Empirical Correlation between Electrical Conductivity and Nitrogen Content in Biochar as Influenced by Pyrolysis Temperature
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Basic Properties of the Biochars
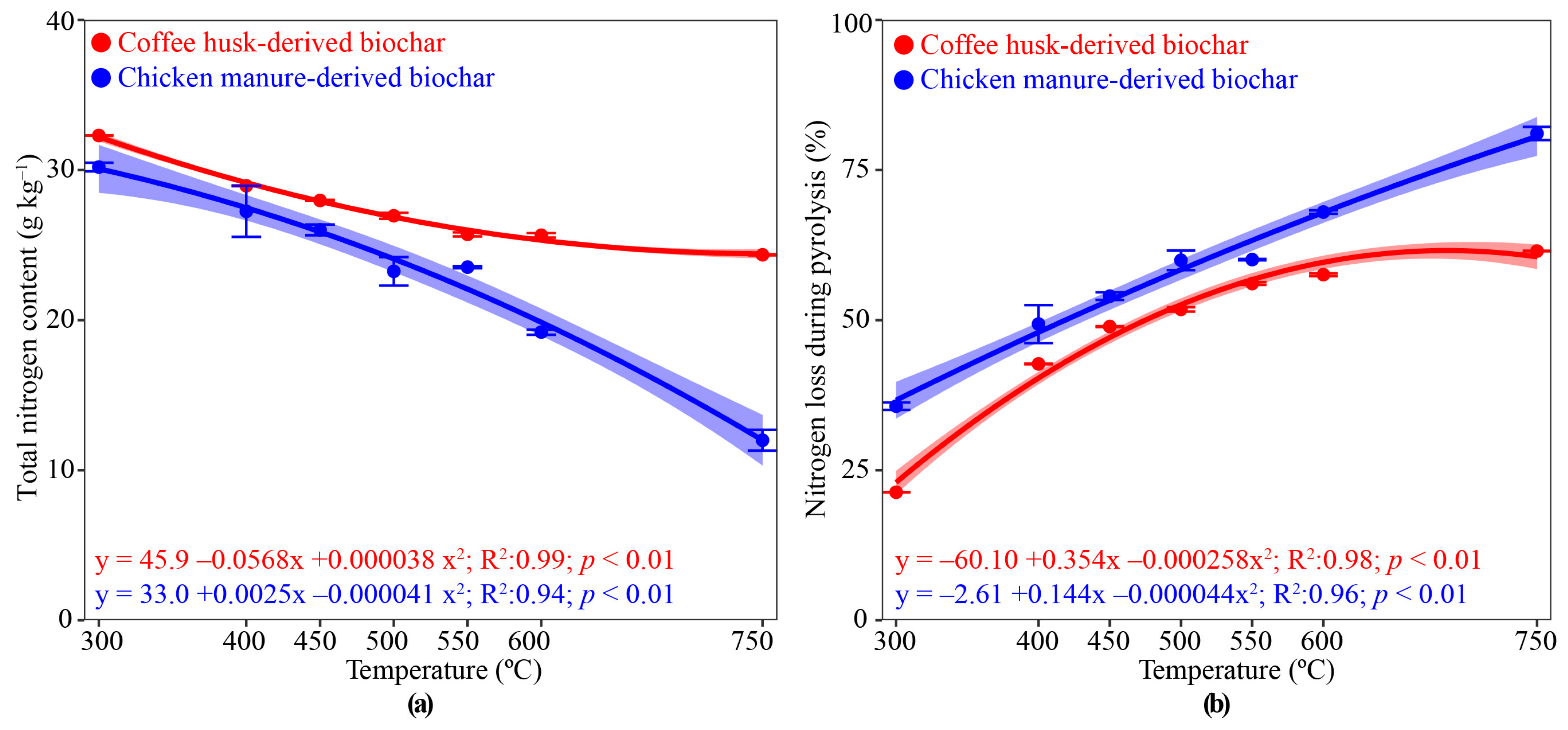
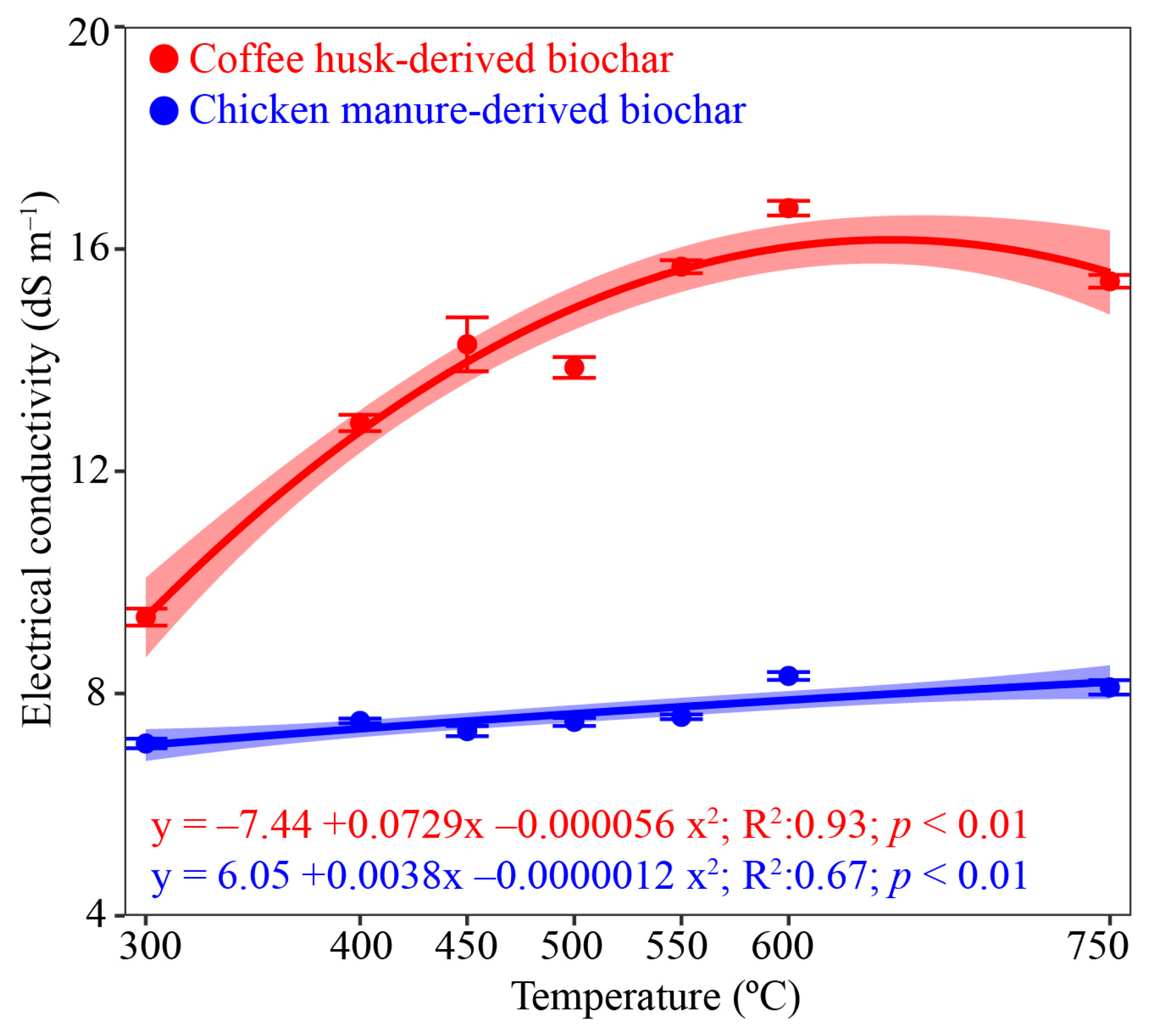
3.2. Biochar Nitrogen and Electrical Conductivity as PT Increased
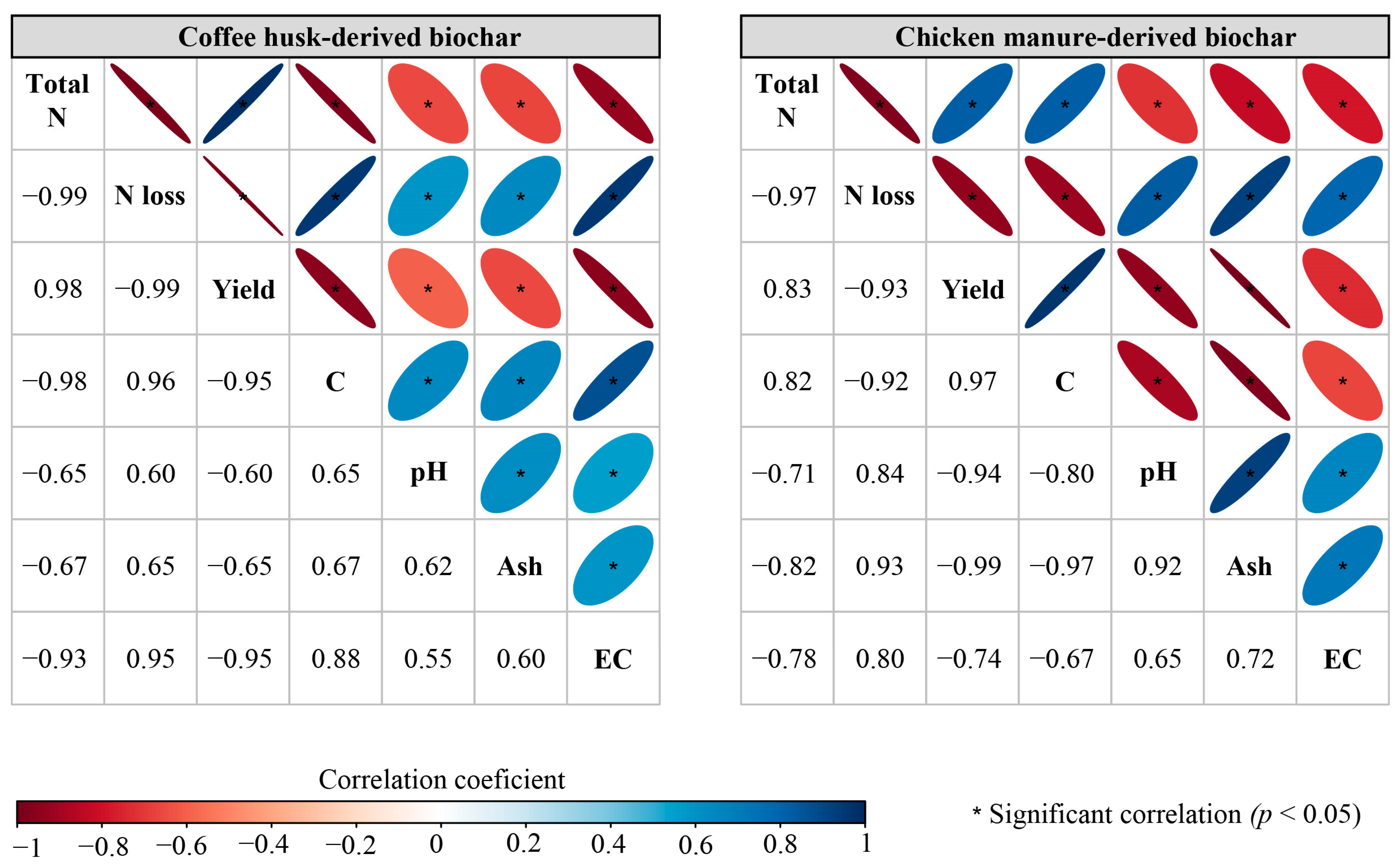
3.3. Correlation Analysis of Biochar Measurements
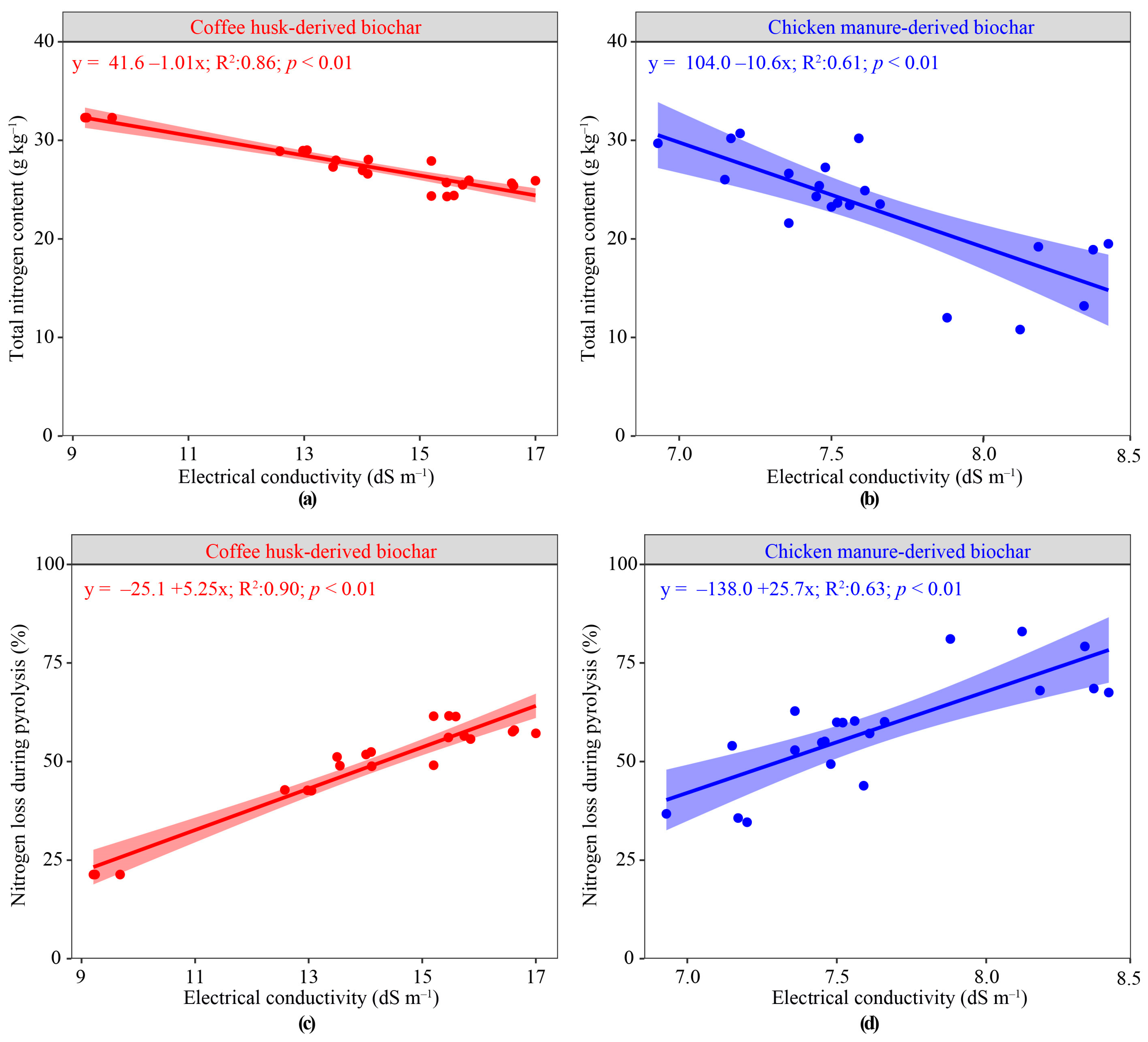
3.4. Total N Content and N Loss Predicted by Electrical Conductivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: An Introduction. In Biochar for Environmental Management; Routledge: New York, USA, 2009; pp. 1–12. [Google Scholar]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How Biochar Works, and When It Doesn’t: A Review of Mechanisms Controlling Soil and Plant Responses to Biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock Choice, Pyrolysis Temperature and Type Influence Biochar Characteristics: A Comprehensive Meta-Data Analysis Review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Lago, B.C.; Silva, C.A.; Melo, L.C.A.; de Morais, E.G. Predicting Biochar Cation Exchange Capacity Using Fourier Transform Infrared Spectroscopy Combined with Partial Least Square Regression. Sci. Total Environ. 