Abstract
The objective of the present paper is to provide a concept for a chemical sensor having a small size, a low power consumption and a high reliability for sensing CO2 under different humidity levels at atmospheric pressure, while maintaining a long term stability under working conditions. A high temperature regeneration process of the sensing layer is unneeded to ensure a long term stability of the sensing material. This objective is achieved by using a hybrid organic-inorganic nanomaterial, consisting of inorganic nanoparticles functionalized with an amine-based polymer.
1. Introduction
Carbon dioxide (CO2) sensors for monitoring the Indoor Air Quality (IAQ) are required in modern building with ventilations to insure the wellbeing of the occupants by adapting the ventilation rates to their needs. As the continuous ventilation of thermally isolated buildings contributes to the energy consumption [1], CO2 sensors will be used to optimize ventilation rates to lower the energy dissipation. Moreover, CO2 sensors are desired for disease detection at early stages by patients, capnography [2] and continuous monitoring of emissions in industrial combustion processes [3]. In our daily life, the amount of exhaled CO2 could be an indicator of occupancy in rooms kept under surveillance [4]. In the security field, the CO2 detector is used to localize the presence of human activity, e.g., to stop human rights violations due to human trafficking and to deliver emergency aid to localized earthquake victims. Although several applications for the monitoring of CO2 are possible, there are only few existing devices to detect CO2 in the desired environments, these devices using either optical or resistive methods. Optical detection based on non-dispersive infrared (NDIR) method is one well-known way to detect gases [5]. CO2 sensors based on the NDIR principle are accurate, allow fast measurements and have a good long-term stability. However, their high price, large device size and high power consumption are clear disadvantages. Conventional CO2 sensors based on solid state electrolytes, metal oxides, were also studied [6]. These materials inevitably show a huge cross sensitivity to other gases and require an operating temperatures higher than 100 °C to reach an acceptable sensitivity. Today, there is only a limited effort dedicated by the scientific community to the development of alternative polymer materials for sensing CO2 [7,8]. This is principally due to the lack of selective, sensitive, reversible, stable sensing materials for low power sensors.
2. Experimental Details
A chemical sensor is a device that transforms chemical information, i.e., the analyte concentration, into an electrically useful signal. Basically, it has two parts: a transducer and a sensing layer. In our study, we fabricated capacitive CO2 sensors, which measure any changes in the dielectric properties of the appropriate sensing material. The transducer is a pair of planar interdigitated electrodes. The sensing materials were Ormocer based materials and a new developed hybrid organic-inorganic material [9].
2.1. Transducer Configuration
A pair of interdigitated microelectrodes was produced by standard photolithography on a 620 µm thick glass substrate. After cleaning the glass substrate wafer, a thin adhesion layer of TiW (40 nm) followed by a thick gold layer (140 nm) were deposited by sputtering technique. The electrodes were patterned by photolithography technique using the desired mask. A resistive heater is made around the gold fingers. Each chip featured two interdigitated gold electrodes, each of them consisting of 83 fingers, filling an area of 3 × 3 mm2. Finger widths and pitches were set to 6 and 12 μm, respectively. The wafer was diced into 4.7 × 3.9 mm2 large individual chips. Each chip was mounted on an adapted PCB and then gold wire bonded thereto. A layer of sensing material was spin coated on top of the planar electrodes. In the case of Ormocer, the layer thickness was 0.4 µm. For the hybrid organic-inorganic material, 4 µm thick layer was used.
2.2. Sensing Materials Preparation
The hybrid organic-inorganic material was prepared by a wet-impregnation method. An amine based polymer, polyethyleneimine, was dissolved in ethanol. Silica nanoparticles were added to the previously prepared polymer solution. The slurry was continuously stirred at room temperature for about 10 min. Then, the slurry was kept under air to remove the solvent excess. The Ormocer material was synthesized via a sol-gel process from 3-aminopropyltrimethoxysilane and propyltrimethoxysilane in a molar ratio of 70:30.
2.3. Electrical Measurements
The electrical properties of the sensors were monitored by impedance spectroscopy using a Solartron 1260 gain-phase frequency analyser model 1260 A controlled by a PC, permitting automated data collection. Up to 10 chips were characterized in the same test chamber under the same gas environments. The impedance measurements can be carried out at different temperatures (from RT to 200 °C) by heating up the chip using the integrated heater and at different frequencies (from 1 MHz to 10 Hz). For this study, the capacitance was recorded between 40 and 100 kHz [10].
