A Compact Robust OWLS System for Biosensing of Multiple Samples †
Abstract
:1. Introduction
2. Device Concepts
3. Materials and Methods.
3.1. Device Design and Fabrication Process
- Deposition of 65 nm of Si3N4 on a fused silica (SiO2) wafer by Low Pressure Chemical Vapor Deposition (LPCVD), at 770 °C, for 18 min.
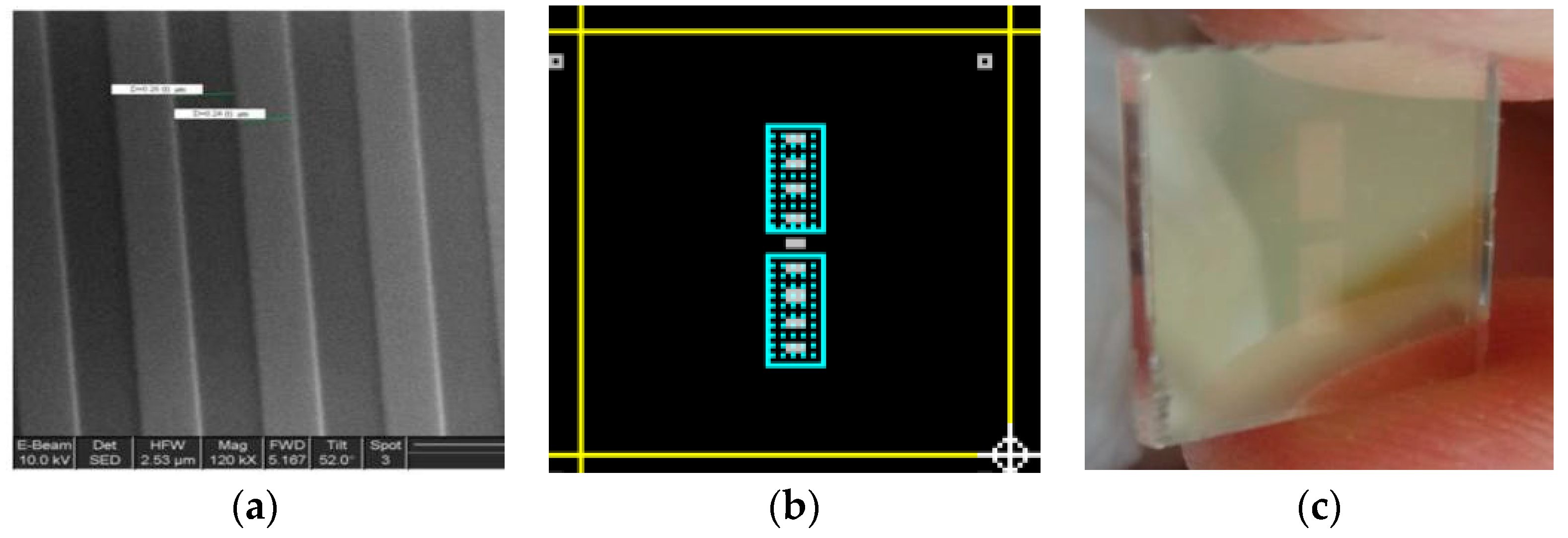
- Grating patterning: a 0.5 µm pitch, 50% duty cycle grating was etched by Electron-Beam Lithography on a photoresist coating. After developing it the wafer was etched by Reactive Ion Etching at an Oxford Plasmalab 100 RIE facility for two minutes to obtain a grating depth of 20 nm (Figure 1a).
- Plasma-Enhanced Chemical Vapor Deposition (PECVD) of a 510 nm SiO2 passivation layer, at 710 °C for 60 min, covering the full wafer.
- Photolithographic definition and HF etching of the SiO2 passivation layer, opening the sensing windows (blue areas on Figure 1b). The central grating remained passivated to act as reference channel.
