Desiccation Tolerance of Aedes aegypti and Aedes albopictus Eggs of Northeastern Argentina Origin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Eggs Collection
2.3. Relative Humidity Experimental Design
2.4. Data Analysis
3. Results
3.1. Egg Survival Under Various RH Conditions in 1 Month of Exposure
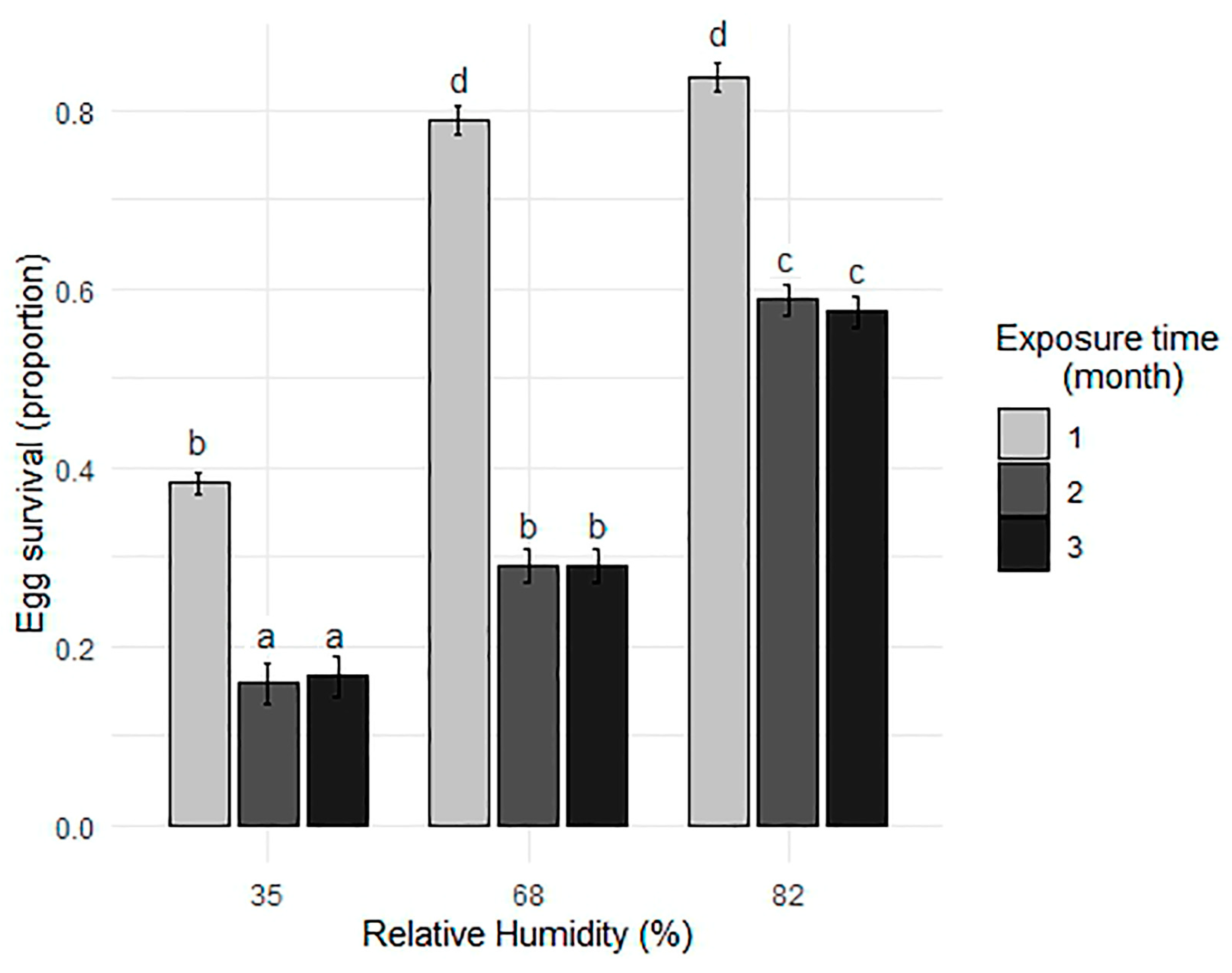
3.2. Egg Survival Under Various RH Conditions During 1, 2, and 3 Months of Exposure
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministerio de Salud de la República Argentina. Boletín Epidemiológico Nacional N° 709, SE 24. 2024. Available online: https://www.argentina.gob.ar/sites/default/files/2024/04/ben-709-se24.pdf (accessed on 1 March 2025).
