Soil–Plant Indicators for Assessing Nutrient Cycling and Ecosystem Functionality in Urban Forestry
Abstract
1. Introduction
1.1. Urban Environment Degradation
1.2. Nature-Based Solutions
1.3. Soil–Plant Health Indicators
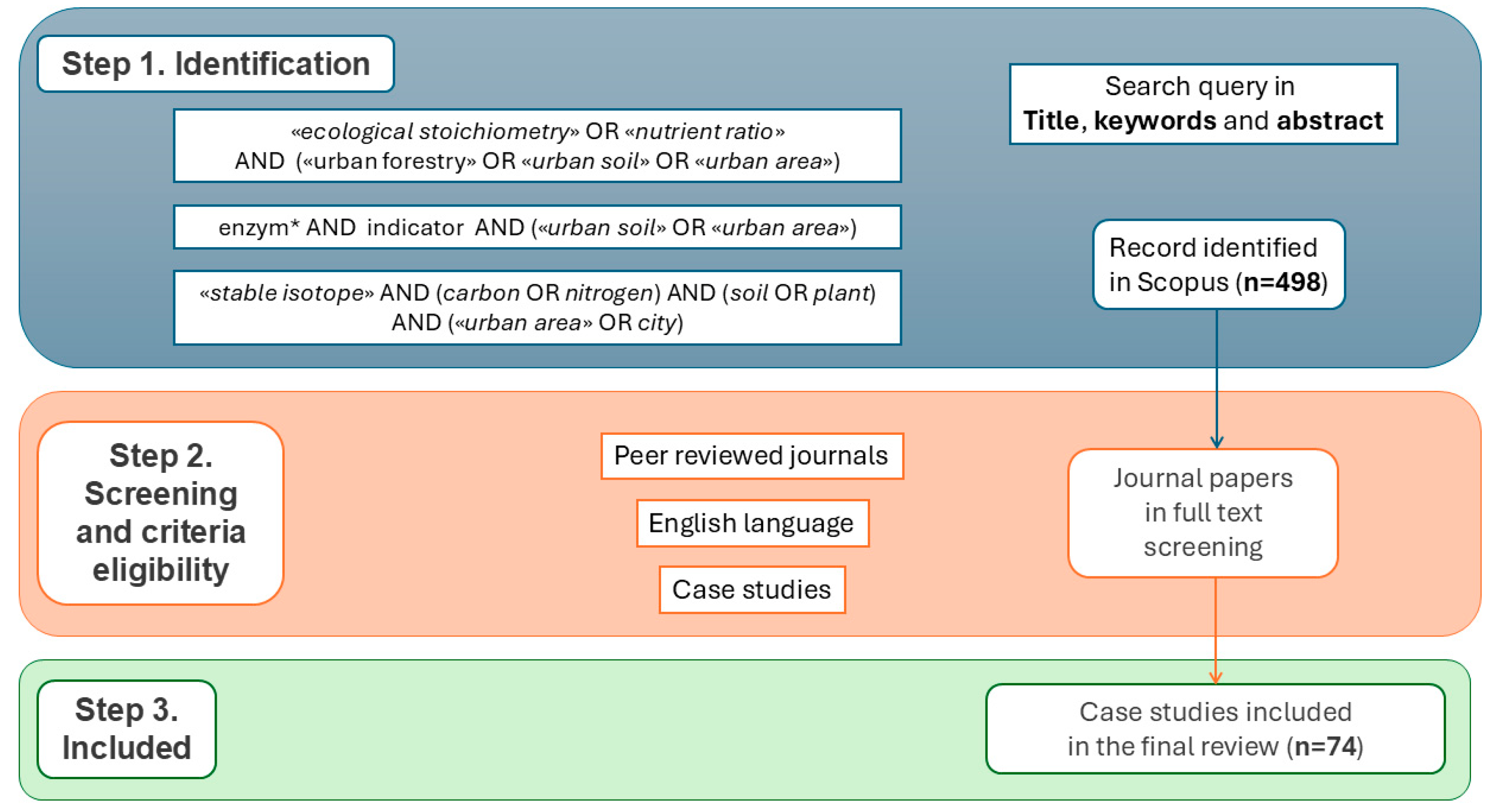
2. Materials and Methods
3. Suitable Indicators of Soil–Plant System Health
3.1. Ecological Stoichiometry
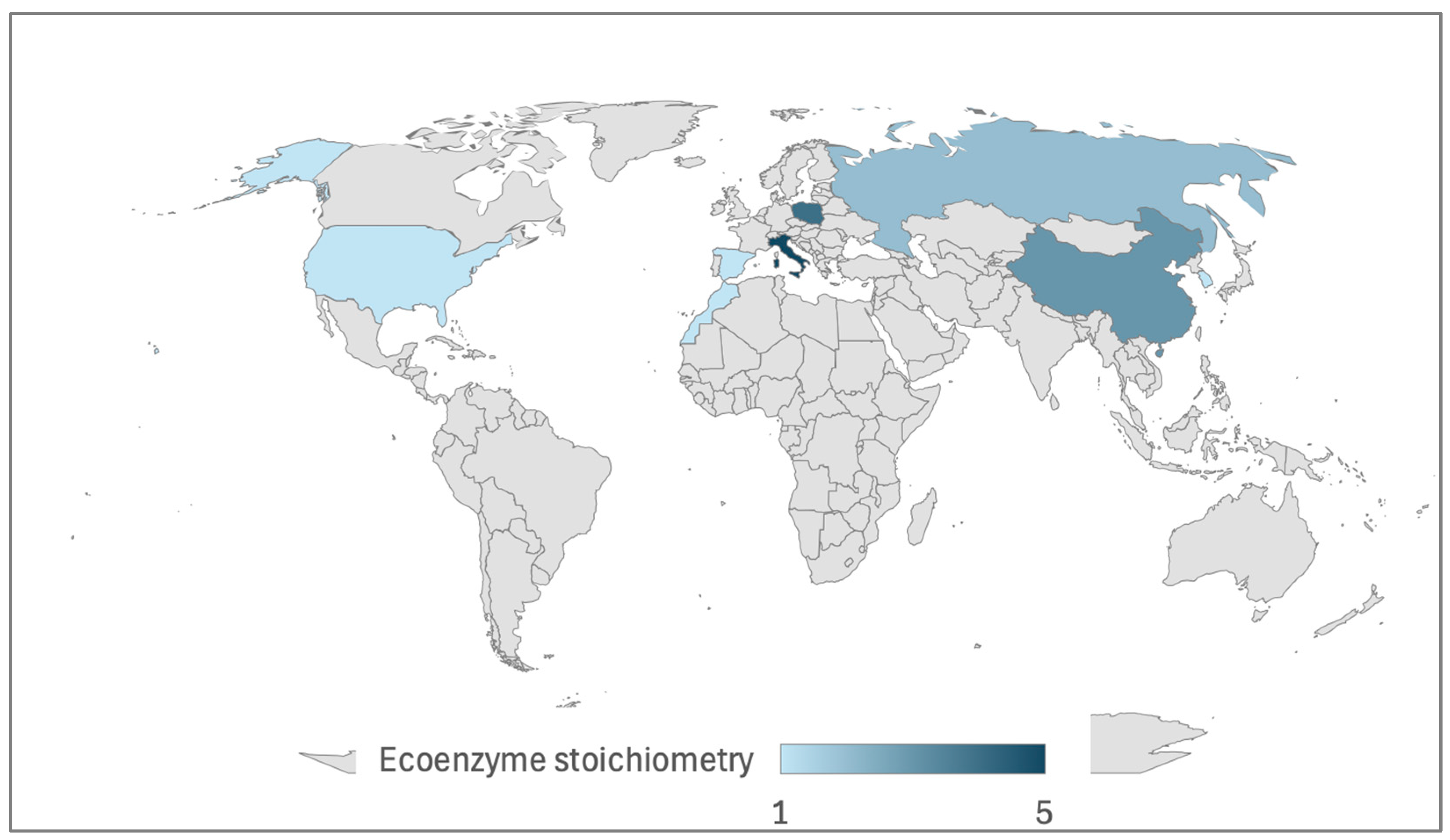
3.2. Soil Enzyme Activities and Ecoenzyme Stoichiometry
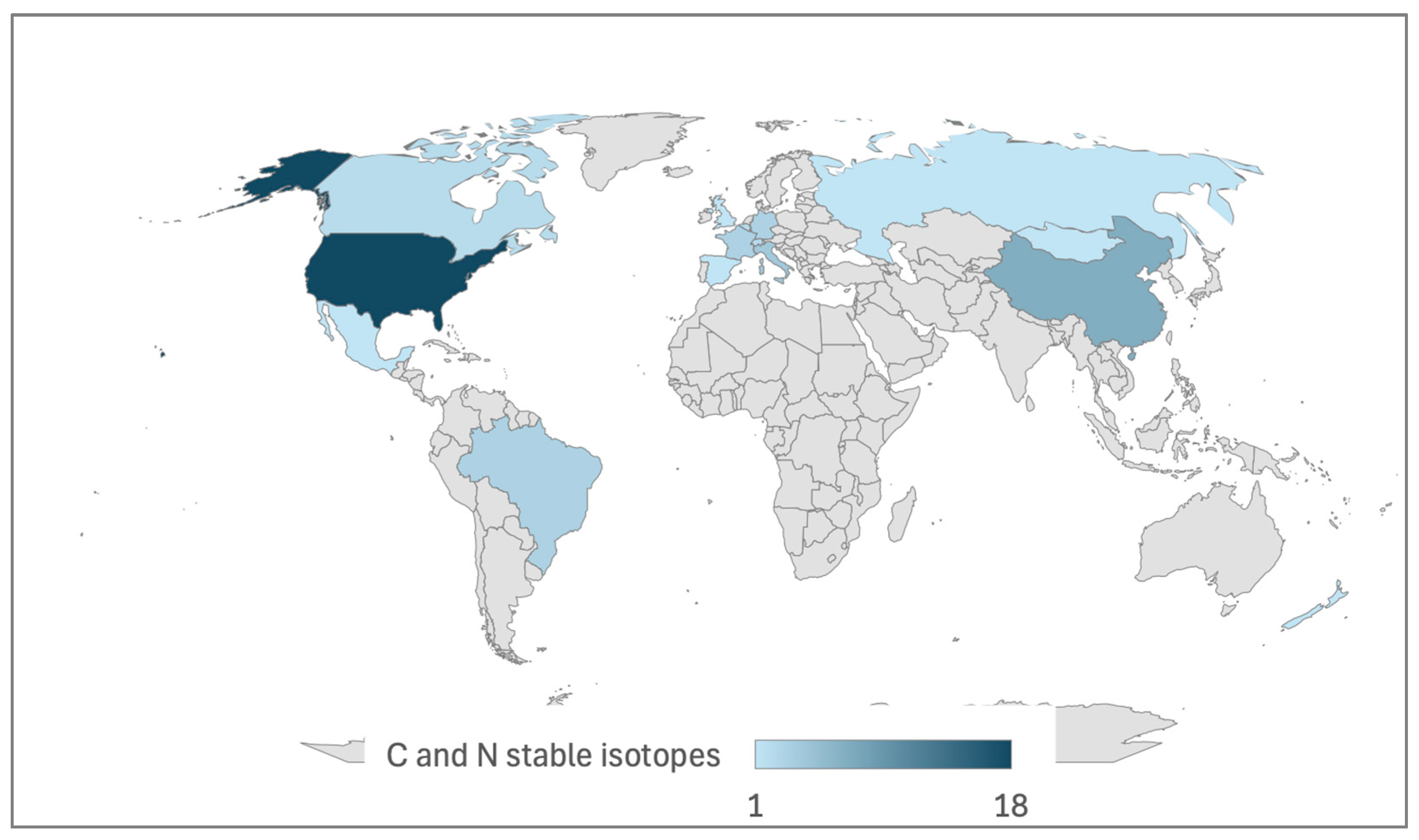
3.3. Carbon and Nitrogen-Stable Isotopes
3.3.1. Case Studies on Carbon Stable Isotopes in the Urban Soil–Plant System
3.3.2. Case Studies on Nitrogen Stable Isotopes in the Urban Soil–Plant System
3.3.3. Relationship Between C and N Stable Isotopes in Urban Contexts and Final Considerations
4. Conclusions and Perspective
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ritchie, H.; Roser, M. Urban Population (%) Long-Run with 2050 Projections (OWID) 2018. Available online: https://ourworldindata.org/urbanization (accessed on 3 December 2024).
- Colsaet, A.; Laurans, Y.; Levrel, H. What drives land take and urban land expansion? A systematic review. Land Use Policy 2018, 79, 339–349. [Google Scholar] [CrossRef]
- Decoville, A.; Feltgen, V. Clarifying the EU objective of no net land take: A necessity to avoid the cure being worse than the disease. Land Use Policy 2023, 131, 106722. [Google Scholar] [CrossRef]
- Peroni, F.; Pappalardo, S.E.; Facchinelli, F.; Crescini, E.; Munafò, M.; Hodgson, M.E.; De Marchi, M. How to map soil sealing, land take and impervious surfaces? A systematic review. Environ. Res. Lett. 2022, 17, 053005. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Biogeochemical C and N cycles in urban soils. Environ. Int. 2009, 35, 1–8. [Google Scholar] [CrossRef]
- Trammell, T.L.; Pataki, D.E.; Pouyat, R.V.; Groffman, P.M.; Rosier, C.; Bettez, N.; Cavender-Bares, J.; Grove, M.J.; Hall, S.J.; Heffernan, J.; et al. Urban soil carbon and nitrogen converge at a continental scale. Ecol. Monogr. 2020, 90, e01401. [Google Scholar] [CrossRef]
- Morel, J.L.; Chenu, C.; Lorenz, K. Ecosystem services provided by soils of urban, industrial, traffic, mining, and military areas (SUITMAs). J. Soils Sediments 2015, 15, 1659–1666. [Google Scholar] [CrossRef]
- Tresch, S.; Moretti, M.; Le Bayon, R.C.; Mäder, P.; Zanetta, A.; Frey, D.; Stehle, B.; Kuhn, A.; Munyangabe, A.; Fliessbach, A. Urban soil quality assessment-a comprehensive case study dataset of urban garden soils. Front. Environ. Sci. 2018, 6, 136. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Trammell, T.L. Climate change and urban forest soils. Dev. Soil Sci. 2019, 36, 189–211. [Google Scholar] [CrossRef]
- O’Riordan, R.; Davies, J.; Stevens, C.; Quinton, J.N.; Boyko, C. The ecosystem services of urban soils: A review. Geoderma 2021, 395, 115076. [Google Scholar] [CrossRef]
- Pavao-Zuckerman, M.A. Urbanization, soils and ecosystem services. In Soil Ecology and Ecosystem Services; Wall, D.H., Ed.; Oxford University Press: Oxford, UK, 2012; pp. 270–278. [Google Scholar]
- Meersmans, J.; Colinet, G.; Negassa, W. Soil health, functions, and ecosystem services: Insights into soil parameters and methods of integration. Front. Environ. Sci. 2024, 12, 1358548. [Google Scholar] [CrossRef]
- Ungaro, F.; Maienza, A.; Ugolini, F.; Lanini, G.M.; Baronti, S.; Calzolari, C. Assessment of joint soil ecosystem services supply in urban green spaces: A case study in Northern Italy. Urban For. Urban Green. 2022, 67, 127455. [Google Scholar] [CrossRef]
- Yang, J.L.; Zhang, G.L. Formation, characteristics and eco-environmental implications of urban soils—A review. Soil Sci. Plant Nutr. 2015, 61, 30–46. [Google Scholar] [CrossRef]