3. Results and Discussions
The dynamic-sensing response of the CO2 sensor was investigated at atmospheric pressure. Figure 1 and Figure 2 show the recorded sensor signal of the interdigitated transducer coated with a 0.4 µm thick Ormocer sensitive layer and a 4 µm amine functionalized nanoparticles layer. Both coated IDT’s were heated to 60 °C by applying a 3.3 V constant voltage to the sensor heater. The recorded signal corresponds to a change in the imaginary part of the impedance, by exposing the sensor to different CO2 concentrations mixed with synthetic air. The exposure times of the sensor to the analyte were fixed to 30 min and 15 min for the Ormocer sensing layer and hybrid organic-inorganic sensing layer respectively. On Figure 1 and Figure 2, the capacitance change dependent on several relative humidity (RH) levels and CO2 concentrations are shown. A capacitance measured in the presence of 400 ppm CO2 is considered as the background sensor capacitance at a specific relative humidity. This CO2 concentration corresponds to the natural level in the atmosphere. The capacitance for both sensing materials increases by raising the relative humidity due to the incorporation of water molecules into the sensing film. For different RH, the sensor is alternatively exposed to different amounts of CO2, which are varied from 400 to 3000 ppm by steps of 400 ppm, mixed in synthetic air. Regarding the amine functionalized silica nanoparticles, see Figure 2, we observed no drift in the capacitance signal at a given relative humidity level. The capacitance decreases by increasing the CO2 amount in synthetic air to reach a steady state within 1–2 min. Once the CO2 concentration is decreased to the background value (400 ppm), the sensor returns to the initial background capacitance in a reversible way without observing any hysteresis. In contrast, thereto, the capacitance signal of the Ormocer sensing layer at a given relative humidity needs a longer time, around 10 min, to reach a signal saturation by changing the CO2 concentration. The recovery time ranges between 10 and 15 min. Consequently, the adsorption process of CO2 sensor is reversible for both sensing materials. However, the hybrid organic-inorganic sensitive layer shows an entire reversibility compared to the Ormocer layer. In the case of CO2 concentration lower that 1500 ppm, the capacitance shows a linearity as a function of the CO2 concentration. For higher CO2 concentrations, this linearity is lost, because the sensitive layers have a limited number of available sites to adsorb CO2. The number of sites available for adsorption drastically decreases and implies a saturation of the capacitance change. It is well known that the interaction of CO2 with the amino group containing materials is basically an acid-base reaction [11]. However, the sensing properties of these sensing materials are affected by the type of the host matrix for the amino groups. The presence of silica nanoparticles seems to stabilize the amine based polymer matrix, increases the CO2 sensor sensitivity and increases the number of available and easily accessible amine groups.
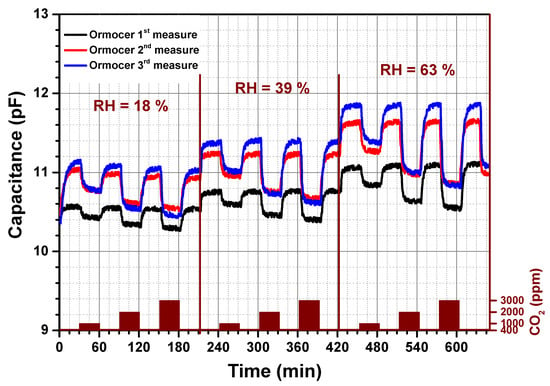
Figure 1.
Capacitance response versus time of Ormocer based CO2 sensor. Capacitance change recorded under different CO2 concentrations and relative humidity levels.
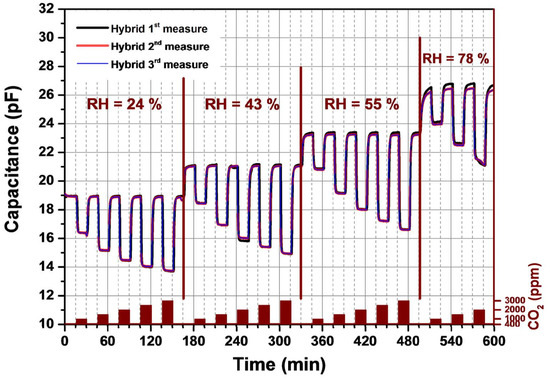
Figure 2.
Capacitance response versus time of amine functionalized silica nanoparticles based CO2 sensor. Capacitance change recorded under different CO2 concentrations and relative humidity levels.
