Abstract
This review article focuses on the application of microwave-assisted techniques in various processes, including microwave-assisted extraction, microwave-assisted pyrolysis, microwave-assisted acid hydrolysis, microwave-assisted organosolv, and microwave-assisted hydrothermal pretreatment. This article discusses the mechanisms behind these techniques and their potential for increasing yield, producing more selectivity, and lowering reaction times while reducing energy usage. It also highlights the advantages and disadvantages of each process and emphasizes the need for further research to scale the processes and optimize conditions for industrial applications. A specific case study is presented on the pretreatment of coffee waste, demonstrating how the choice of microwave-assisted processes can lead to different by-products depending on the initial composition of the biomass.
1. Introduction
Biomass residues, comprising organic materials from agricultural, forestry, and industrial processes, are essential resources for bioenergy production and sustainable waste management [1]. However, the lack of proper management of biomass residues can lead to contamination of the environment [2]. When biomass residues are left unmanaged or disposed of improperly, they can release pollutants such as heavy metals, pesticides, and organic compounds into the soil, water, and air, posing risks to ecosystems and human health [3]. Biomass residues not only contribute to environmental pollution but also hinder the potential for energy generation and bio-based products, emphasizing the critical need for effective waste management practices [4,5].
The valorization of biomass residues plays a crucial role in mitigating environmental contamination and promoting resource efficiency. By converting biomass residues into valuable products through innovative technologies such as pyrolysis [6], gasification [7], hydrothermal valorization [8,9], bioconversion [10,11] and extraction [12], the environmental impact of unmanaged biomass residues can be minimized. Valorization processes can transform contaminated biomass into biofuels, biochar, and other high-value materials, reducing pollution and enhancing resource recovery. Proper valorization of biomass residues not only helps in addressing environmental contamination but also contributes to sustainable waste management practices, highlighting the importance of utilizing biomass residues efficiently to mitigate pollution and promote environmental sustainability [13].
Microwave-assisted extraction (MAE) has emerged as a promising technology for extracting bioactive compounds from biomass residues efficiently and sustainably. This innovative method utilizes microwaves to generate high-frequency waves that cause dielectric heating in wet biomass, leading to the disruption of cell walls and the release of valuable biochemical components such as lipids, pigments, proteins, and carbohydrates. Compared to conventional extraction methods, MAE offers several advantages, including quicker heating, selective energy dissipation, and enhanced heat and mass transfer, resulting in reduced working times, increased yield, and improved purity of the extracted compounds. The environmentally friendly nature of MAE, with reduced solvent requirements and effective cell disruption, positions it as a viable option for extracting bioactive compounds from biomass residues, contributing to sustainable waste management practices and the efficient utilization of biomass resources [14].
Microwave technology in biomass extraction not only offers a green and efficient method for obtaining bioactive compounds but also presents opportunities for industrial-scale applications. By utilizing microwaves for extraction processes, researchers have observed effective cell wall disruption with low energy input, rapid treatment times, and the avoidance of hazardous substances [15]. This technology enables the extraction of a wide range of compounds from biomass residues, including lipids, pigments, and proteins, with high selectivity and efficiency. The benefits of microwave-assisted extraction extend to the production of biofuels, high-value biochemicals, and other bioproducts, showcasing its potential to revolutionize the extraction of valuable compounds from biomass residues while promoting sustainability and environmental stewardship in the bioenergy and bioproduct industries [16].
The scope of this article aims to provide a comprehensive understanding of microwave-assisted processes in the context of biomass valorization. It will delve into the working principles, elucidating how microwaves facilitate the extraction of bioactive compounds from biomass efficiently and sustainably. This article will focus on optimizing pretreatment by exploring various parameters and conditions that influence the extraction/reaction process, such as power levels, irradiation time, and choice of solvents. Additionally, it will highlight the specific application of microwaves for biomass valorization, emphasizing its role in extracting valuable components like lipids, pigments, proteins, and carbohydrates from biomass residues for various industrial purposes.