- Muñoz-Rojas, M.; Jordán, A.; Zavala, L.M.; De la Rosa, D.; Abd-Elmabod, S.K.; Anaya-Romero, M. Impact of land use and land cover changes on organic carbon stocks in Mediterranean soils (1956–2007). Land Degrad. Dev. 2015, 26, 168–179. [Google Scholar] [CrossRef]
- Adhikari, K.; Owens, P.R.; Libohova, Z.; Miller, D.M.; Wills, S.A.; Nemecek, J. Assessing soil organic carbon stock ofWisconsin, USA and its fate under future land use and climate change. Sci. Total Environ. 2019, 667, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Adhikari, K.; Zhuang, Q.; Gu, H.; Jin, X. Impacts of urbanization on soil organic carbon stocks in the northeast coastal agricultural areas of China. Sci. Total Environ. 2020, 721, 137814. [Google Scholar] [CrossRef]
- Escalas, A.; Hale, L.; Voordeckers, J.W.; Yang, Y.; Firestone, M.K.; Alvarez-Cohen, L.; Zhou, J.Z. Microbial functional diversity: From concepts to applications. Ecol. Evol. 2019, 9, 12000–12016. [Google Scholar] [CrossRef]
- Korneykova, M.V.; Vasenev, V.I.; Nikitin, D.A.; Soshina, A.S.; Dolgikh, A.V.; Sotnikova, Y.L. Urbanization affects soil microbiome profile distribution in the Russian Arctic region. Int. J. Environ. Res. Public Health 2021, 18, 11665. [Google Scholar] [CrossRef]
- Fu, B.; Fang, C.; Xia, J.; Pan, S.; Zhou, L.; Peng, Y.; Yan, Y.; Yang, Y.; He, Y.; Chen, S.; et al. Urbanization alters soil bacterial communities in southern China coastal cities. Ecotoxicol. Environ. Saf. 2023, 250, 114492. [Google Scholar] [CrossRef]
- Beesley, L.; Dickinson, N. Carbon and trace element fluxes in the pore water of an urban soil following green waste compost, woody and biochar amendments, inoculated with the earthworm Lumbricus terrestris. Soil Biol. Biochem. 2011, 43, 188–196. [Google Scholar] [CrossRef]
- Chen, Y.J.; Day, S.D.; Wick, A.F.; McGuire, K.J. Influence of urban land development and subsequent soil rehabilitation on soil aggregates, carbon, and hydraulic conductivity. Sci. Total Environ. 2014, 494, 329–336. [Google Scholar] [CrossRef]
- Riggs, C.E.; Hobbie, S.E.; Bach, E.M.; Hofmockel, K.S.; Kazanski, C.E. Nitrogen addition changes grassland soil organic matter decomposition. Biogeochemistry 2015, 125, 203–219. [Google Scholar] [CrossRef]
- Wang, S.; Adhikari, K.; Wang, Q.; Jin, X.; Li, H. Role of environmental variables in the spatial distribution of soil carbon (C), nitrogen (N), and C: N ratio from the northeastern coastal agroecosystems in China. Ecol. Indic. 2018, 84, 263–272. [Google Scholar] [CrossRef]
- Zhao, M.S.; Zhang, G.L.; Wu, Y.J.; Li, D.C.; Zhao, Y.G. Driving forces of soil organic matter change in Jiangsu Province of China. Soil Use Manag. 2015, 31, 440–449. [Google Scholar] [CrossRef]
- Stumpf, F.; Keller, A.; Schmidt, K.; Mayr, A.; Gubler, A.; Schaepman, M. Spatiotemporal land use dynamics and soil organic carbon in Swiss agroecosystems. Agric. Ecosyst. Environ. 2018, 258, 129–142. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Huang, M.; Fan, H.; Wang, J.; Jiang, D.; Zhang, M.; Huang, Y. Urbanization altered regional soil organic matter quantity and quality: Insight from excitation emission matrix (EEM) and parallel factor analysis (PARAFAC). Chemosphere 2019, 220, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Edmonson, J.L.; Davies, Z.G.; McHugh, N.; Gaston, K.J.; Leake, J.R. Organic carbon hidden in urban ecosystems. Sci. Rep. 2012, 2, 963. [Google Scholar] [CrossRef]
- Xu, N.; Liu, H.; Feng, W.; Zhu, Y. Urban expanding pattern and soil organic, inorganic carbon distribution in Shanghai, China. Environ. Earth Sci. 2012, 66, 1233–1238. [Google Scholar] [CrossRef]