- Rubio, A.; Cardo, M.V.; Vezzani, D.; Carbajo, A.E. Aedes aegypti spreading in South America: New coldest and southernmost records. Mem. Inst. Oswaldo Cruz 2020, 115, e190496. [Google Scholar] [CrossRef] [PubMed]
- Goenaga, S.; Chuchuy, A.; Micieli, M.V.; Natalini, B.; Kuruc, J.; Kowalewski, M. Expansion of the Distribution of Aedes albopictus (Diptera: Culicidae): New Records in Northern Argentina and Their Implications From an Epidemiological Perspective. J. Med. Entomol. 2020, 57, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rejon, J.E.; Navarro, J.C.; Cigarroa-Toledo, N.; Baak-Baak, C.M. An Updated Review of the Invasive Aedes albopictus in the Americas; Geographical Distribution, Host Feeding Patterns, Arbovirus Infection, and the Potential for Vertical Transmission of Dengue Virus. Insects 2021, 12, 967. [Google Scholar] [CrossRef] [PubMed]
- Christophers, S. Its life history, bionomics, and structure. In Aedes aegypti (L.), the Yellow Fever Mosquito; Cambridge University Press: Cambridge, UK, 1960. [Google Scholar]
- Higa, Y. Dengue vectors and their spatial distribution. Trop. Med. Health 2011, 39 (Suppl. 4), 17–27. [Google Scholar] [CrossRef]
- Vitek, C.J.; Livdahl, T.P. Field and laboratory comparison of hatch rates in Aedes albopictus (Skuse). J. Am. Mosq. Control. Assoc. 2006, 22, 609–614. [Google Scholar] [CrossRef]
- Sota, T.; Mogi, M. Survival time and resistance to desiccation of diapause and non-diapause eggs of temperate Aedes (Stegomyia) mosquitoes. Entomol. Exp. Appl. 1992, 63, 155–161. [Google Scholar] [CrossRef]
- Juliano, S.A.; Lounibos, P.L. Ecology of invasive mosquitoes: Effects on resident species and on human health. Ecol. Lett. 2005, 8, 558–574. [Google Scholar] [CrossRef]
- Juliano, S.A.; O’Meara, G.F.; Morrill, J.R.; Cutwa, M.M. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia 2002, 130, 458. [Google Scholar] [CrossRef]
- Ponce, G.; Flores, A.E.; Badii, M.H.; Fernández, I. Bionomía de Aedes albopictus (Skuse). Rev. Salud Pública Nutr. 2004, 5. Available online: https://respyn.uanl.mx/index.php/respyn/article/view/127 (accessed on 15 February 2025).
