chen, W.Y.; Chen, D.; Yan, J.; Yin, S. Quantifying the Capacity of Tree Branches for Retaining Airborne Submicron Particles. Environ. Pollut. 2022, 310, 119873. [Google Scholar] [CrossRef]
- Zhao, L.; Lee, X.; Smith, R.B.; Oleson, K. Strong Contributions of Local Background Climate to Urban Heat Islands. Nature 2014, 511, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Bickford, C.P. Ecophysiology of Leaf Trichomes. Funct. Plant Biol. 2016, 43, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Hami, A.; Abdi, B.; Zarehaghi, D.; Maulan, S. Bin Assessing the Thermal Comfort Effects of Green Spaces: A Systematic Review of Methods, Parameters, and Plants’ Attributes. Sustain. Cities Soc. 2019, 49, 101634. [Google Scholar] [CrossRef]
- Stav, Y.; Lawson, G.M. Vertical Vegetation Design Decisions and Their Impact on Energy Consumption in Subtropical Cities. In The Sustainable City VII: Urban Regeneration and Sustainability; Passerini, G., Brebbia, C.A., Latini, G., Eds.; WIT Press: Ancona, Italy, 2012; pp. 489–500. [Google Scholar]
- Livesley, S.J.; McPherson, E.G.; Calfapietra, C. The Urban Forest and Ecosystem Services: Impacts on Urban Water, Heat, and Pollution Cycles at the Tree, Street, and City Scale. J. Environ. Qual. 2016, 45, 119–124. [Google Scholar] [CrossRef]
- Baptista, M.D.; Livesley, S.J.; Parmehr, E.G.; Neave, M.; Amati, M. Variation in Leaf Area Density Drives the Rainfall Storage Capacity of Individual Urban Tree Species. Hydrol. Process. 2018, 32, 3729–3740. [Google Scholar] [CrossRef]
- Holder, C.D.; Gibbes, C. Influence of Leaf and Canopy Characteristics on Rainfall Interception and Urban Hydrology. Hydrol. Sci. J. 2017, 62, 182–190. [Google Scholar] [CrossRef]
- Yan, T.; Wang, Z.; Liao, C.; Xu, W.; Wan, L. Effects of the Morphological Characteristics of Plants on Rainfall Interception and Kinetic Energy. J. Hydrol. 2021, 592, 125807. [Google Scholar] [CrossRef]
- Yang, J.; Chang, Y.; Yan, P. Ranking the Suitability of Common Urban Tree Species for Controlling PM2.5 Pollution. Atmos. Pollut. Res. 2015, 6, 267–277. [Google Scholar] [CrossRef]
- Hallam, N.D. Growth and Regeneration of Waxes on the Leaves of Eucalyptus. Planta 1970, 93, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Weerakkody, U.; Dover, J.W.; Mitchell, P.; Reiling, K. Evaluating the Impact of Individual Leaf Traits on Atmospheric Particulate Matter Accumulation Using Natural and Synthetic Leaves. Urban For. Urban Green. 2018, 30, 98–107. [Google Scholar] [CrossRef]
- Barwise, Y.; Kumar, P. Designing Vegetation Barriers for Urban Air Pollution Abatement: A Practical Review for Appropriate Plant Species Selection. NPJ Clim. Atmos. Sci. 2020, 3, 12. [Google Scholar] [CrossRef]
- Moura, B.B.; Zammarchi, F.; Manzini, J.; Yasutomo, H.; Brilli, L.; Vagnoli, C.; Gioli, B.; Zaldei, A.; Giordano, T.; Martinelli, F.; et al. Assessment of Seasonal Variations in Particulate Matter Accumulation and Elemental Composition in Urban Tree Species. Environ. Res. 2024, 252, 118782. [Google Scholar] [CrossRef] [PubMed]
- Viecco, M.; Vera, S.; Jorquera, H.; Bustamante, W.; Gironás, J.; Dobbs, C.; Leiva, E. Potential of Particle Matter Dry Deposition on Green Roofs and Living Walls Vegetation for Mitigating Urban Atmospheric Pollution in Semiarid Climates. Sustainability 2018, 10, 2431. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, B.; Niu, X. Relationship between Leaf Surface Characteristics and Particle Capturing Capacities of Different Tree Species in Beijing. Forests 2017, 8, 92. [Google Scholar] [CrossRef]
- Chen, L.; Liu, C.; Zhang, L.; Zou, R.; Zhang, Z. Variation in Tree Species Ability to Capture and Retain Airborne Fine Particulate Matter (PM2.5). Sci. Rep. 2017, 7, 3206. [Google Scholar] [CrossRef]