2021, 794, 148762. [Google Scholar] [CrossRef] [PubMed]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for Soil Applications-Sustainability Aspects, Challenges and Future Prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K.-H. Benefits and Limitations of Biochar Amendment in Agricultural Soils: A Review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; De Melo, I.C.N.A.; Melo, L.C.A.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of Biochar Derived from Wood and High-Nutrient Biomasses with the Aim of Agronomic and Environmental Benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A Critical Review on the Biochar Production Techniques, Characterization, Stability and Applications for Circular Bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Y.; Singh, B.P.; Wang, H.; Cai, X.; Chen, J.; Qin, H.; Li, Y.; Chang, S.X. Contrasting Short-Term Responses of Soil Heterotrophic and Autotrophic Respiration to Biochar-Based and Chemical Fertilizers in a Subtropical Moso Bamboo Plantation. Appl. Soil Ecol. 2021, 157, 103758. [Google Scholar] [CrossRef]
- De Morais, E.G.; Silva, C.A.; Jindo, K. Biochar-Based Phosphate Fertilizers: Synthesis, Properties, Kinetics of P Release and Recommendation for Crops Grown in Oxisols. Agronomy 2023, 13, 326. [Google Scholar] [CrossRef]
- de oliveira Paiva, I.; de Morais, E.G.; Jindo, K.; Silva, C.A. Biochar N Content, Pools and Aromaticity as Affected by Feedstock and Pyrolysis Temperature. J. Soil Sci. Plant Nutr. 2024, 1, 1–21. [Google Scholar] [CrossRef]