Furthermore, this article will introduce an innovative design of a microwave system tailored specifically for biomass valorization. This design will showcase advancements in microwave technology that enhance the efficiency and effectiveness of biomass extraction processes. The innovative design will address the unique requirements of biomass valorization, considering factors like heating uniformity, energy efficiency, and scalability for industrial applications. By presenting this novel microwave system, this article aims to contribute to the advancement of sustainable practices in biomass valorization and promote the utilization of biomass resources for the production of biofuels, biochemicals, and other high-value products.
2. Microwave-Assisted Processes
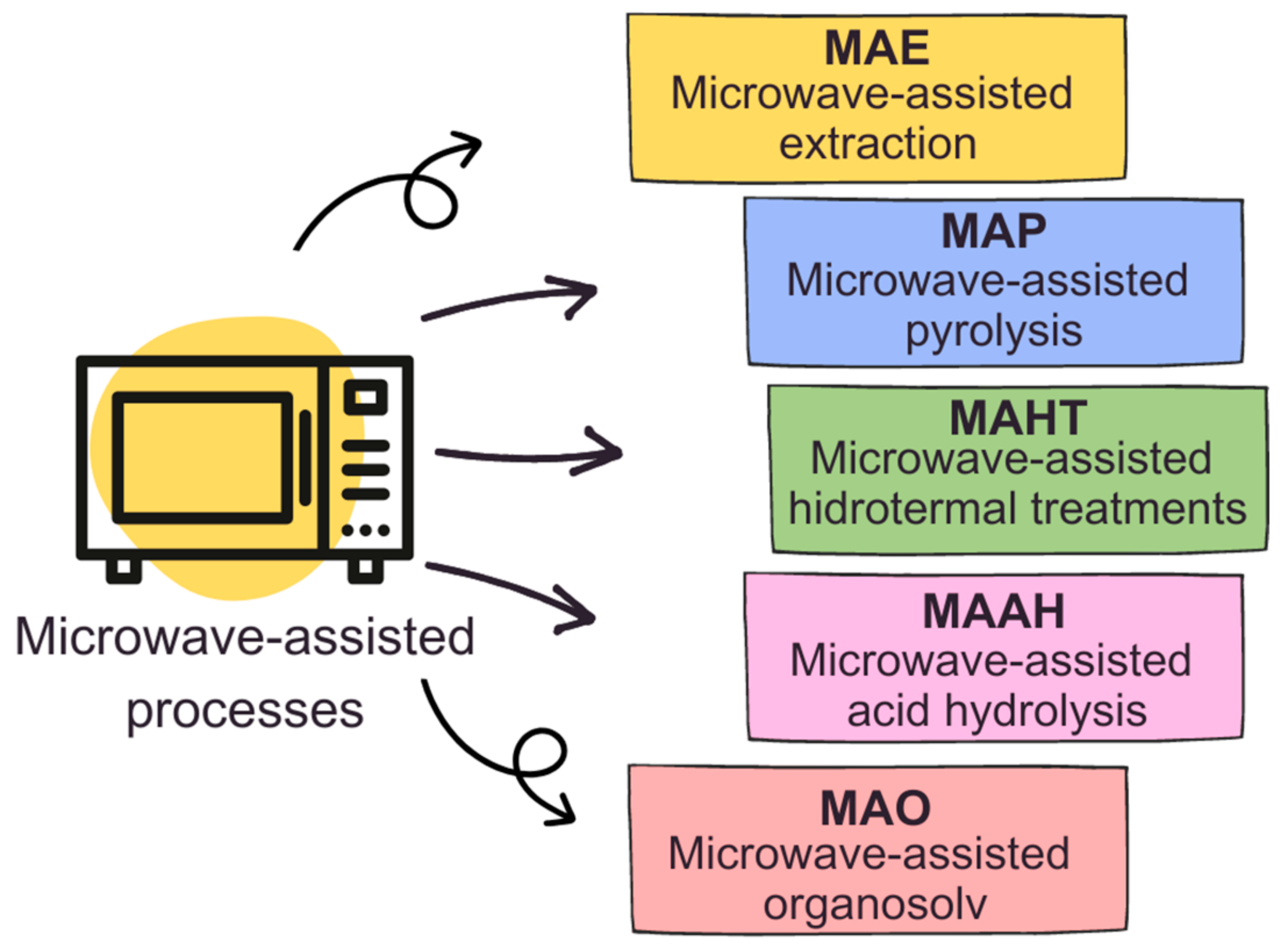
Microwaves are a form of electromagnetic radiation with wavelengths shorter than radio waves but longer than infrared waves, typically ranging from 1 mm to 1 m, with frequencies between 300 MHz and 300 GHz [17]. These waves travel in a straight line, known as the line of sight, and are readily reflected by obstructions (metals) but can be absorbed [18]. In chemical processes, microwaves are utilized in microwave chemistry, where they act as high-frequency electric fields capable of heating materials containing mobile electric charges, such as polar molecules in solvents or conducting ions in solids [19]. When microwaves interact with these materials, they induce molecular rotations and collisions, leading to energy loss and heating. This somewhat selective heating effect allows for rapid and uniform heating of specific components within a reaction mixture, resulting in accelerated reaction rates [20,21], milder reaction conditions, higher chemical yields, and different reaction selectivities compared to conventional heating methods [22,23,24,25]. It is worth highlighting that said uniform heating is only true for small samples, and in single-mode devices; the turntable and the convection in liquid media play major roles in averaging the heating. Microwave chemistry has gained popularity in various fields, including organic and inorganic chemistry, due to its ability to intensify processes, improve efficiency, and enable unique reaction pathways not easily achievable through traditional heating methods [26]. Figure 1 presents some of the microwave-assisted processes that will be presented in the current review.