- Liu, R.; Wang, M.; Chen, W. The influence of urbanization on organic carbon sequestration and cycling in soils of Beijing. Landsc. Urban Plan 2018, 169, 241–249. [Google Scholar] [CrossRef]
- Zhu, W.X.; Dillard, N.D.; Grimm, N.B. Urban nitrogen biogeochemistry: Status and processes in green retention basins. Biogeochemistry 2005, 71, 177–196. [Google Scholar] [CrossRef]
- Grimm, N.B.; Grove, J.M.; Pickett, S.T.; Redman, C.L. Integrated Approaches to Long-Term Studies of Urban Ecological Systems. In Urban Ecology; Marzluff, J.M., Shulenberger, E., Endlicher, W., Alberti, M., Bradley, G., Ryan, C., Simon, U., ZumBrunnen, C., Eds.; Springer: Boston, MA, USA, 2008; pp. 123–141. [Google Scholar] [CrossRef]
- Filonchyk, M.; Yan, H. The characteristics of air pollutants during different seasons in the urban area of Lanzhou, Northwest China. Environ. Earth Sci. 2018, 77, 1–17. [Google Scholar] [CrossRef]
- United Nations Habitat. World Cities Report 2020: The Value of Sustainable Urbanization. United Nations Human Settlements Programme, London: Earthscan 2020. Available online: https://unhabitat.org/sites/default/files/2020/10/wcr_2020_report.pdf (accessed on 3 December 2024).
- Gokhale, S.; Khare, M. A theoretical framework for the episodic-urban air quality management plan (e-UAQMP). Atmos. Environ. 2007, 41, 7887–7894. [Google Scholar] [CrossRef]
- Gulia, S.; Nagendra, S.S.; Khare, M.; Khanna, I. Urban air quality management-A review. Atmos. Pollut. Res. 2015, 6, 286–304. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and health impacts of air pollution: A review. Front. Public Health 2020, 8, 00014. [Google Scholar] [CrossRef] [PubMed]
- Lovett, G.M.; Tear, T.H.; Evers, D.C.; Findlay, S.E.; Cosby, B.J.; Dunscomb, J.K.; Driscoll, C.T.; Weathers, K.C. Effects of air pollution on ecosystems and biological diversity in the eastern United States. Ann. N. Y. Acad. Sci. 2009, 1162, 99–135. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohammad, N.; Roychoudhury, N. Soil pollution: Causes, effects and control. Van Sangyan 2016, 3, 1–14. [Google Scholar]
- Cohen-Shacham, E.; Walters, G.; Janzen, C.; Maginnis, S. Nature-Based Solutions to Address Global Societal Challenges; IUCN: Gland, Switzerland, 2016. [Google Scholar] [CrossRef]
- European Commission: Directorate-General for Research and Innovation. Biodiversity and Nature-Based Solutions—Analysis of EU-Funded Projects; Publications Office of the European Union: Luxembourg, 2020; Available online: https://data.europa.eu/doi/10.2777/183298 (accessed on 3 December 2024).
- European Commission: Directorate-General for Research and Innovation; Bulkeley, H.; Naumann, S.; Vojinovic, Z.; Calfapietra, C.; Whiteoak, K.; Freitas, T.; Vandewoestijne, S.; Wild, T. Nature-Based Solutions: State of the Art in EU-Funded Projects; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar] [CrossRef]
- Collier, M.J.; Frantzeskaki, N.; Connop, S.; Dick, G.; Dumitru, A.; Dziubała, A.; Fletcher, I.; Georgiou, P.; Holscher, K.; Kooijman, E.; et al. An integrated process for planning, delivery, and stewardship of urban nature-based solutions: The Connecting Nature Framework. Nat.-Based Solut. 2023, 3, 100060. [Google Scholar] [CrossRef]
- Seddon, N.; Chausson, A.; Berry, P.; CaJ, G.; Smith, A.; Turner, B. Understanding the value and limits of nature-based solutions to climate change and other global challenges. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190120. [Google Scholar] [CrossRef]
- C.B.D. CBD/COP/DEC/15/4, Decision Adopted by the Conference of the Parties to the Convention on Biological Diversity 15/4. Kunming-Montreal Global Biodiversity Framework. 2022. Available online: https://www.cbd.int/doc/decisions/cop-15/cop-15-dec-04-en.pdf (accessed on 3 December 2024).
