Silicon-Quartz Microcapillary Opto-Fluidic Platform Obtained by CMOS-Compatible Die to Wafer 200 mm Dual Bonding Process †
Abstract
:1. Introduction
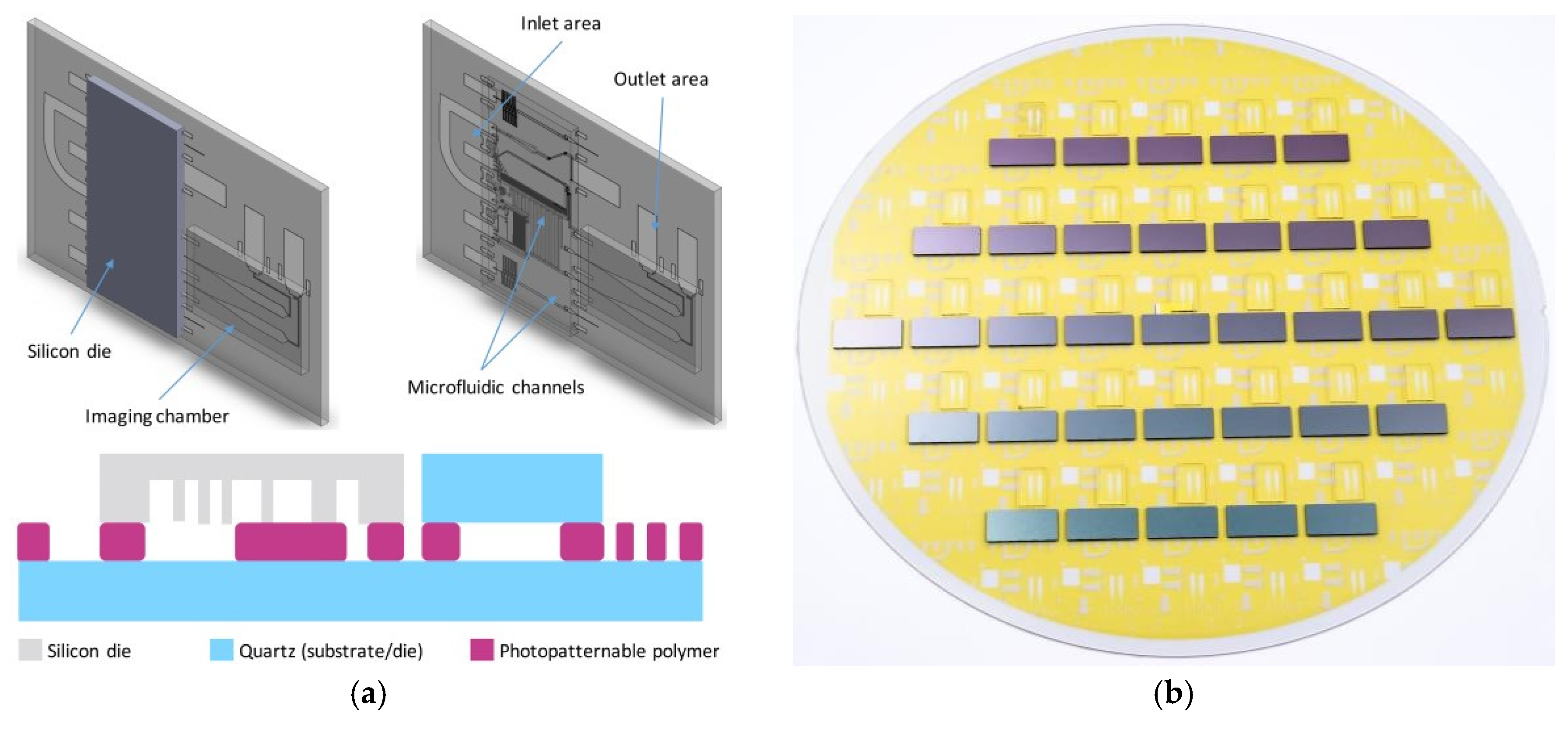
2. The Silicon-Quartz Opto-Fluidic Platform
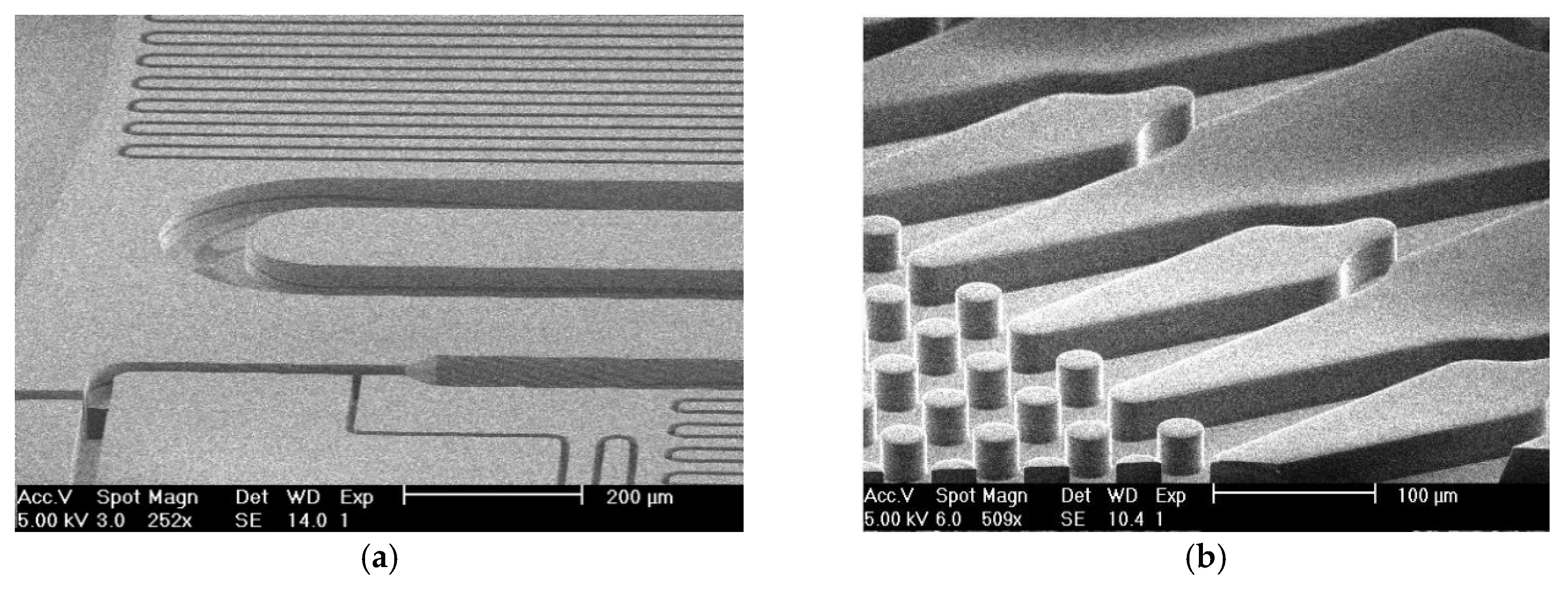
2.1. Device Fabrication Process
2.2. The Bonding Process
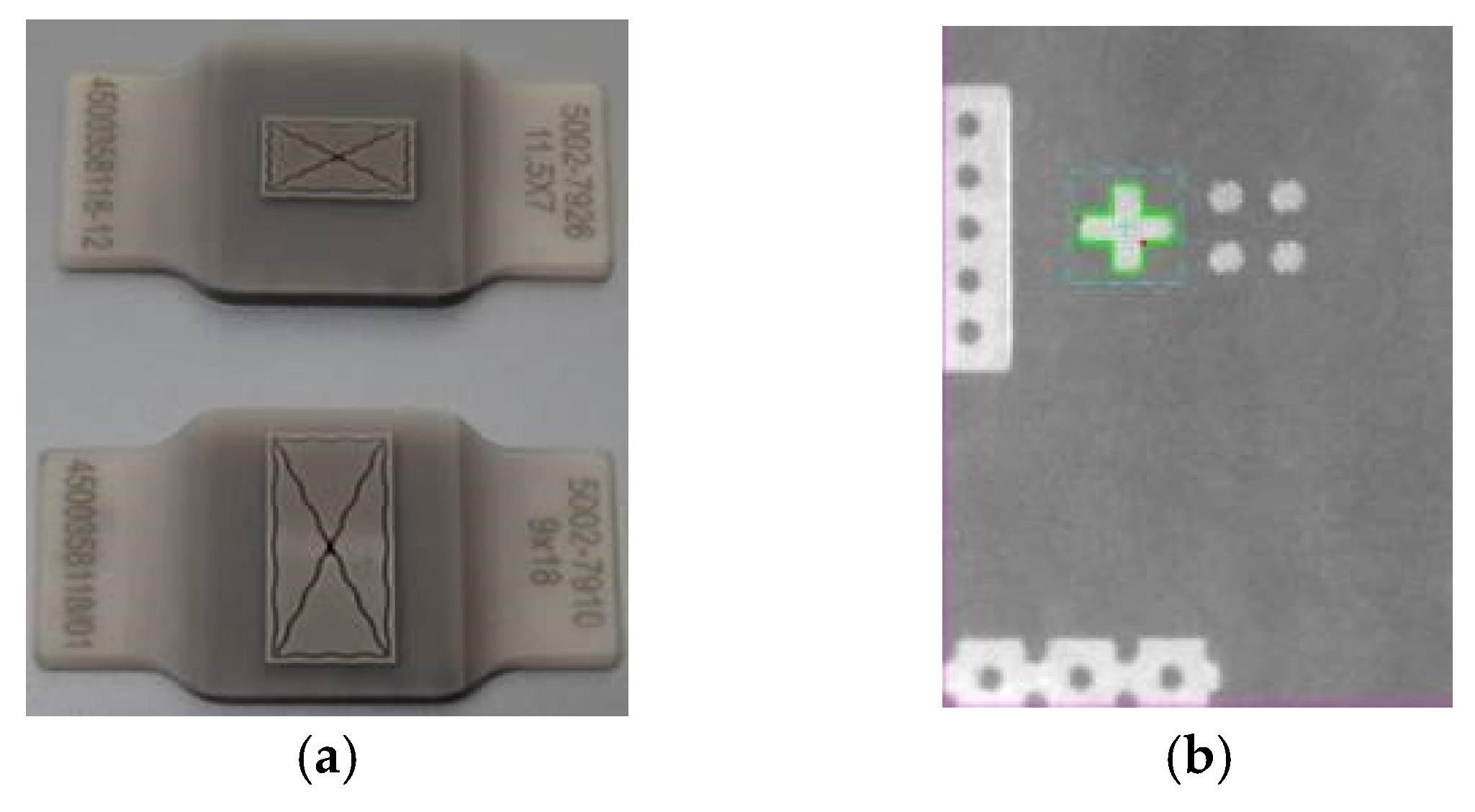
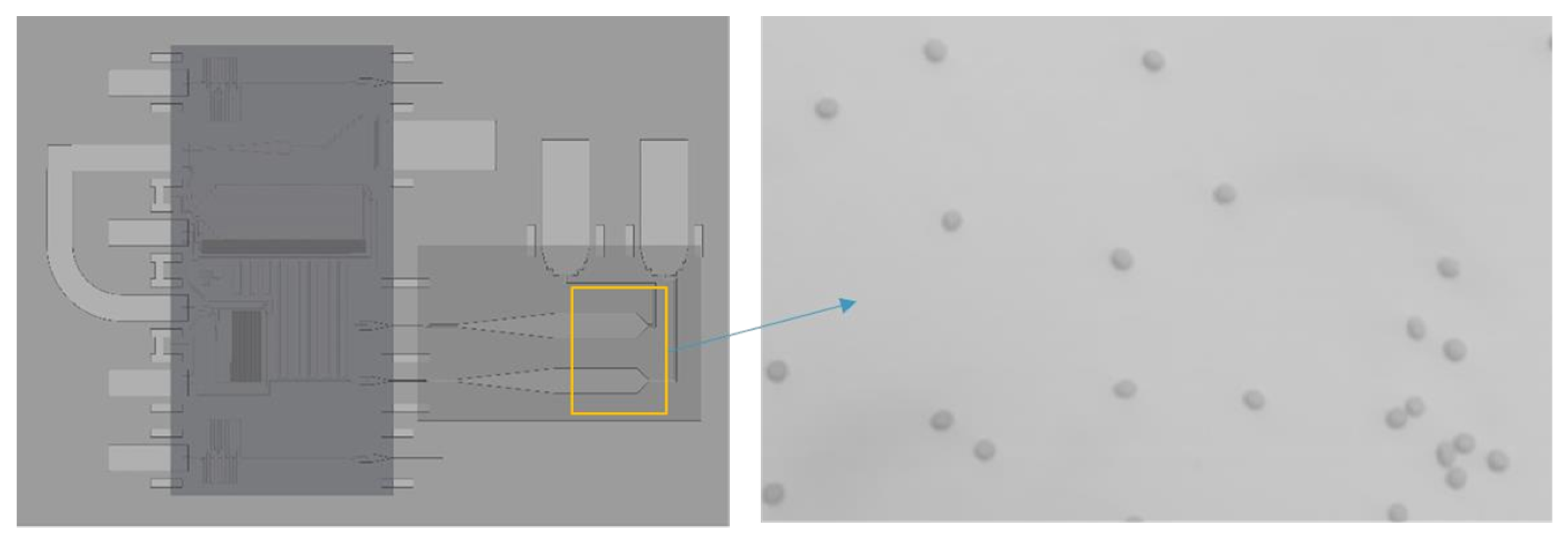
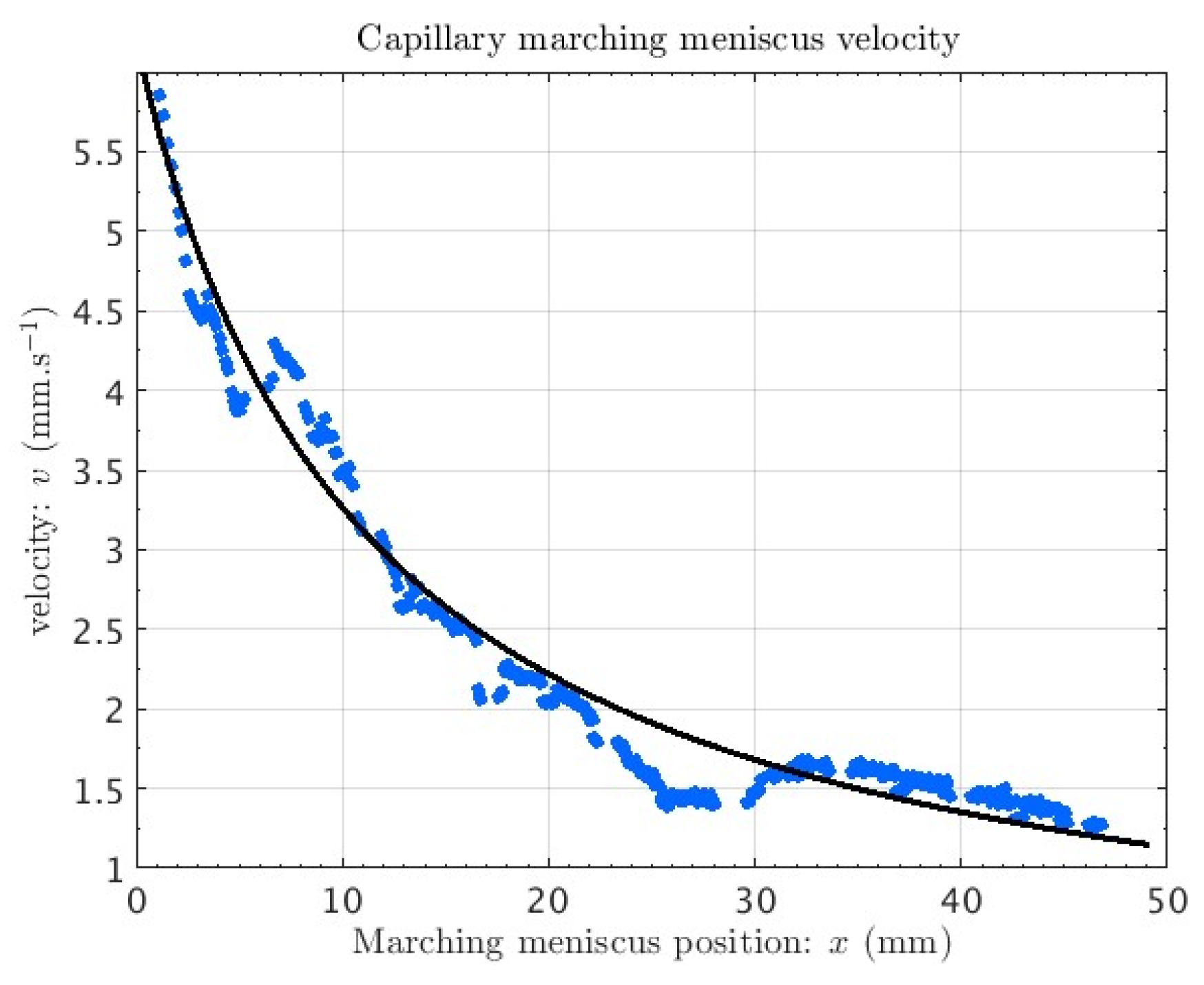