- Liu, W.J.; Li, W.W.; Jiang, H.; Yu, H.Q. Fates of Chemical Elements in Biomass during Its Pyrolysis. Chem. Rev. 2017, 117, 6367–6398. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tan, Z.; Zhang, L.; Huang, Q. Influence of Pyrolysis Conditions on Nitrogen Speciation in a Biochar ‘Preparation-Application’ Process. J. Energy Inst. 2018, 91, 916–926. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Wang, T.; Camps Arbestain, M.; Hedley, M.; Bishop, P. Chemical and Bioassay Characterisation of Nitrogen Availability in Biochar Produced from Dairy Manure and Biosolids. Org. Geochem. 2012, 51, 45–54. [Google Scholar] [CrossRef]
- Leng, L.; Xu, S.; Liu, R.; Yu, T.; Zhuo, X.; Leng, S.; Xiong, Q.; Huang, H. Nitrogen Containing Functional Groups of Biochar: An Overview. Bioresour. Technol. 2020, 298, 122286. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Patra, B.R.; Podder, J.; Dalai, A.K. Synthesis of Biochar From Lignocellulosic Biomass for Diverse Industrial Applications and Energy Harvesting: Effects of Pyrolysis Conditions on the Physicochemical Properties of Biochar. Front. Mater. 2022, 9, 870184. [Google Scholar] [CrossRef]