- Weber, F.; Kowarik, I.; Säumel, I. Herbaceous Plants as Filters: Immobilization of Particulates along Urban Street Corridors. Environ. Pollut. 2014, 186, 234–240. [Google Scholar] [CrossRef]
- Corada, K.; Woodward, H.; Alaraj, H.; Collins, C.M.; de Nazelle, A. A Systematic Review of the Leaf Traits Considered to Contribute to Removal of Airborne Particulate Matter Pollution in Urban Areas. Environ. Pollut. 2021, 269, 116104. [Google Scholar] [CrossRef]
- Charoenkit, S.; Yiemwattana, S. Living Walls and Their Contribution to Improved Thermal Comfort and Carbon Emission Reduction: A Review. Build. Environ. 2016, 105, 82–94. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, F. Leaf Traits Are Good Predictors of Plant Performance across 53 Rain Forest Species. Ecology 2006, 87, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Alegre, L.; Tapia, L.; Calafell, R.; Serret, M.D. Relationships between photosynthetic capacity and leaf structure in several shade plants. Am. J. Bot. 1986, 73, 1760–1770. [Google Scholar] [CrossRef]
- Soliveres, S.; van der Plas, F.; Manning, P.; Prati, D.; Gossner, M.M.; Renner, S.C.; Alt, F.; Arndt, H.; Baumgartner, V.; Binkenstein, J.; et al. Biodiversity at Multiple Trophic Levels Is Needed for Ecosystem Multifunctionality. Nature 2016, 536, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Youngsteadt, E.; Keighron, M.C. Urban Pollination Ecology. Annu. Rev. Ecol. Evol. Syst. 2023, 54, 21–42. [Google Scholar] [CrossRef]
- Dietzel, S.; Rojas-Botero, S.; Kollmann, J.; Fischer, C. Enhanced Urban Roadside Vegetation Increases Pollinator Abundance Whereas Landscape Characteristics Drive Pollination. Ecol. Indic. 2023, 147, 109980. [Google Scholar] [CrossRef]
- Wang, H.; Ran, N.; Jiang, H.-Q.; Wang, Q.-Q.; Ye, M.; Bowler, P.A.; Jin, X.-F.; Ye, Z.-M. Complex Floral Traits Shape Pollinator Attraction to Flowering Plants in Urban Greenspaces. Urban For. Urban Green. 2024, 91, 128165. [Google Scholar] [CrossRef]
- Cabon, V.; Kracht, A.; Seitz, B.; Kowarik, I.; von der Lippe, M.; Buchholz, S. Urbanisation Modulates the Attractiveness of Plant Communities to Pollinators by Filtering for Floral Traits. Oikos 2022, 2022, e09071. [Google Scholar] [CrossRef]
- Blowers, C.J.; Cunningham, H.M.; Wilcox, A.; Randall, N.P. What Specific Plant Traits Support Ecosystem Services Such as Pollination, Bio-Control and Water Quality Protection in Temperate Climates? A Systematic Map Protocol. Environ. Evid. 2017, 6, 3. [Google Scholar] [CrossRef]
- Sikora, A.; Michołap, P.; Sikora, M. What Kind of Flowering Plants Are Attractive for Bumblebees in Urban Green Areas? Urban For. Urban Green. 2020, 48, 126546. [Google Scholar] [CrossRef]
- Menzel, R. Untersuchungen Zum Erlernen von Spektralfarben Durch Die Honigbiene (Apis Mellifica). Z. Vgl. Physiol. 1967, 56, 22–62. [Google Scholar] [CrossRef]
- Giurfa, M.; Núñez, J.; Chittka, L.; Menzel, R. Colour Preferences of Flower-Naive Honeybees. J. Comp. Physiol. A 1995, 177, 247–259. [Google Scholar] [CrossRef]
- Sardeshpande, M.; Shackleton, C. Fruits of the City: The Nature, Nurture and Future of Urban Foraging. People Nat. 2023, 5, 213–227. [Google Scholar] [CrossRef]
- Koike, S.; Masaki, T. Characteristics of Fruits Consumed by Mammalian Frugivores in Japanese Temperate Forest. Ecol. Res. 2019, 34, 246–254. [Google Scholar] [CrossRef]
- Gobster, P.H.; Nassauer, J.I.; Daniel, T.C.; Fry, G. The Shared Landscape: What Does Aesthetics Have to Do with Ecology? Landsc. Ecol. 2007, 22, 959–972. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, T.; Liu, F.; Zhang, Y.; Song, Y. How Does Urban Green Space Impact Residents’ Mental Health: A Literature Review of Mediators. Int. J. Environ. Res. Public Health 2021, 18, 11746. [Google Scholar] [CrossRef]
- Hůla, M.; Flegr, J. Habitat Selection and Human Aesthetic Responses to Flowers. Evol. Hum. Sci. 2021, 3, e5. [Google Scholar] [CrossRef]