3. Device Testing
References
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Gervais, L.; Delamarche, E. Toward one-step point-of-care immunodiagnostics using capillary-driven microfluidics and PDMS substrates. Lab Chip 2009, 9, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.M.; Lindahl, T.L.; Killard, A.J. Development of a point of care lateral flow device for measuring human plasma fibrinogen. Anal. Chem. 2010, 82, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorentino, G.; Jones, B.; Roth, S.; Grac, E.; Jayapala, M.; Bex, P.; Almeida, D.D.D.; Humbert, A.; Severi, S. Silicon-Quartz Microcapillary Opto-Fluidic Platform Obtained by CMOS-Compatible Die to Wafer 200 mm Dual Bonding Process. Proceedings 2018, 2, 1018. https://doi.org/10.3390/proceedings2131018
Fiorentino G, Jones B, Roth S, Grac E, Jayapala M, Bex P, Almeida DDD, Humbert A, Severi S. Silicon-Quartz Microcapillary Opto-Fluidic Platform Obtained by CMOS-Compatible Die to Wafer 200 mm Dual Bonding Process. Proceedings. 2018; 2(13):1018. https://doi.org/10.3390/proceedings2131018
Chicago/Turabian StyleFiorentino, Giuseppe, Ben Jones, Sophie Roth, Edith Grac, Murali Jayapala, Pieter Bex, Daniel D. De Almeida, Aurelie Humbert, and Simone Severi. 2018. "Silicon-Quartz Microcapillary Opto-Fluidic Platform Obtained by CMOS-Compatible Die to Wafer 200 mm Dual Bonding Process" Proceedings 2, no. 13: 1018. https://doi.org/10.3390/proceedings2131018
APA StyleFiorentino, G., Jones, B., Roth, S., Grac, E., Jayapala, M., Bex, P., Almeida, D. D. D., Humbert, A., & Severi, S. (2018). Silicon-Quartz Microcapillary Opto-Fluidic Platform Obtained by CMOS-Compatible Die to Wafer 200 mm Dual Bonding Process. Proceedings, 2(13), 1018. https://doi.org/10.3390/proceedings2131018