- Trpis, M. Dry season survival of Aedes aegypti eggs in various breeding sites in the Dar es Salaam area, Tanzania. Bull. World Health Organ. 1972, 47, 433. [Google Scholar]
- Sota, T.; Mogi, M. Interspecific variation in desiccation survival time of Aedes (Stegomyia) mosquito eggs is correlated with habitat and egg size. Oecologia 1992, 90, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.; Ritchie, S.A.; Russell, R.C.; Zalucki, M.P.; Den Hurk, A.F.V. Ability for Aedes albopictus (Diptera: Culicidae) to survive at the climatic limits of its potential range in Eastern Australia. J. Med. Entomol. 2014, 51, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhao, J.; Deng, H.; Xiao, J.; Liu, T.; Zeng, W.; Li, X.; Hu, J.; Huang, C.; Zhu, G.; et al. Effects of temperature, relative humidity, and illumination on the entomological parameters of Aedes albopictus: An experimental study. Int. J. Biometeorol. 2023, 67, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Alem, I.S.; De Majo, M.S.; Campos, R.E.; Schweigmann, N. Cold season mortality and hatching behavior of Aedes aegypti L. (Diptera: Culicidae) eggs in Buenos Aires City, Argentina. J. Vector Ecol. 2011, 36, 94–99. [Google Scholar] [CrossRef]
- Giménez, J.O.; Fischer, S.; Zalazar, L.; Stein, M. Cold Season Mortality under Natural Conditions and Subsequent Hatching Response of Aedes (Stegomyia) aegypti (Diptera: Culicidae) Eggs in a Subtropical City of Argentina. J. Med. Entomol. 2015, 52, 879–885. [Google Scholar] [CrossRef]
- Obholz, G.; San Blas, G.; Fischer, S.; Diaz, A. Winter survival of Aedes aegypti (Diptera: Culicidae) eggs at its southern limit distribution. Acta Trop. 2022, 231, 106471. [Google Scholar] [CrossRef]
- Garzón, M.J.; Maffey, L.; Lizuain, A.; Soto, D.; Diaz, P.C.; Leporace, M.; Salomón, O.D.; Schweigmann, N.J. Temperature and photoperiod effects on dormancy status and life cycle parameters in Aedes albopictus and Aedes aegypti from subtropical Argentina. Med. Vet. Entomol. 2020, 35, 97–105. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística y Censos (INDEC). Censo Nacional de Población, Hogares y Viviendas 2010. Nomenclador Nacional de Vías de Circulación. 2022. Available online: https://www.indec.gob.ar/ (accessed on 15 February 2025).
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Lizuain, A.A.; Leporace, M.; Santini, M.S.; Utgés, M.E.; Schweigmann, N. Update on the distribution of Aedes albopictus (Diptera: Culicidae) in Misiones, Argentina. Rev. Inst. Med. Trop. Sao Paulo 2019, 61, e46. [Google Scholar] [CrossRef]
- Winston, P.W.; Bates, D.H. Saturated Solutions For the Control of Humidity in Biological Research. Ecology 1960, 41, 232–237. [Google Scholar] [CrossRef]
- Isoe, J.; Koch, L.E.; Isoe, Y.E.; Rascón, A.A.; Brown, H.E.; Massani, B.B.; Miesfeld, R.L. Identification and characterization of a mosquito-specific eggshell organizing factor in Aedes aegypti mosquitoes. PLoS Biol. 2019, 17, e3000068. [Google Scholar] [CrossRef] [PubMed]
- McHaffey, D.G.; Harwood, R.F. Photoperiod and temperature influences on diapause in eggs of the floodwater mosquito, Aedes dorsalis (Meigen) (Diptera: Culicidae). J. Med. Entomol. 1970, 7, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Conover, W.J. Practical Nonparametric Statistical, 3rd ed.; John Wiley & Sons Inc.: New York, NY, USA, 1999; pp. 428–433. [Google Scholar]
- Mayilsamy, M. Extremely Long Viability of Aedes aegypti (Diptera: Culicidae) Eggs Stored Under Normal Room Condition. J. Med. Entomol. 2019, 56, 878–880. [Google Scholar] [CrossRef]
- Hosch, S.A. A Comparison of Egg Desiccation Tolerance and Development Under Different Temperatures for Three Common Aedes mosquitoes. Ph.D. Thesis, The University of Southern Mississippi, Hattiesburg, MS, USA, 2020. Available online: https://aquila.usm.edu/honors_theses/719 (accessed on 15 February 2025).