Figure 1.
Microwave-assisted processes.
2.1. Microwave-Assisted Extraction (MAE)
Microwave-assisted extraction (MAE) is an innovative extraction technique that combines microwave energy with traditional solvent extraction methods. In MAE, microwaves are used to heat solvents and plant tissues, increasing the kinetic of extraction [27]. This method offers several advantages over traditional extraction methods, including shorter extraction times, reduced solvent usage, higher extraction rates, and lower costs [28]. MAE has become popular in extracting compounds from various matrices, especially natural products, due to its efficiency and effectiveness [29]. Advanced MAE techniques like pressurized microwave-assisted extraction (PMAE) [27] and solvent-free microwave-assisted extraction (SFMAE) [30]. have further enhanced the extraction process, making MAE a valuable tool in the extraction of bioactive compounds from plants.
The mechanism of microwave-assisted extraction (MAE), and most of the microwave-assisted processes, involve several sequential steps:
Separation of Solute: The process begins with the separation of solutes from the sample matrix under increased pressure and temperature. The application of microwave energy initiates the heating process, leading to the disruption of the sample matrix and the release of solutes.
Solvent Penetration: As the sample is heated, the solvent penetrates into the plant material through diffusion. This penetration causes the dissolution of solutes into the solvent until saturation is reached.
Effective Diffusion: The solution containing the dissolved solutes diffuses to the plant surface through effective diffusion mechanisms. This movement allows the solutes to transfer to the bulk solution, where they can be collected for further processing.
Overall, the mechanism of microwave-assisted extraction involves the application of microwave energy to heat the sample, facilitate the dissolution of solutes into the solvent, and promote the transfer of solutes to the bulk solution through effective diffusion processes [31,32].