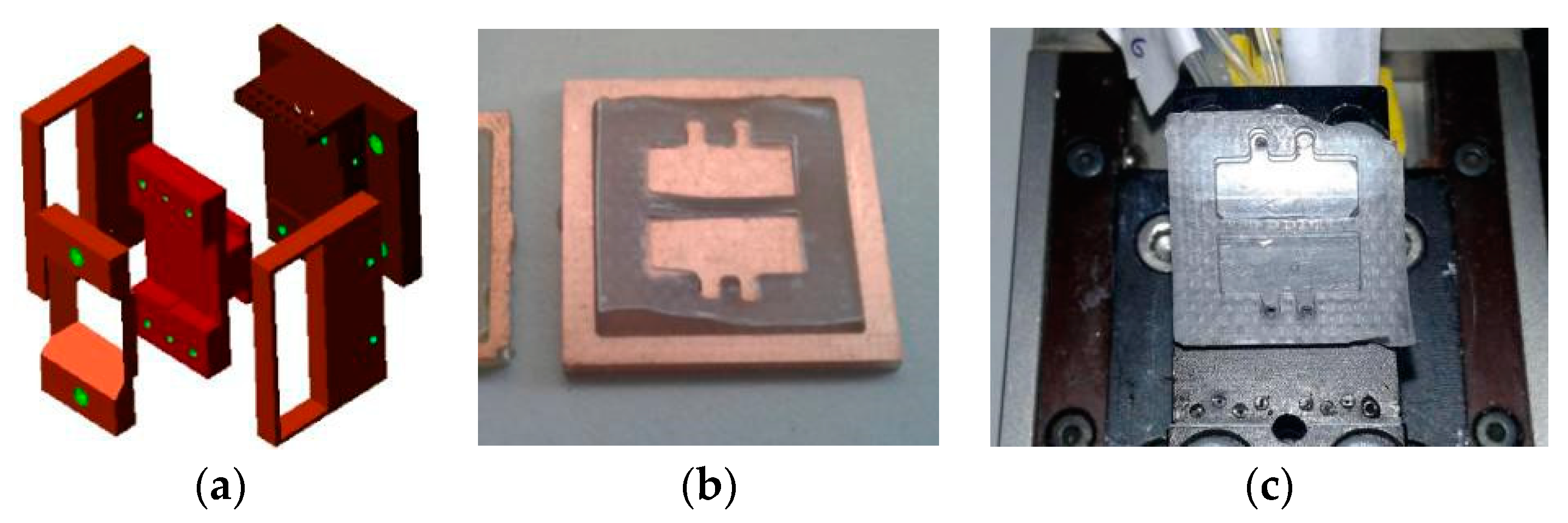
3.2. Holder Design and Fluidics
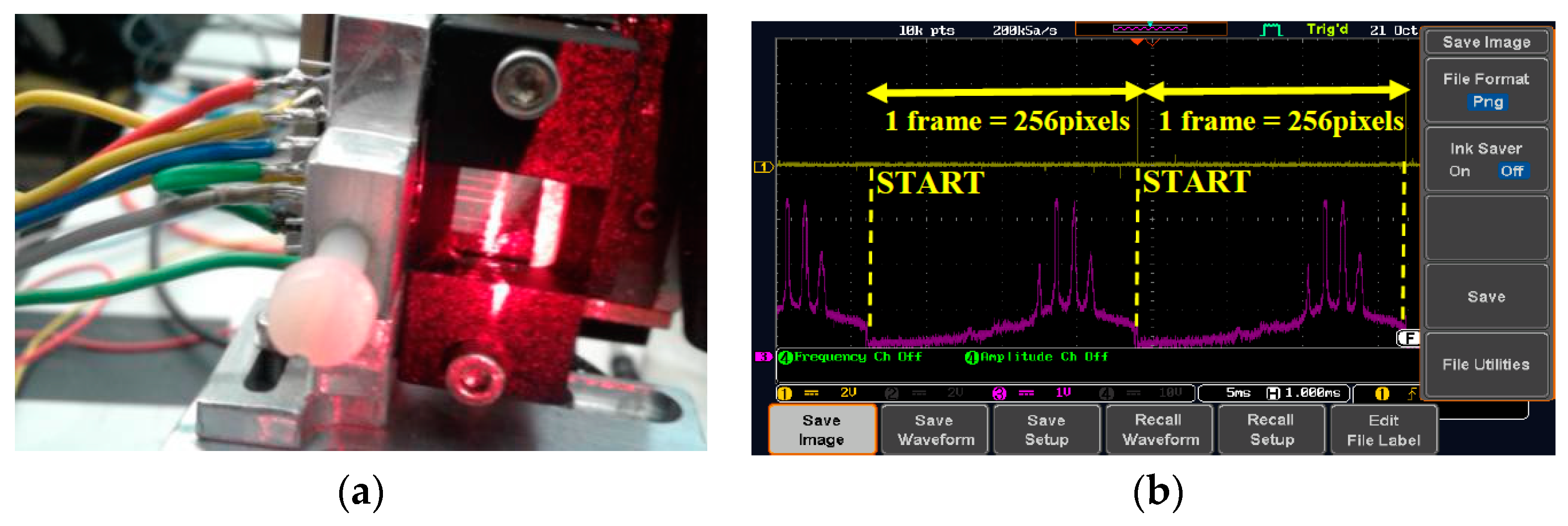
3.3. Instrumentation
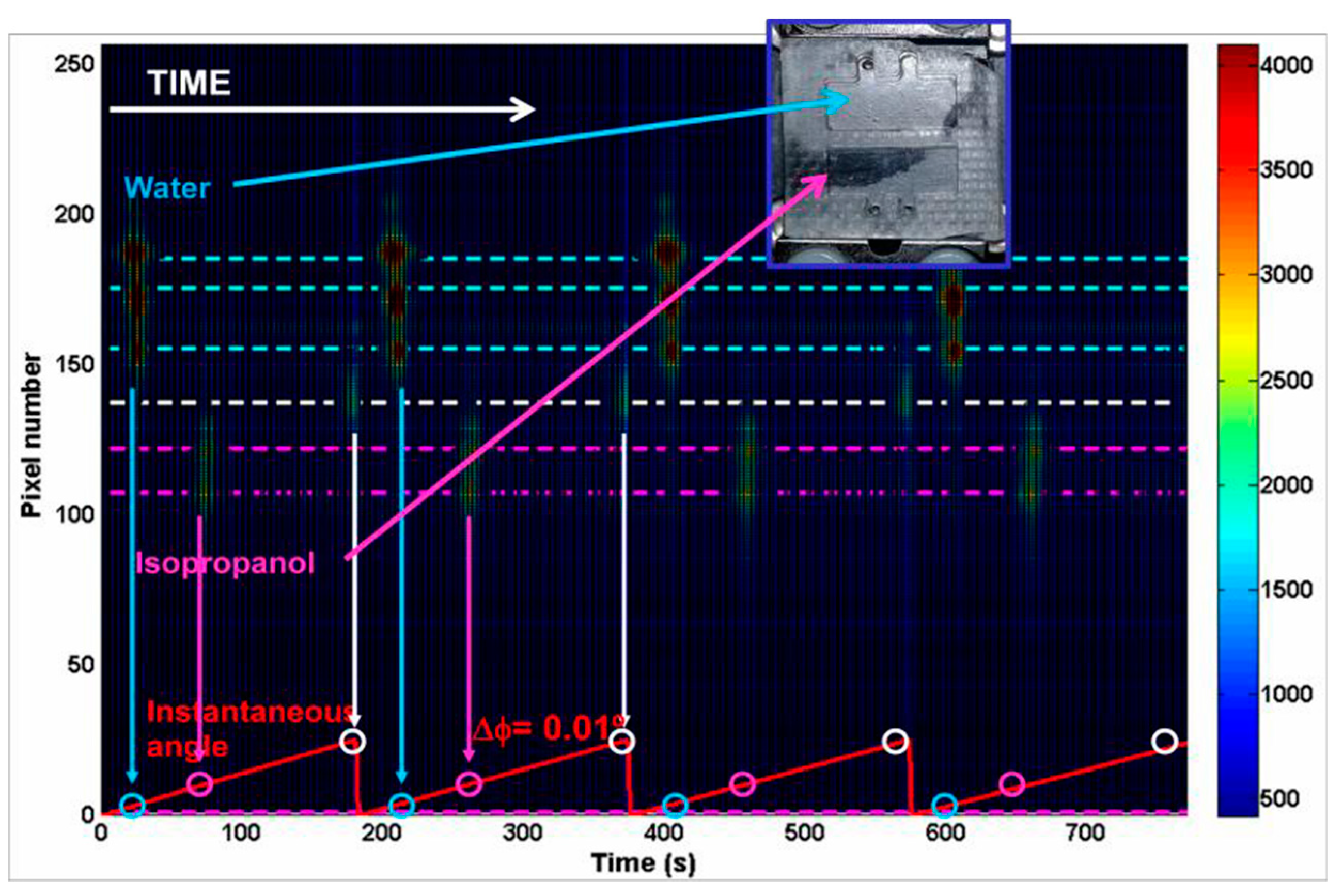
4. Results
5. Conclusions
Author Contributions
Acknowledgments
References
- Székács, I.; Kaszás, N.; Gróf, P.; Erdélyi, K.; Szendrő, I.; Mihalik, B.; Pataki, Á.; Antoni, F.A.; Madarász, E. Optical Waveguide Lightmode Spectroscopic Techniques for Investigating Membrane-Bound Ion Channel Activities. PLoS ONE 2013, 8, e81398. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S. Optical Biosensors for Label-Free Detection of Biomolecular Interactions. Instrum. Sci. Technol. 2014, 42, 109–127. [Google Scholar] [CrossRef]
- Heideman, R.G.; Hoekman, M.; Schreuder, E. TriPleX-based integrated optical ring resonators for lab-on-a-chip and environmental detection. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1583–1596. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Fan, T.; Hu, N.; Yang, Z.; Chen, X.; Wang, Z.-Y.; Yang, J. Design and Performance of a Portable and Multichannel SPR Device. Sensors 2017, 17, 1435. [Google Scholar] [CrossRef]