- Faull, K.J.; Williams, C.R. Intraspecific variation in desiccation survival time of Aedes aegypti (L.) mosquito eggs of Australian origin. J. Vector Ecol. 2015, 40, 292–300. [Google Scholar] [CrossRef]
- Rezende, G.L.; Martins, A.J.; Gentile, C.; Farnesi, L.C.; Pelajo-Machado, M.; Peixoto, A.A.; Valle, D. Embryonic desiccation resistance in Aedes aegypti: Presumptive role of the chitinized Serosal Cuticle. BMC Dev. Biol. 2008, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Vargas, H.C.M.; Farnesi, L.C.; Martins, A.J.; Valle, D.; Rezende, G.L. Serosal cuticle formation and distinct degrees of desiccation resistance in embryos of the mosquito vectors Aedes aegypti, Anopheles aquasalis and Culex quinquefasciatus. J. Insect Physiol. 2014, 62, 54–60. [Google Scholar] [CrossRef]
- Lounibos, L.P.; O’Meara, G.F.; Juliano, S.A.; Nishimura, N.; Escher, R.L.; Reiskind, M.H.; Cutwa, M.; Greene, K. Differential Survivorship of Invasive Mosquito Species in South Florida Cemeteries: Do Site-Specific Microclimates Explain Patterns of Coexistence and Exclusion? Ann. Entomol. Soc. Am. 2010, 103, 757–770. [Google Scholar] [CrossRef]
- Urbanski, J.M.; Benoit, J.B.; Michaud, M.R.; Denlinger, D.L.; Armbruster, P. The molecular physiology of increased egg desiccation resistance during diapause in the invasive mosquito, Aedes albopictus. Proc. R. Soc. B Biol. Sci. 2010, 277, 2683–2692. [Google Scholar] [CrossRef]
- Yee, D.A.; Juliano, S.A.; Vamosi, S.M. Seasonal Photoperiods Alter Developmental Time and Mass of an Invasive Mosquito, Aedes albopictus (Diptera: Culicidae), Across Its North-South Range in the United States. J. Med. Entomol. 2012, 49, 825–832. [Google Scholar] [CrossRef]
- Costanzo, K.S.; Kesavaraju, B.; Juliano, S.A. Condition-specific competition in container mosquitoes: The role of noncompeting life-history stages. Ecology 2005, 86, 3289–3295. [Google Scholar] [CrossRef]
- Braks, M.A.H.; Honório, N.A.; Lourenço-De-Oliveira, R.; Juliano, S.A.; Lounibos, L.P. Convergent Habitat Segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Southeastern Brazil and Florida. J. Med. Entomol. 2003, 40, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.R.; Nishimura, N.; Wagner, B.; Braks, M.A.H.; O’Connell, S.M.; Philip Lounibos, L. Habitat Segregation of Mosquito Arbovirus Vectors in South Florida. J. Med. Entomol. 2006, 43, 1134. [Google Scholar] [CrossRef] [PubMed]