- Morais, E.G.; Silva, C.A.; Maluf, H.J.G.M.; de oliveira Paiva, I.; de Paula, L.H.D. Effects of Compost-Based Organomineral Fertilizers on the Kinetics of NPK Release and Maize Growth in Contrasting Oxisols. Waste Biomass Valoriz. 2022, 14, 2299–2321. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of Pyrolysis Temperature and Manure Source on Physicochemical Characteristics of Biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Carnier, R.; Coscione, A.R.; Delaqua, D.; Abreu, C.A.D. Coffee Industry Waste-Derived Biochar: Characterization and Agricultural Use Evaluation According to Brazilian Legislation. Bragantia 2021, 80, e5721. [Google Scholar] [CrossRef]
- Başer, B.; Yousaf, B.; Yetis, U.; Abbas, Q.; Kwon, E.E.; Wang, S.; Bolan, N.S.; Rinklebe, J. Formation of Nitrogen Functionalities in Biochar Materials and Their Role in the Mitigation of Hazardous Emerging Organic Pollutants from Wastewater. J. Hazard. Mater. 2021, 416, 126131. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, Z.; Van Zwieten, L.; Bolan, N.; Dong, D.; Quin, B.F.; Meng, J.; Li, F.; Wu, F.; Wang, H.; et al. A Critical Review of Biochar-Based Nitrogen Fertilizers and Their Effects on Crop Production and the Environment. Biochar 2022, 4, 36. [Google Scholar] [CrossRef]
- Mainali, K.; Garcia-Perez, M. Understanding Organic N Interactions with Cellulose during Pyrolysis to Produce N-Doped Carbonaceous Materials. J. Anal. Appl. Pyrolysis 2023, 169, 105791. [Google Scholar] [CrossRef]