- E.C. Proposal for a Regulation of the European Parliament and of the Council on Nature Restoration COM/2022/304 final. 2022. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52022PC0304&qid=1705574472724 (accessed on 3 December 2024).
- E.U. Regulation of the European Parliament and of the Council on Nature Restoration 2022/0195(COD). 2022. Available online: https://www.europarl.europa.eu/doceo/document/TA-9-2023-07-12_EN.html#sdocta6 (accessed on 3 December 2024).
- E.C. Communication from the Commission to the European Parliament, the Council, The European Economic and Social Committee and the Committee of the Regions EU Biodiversity Strategy for 2030 Bringing Nature Back into Our Lives. COM/2020/380 final. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52020DC0380 (accessed on 3 December 2024).
- Banzhaf, E.; Pedersen, A.B.; Fitch, A.; Fletcher, D.; Hutchins, M.; Iversen, S.; Jones, L.; Knopp, J.; Levin, G.; Russel, D.; et al. Recommendations for Potential Target Values in Cities. Deliverable 3.5. REGREEN—Fostering Nature‐Based Solutions for Smart, Green and Healthy Urban Transitions in Europe and China. Horizon2020 Grant No. 821016. Available online: https://www.regreen-project.eu/wp-content/uploads/REGREEN-D3.5_Recommendations-for-potential-target-values-for-cities_.pdf (accessed on 3 December 2024).
- Almenar, J.B.; Elliot, T.; Rugani, B.; Philippe, B.; Navarrete Gutierrez, T.; Sonnemann, G.; Geneletti, D. Nexus between nature-based solutions, ecosystem services and urban challenges. Land Use Policy 2021, 100, 104898. [Google Scholar] [CrossRef]
- Vannucchi, F.; Buoncristiano, A.; Scatena, M.; Caudai, C.; Bretzel, F. Low productivity sub-strate leads to functional diversification of green roof plant assemblage. Ecol. Eng. 2022, 176, 106547. [Google Scholar] [CrossRef]
- Anderson, V.; Gough, W.A. Evaluating the potential of nature-based solutions to reduce ozone, nitrogen dioxide, and carbon dioxide through a multi-type green infrastructure study in Ontario. Can. City Environ. Interact. 2020, 6, 100043. [Google Scholar] [CrossRef]
- Oukawa, G.Y.; Krecl, P.; Targino, A.C.; Batista, L.F.A. Advantages of modeling the urban heat island intensity: A tool for implementing nature-based solutions. Sustain. Cities Soc. 2024, 102, 105204. [Google Scholar] [CrossRef]
- Ale, S.; Adjonou, K.; Segla, K.N.; Komi, K.; Zoungrana, J.-B.B.; Aholou, C.; Kokou, K. Urban Forestry in Sub-Saharan Africa: Challenges, Contributions, and Future Directions for Combating Climate Change and Restoring Forest Landscapes. Sustainability 2025, 17, 24. [Google Scholar] [CrossRef]
- Ramon, M.; Lafortezza, R.; Ribeiro, A.P.; de Camargo, P.B.; Domingos, M.; Gomes, E.P.C.; dos Reis Tavares, A.; Dias, A.G.; Kniess, C.T.; Ferreira, M.L. Carbon and nitrogen stock in soils of subtropical urban forests: Isotopic d13C and d15N indicators for nature-based solutions in a megacity. Ecol. Indic. 2024, 160, 111743. [Google Scholar] [CrossRef]
- Bretzel, F.; Vannucchi, F.; Pini, R.; Scatena, M.; Marradi, A.; Cinelli, F. Use of coarse substrate to increase the rate of water infiltration and the bearing capacity in tree plantings. Ecol. Eng. 2020, 148, 105789. [Google Scholar] [CrossRef]
- Vannucchi, F.; Scartazza, A.; Scatena, M.; Rosellini, I.; Tassi, E.; Cinelli, F.; Bretzel, F. Deinked paper sludge and mature compost as high-value components of soilless substrate to support tree growth. J. Clean. Prod. 2021, 290, 125176. [Google Scholar] [CrossRef]
- Layman, R.M.; Day, S.D.; Mitchell, D.K.; Chen, Y.; Harris, J.R.; Daniels, W.L. Below ground matters: Urban soil rehabilitation increases tree canopy and speeds establishment. Urban For. Urban Green. 2016, 16, 25–35. [Google Scholar] [CrossRef]
- Lu, T.; Ke, M.; Lavoie, M.; Jin, Y.; Fan, X.; Zhang, Z.; Fu, Z.; Sun, L.; Gillings, M.; Penuelas, J. Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 2018, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Huang, X.B.; Lang, X.D.; Tang, R.; Zhang, R.; Li, S.F.; Su, J.R. Effects of plant diversity, soil microbial diversity, and network complexity on ecosystem multifunctionality in a tropical rainforest. Front. Plant Sci. 2023, 14, 1238056. [Google Scholar] [CrossRef]
- Wu, W.; Ke, T.; Zhou, X.; Li, Q.; Tao, Y.; Zhang, Y.; Zeng, Y.; Cao, J.; Chen, L. Synergistic remediation of copper mine tailing sand by microalgae and fungi. Appl. Soil Ecol. 2022, 175, 104453. [Google Scholar] [CrossRef]
- Jia, P.; Liang, J.; Yang, S.; Zhang, S.; Liu, J.; Liang, Z.; Li, F.; Zeng, Q.; Fang, Z.; Liao, B.; et al. Plant diversity enhances the reclamation of degraded lands by stimulating plant-soil feedbacks. J. Appl. Ecol. 2020, 57, 1258–1270. [Google Scholar] [CrossRef]
- Omar, M.; Sayed, N.A.; Barre, K.; Halwani, J.; Machon, N. Drivers of the distribution of spontaneous plant communities and species within urban tree bases. Urban For. Urban Green. 2018, 35, 174–191. [Google Scholar] [CrossRef]
- Duan, P.; Fu, R.; Nottingham, A.T.; Domeignoz-Horta, L.A.; Yang, X.; Du, H.; Wang, K.; Li, D. Tree species diversity increases soil microbial carbon use efficiency in a subtropical forest. Glob. Change Biol. 2023, 29, 7131–7144. [Google Scholar] [CrossRef]
- Miyamoto, T.; Hiura, T. Decomposition and nitrogen release from the foliage litter of fir (Abies sachalinensis) and oak (Quercus crispula) under different forest canopies in Hokkaido, Japan. Ecol. Res. 2008, 23, 673–680. [Google Scholar] [CrossRef]
- Gaublomme, E.; Vos, B.D.; Cools, N. An Indicator for Microbial Biodiversity in Forest Soils; INBO. R.2006.40; Instituut voor Natuur-en Bosonderzoek: Brussels, Belgium, 2006; ISSN 1782-9054. [Google Scholar]
- Otero-Durán, L.; Torres, A. Trees and sidewalks: Toward an infrastructure protection approach. Front. Sustain. Cities 2024, 6, 1336472. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Rinot, O.; Levy, G.J.; Steinberger, Y.; Svoray, T.; Eshel, G. Soil health assessment: A critical review of current methodologies and a proposed new approach. Sci. Total Environ. 2019, 648, 1484–1491. [Google Scholar] [CrossRef]
- Kunito, T.; Moro, H.; Mise, K.; Sawada, K.; Otsuka, S.; Nagaoka, K.; Fujita, K. Ecoenzymatic stoichiometry as a temporally integrated indicator of nutrient availability in soils. Soil Sci. Plant Nutr. 2024, 70, 246–269. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Fu, S. Biological indices for soil quality evaluation: Perspectives and limitations. Land Degrad. Dev. 2016, 27, 14–25. [Google Scholar] [CrossRef]