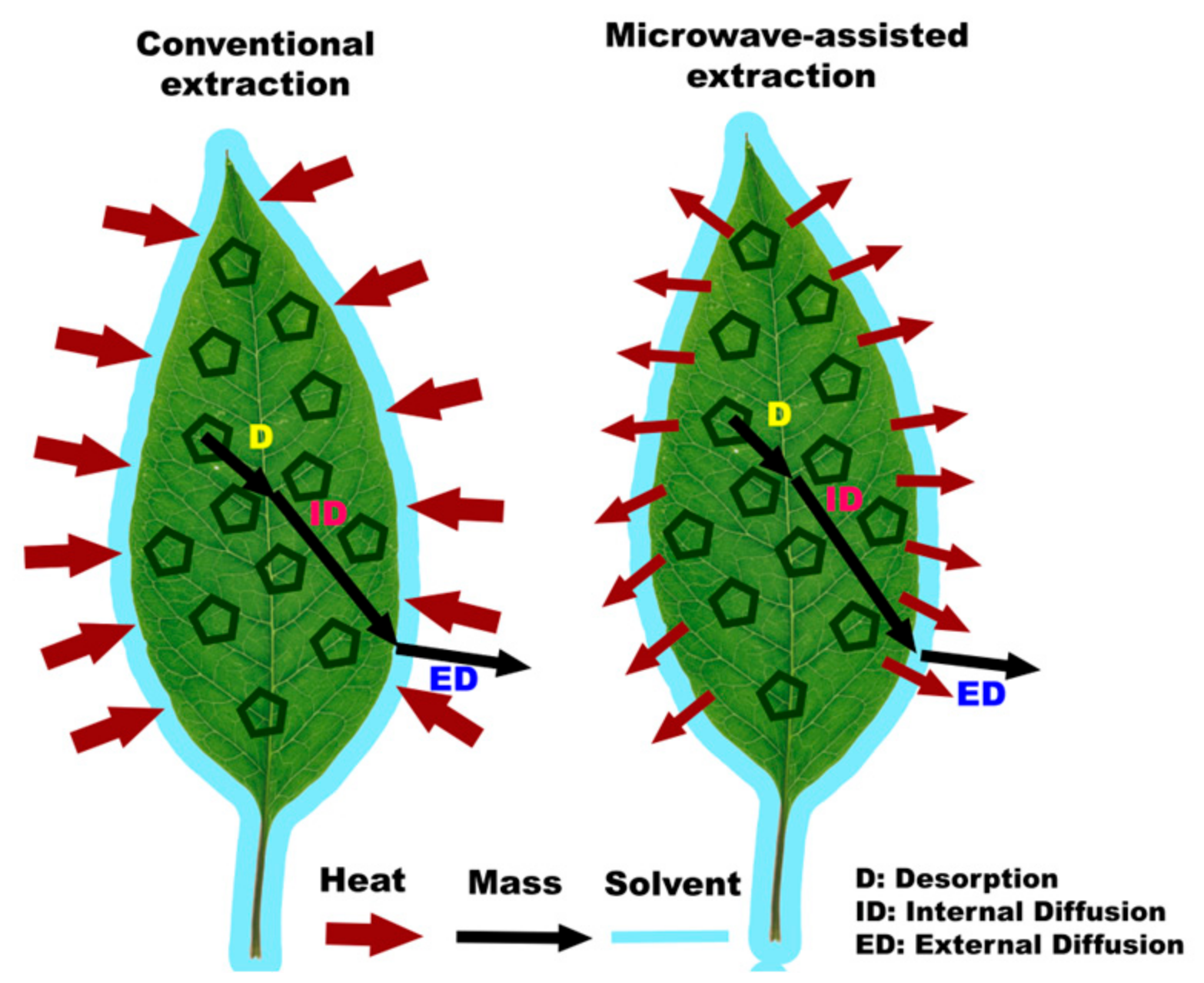
The main difference between conventional extraction methods and microwave-assisted extraction (MAE) lies in the heating process used to extract compounds from the samples. In conventional extraction methods like maceration or soxhletation, the extraction relies on the slow diffusion of solutes into the solvent over an extended period, requiring more time for the extraction process to complete. On the other hand, MAE utilizes microwave radiation to rapidly and efficiently heat the solvent and sample, creating high vapor and pressure and the disruption of the cell wall of matrices (Figure 2), significantly reducing the extraction time. This quick and targeted heating in MAE enhances the kinetic of extraction, leading to shorter extraction times, higher extraction rates, and lower solvent usage compared to conventional methods.