- Xu, S.; Chen, J.; Peng, H.; Leng, S.; Li, H.; Qu, W.; Hu, Y.; Li, H.; Jiang, S.; Zhou, W.; et al. Effect of Biomass Type and Pyrolysis Temperature on Nitrogen in Biochar, and the Comparison with Hydrochar. Fuel 2021, 291, 120128. [Google Scholar] [CrossRef]
- Zhan, H.; Zhuang, X.; Song, Y.; Chang, G.; Wang, Z.; Yin, X.; Wang, X.; Wu, C. Formation and Regulatory Mechanisms of N-Containing Gaseous Pollutants during Stage-Pyrolysis of Agricultural Biowastes. J. Clean. Prod. 2019, 236, 117706. [Google Scholar] [CrossRef]
- Zhan, H.; Zhuang, X.; Song, Y.; Huang, Y.; Liu, H.; Yin, X.; Wu, C. Evolution of Nitrogen Functionalities in Relation to NO Precursors during Low-Temperature Pyrolysis of Biowastes. Fuel 2018, 218, 325–334. [Google Scholar] [CrossRef]
- Chacón, F.J.; Cayuela, M.L.; Roig, A.; Sánchez-Monedero, M.A. Understanding, Measuring and Tuning the Electrochemical Properties of Biochar for Environmental Applications. Rev. Environ. Sci. Bio/Technol. 2017, 16, 695–715. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Cao, L.; Ok, Y.S.; Cao, X. Characterization and Quantification of Electron Donating Capacity and Its Structure Dependence in Biochar Derived from Three Waste Biomasses. Chemosphere 2018, 211, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Gabhi, R.S.; Kirk, D.W.; Jia, C.Q. Preliminary Investigation of Electrical Conductivity of Monolithic Biochar. Carbon N. Y. 2017, 116, 435–442. [Google Scholar] [CrossRef]