- Deb, S.; Bhadoria, P.B.S.; Mandal, B.; Rakshit, A.; Singh, H.B. Soil organic carbon: Towards better soil health, productivity and climate change mitigation. Clim. Chang. Environ. Sustain. 2015, 3, 26. [Google Scholar] [CrossRef]
- Paustian, K.; Collier, S.; Baldock, J.; Burgess, R.; Creque, J.; Delonge, M.; Dungait, J.; Ellert, B.; Frank, S.; Goddard, T.; et al. Quantifying carbon for agricultural soil management: From the current status toward a global soil information system. Carbon Manag. 2019, 10, 567–587. [Google Scholar] [CrossRef]
- Shikuku, K.M.; Valdivia, R.O.; Paul, B.K.; Mwongera, C.; Winowiecki, L.; Läderach, P.; Herrero, M.; Silvestri, S. Prioritizing climate-smart livestock technologies in rural Tanzania: A minimum data approach. Agric. Syst. 2017, 151, 204–216. [Google Scholar] [CrossRef]
- Sardans, J.; Janssens, I.A.; Ciais, P.; Obersteiner, M.; Peñuelas, J. Recent advances and future research in ecological stoichiometry. Perspect. Plant Ecol. Evol. Syst. 2021, 50, 125611. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A.; Marxsen, J.; Sinsabaugh, R.L.; et al. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Bu, W.S.; Chen, F.S.; Wang, F.C.; Fang, X.M.; Mao, R.; Wang, H.M. The species- specific responses of nutrient resorption and carbonhydrate accumulation in leaves and roots to nitrogen addition in a subtropical mixed plantation. Can. J. For Res. 2019, 49, 826–835. [Google Scholar] [CrossRef]
- Cotrufo, F.M.; Lavallee, J.M.; Zhang, Y.; Hansen, P.M.; Paustian, K.H.; Schipanski, M.; Wallenstein, M.D. In-N-Out: A hierarchical framework to understand and predict soil carbon storage and nitrogen recycling. Glob. Change Biol. 2021, 27, 4456–4468. [Google Scholar] [CrossRef]
- Xue, Y.; Kang, H.; Cui, Y.; Lu, S.; Yang, H.; Zhu, J.; Fu, Z.; Yan, C.; Wang, D. Consistent plant and microbe nutrient limitation patterns during natural vegetation restoration. Front. Plant Sci. 2022, 13, 885984. [Google Scholar] [CrossRef]
- Du, E.; Xia, N.; Guo, Y.; Tian, Y.; Li, B.; Liu, X.; De Vries, W. Ecological effects of nitrogen deposition on urban forests: An overview. Front. Agric. Sci. Eng. 2022, 9, 445–456. [Google Scholar] [CrossRef]
- Yoon, T.K. Urban Soil Carbon: Processes and Patterns. In Soils in Urban Ecosystem; Rakshit, A., Ghosh, S., Vasenev, V., Pathak, H., Rajput, V.D., Eds.; Springer: Singapore, 2022; pp. 65–100. [Google Scholar]
- Hu, X.F.; Chen, F.S.; Nagle, G.; Fang, Y.T.; Yu, M.Q. Soil phosphorus fractions and tree phosphorus resorption in pine forests along an urban-to-rural gradient in Nanchang, China. Plant Soil 2011, 346, 97–106. [Google Scholar] [CrossRef]
- Yang, J.L.; Yuan, D.G.; Zhao, Y.G.; He, Y.; Zhang, G.L. Stoichiometric relations of C, N, and P in urban top soils in Nanjing, China, and their biogeochemical implications. J Soils Sediments 2021, 21, 2154–2164. [Google Scholar] [CrossRef]
- Boccuzzi, G.; Nakazato, R.K.; Pereira, M.A.G.; Rinaldi, M.C.; Lopes, M.I.; Domingos, M. Anthropogenic deposition increases nitrogen-phosphorus imbalances in tree vegetation, litter and soil of Atlantic Forest remnants. Plant Soil 2021, 461, 341–354. [Google Scholar] [CrossRef]
- Vannucchi, F.; Scartazza, A.; Macci, C.; Bretzel, F.; Doni, S.; Rosellini, T.; Tassi, E.; Pini, R.; Masciandaro, G.; Peruzzi, E. Isotope signature and ecoenzymatic stoichiometry as key indicators of urban soil functionality. J. Soils Sediments 2024, 25, 451–461. [Google Scholar] [CrossRef]
- Kempf, P.; Lasota, J.; Kempf, M.; Blonska, E. Soil properties of Kraków’s urban forests with selected alien tree species. Sylwan 2023, 167, 521–534. [Google Scholar] [CrossRef]
- Huang, R.; Lan, T.; Song, X.; Li, J.; Ling, J.; Deng, O.; Wang, C.; Gao, X.; Li, Q.; Tang, X.; et al. Soil labile organic carbon impacts C: N: P stoichiometry in urban park green spaces depending on vegetation types and time after planting. Appl. Soil Ecol. 2021, 163, 103926. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Prażuch, W.; Ilek, A. Vertical variations in enzymatic activity and C: N: P stoichiometry in forest soils under the influence of different tree species. Eur. J. For. Res. 2025, 144, 83–94. [Google Scholar] [CrossRef]
- Wang, Z.; Tao, T.; Wang, Y.; Small, G.E.; Chen, J.; Sun, X. Soil quality in urban forests under different understory management practices. Land Degrad. Dev. 2022, 34, 899–910. [Google Scholar] [CrossRef]
- Scartazza, A.; Vannucchi, F.; Peruzzi, E.; Macci, C.; Scatena, M.; Manzini, J.; Masciandaro, G.; Hoshika, Y.; Paoletti, E. Nutrient interaction in the soil-plant system and tree physiological functional traits in an urban green infrastructure. J. Soil Sci. Plant Nutr. 2025, in press. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Piaszczyk, W. Dissolved Carbon and Nitrogen Release from Deadwood of Different Tree Species in Various Stages of Decomposition. Soil Sci. Plant Nutr. 2018, 65, 100–107. [Google Scholar] [CrossRef]
- Błońska, E.; Piaszczyk, W.; Staszel, K.; Lasota, J. Enzymatic Activity of Soils and Soil Organic Matter Stabilization as an Effect of Components Released from the Decomposition of Litter. Appl. Soil Ecol. 2021, 157, 103723. [Google Scholar] [CrossRef]
- Paul, S.; Rakshit, A. Classification and Functional Characteristics of Urban Soil. In Soils in Urban Ecosystem; Springer: Singapore, 2022; pp. 11–23. [Google Scholar] [CrossRef]
- Uzarowicz, Ł.; Wolińska, A.; Błońska, E.; Szafranek-Nakonieczna, A.; Kuźniar, A.; Słodczyk, Z.; Kwasowski, W. Technogenic Soils (Technosols) Developed from Mine Spoils Containing Fe Sulphides: Microbiological Activity as an Indicator of Soil Development Following Land Reclamation. Appl. Soil Ecol. 2020, 156, 103699. [Google Scholar] [CrossRef]
- Malek, K.; Malek, K.; Khanmohammadi, F. Response of Soil Thermal Conductivity to Various Soil Properties. Int. Commun. Heat Mass Transf. 2021, 127, 105516. [Google Scholar] [CrossRef]
- Shan, Q.; Yu, Y.; Yu, J.; Zhang, J. Soil enzyme activities and their indication for fertility of urban forest soil. Front. Environ. Sci. Eng. China 2008, 2, 218–223. [Google Scholar] [CrossRef]
- Li, T.; Meng, L.; Herman, U.; Lu, Z.; Crittenden, J. A Survey of Soil Enzyme Activities along Major Roads in Beijing: The Implications for Traffic Corridor Green Space Management. Int. J. Environ. Res. Public Health 2015, 12, 12475–12488. [Google Scholar] [CrossRef] [PubMed]
- Cardelli, R.; Vanni, G.; Guidi, L.; Marchini, F.; Saviozzi, A. Antioxidant Capacity in Urban Soils. Landsc. Urban Plan. 2014, 124, 66–75. [Google Scholar] [CrossRef]
- Castaldi, S.; Rutigliano, F.A.; Virzo de Santo, A. Suitability of Soil Microbial Parameters as Indicators of Heavy Metal Pollution. Water Air Soil Pollut. 2004, 158, 21–35. [Google Scholar] [CrossRef]