- Kaplan, S. The Restorative Benefits of Nature: Toward an Integrative Framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Neale, C.; Griffiths, A.; Chalmin-Pui, L.S.; Mendu, S.; Boukhechba, M.; Roe, J. Color Aesthetics: A Transatlantic Comparison of Psychological and Physiological Impacts of Warm and Cool Colors in Garden Landscapes. Wellbeing Space Soc. 2021, 2, 100038. [Google Scholar] [CrossRef]
- Quan, Q.; He, N.; Zhang, R.; Wang, J.; Luo, Y.; Ma, F.; Pan, J.; Wang, R.; Liu, C.; Zhang, J.; et al. Plant Height as an Indicator for Alpine Carbon Sequestration and Ecosystem Response to Warming. Nat. Plants 2024, 10, 890–900. [Google Scholar] [CrossRef]
- Zhang, L.; Dempsey, N.; Cameron, R. Flowers—Sunshine for the Soul! How Does Floral Colour Influence Preference, Feelings of Relaxation and Positive up-Lift? Urban For. Urban Green. 2023, 79, 127795. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, C.; Han, Q.; Lu, C. Structure and Characteristics of Plant-Frugivore Network in an Urban Park: A Case Study in Nanjing Botanical Garden Mem. Sun Yat-Sen. Diversity 2022, 14, 71. [Google Scholar] [CrossRef]
- Petter, J.; Ries, P.; D’Antonio, A.; Contreras, R. A Tree Selection Survey of Tree City USA Designated Cities in the Pacific Northwest. Arboric. Urban For. (AUF) 2020, 46, 371–384. [Google Scholar] [CrossRef]
- Sjöman, H.; Watkins, J.H.R. What Do We Know about the Origin of Our Urban Trees?—A North European Perspective. Urban For. Urban Green. 2020, 56, 126879. [Google Scholar] [CrossRef]
- Silva-Luz, C.L.; Pirani, J.R.; Pell, S.K.; Mitchell, J.D. Anacardiaceae. Available online: https://floradobrasil.jbrj.gov.br/FB4380 (accessed on 4 September 2024).
- Radford, A.E. Vascular Plant Systematics; Harper & Row: New York, NY, USA, 1974.
- Evert RF, E.S. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Bell, A.D. Plant Form: An Illustrated Guide to Flowering Plant Morphology; Oxford University Press: New York, NY, USA, 1990. [Google Scholar]
- López-Martínez, A.M.; Magallón, S.; von Balthazar, M.; Schönenberger, J.; Sauquet, H.; Chartier, M. Angiosperm Flowers Reached Their Highest Morphological Diversity Early in Their Evolutionary History. New Phytol. 2024, 241, 1348–1360. [Google Scholar] [CrossRef]
- Dmitruk, M.; Strzałkowska-Abramek, M.; Bożek, M.; Denisow, B. Plants Enhancing Urban Pollinators: Nectar Rather than Pollen Attracts Pollinators of Cotoneaster Species. Urban For Urban Green 2022, 74, 127651. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Cheung, K.K.S.; Chu, L.M. Thermal Performance of a Vegetated Cladding System on Facade Walls. Build Environ 2010, 45, 1779–1787. [Google Scholar] [CrossRef]
- Feng, H.; An, J.; Wang, H.; Miao, X.; Yang, G.; Feng, H.; Wu, Y.; Ma, X. The Ecological Healthcare Benefits and Influences of Plant Communities in Urban Wetland Parks. Forests 2023, 14, 2257. [Google Scholar] [CrossRef]
- Wang, H.; Shi, H. Particle Retention Capacity, Efficiency, and Mechanism of Selected Plant Species: Implications for Urban Planting for Improving Urban Air Quality. Plants 2021, 10, 2109. [Google Scholar] [CrossRef]
- Ma, X.Y.; Shu, Y.; Shi, Y.; Bao, Z.Y. Analysis of plant selection and landscaping design for rain garden: A case study of Gongkang Rainwater Garden in Shanghai. Acta Agric. Zhejiangensis 2018, 30, 1526–1533. [Google Scholar]
- Yang, B.; Lee, D.K.; Heo, H.K.; Biging, G. The Effects of Tree Characteristics on Rainfall Interception in Urban Areas. Landsc. Ecol. Eng. 2019, 15, 289–296. [Google Scholar] [CrossRef]
- Selmi, W.; Weber, C.; Rivière, E.; Blond, N.; Mehdi, L.; Nowak, D. Air Pollution Removal by Trees in Public Green Spaces in Strasbourg City, France. Urban For Urban Green 2016, 17, 192–201. [Google Scholar] [CrossRef]