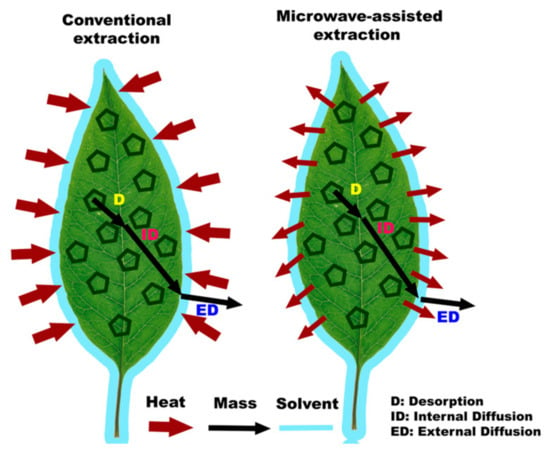
Figure 2.
Explanation of microwave radiation.
To provide a comprehensive overview of the diverse applications and outcomes of microwave-assisted extraction, a comparative Table 1 has been curated. This table presents a detailed analysis of different initial plant materials, the specific microwave extraction conditions employed, the extracted products, and the corresponding yields. By examining these key parameters across a range of studies, valuable insights into the effectiveness and versatility of microwave-assisted extraction in obtaining bioactive compounds from different botanical sources can be gleaned.

Table 1.
Microwave-assisted extraction reported in the literature.
Numerous factors, such as the solvent-to-feed ratio, solvent composition, water content, plant sample characteristics, irradiation period, stirring impact, microwave energy density, and extraction temperature, all affect the efficiency of MAE.
Solvent-to-feed ratio and solvent composition: In microwave-assisted extraction (MAE), the solvent-to-feed ratio is a critical component that greatly affects the extraction performance. The most important component influencing MAE is solvent selection, and a well-chosen solvent will result in a productive extraction procedure. The solvent-to-feed ratio has an impact on the solubility of the target molecule, the mass transfer kinetics of the procedure, and the solvent penetration that results from the interaction of the dielectric effect and sample matrix [40].
The solvent will be able to absorb microwave energy if it has a high dielectric constant and dielectric loss [41]. Ethanol, methanol, and water are good examples of solvents that absorb microwaves well and have enough polarity to be cooked in a microwave. A tiny amount of water added to a polar solvent causes more water to diffuse into the plant sample, which effectively heats the plant and speeds up the mass transfer rates of chemicals into the solvent [42]. The solvent-to-feed ratio significantly influences MAE extraction efficiency, as demonstrated by Alara et al. (2019), where the authors optimized the extraction conditions for phenolic compound-rich extracts from Chromolaena odorata [29]. The composition of the solvent used in MAE plays a vital role in the extraction process. The polarity of the solvent affects its ability to absorb microwave energy effectively, thereby influencing the extraction efficiency. Research has demonstrated that an increase in solvent polarity can enhance the extraction rate and yield of bioactive compounds [40].
Microwave power: The heating rate and extraction kinetics are affected by the intensity of the microwave radiation used during extraction, which is determined by the microwave power. Because heat-sensitive chemicals are degraded by strong microwave power, extraction yield may be reduced [43]. Higher microwave power often results in an extraction yield that increases proportionately until it reaches a point where it either becomes negligible or begins to decrease. Because microwave power causes localized heating inside the sample and drives MAE, it is essential for the analyte to diffuse and dissolve in the solvent [44]. However, because compounds are more susceptible to heat degradation, it is crucial to remember that excessive power might also result in a loss in yield. To yield compounds, a longer irradiation period will be required while using low power [45]. The relationship between extraction temperature and microwave power is crucial, with higher microwave power resulting in increased extraction temperature. This enhances solubility and solvent potency, but a more effective approach is to use low and medium power with longer exposure [46,47]. Microwave power significantly impacts the extraction yield and extraction time. Higher power generally leads to better extraction yields and faster extraction times. However, excessively high microwave power can lead to degradation of thermally sensitive chemicals in the plant matrix. Therefore, selecting the right microwave power is crucial to shorten extraction time and achieve desired yields [47];
Extraction time: The extraction time in microwave-assisted extraction (MAE) is a critical parameter that significantly influences the efficiency and outcomes of the extraction process. Research has indicated that the extraction time directly affects the extraction yield and quality of bioactive compounds. Optimal extraction times need to be determined to prevent under-extraction or degradation of compounds.