- Teng, Y.; Yang, J.; Wang, J.; Song, L. Bioavailability of Vanadium Extracted by EDTA, HCl, HOAC, and NaNO3 in Topsoil in the Panzhihua Urban Park, Located in Southwest China. Biol. Trace Elem. Res. 2011, 144, 1394–1404. [Google Scholar] [CrossRef]
- Hagmann, D.F.; Goodey, N.M.; Mathieu, C.; Evans, J.; Aronson, M.F.J.; Gallagher, F.; Krumins, J.A. Effect of Metal Contamination on Microbial Enzymatic Activity in Soil. Soil Biol. Biochem. 2015, 91, 291–297. [Google Scholar] [CrossRef]
- Jaworska, H.; Lemanowicz, J. Heavy Metal Contents and Enzymatic Activity in Soils Exposed to the Impact of Road Traffic. Sci. Rep. 2019, 9, 19981. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Lemanowicz, J.; Rydlewska, M.; Sowiński, P. The Impact of Proximity to Road Traffic on Heavy Metal Accumulation and Enzyme Activity in Urban Soils and Dandelion. Sustainability 2024, 16, 812. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Herbón, C.; Probst, M.; Fornasier, F.; Barral, M.T.; Paradelo, R. Influence of Land Use on the Microbiological Properties of Urban Soils. Appl. Soil Ecol. 2022, 175, 104452. [Google Scholar] [CrossRef]
- Gómez-Sagasti, M.T.; Garbisu, C.; Urra, J.; Míguez, F.; Artetxe, U.; Hernández, A.; Vilela, J.; Alkorta, I.; Becerril, J.M. Mycorrhizal-assisted phytoremediation and intercropping strategies improved the health of contaminated soil in a peri-urban area. Front. Plant Sci. 2021, 12, 693044. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matinian, N.N.; Gusareva, A.L.; Bakhmatova, K.A.; Sheshukova, A.A. Microbiological Indicators and Heavy Metal Concentration in Ecological Assessment of Urban Soils of Saint Petersburg, Russia. Geogr. Environ. Sustain. 2020, 13, 214–223. [Google Scholar] [CrossRef][Green Version]
- Ao, G.; Qin, W.; Wang, X.; Yu, M.; Feng, J.; Han, M.; Zhu, B. Linking the Rhizosphere Effects of 12 Woody Species on Soil Microbial Activities with Soil and Root Nitrogen Status. Rhizosphere 2023, 28, 100809. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Haddad, S.A.; Bartkowiak, A.; Lamparski, R.; Wojewódzki, P. The Role of an Urban Park’s Tree Stand in Shaping the Enzymatic Activity, Glomalin Content and Physicochemical Properties of Soil. Sci. Total Environ. 2020, 741, 140446. [Google Scholar] [CrossRef] [PubMed]
- Maienza, A.; Ungaro, F.; Baronti, S.; Colzi, I.; Giagnoni, L.; Gonnelli, C.; Renella, G.; Ugolini, F.; Calzolari, C. Biological Restoration of Urban Soils after De-Sealing Interventions. Agriculture 2021, 11, 190. [Google Scholar] [CrossRef]
- Gorbov, S.N.; Gorovtsov, A.V.; Bezuglova, O.S.; Anisimova, M.A.; Skripnikov, P.N.; Tishchenko, S.A.; Marschner, B. Enzyme Activity of Soils in Urban Landscapes of the Lower Don Area, Southern Russia. Land Degrad. Dev. 2021, 32, 1618–1631. [Google Scholar] [CrossRef]
- Beroigui, M.; Naylo, A.; Walczak, M.; Hafidi, M.; Charzyński, P.; Świtoniak, M.; Różański, S.; Boularbah, A. Physicochemical and Microbial Properties of Urban Park Soils of the Cities of Marrakech, Morocco and Toruń, Poland: Human Health Risk Assessment of Fecal Coliforms and Trace Elements. CATENA 2020, 194, 104673. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Farooq, M.; Kim, K.-H.; Lee, Y.-H.; Qayyum, M.F.; Al-Wabel, M.I.; Lee, S.S.; Ok, Y.S. Determining Soil Quality in Urban Agricultural Regions by Soil Enzyme-Based Index. Environ. Geochem. Heal. 2017, 39, 1531–1544. [Google Scholar] [CrossRef]
- Ananyeva, N.D.; Ivashchenko, K.V.; Sushko, S.V. Microbial Indicators of Urban Soils and Their Role in the Assessment of Ecosystem Services: A Review. Eurasian Soil Sci. 2021, 54, 1517–1531. [Google Scholar] [CrossRef]
- Bierza, W.; Czarnecka, J.; Błońska, A.; Kompała-Bąba, A.; Hutniczak, A.; Jendrzejek, B.; Bakr, J.; Jagodziński, A.M.; Prostański, D.; Woźniak, G. Plant Diversity and Species Composition in Relation to Soil Enzymatic Activity in the Novel Ecosystems of Urban–Industrial Landscapes. Sustainability 2023, 15, 7284. [Google Scholar] [CrossRef]
- Séré, G.; Guern, C.L.; Bispo, A.; Layet, C.; Ducommun, C.; Clesse, M.; Schwartz, C.; Vidal-Beaudet, L. Selection of Soil Health Indicators for Modelling Soil Functions to Promote Smart Urban Planning. Sci. Total Environ. 2024, 924, 171347. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Hill, B.H.; Shah, J.J.F. Ecoenzymatic Stoichiometry of Microbial Organic Nutrient Acquisition in Soil and Sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Guan, H.L.; Fan, J.W.; Lu, X. Soil Specific Enzyme Stoichiometry Reflects Nitrogen Limitation of Microorganisms under Different Types of Vegetation Restoration in the Karst Areas. Appl. Soil Ecol. 2022, 169, 104253. [Google Scholar] [CrossRef]
- Xie, Y.; Ouyang, Y.; Han, S.; Se, J.; Tang, S.; Yang, Y.; Ma, Q.; Wu, L. Crop Rotation Stage Has a Greater Effect than Fertilisation on Soil Microbiome Assembly and Enzymatic Stoichiometry. Sci. Total Environ. 2022, 815, 152956. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Moorhead, D.L.; Guo, X.; Peng, S.; Wang, Y.; Zhang, X.; Fang, L. Stoichiometric Models of Microbial Metabolic Limitation in Soil Systems. Glob. Ecol. Biogeogr. 2021, 30, 2297–2311. [Google Scholar] [CrossRef]
- Cui, Y.; Peng, S.; Delgado-Baquerizo, M.; Rillig, M.C.; Terrer, C.; Zhu, B.; Jing, X.; Chen, J.; Li, J.; Feng, J.; et al. Microbial Communities in Terrestrial Surface Soils Are Not Widely Limited by Carbon. Glob. Chang. Biol. 2023, 29, 4412–4429. [Google Scholar] [CrossRef]
- Mori, T. Microbial Nutrient Limitation in Tropical Forest Soils Determined Using the V-T Model Contradicts the Traditional View That C Is the Major Limiting Element. Tropics 2022, 31, 59–63. [Google Scholar] [CrossRef]
- Pataki, D.E.; Bush, S.E.; Ehleringer, J.R. Stable isotopes as a tool in urban ecology. In Stable Isotopes and Biosphere-Atmosphere Interactions: Processes and Biological Controls; Flanagan, L.B., Ehleringer, J.R., Pataki, D.E., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2005; pp. 199–214. [Google Scholar] [CrossRef]
- Pataki, D.E.; Randerson, J.T.; Wang, W.; Herzenach, M.; Grulke, N.E. The carbon isotope composition of plants and soils as biomarkers of pollution. In Isoscapes: Understanding Movement, Pattern, and Process on Earth Through Isotope Mapping; West, J., Bowen, G., Dawson, T., Tu, K., Eds.; Springer: Dordrecht, The Netherland, 2010; pp. 407–423. [Google Scholar] [CrossRef]