- Chaparro, L.; Terradas, J. Ecological Services of Urban Forest in Barcelona; CREAF: Bellaterra, Spain, 2009. [Google Scholar] [CrossRef]
- Kong, L.; Lau, K.K.L.; Yuan, C.; Chen, Y.; Xu, Y.; Ren, C.; Ng, E. Regulation of Outdoor Thermal Comfort by Trees in Hong Kong. Sustain Cities Soc 2017, 31, 12–25. [Google Scholar] [CrossRef]
- Nooraei Beidokhti, A.; Moore, T.L. The Effects of Precipitation, Tree Phenology, Leaf Area Index, and Bark Characteristics on Throughfall Rates by Urban Trees: A Meta-Data Analysis. Urban For Urban Green 2021, 60, 127052. [Google Scholar] [CrossRef]
- Barwise, Y.; Kumar, P.; Abhijith, K.V.; Gallagher, J.; McNabola, A.; Watts, J.F. A Trait-Based Investigation into Evergreen Woody Plants for Traffic-Related Air Pollution Mitigation over Time. Sci. Total Environ. 2024, 914, 169713. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; McPherson, G.G. Rainfall Interception of Three Trees in Oakland, California. Urban Ecosyst. 2011, 14, 755–769. [Google Scholar] [CrossRef]

| Thermal Comfort | Rainfall Interception | Air-Filtering | CO2 Sequestration | Biodiversity Support | Cultural Services | |
|---|---|---|---|---|---|---|
| Life form and size | All life forms: ↑ albedo, evaporative cooling, convective turbulence. Trees: ↑ shading | All life forms: ↓ kinetic energy. Trees and shrubs: ↑ throughfall and stemflow | All life forms: ↑ passive deposition on leaves. Trees and shrubs: ↑ deposition on bark. | All life forms: ↑ carbon sink. Trees: ↑ carbon residence time | All life forms | All life forms |
| Leaf phenology | Evergreen: ↑ provision year-round. Deciduous: ↑ resilience. | Evergreen: ↑ provision year-round. Deciduous: ↑ resilience. | Evergreen: ↑ provision year-round. Deciduous: ↑ resilience. | Evergreen: ↑ provision year-round. Deciduous: ↑ resilience. | ||
| Bark morphology | Rough: ↓ stemflow Lenticels: ↓ stemflow | Rough: ↑ deposition of PM Lenticels: ↑ deposition of PM | ||||
| Leaf morphology | Thicker: ↓ transmittance. Light colored: ↑ albedo. | Rough: ↑ water storage, ↓ throughfall. Compound: ↑ water storage, ↓ throughfall. Small leaves and leaflets: ↑ water storage, ↓ throughfall. Trichomes: ↑ water storage, ↓ throughfall. | Rough: ↑ deposition of PM Epicuticular wax: ↑ deposition of PM Trichomes: ↑ deposition of PM | Thicker: ↑ assimilation. Leaf area: ↑ assimilation. | ||
| Reproductive morphology and phenology | Flower: ↑ species reproduction. Fruit: ↑ food provision. Inflorescence: ↑ different pollinators. | Flower: ↑ aesthetics and emotional connection. Fruit: ↑ food provision. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva Luz, C.L.d.; Reale, R.; Candido, L.F.; Zappi, D.; Locosselli, G.M. Using Morphological Characters to Support Decision-Making in Nature-Based Solutions: A Shortcut to Promote Urban Plant Biodiversity. Urban Sci. 2024, 8, 233. https://doi.org/10.3390/urbansci8040233
Silva Luz CLd, Reale R, Candido LF, Zappi D, Locosselli GM. Using Morphological Characters to Support Decision-Making in Nature-Based Solutions: A Shortcut to Promote Urban Plant Biodiversity. Urban Science. 2024; 8(4):233. https://doi.org/10.3390/urbansci8040233
Chicago/Turabian StyleSilva Luz, Cíntia Luiza da, Ricardo Reale, Leticia Figueiredo Candido, Daniela Zappi, and Giuliano Maselli Locosselli. 2024. "Using Morphological Characters to Support Decision-Making in Nature-Based Solutions: A Shortcut to Promote Urban Plant Biodiversity" Urban Science 8, no. 4: 233. https://doi.org/10.3390/urbansci8040233
APA StyleSilva Luz, C. L. d., Reale, R., Candido, L. F., Zappi, D., & Locosselli, G. M. (2024). Using Morphological Characters to Support Decision-Making in Nature-Based Solutions: A Shortcut to Promote Urban Plant Biodiversity. Urban Science, 8(4), 233. https://doi.org/10.3390/urbansci8040233