Both types of sensitive layers were aged at ambient conditions for several weeks. Each graph in Figure 1 and Figure 2 contains three curves: 1st measure, 2nd measure and 3rd measure. All measurement are spaced by some weeks. The sensor signal, named 2nd measure and 3rd measure, was monitored in the same conditions as the initial measurement (1st measure). This allowed us to estimate the layer properties regarding its durability and stability. No change in the sensor signal was observed for the hybrid organic-inorganic materials compared to the Ormocer material. These results indicate a good stability of a CO2 sensor based on amine functionalized silica nanoparticles material. Consequently, the new developed sensitive layer are promising for future commercialization.
4. Conclusions
Interdigitated transducers coated with amino group-functionalized silica nanoparticles react with CO2 following the acid-base reaction as it is the case for Ormocer sensing material. The sensors show good selectivity, sensitivity, reversibility and reproducibility by a continuous monitoring of carbon dioxide. The response of amino functionalized silica nanoparticles towards carbon dioxide is fast and reversible. Such sensor elements show advantages because of their easy handling, low costs and fast responses. A carbon dioxide levels ranging between 400 and 2000 ppm are easily detected by amine functionalized nanoparticles at sensor working temperature lower than 60 °C. The sensors outstands operating conditions under relative humidity levels up to 80% RH. The sensing coatings show no cross sensitivity to other gases like acetone.
Author Contributions
J.B. provided the main idea of using amine functionalized nanoparticles as sensing materials, designed and performed the experiments and wrote the paper. A.K. and H.-E.E. and I.E. contributed to the discussion of the obtained data and to the final version of the manuscript.
Acknowledgments
Andreas Drost, Vu-Chi. Hung, Robert Faul and Dennise Linke for designing/realizing IDTs.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, J.; Wall, J.; Platt, G. Indoor Air Quality Control of HVAC System. In Proceedings of the 2010 International Conference Modelling, Identification and Control, Okayama, Japan, 17–19 July 2010. [Google Scholar]
- Siobal, M.S.; Ong, H.; Valdes, J.; Tang, J. Calculation of physiologic dead space: Comparison of ventilator volumetric capnography to measurements by metabolic analyzer and volumetric CO2 monitor. Respir. Care 2013, 58, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- United Nations Intergovernmental Panel on Climate Change (IPCC). Available online: http://www.ipcc.ch/ (accessed on 30 June 2017).
- Labeodan, T.; Zeiler, W.; Boxem, G.; Zhao, Y. Occupancy measurement in commercial office buildings for demand driven control applications—A survey and detection system evaluation. Energy Build. 2015, 93, 303–314. [Google Scholar] [CrossRef]
- Hodgkinsona, J.; Smith, R.; Wah-On, H.; Saffell, J.R.; Tatama, R.P. Non-dispersive infra-red (NDIR) measurement of carbon dioxide at 4.2 μm in a compact and optically efficient sensor. Sens. Actuators B Chem. 2013, 186, 580–588. [Google Scholar] [CrossRef]
- Moos, R.; Sahner, K.; Fleischer, M.; Guth, U.; Barsan, N.; Weimar, U. Solid State Gas Sensor Research in Germany—A Status Report. Sensors 2009, 9, 4323–4365. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, K.K.; Kupnik, M.; Khuri-Yaku, B.T. Functionalization layers for CO2 sensing using capacitive micromachined ultrasonic transducers. Sens. Actuators B Chem. 2012, 174, 87–93. [Google Scholar] [CrossRef]
- Willa, C.; Yuan, J.; Niederberger, M.; Koziej, D. When Nanoparticles Meet Poly(Ionic Liquid)s: Chemoresistive CO2 Sensing at Room Temperature. Adv. Funct. Mater. 2015, 25, 2537–2542. [Google Scholar] [CrossRef]
- Boudaden, J.; Klumpp, A.; Eisele, I.; Kutter, C. Smart capacitive CO2 sensor. In Proceedings of the IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016; pp. 775–777. [Google Scholar]
- Endres, H.E.; Jander, H.J.; Göttler, W. A test system for gas sensors. Sens. Actuators B 1995, 23, 163–172. [Google Scholar]
- Kortunov, P.V.; Siskin, M.; Paccagnini, M.; Thomann, H. CO2 Reaction Mechanisms with Hindered Alkanolamines: Control and Promotion of Reaction Pathways. Energy Fuels 2016, 30, 1223–1236. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).