- Soba, D.; Gámez, A.L.; Úriz, N.; de Larrinaga, L.R.; Gonzalez-Murua, C.; Becerril, J.M.; Esteban, R.; Serret, D.; Araus, J.L.; Aranjuelo, I. Foliar heavy metals and stable isotope (d13C, d15N) profiles as reliable urban pollution biomonitoring tools. Urban For. Urban Green. 2021, 57, 126918. [Google Scholar] [CrossRef]
- Wang, W.; Pataki, D.E. Spatial patterns of plant isotope tracers in the Los Angeles urban region. Landsc. Ecol. 2010, 25, 35–52. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Lichtfouse, M.; Jaffrézic, A. d13C values of grasses as a novel indicator of pollution by fossil-fuel-derived greenhouse gas CO2 in urban areas. Environ. Sci. Technol. 2003, 37, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, Y.; Xia, N.; Terrer, C.; Guo, H.; Du, E. Urban CO2 imprints on carbon isotope and growth of Chinese pine in the Beijing metropolitan region. Sci. Total Environ. 2023, 866, 161389. [Google Scholar] [CrossRef] [PubMed]
- Balasooriya, B.L.W.K.; Samson, R.; Mbikwa, F.; Boeckx, P.; Van Meirvenne, M. Biomonitoring of urban habitat quality by anatomical and chemical leaf characteristics. Environ. Exp. Bot. 2009, 65, 386–394. [Google Scholar] [CrossRef]
- Sonti, N.F.; Hallett, R.A.; Griffin, K.L.; Trammell, T.L.; Sullivan, J.H. Chlorophyll fluorescence parameters, leaf traits and foliar chemistry of white oak and red maple trees in urban forest patches. Tree Physiol. 2021, 41, 269–279. [Google Scholar] [CrossRef]
- Dongarra, G.; Varrica, D. d13C variations in tree rings as an indication of severe changes in the urban air quality. Atmos. Environ. 2002, 36, 5887–5896. [Google Scholar] [CrossRef]
- Wang, W.; Pataki, D.E. Drivers of spatial variability in urban plant and soil isotopic composition in the Los Angeles basin. Plant Soil 2012, 350, 323–338. [Google Scholar] [CrossRef]
- Díaz-Álvarez, E.A.; de la Barrera, E. Isotopic biomonitoring of anthropic carbon emissions in a megalopolis. PeerJ 2020, 8, e9283. [Google Scholar] [CrossRef] [PubMed]
- Batkhuyag, E.U.; Lehmann, M.M.; Cherubini, P.; Ulziibat, B.; Soyol-Erdene, T.O.; Schaub, M.; Saurer, M. Combination of multiple stable isotope and elemental analyses in urban trees reveals air pollution and climate change effects in Central Mongolia. Ecol. Indic. 2023, 154, 110719. [Google Scholar] [CrossRef]
- Battipaglia, G.; Marzaioli, F.; Lubritto, C.; Altieri, S.; Strumia, S.; Cherubini, P.; Cotrufo, M.F. Traffic pollution affects tree-ring width and isotopic composition of Pinus pinea. Sci. Total Environ. 2010, 408, 586–593. [Google Scholar] [CrossRef]
- Hirsch, M.; Böddeker, H.; Albrecht, A.; Saha, S. Drought tolerance differs between urban tree species but is not affected by the intensity of traffic pollution. Trees 2023, 37, 111–131. [Google Scholar] [CrossRef]
- Norra, S.; Handley, L.L.; Berner, Z.; Stüben, D. 13C and 15N natural abundances of urban soils and herbaceous vegetation in Karlsruhe, Germany. Eur. J. Soil Sci. 2005, 56, 607–620. [Google Scholar] [CrossRef]
- Boeckx, P.; Van Meirvenne, M.; Raulo, F.; Van Cleemput, O. Spatial patterns of d13C and d15N in the urban topsoil of Gent, Belgium. Org. Geochem. 2006, 37, 1383–1393. [Google Scholar] [CrossRef]
- Xu, N.; Bai, X. Spatial distribution of organic carbon fractions and 13C in urban soils, Shanghai, China. Isr. J. Ecol. Evol. 2017, 63, 78–84. [Google Scholar] [CrossRef]
- Konstantinov, A.; Konstantinova, E.; Smirnov, P.; Minkina, T.; Batalin, G.; Gareev, B.; Mingazov, G.; Loiko, S. Assessment of soil development during rapid urbanization using the carbon and nitrogen stable isotope composition. Environ. Geochem. Health 2023, 45, 9123–9134. [Google Scholar] [CrossRef]
- Lambrecht, S.C.; Mahieu, S.; Cheptou, P.O. Natural selection on plant physiological traits in an urban environment. Acta Oecol. 2016, 77, 67–74. [Google Scholar] [CrossRef]
- Li, C.; Huang, M.; Liu, J.; Ji, S.; Zhao, R.; Zhao, D.; Sun, R. Isotope-based water-use efficiency of major greening plants in a sponge city in northern China. PLoS ONE 2019, 14, e0220083. [Google Scholar] [CrossRef]
- Vallano, D.M.; Sparks, J.P. Foliar d15N is affected by foliar nitrogen uptake, soil nitrogen, and mycorrhizae along a nitrogen deposition gradient. Oecologia 2013, 172, 47–58. [Google Scholar] [CrossRef]
- Ammann, M.; Siegwolf, R.; Pichlmayer, F.; Suter, M.; Saurer, M.; Brunold, C. Estimating the uptake of traffic-derived NO2 from 15N abundance in Norway spruce needles. Oecologia 1999, 118, 124–131. [Google Scholar] [CrossRef]
- Guerrieri, M.R.; Siegwolf, R.T.W.; Saurer, M.; Jäggi, M.; Cherubini, P.; Ripullone, F.; Borghetti, M. Impact of different nitrogen emission sources on tree physiology as assessed by a triple stable isotope approach. Atmos. Environ. 2009, 43, 410–418. [Google Scholar] [CrossRef]
- Gong, C.; Xian, C.; Su, Y.; Ouyang, Z. Estimating the nitrogen source apportionment of Sophora japonica in roadside green spaces using stable isotope. Sci. Total Environ. 2019, 689, 1348–1357. [Google Scholar] [CrossRef]
- Gong, C.; Xian, C.; Cui, B.; He, G.; Wei, M.; Zhang, Z.; Ouyang, Z. Estimating NOx removal capacity of urban trees using stable isotope method: A case study of Beijing, China. Environ. Pollut. 2021, 290, 118004. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Xian, C.; Ouyang, Z. Isotopic Composition (d15N and d18O) of Urban Forests in Different Climate Types Indicates the Potential Influences of Traffic Exhaust and Relative Humidity. Forests 2022, 13, 2060. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, H.; Qu, L. Nitrogen concentrations and nitrogen isotopic compositions in leaves of Cinnamomum camphora and Pinus massoniana (Lamb.) for indicating atmospheric nitrogen deposition in Guiyang (SW China). Atmos. Environ. 2017, 159, 1–10. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, H.; Wu, D. Traffic-related dustfall and NOx, but not NH3, seriously affect nitrogen isotopic compositions in soil and plant tissues near the roadside. Environ. Pollut. 2019, 249, 655–665. [Google Scholar] [CrossRef]
- Kuang, Y.; Sun, F.; Wen, D.; Xu, Z.; Huang, L.; Li, J. Nitrogen deposition influences ni-trogen isotope composition in soil and needles of Pinus massoniana forests along an urban-rural gradient in the Pearl River Delta of south China. J. Soils Sediments 2011, 11, 589–595. [Google Scholar] [CrossRef]
- McDermot, C.R.; Minocha, R.; D’Amico III, V.; Long, S.; Trammell, T.L. Red maple (Acer rubrum L.) trees demonstrate acclimation to urban conditions in deciduous forests embedded in cities. PLoS ONE 2020, 15, e0236313. [Google Scholar] [CrossRef]
- Scartazza, A.; Huarancca Reyes, T.; Bretzel, F.; Pini, R.; Guglielminetti, L.; Calfapietra, C. Has COVID-19 Lockdown Affected C and N Level and Isotope Composition in Urban Soils and Plant Leaves? Ecosyst. Health Sustain. 2023, 9, 0117. [Google Scholar] [CrossRef]