- Bartoli, M.; Troiano, M.; Giudicianni, P.; Amato, D.; Giorcelli, M.; Solimene, R.; Tagliaferro, A. Effect of Heating Rate and Feedstock Nature on Electrical Conductivity of Biochar and Biochar-Based Composites. Appl. Energy Combust. Sci. 2022, 12, 100089. [Google Scholar] [CrossRef]
- Chintala, R.; Clay, D.E.; Schumacher, T.E.; Malo, D.D.; Julson, J.L. Optimization of Oxygen Parameters for Determination of Carbon and Nitrogen in Biochar Materials. Anal. Lett. 2013, 46, 532–538. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Mandal, S.; Niazi, N.K.; Vithanage, M.; Parikh, S.J.; Mukome, F.N.D.; Rizwan, M.; Oleszczuk, P.; Al-Wabel, M.; Bolan, N.; et al. Advances and Future Directions of Biochar Characterization Methods and Applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2275–2330. [Google Scholar] [CrossRef]
- Singh, B.; Camps-Arbestain, M.; Lehmann, J.; CSIRO (Australia). Biochar: A Guide to Analytical Methods; CRC Press: Boca Raton, FL, USA, 2017; ISBN 1486305105. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses 2020. Available online: https://rpkgs.datanovia.com/factoextra/index.html (accessed on 11 December 2023).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; 2023; Available online: https://www.r-project.org/ (accessed on 11 December 2023).