In MAE, the extraction time is often significantly shorter compared to traditional extraction methods. For instance, a study comparing MAE with conventional methods found that MAE only required 2 min for extraction, while traditional methods like heat reflux extraction, ultrasonic extraction, and Soxhlet extraction needed significantly longer extraction times ranging from 24 h to 90 min [42]. This highlights the rapid and efficient nature of MAE in extracting bioactive compounds from plant materials.
Moreover, the influence of extraction time on the extraction yield in MAE has been demonstrated in various studies. For example, in the optimization of MAE conditions, researchers found that an extraction time of 5–6 min resulted in optimal extraction efficiency for phenolic compounds, with ethanol 80% as the solvent and a liquid/solid ratio of 12.7:1 [47]. This indicates that the duration of extraction plays a crucial role in determining the yield and quality of the extracted compounds in MAE.
Temperature: Temperature increases solvent power due to decreased surface tension and viscosity, enhancing solubilization and matrix wetting. MAE efficiency increases until an optimum temperature is reached. Extraction temperature and microwave power are interrelated, with higher microwave power leading to an increase in extraction temperature. The recommended temperature range should be from 30 to 60–140 °C, considering the stability of the compound of interest [48]. The extraction period of the MAE needs to be regulated, often lasting between minutes and seconds, to reduce the possibility of heat deterioration and oxidation. Reduced water content helps prevent the thermal and chemical breakdown of compounds. Cyclic extraction is used to prevent thermal degradation of compounds. Polar chemicals are heated fast with microwaves [49].
There are other forms of MAE that involve minor adjustments to the fundamental configuration, such as the addition of gases, pressure, distillation, and other additional methods that enable the extraction of certain compounds from the vegetal sample. These are discussed in brief next [50].
Nitrogen-protected microwave-assisted extraction (NMAE) is a type of microwave-assisted extraction that uses nitrogen gas to protect the sample and solvent from oxidation during the extraction process [51]. This technique is particularly useful for extracting heat-sensitive and oxygen-sensitive compounds, as it minimizes the risk of degradation and oxidation. It works by placing the sample and solvent mixture in an extraction vessel that is pressurized with nitrogen gas, creating an inert atmosphere to minimize oxidation of extracted compounds. The sealed vessel is then subjected to microwave energy inside a microwave cavity, heating the solvent and sample to facilitate extraction. The nitrogen atmosphere helps reduce or prevent oxidation of heat-sensitive and oxygen-sensitive compounds, resulting in higher extraction yields compared to conventional methods. Nitrogen-protected microwave-assisted extraction (NPMAE) has been applied for the extraction of ascorbic acid (AA) from various fruit and vegetable samples, such as guava, yellow pepper, green pepper, and cayenne pepper [51]. Vacuum microwave-assisted extraction (VMAE) is a type of microwave-assisted extraction that is performed under vacuum conditions. This technique allows for the extraction of thermally labile compounds at lower temperatures, reducing the risk of degradation [52]. The vacuum also helps to increase the solvent’s penetration into the sample matrix, improving the extraction efficiency [53]. This method has been applied for the extraction of antioxidants from plant samples, including the extraction of polyphenolic compounds and pigments from Chinese herbs. VMAE has also been used for the extraction of functional compounds from plants, leveraging the reduced boiling point of the extraction solvent under vacuum conditions to prevent the degradation of heat-sensitive compounds [52].