- Xia, N.; Du, E.; Tang, Y.; Guo, H. A distinctive latitudinal trend of nitrogen isotope signature across urban forests in eastern China. Glob. Change Biol. 2023, 29, 5666–5676. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, H.; Guan, H.; Long, C. Monitoring atmospheric nitrogen pollution in Guiyang (SW China) by contrasting use of Cinnamomum camphora leaves, branch bark and bark as biomonitors. Environ. Pollut. 2018, 233, 1037–1048. [Google Scholar] [CrossRef]
- Stewart, G.R.; Aidar, M.P.; Joly, C.A.; Schmidt, S. Impact of point source pollution on nitrogen isotope signatures (d15N) of vegetation in SE Brazil. Oecologia 2022, 131, 468–472. [Google Scholar] [CrossRef]
- Niepsch, D.; Clarke, L.J.; Newton, J.; Tzoulas, K.; Cavan, G. High spatial resolution assessment of air quality in urban centres using lichen carbon, nitrogen and sulfur contents and stable-isotope-ratio signatures. Environ. Sci. Pollut. Res. 2023, 30, 58731–58754. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Yoh, M.; Koba, K.; Zhu, W.; Takebayashi, Y.U.; Xiao, Y.; Lei, C.; Mo, J.; Zhang, W.; Lu, X. Nitrogen deposition and forest nitrogen cycling along an urban–rural transect in southern China. Glob. Change Biol. 2011, 17, 872–885. [Google Scholar] [CrossRef]
- Bukata, A.R.; Kyser, T.K. Carbon and nitrogen isotope variations in tree-rings as records of perturbations in regional carbon and nitrogen cycles. Environ. Sci. Technol. 2007, 41, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Bai, S.; Xu, Z.; Blumfield, T.J.; Reverchon, F. Human footprints in urban forests: Implication of nitrogen deposition for nitrogen and carbon storage. J. Soils Sediments 2015, 15, 1927–1936. [Google Scholar] [CrossRef]
- Pereira, M.A.G.; Domingos, M.; da Silva, E.A.; Aragaki, S.; Ramon, M.; de Camargo, P.B.; Ferreira, M.L. Isotopic composition (d13C and d15N) in the soil-plant system of subtropical urban forests. Sci. Total Environ. 2022, 851, 158052. [Google Scholar] [CrossRef] [PubMed]
- Trammell, T.L.E.; Pataki, D.E.; Cavender-Bares, J.; Groffman, P.M.; Hall, S.J.; Heffernan, J.B.; Hobbie, S.E.; Morse, J.L.; Neill, C.; Nelson, K.C. Plant nitrogen concentration and isotopic composition in residential lawns across seven US cities. Oecologia 2016, 181, 271–285. [Google Scholar] [CrossRef]
- Smith, R.M.; Williamson, J.C.; Pataki, D.E.; Ehleringer, J.; Dennison, P. Soil carbon and nitrogen accumulation in residential lawns of the Salt Lake Valley, Utah. Oecologia 2018, 187, 1107–1118. [Google Scholar] [CrossRef]
- Alagich, R.; Gardeisen, A.; Alonso, N.; Rovira, N.; Bogaard, A. Using stable isotopes and functional weed ecology to explore social differences in early urban contexts: The case of Lattara in mediterranean France. J. Archaeol. Sci. 2018, 93, 135–149. [Google Scholar] [CrossRef]
- Bijoor, N.S.; Czimczik, C.I.; Pataki, D.E.; Billings, S.A. Effects of temperature and fertilization on nitrogen cycling and community composition of an urban lawn. Glob. Change Biol. 2008, 14, 2119–2131. [Google Scholar] [CrossRef]
- Cobley, L.A.E.; Pataki, D.E.; McCarthy, H.R.; Martin, S.A.; Ehleringer, J.R. Housing age and affluence influence plant and soil nitrogen and carbon cycles in two semiarid cities. J. Geophys. Res.-Biogeo. 2018, 123, 3178–3192. [Google Scholar] [CrossRef]
- Cobley, L.A.E.; Pataki, D.E. Vehicle emissions and fertilizer impact the leaf chemistry of urban trees in Salt Lake Valley, UT. Environ. Pollut. 2019, 254, 112984. [Google Scholar] [CrossRef] [PubMed]
- Cobley, L.A.E.; Pataki, D.E.; Adler, F.R.; Hinners, S.J. Using traffic density and foliar chemistry variables to understand interactions between air pollution and household income. J. Geophys. Res.-Atmos. 2021, 126, e2021JD034942. [Google Scholar] [CrossRef]
- Rogers, K.M.; Turnbull, R.E.; Martin, A.P.; Baisden, W.T.; Rattenbury, M.S. Stable isotopes reveal human influences on southern New Zealand soils. Appl. Geochem. 2017, 82, 15–24. [Google Scholar] [CrossRef]
- Ding, Q.; Shao, H.; Zhang, C.; Fang, X. The patterns of soil nitrogen stocks and C: N stoichiometry under impervious surfaces in China. Earth Syst. Sci. Data 2023, 15, 4599–4612. [Google Scholar] [CrossRef]
- Li, F.; Sun, B.; Shi, Z.; Pei, N. Changes in ecological stoichiometry and nutrient resorption in Castanopsis hystrix plantations along an urbanization gradient in the lower subtropics. J. For. Res. 2021, 32, 2323–2331. [Google Scholar] [CrossRef]
- Shi, L.; Li, Q.; Fu, X.; Kou, L.; Dai, X.; Wang, H. Foliar, root and rhizospheric soil C: N: P stoichiometries of overstory and understory species in subtropical plantations. Catena 2021, 198, 105020. [Google Scholar] [CrossRef]
- Filipiak, Z.M.; Mayoral, C.; Mills, S.A.; Hayward, S.A.; Ullah, S. Elevated atmospheric CO2 alters the multi-element stoichiometry of pollen-bearing oak flowers, with possible negative effects on bees. Oecologia 2024, 206, 101–114. [Google Scholar] [CrossRef]
- Dong, L.; Song, A.; Zhang, J.; Peng, L.; Cheng, N.; Cao, B. Comparison of C, N and P Stoichiometry in Different Organs of Fraxinus velutina. Forests 2023, 14, 64. [Google Scholar] [CrossRef]
- Hou, X.; Wu, X.; Ma, C.; Tian, D.; Yan, Z.; Li, P. Effect of the elevated ozone on greening tree species of urban: Alterations in CNP stoichiometry and nutrient stock allocation to leaves and fine roots. Urban For. Urban Green. 2022, 76, 127735. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macci, C.; Vannucchi, F.; Scartazza, A.; Masciandaro, G.; Doni, S.; Peruzzi, E. Soil–Plant Indicators for Assessing Nutrient Cycling and Ecosystem Functionality in Urban Forestry. Urban Sci. 2025, 9, 82. https://doi.org/10.3390/urbansci9030082
Macci C, Vannucchi F, Scartazza A, Masciandaro G, Doni S, Peruzzi E. Soil–Plant Indicators for Assessing Nutrient Cycling and Ecosystem Functionality in Urban Forestry. Urban Science. 2025; 9(3):82. https://doi.org/10.3390/urbansci9030082
Chicago/Turabian StyleMacci, Cristina, Francesca Vannucchi, Andrea Scartazza, Grazia Masciandaro, Serena Doni, and Eleonora Peruzzi. 2025. "Soil–Plant Indicators for Assessing Nutrient Cycling and Ecosystem Functionality in Urban Forestry" Urban Science 9, no. 3: 82. https://doi.org/10.3390/urbansci9030082
APA StyleMacci, C., Vannucchi, F., Scartazza, A., Masciandaro, G., Doni, S., & Peruzzi, E. (2025). Soil–Plant Indicators for Assessing Nutrient Cycling and Ecosystem Functionality in Urban Forestry. Urban Science, 9(3), 82. https://doi.org/10.3390/urbansci9030082