- Kotsakiozi, P.; Richardson, J.B.; Pichler, V.; Favia, G.; Martins, A.J.; Urbanelli, S.; Armbruster, P.A.; Caccone, A. Population genomics of the Asian tiger mosquito, Aedes albopictus: Insights into the recent worldwide invasion. Ecol. Evol. 2017, 7, 10143–10157. [Google Scholar] [CrossRef] [PubMed]
- Birungi, J.; Munstermann, L.E. Genetic Structure of Aedes albopictus (Diptera: Culicidae) Populations Based on Mitochondrial ND5 Sequences: Evidence for an Independent Invasion into Brazil and United States. Ann. Entomol. Soc. Am. 2002, 95, 125–132. [Google Scholar] [CrossRef]
- Judson, C.L. The Physiology of Hatching of Aedine Mosquito Eggs: Hatching Stimulus. Ann. Entomol. Soc. Am. 1960, 53, 688–691. [Google Scholar] [CrossRef]
- da Silva, A.C.; Scalize, P.S. Environmental Variables Related to Aedes aegypti Breeding Spots and the Occurrence of Arbovirus Diseases. Sustainability 2023, 15, 8148. [Google Scholar] [CrossRef]
- Egid, B.R.; Coulibaly, M.; Dadzie, S.K.; Kamgang, B.; McCall, P.J.; Sedda, L.; Toe, K.H.; Wilson, A.L. Review of the ecology and behaviour of Aedes aegypti and Aedes albopictus in Western Africa and implications for vector control. Curr. Res. Parasitol. Vector-Borne Dis. 2022, 2, 100074. [Google Scholar] [CrossRef]
- Guo, X.; Luo, L.; Long, Y.; Teng, P.; Wei, Y.; Xie, T.; Li, L.; Yin, Q.; Li, Z.; Wang, Y.; et al. Field investigation combined with modeling uncovers the ecological heterogeneity of Aedes albopictus habitats for strategically improving systematic management during urbanization. Parasites Vectors 2023, 16, 382. [Google Scholar] [CrossRef]
- Faraone, J.; Fischer, S.; Aponte, C.A.; Etchepare, E.; Stechina, O.S.; Stein, M. Hatching pattern and coexistence of Aedes aegypti and Aedes albopictus (Culicidae) in a subtropical city, Argentina, after three decades of coexistence. Acta Trop. 2021, 218, 105885. [Google Scholar] [CrossRef]
- Martín, M.E.; Alonso, A.C.; Faraone, J.; Stein, M.; Estallo, E.L. Satellite observation to assess dengue risk due to Aedes aegypti and Aedes albopictus in a subtropical city. Med. Vet. Entomol. 2023, 37, 27–36. [Google Scholar] [CrossRef]

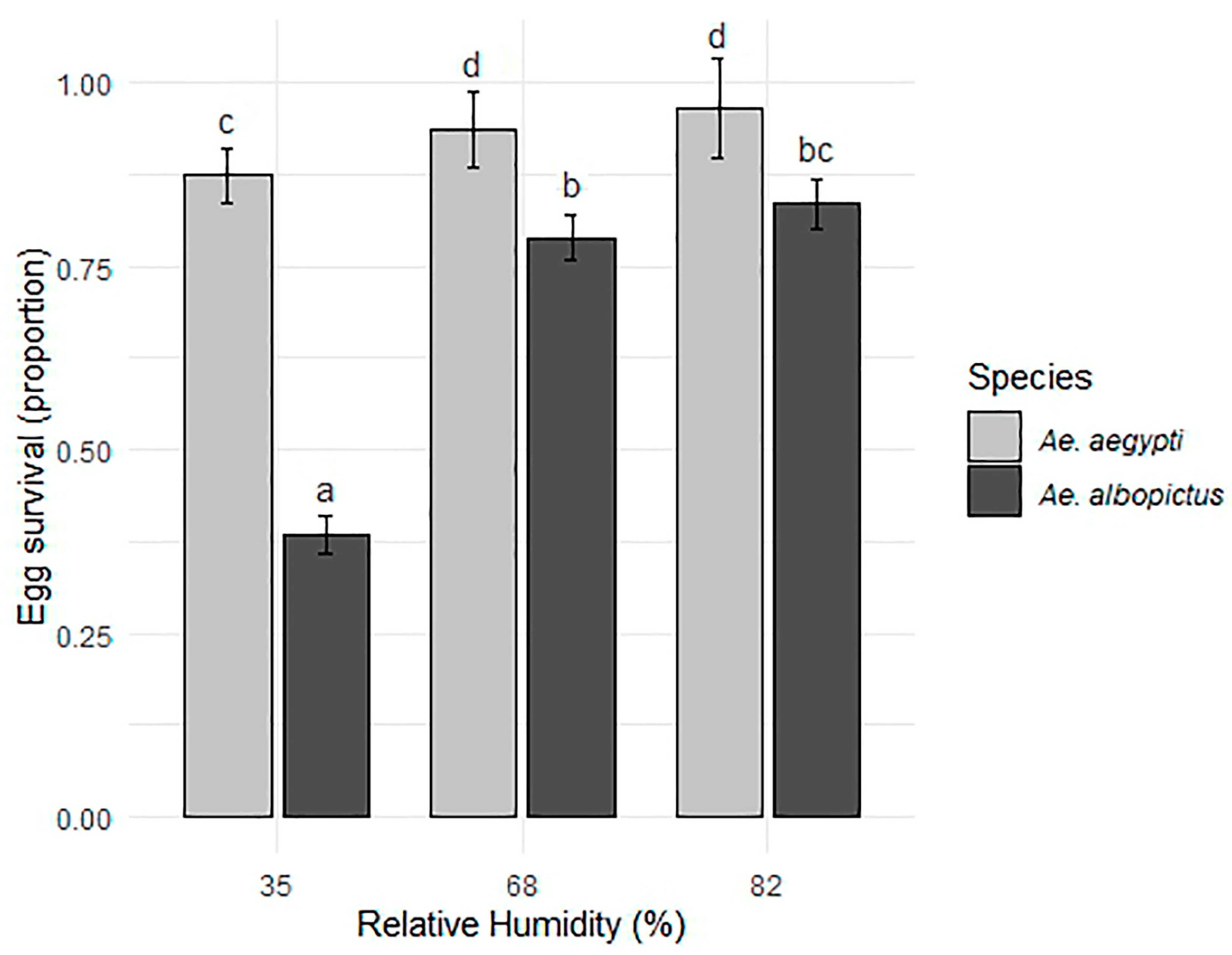

| Species | Relative Humidity (%) | N | Survival | SD | CI |
|---|---|---|---|---|---|