Focused microwave-assisted Soxhlet extraction (FMASE) is a type of microwave-assisted extraction that combines the principles of Soxhlet extraction and microwave-assisted extraction [54]. This technique uses focused microwave energy to heat the solvent and sample matrix, promoting the transfer of solutes from the sample into the solvent. The FMASE system typically consists of a microwave cavity with a rotating sample holder, allowing for continuous solvent flow and efficient extraction [55]. FMASE has been successfully used for the extraction of natural products, pharmaceuticals, and environmental contaminants from complex samples, demonstrating its versatility and potential for automation and simultaneous extraction of multiple samples [54].
Ultrasonic microwave-assisted extraction (UMAE) is a type of microwave-assisted extraction that combines the principles of ultrasonic extraction and microwave-assisted extraction [56]. This technique uses ultrasonic waves to create cavitation in the solvent, promoting the transfer of solutes from the sample into the solvent. Microwave energy is used to heat the solvent and sample matrix, further enhancing the extraction efficiency [57]. Ultrasonic microwave-assisted extraction (UMAE) has been applied for the extraction of lycopene from tomato paste, with optimized conditions of 98 W microwave power, 40 KHz ultrasonic processing, a solvent-to-tomato paste ratio of 10.6:1 (v/w), and an extraction time of 367 s, resulting in a lycopene yield of 97.4%. This technique offers advantages in terms of efficiency and yield compared to ultrasonic-assisted extraction (UAE), showcasing its potential as a highly efficient extraction method for lycopene and potentially other compounds from complex matrices like tomato paste [58]. Microwave hydro-distillation (MWHD or MAHD) is a type of microwave-assisted extraction that uses microwave energy to heat the sample matrix and water, promoting the release of volatile compounds [59] The vapor is then condensed and collected, allowing for the isolation of essential oils and other volatile compounds [60]. MWHD has shown advantages in terms of energy efficiency, extraction speed, and improved quality of essential oils, making it a promising alternative to conventional hydrodistillation for the extraction of aromatic compounds from botanical sources.
Microwave steam distillation (MSD) is a type of microwave-assisted extraction that uses microwave energy to heat the sample matrix and water, promoting the release of volatile compounds [61]. The steam is then condensed and collected, allowing for the isolation of essential oils and other volatile compounds [62]. Solvent-free microwave extraction (SFME) is a type of microwave-assisted extraction that does not use any solvent. This technique uses microwave energy to heat the sample matrix, promoting the release of volatile compounds [63]. The vapor is then condensed and collected, allowing for the isolation of essential oils and other volatile compounds [63].
Vacuum microwave hydro-distillation (VMHD) is a type of microwave-assisted extraction that combines the principles of vacuum distillation and microwave-assisted extraction. This technique uses microwave energy to heat the sample matrix and water, promoting the release of volatile compounds [50]. The vacuum helps to reduce the boiling point of the water, allowing for the extraction of thermally labile compounds at lower temperatures. Microwave hydro-diffusion and gravity (MHG) is a type of microwave-assisted extraction that uses microwave energy to heat the sample matrix, promoting the release of volatile compounds [64]. The vapor is then allowed to diffuse through a porous membrane, separating the volatile compounds from the sample matrix. The volatile compounds are then collected via gravity, allowing for the isolation of essential oils and other volatile compounds [65].
Microwave-assisted extraction is a versatile technique that can be used to extract a wide range of compounds from various sample matrices. The different types of microwave-assisted extraction, such as NMAE, VMAE, FMASE, UMAE, MWHD, MSD, SFME, VMHD, and MHG, offer unique advantages and applications, depending on the specific needs of the extraction process.
Plant sample characteristics and water content:
Water content and other variables of the plant sample have a major impact on microwave-assisted extraction (MAE). The effectiveness of extraction rises with the plant sample’s contact surface area; finer samples enable a deeper penetration of microwave irradiation. On the other hand, excessive fineness might cause technical issues and necessitate centrifugation or filtering while preparing the plant samples [66]. In order to enhance interaction between the solvent and the plant matrix, the ground sample is homogenized during sample preparation; the average size of the plant particles is between 2 and 100 mm. Pre-leaching is the practice of soaking the plant matrix before extraction to increase yield. Because of the moisture in the plant matrix, which functions as a solvent and increases cell pressure and solute dispensation by breaking down the cell wall, the recovery of bioactive chemicals from the matrix tends to rise [29]. Water is positively impacted by an increase in solvent polarity, which promotes the heating process. Additionally, additional water produces hydrolyzation and lessens the oxidation of bioactive substances. A plant sample’s water content has a major impact on how well it extracts, with higher moisture content producing more effective extraction [67].