- Zhang, X.; Tsuji, S.; Kitaoka, H.; Kobayashi, H.; Tamai, M.; Honjoh, K.I.; Miyamoto, T. Simultaneous Detection of Escherichia coli O157:H7, Salmonella enteritidis, and Listeria monocytogenes at a Very Low Level Using Simultaneous Enrichment Broth and Multichannel SPR Biosensor. J. Food Sci. 2017, 82, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Homola, J.M. (Ed.) Surface Plasmon Resonance Based Sensors; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2006; Volume 4. [Google Scholar]
- Voros, J.; Ramsden, J.J.; Csucs, G.; Szendro, I.; De Paul, S.M.; Textor, M.; Spencer, N.D. Optical grating coupler biosensors. Biomaterials 2002, 23, 3699–3710. [Google Scholar] [CrossRef]
- Banuls, M.J.; Puchades, R.; Maquieira, A. Chemical surface modifications for the development of silicon-based label-free integrated optical (IO) biosensors: A review. Anal. Chim. Acta 2013, 777, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tiefenthaler, K. Integrated optical couplers as chemical waveguide sensors. Adv. Biosens. 1992, 2, 261–289. [Google Scholar]
- Darwish, N.; Caballero, D.; Moreno, M.; Errachid, A.; Samitier, J. Multi-analytic grating coupler biosensor for differential binding analysis. Sens. Actuators B Chem. 2010, 144, 413–417. [Google Scholar] [CrossRef]
- Wei, X. Weiss, S.M. Guided mode biosensor based on grating coupled porous silicon waveguide. Opt. Express 2011, 19, 11330–11339. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Sereno, M.; Darwish-Miranda, N.; Giménez-Conejo, M.; Palacio-Bonet, F.; Bernat-Ubiaga, I.; Romano-Rodríguez, A. A Compact Robust OWLS System for Biosensing of Multiple Samples. Proceedings 2018, 2, 863. https://doi.org/10.3390/proceedings2130863
Moreno-Sereno M, Darwish-Miranda N, Giménez-Conejo M, Palacio-Bonet F, Bernat-Ubiaga I, Romano-Rodríguez A. A Compact Robust OWLS System for Biosensing of Multiple Samples. Proceedings. 2018; 2(13):863. https://doi.org/10.3390/proceedings2130863
Chicago/Turabian StyleMoreno-Sereno, Mauricio, Nasser Darwish-Miranda, Miquel Giménez-Conejo, Francisco Palacio-Bonet, Iván Bernat-Ubiaga, and Albert Romano-Rodríguez. 2018. "A Compact Robust OWLS System for Biosensing of Multiple Samples" Proceedings 2, no. 13: 863. https://doi.org/10.3390/proceedings2130863