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix; 2017; Available online: https://cran.r-project.org/web/packages/corrplot/ (accessed on 11 December 2023).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, S.; Yin, M.; He, Z.; Bi, D. Co-Pyrolysis of Cellulose with Urea and Chitosan to Produce Nitrogen-Containing Compounds and Nitrogen-Doped Biochar: Product Distribution Characteristics and Reaction Path Analysis. J. Anal. Appl. Pyrolysis 2023, 169, 105795. [Google Scholar] [CrossRef]
- Raclavská, H.; Růžičková, J.; Raclavský, K.; Juchelková, D.; Kucbel, M.; Švédová, B.; Slamová, K.; Kacprzak, M. Effect of Biochar Addition on the Improvement of the Quality Parameters of Compost Used for Land Reclamation. Environ. Sci. Pollut. Res. 2021, 30, 8563–8581. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A Bayesian Extension of the Minimum AIC Procedure of Autoregressive Model Fitting. Biometrika 1979, 66, 237. [Google Scholar] [CrossRef]
- Broer, K.M.; Brown, R.C. The Role of Char and Tar in Determining the Gas-Phase Partitioning of Nitrogen during Biomass Gasification. Appl. Energy 2015, 158, 474–483. [Google Scholar] [CrossRef]
- Wang, B.; Lehmann, J.; Hanley, K.; Hestrin, R.; Enders, A. Ammonium Retention by Oxidized Biochars Produced at Different Pyrolysis Temperatures and Residence Times. RSC Adv. 2016, 6, 41907–41913. [Google Scholar] [CrossRef]
- Liu, S.; He, Z.; Dong, Q.; Zhao, A.; Huang, F.; Bi, D. Comparative Assessment of Water and Organic Acid Washing Pretreatment for Nitrogen-Rich Pyrolysis: Characteristics and Distribution of Bio-Oil and Biochar. Biomass Bioenergy 2022, 161, 106480. [Google Scholar] [CrossRef]
- Gabhi, R.; Basile, L.; Kirk, D.W.; Giorcelli, M.; Tagliaferro, A.; Jia, C.Q. Electrical Conductivity of Wood Biochar Monoliths and Its Dependence on Pyrolysis Temperature. Biochar 2020, 2, 369–378. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. Modification of Biochar Surface by Air Oxidation: Role of Pyrolysis Temperature. Biomass Bioenergy 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Shi, J.; Fan, X.; Tsang, D.C.W.; Wang, F.; Shen, Z.; Hou, D.; Alessi, D.S. Removal of Lead by Rice Husk Biochars Produced at Different Temperatures and Implications for Their Environmental Utilizations. Chemosphere 2019, 235, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Maluf, H.J.G.M.; Silva, C.A.; de Morais, E.G.; Paula, L.H.D.D. Is Composting a Route to Solubilize Low-Grade Phosphate Rocks and Improve MAP-Based Composts? Rev. Bras. Ciência do Solo 2018, 42, e0170079. [Google Scholar] [CrossRef]
- Kane, S.; Ulrich, R.; Harrington, A.; Stadie, N.P.; Ryan, C. Physical and Chemical Mechanisms That Influence the Electrical Conductivity of Lignin-Derived Biochar. Carbon Trends 2021, 5, 100088. [Google Scholar] [CrossRef]
| Raw Biomass | Carbon Content (g kg−1) * | Nitrogen Content (g kg−1) * | pH * | Electrical Conductivity (dS m−1) * | Ash * %, w/w |