| Aedes aegypti | 35 | 11 | 0.880 | 0.017 | 0.039 |
| 68 | 11 | 0.952 | 0.016 | 0.036 | |
| 82 | 11 | 0.973 | 0.007 | 0.016 | |
| Aedes albopictus | 35 | 11 | 0.376 | 0.045 | 0.101 |
| 68 | 11 | 0.791 | 0.016 | 0.036 | |
| 82 | 11 | 0.841 | 0.024 | 0.053 |
| Exposure Time (Month) | Relative Humidity (%) | N | Survival | SD | CI |
|---|---|---|---|---|---|
| 1 | 35 | 6 | 0.879 | 0.019 | 0.050 |
| 1 | 68 | 6 | 0.953 | 0.014 | 0.037 |
| 1 | 82 | 6 | 0.957 | 0.011 | 0.028 |
| 2 | 35 | 6 | 0.886 | 0.042 | 0.109 |
| 2 | 68 | 6 | 0.933 | 0.020 | 0.051 |
| 2 | 82 | 6 | 0.941 | 0.023 | 0.058 |
| 3 | 35 | 6 | 0.310 | 0.074 | 0.189 |
| 3 | 68 | 6 | 0.739 | 0.105 | 0.267 |
| 3 | 82 | 6 | 0.913 | 0.029 | 0.074 |
| Exposure Time (Month) | Relative Humidity (%) | N | Survival | SD | CI |
|---|---|---|---|---|---|
| 1 | 35 | 6 | 0.397 | 0.061 | 0.156 |
| 1 | 68 | 6 | 0.790 | 0.018 | 0.047 |
| 1 | 82 | 6 | 0.819 | 0.040 | 0.102 |
| 2 | 35 | 6 | 0.159 | 0.007 | 0.017 |
| 2 | 68 | 6 | 0.290 | 0.018 | 0.046 |
| 2 | 82 | 6 | 0.588 | 0.034 | 0.089 |
| 3 | 35 | 6 | 0.167 | 0.026 | 0.067 |
| 3 | 68 | 6 | 0.290 | 0.045 | 0.117 |
| 3 | 82 | 6 | 0.574 | 0.053 | 0.136 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, M.E.; Estallo, E.L.; Estrada, L.G.; Matiz Enriquez, C.; Stein, M. Desiccation Tolerance of Aedes aegypti and Aedes albopictus Eggs of Northeastern Argentina Origin. Trop. Med. Infect. Dis. 2025, 10, 116. https://doi.org/10.3390/tropicalmed10040116
Martín ME, Estallo EL, Estrada LG, Matiz Enriquez C, Stein M. Desiccation Tolerance of Aedes aegypti and Aedes albopictus Eggs of Northeastern Argentina Origin. Tropical Medicine and Infectious Disease. 2025; 10(4):116. https://doi.org/10.3390/tropicalmed10040116
Chicago/Turabian StyleMartín, Mía E., Elizabet L. Estallo, Luis G. Estrada, Carolina Matiz Enriquez, and Marina Stein. 2025. "Desiccation Tolerance of Aedes aegypti and Aedes albopictus Eggs of Northeastern Argentina Origin" Tropical Medicine and Infectious Disease 10, no. 4: 116. https://doi.org/10.3390/tropicalmed10040116
APA StyleMartín, M. E., Estallo, E. L., Estrada, L. G., Matiz Enriquez, C., & Stein, M. (2025). Desiccation Tolerance of Aedes aegypti and Aedes albopictus Eggs of Northeastern Argentina Origin. Tropical Medicine and Infectious Disease, 10(4), 116. https://doi.org/10.3390/tropicalmed10040116