|---|---|---|---|---|---|
| Coffee husk | 40.7 | 18.8 ± 0.4 | 4.7 ± 0.03 | 6.93 ± 0.04 | 6.93 ± 0.73 |
| Chicken manure | 24.7 | 33.1 ± 0.1 | 7.7± 0.12 | 8.00 ± 0.04 | 34.2 ± 0.75 |
| Biochar | Temperature (°C) | Yield (%) * | Carbon Content (g kg−1) * | pH ** | Ash (%) ** |
|---|---|---|---|---|---|
| Coffee husk | 300 | 45.8 | 562 | 9.8 ± 2.02 | 13.1 ± 0.09 |
| 400 | 37.2 | 587 | 10.2 ± 1.37 | 15.1 ± 0.08 | |
| 450 | 34.3 | 601 | 9.9 ± 1.13 | 16.2 ± 0.02 | |
| 500 | 33.6 | 606 | 9.7 ± 1.33 | 15.7± 0.05 | |
| 550 | 32.1 | 624 | 10.2 ± 1.33 | 16.9 ± 0.07 | |
| 600 | 31.1 | 611 | 10.4 ± 1.30 | 18.0 ± 0.10 | |
| 750 | 29.7 | 634 | 10.9 ± 0.68 | 20.0 ± 0.03 | |
| Chicken manure | 300 | 70.5 | 274 | 8.7 ± 0.03 | 44.8 ± 0.03 |
| 400 | 61.5 | 258 | 10.53 ± 0.06 | 50.9 ± 0.06 | |
| 450 | 58.5 | 239 | 10.43 ± 0.08 | 53.9 ± 0.02 | |
| 500 | 57.0 | 239 | 10.51 ± 0.39 | 54.3 ± 0.05 | |
| 550 | 56.2 | 243 | 10.52 ± 0.31 | 55.5 ± 0.05 | |
| 600 | 55.2 | 238 | 10.59 ± 0.31 | 55.7 ± 0.01 | |
| 750 | 52.2 | 227 | 11.14 ± 0.42 | 58.4 ± 0.07 |
| Variable | Biochar | Linear | Quadratic | Elovich | Power | Hyperbolic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | AIC | R2 | RMSE | AIC | R2 | RMSE | AIC | R2 | RMSE | AIC | R2 | RMSE | AIC | ||
| Total N Content | CH | 0.89 | 0.8 | 57 | 0.99 | 0.2 | 9 | 0.96 | 0.5 | 35 | 0.98 | 0.4 | 25 | 0.98 | 0.3 | 18 |
| CM | 0.92 | 1.6 | 85 | 0.94 | 1.3 | 80 | 0.86 | 2.1 | 97 | 0.79 | 2.6 | 106 | 0.63 | 3.5 | 118 | |
| N loss | CH | 0.80 | 5.6 | 138 | 0.98 | 1.6 | 88 | 0.91 | 3.8 | 122 | 0.82 | 5.4 | 136 | 0.85 | 5.1 | 134 |
| CM | 0.96 | 2.7 | 108 | 0.96 | 2.6 | 107 | 0.96 | 2.7 | 107 | 0.96 | 2.6 | 106 | 0.96 | 2.6 | 105 | |
| Electrical conductivity | CH | 0.66 | 1.3 | 77 | 0.93 | 0.6 | 46 | 0.78 | 1.1 | 68 | 0.72 | 1.2 | 73 | 0.78 | 1.1 | 68 |
| CM | 0.67 | 0.2 | 6 | 0.67 | 0.2 | 7 | 0.66 | 0.2 | 6 | 0.66 | 0.2 | 6 | 0.63 | 0.3 | 8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, E.G.d.; Silva, C.A.; Gao, S.; Melo, L.C.A.; Lago, B.C.; Teodoro, J.C.; Guilherme, L.R.G. Empirical Correlation between Electrical Conductivity and Nitrogen Content in Biochar as Influenced by Pyrolysis Temperature. Nitrogen 2024, 5, 288-300. https://doi.org/10.3390/nitrogen5020019
Morais EGd, Silva CA, Gao S, Melo LCA, Lago BC, Teodoro JC, Guilherme LRG. Empirical Correlation between Electrical Conductivity and Nitrogen Content in Biochar as Influenced by Pyrolysis Temperature. Nitrogen. 2024; 5(2):288-300. https://doi.org/10.3390/nitrogen5020019
Chicago/Turabian StyleMorais, Everton Geraldo de, Carlos Alberto Silva, Suduan Gao, Leônidas Carrijo Azevedo Melo, Bruno Cocco Lago, Jéssica Cristina Teodoro, and Luiz Roberto Guimarães Guilherme. 2024. "Empirical Correlation between Electrical Conductivity and Nitrogen Content in Biochar as Influenced by Pyrolysis Temperature" Nitrogen 5, no. 2: 288-300. https://doi.org/10.3390/nitrogen5020019