Stirring: Stirring plays a crucial role in enhancing the mass transfer kinetics during extraction by promoting the contact between the solvent and the plant matrix. Effective stirring ensures uniform distribution of heat and solvent within the sample, leading to improved extraction rates and yields. Studies have shown that stirring can help in achieving better extraction efficiency by facilitating the penetration of the solvent into the plant material, thereby enhancing the extraction of bioactive compounds. Proper stirring can also prevent localized heating and ensure consistent extraction throughout the sample, contributing to the overall effectiveness of the MAE process [68].
2.2. Microwave-Assisted Pyrolysis (MAP)
Microwave-assisted pyrolysis is a novel method for the in situ processing of biomass waste using microwave heating. It is a thermal degradation process that converts biomass into solid, liquid, and gaseous products under controlled temperature and pressure conditions. The microwave-assisted pyrolysis of biomass waste has several advantages over conventional pyrolysis methods, such as faster heating rates, higher reaction rates, and improved product selectivity [69].
The process of microwave-assisted pyrolysis involves the application of microwave radiation to heat the biomass material, which leads to the breakdown of complex organic molecules into simpler compounds. The microwave radiation causes the polar molecules in the biomass to rotate and vibrate, generating heat within the material. This heat leads to the breakdown of the biomass into volatile gases, liquids, and solid residues [70].
The mechanism of microwave-assisted pyrolysis involves several steps. First, the material is placed in a microwave reactor, and microwave energy is applied. The microwave energy causes the material to heat up, leading to thermal degradation and the formation of volatile gases [71]. The temperature variation of the material can be explained in four stages: the initial step is the dielectric relaxation of the water molecules, which leads to heating of the material; the second step involves the heating of the material up to 200 °C, where the main products are water and volatile gases; the third step is the formation of bio-oil and biochar, which occurs between 200 °C and 600 °C; and the final step is the decomposition of the biochar, which occurs at temperatures above 600 °C [72].
Numerous variables, such as the material’s characteristics, the parameters of the microwave treatment, the microwave absorber, co-pyrolysis, catalysts, and reactor design, influence the distribution of pyrolysis products. By modifying the catalysts’ composition, morphology, structure, and proportion through thoughtful design, the selectivity of the resulting products may be regulated. The primary determinant of catalyst activity is acidity. Higher acidity facilitates the pyrolysis of macromolecules into smaller gaseous molecules, but it also increases the risk of thermally sensitive chemicals in the plant matrix breaking down [73].
Microwave-assisted pyrolysis of plastic waste and biomass involves breaking down the plastic polymers and complex structure of biomass materials into smaller molecules using microwave radiation [74]. The resulting products include high-value-added liquid oil, gas, and solid carbon. The distribution of pyrolysis products is affected by several factors, including the properties of the plastics, biomass, microwave treatment parameters, microwave absorber, co-pyrolysis, catalysts, and reactor design [75].
Table 2 presents different conditions and value-added products that were obtained by previous researchers using different conditions of microwave-assisted pyrolysis.

Table 2.
Microwave-assisted pyrolysis conditions reported in the literature.
Microwave-assisted pyrolysis is influenced by various parameters that play a crucial role in determining the efficiency and outcomes of the process. Some key parameters include microwave power, pyrolysis temperature, residence time, microwave exposure time, and the addition of microwave absorbers.
Microwave Power: The level of microwave power applied during pyrolysis affects the heating rate, temperature, and overall efficiency of the process. Higher microwave power can lead to faster heating rates and improved conversion of the feedstock into desired products [73]. In Table 2, and in other researchers’ work, the microwave power levels range from 300 W to 800 W [81]. These power levels are crucial in determining the heating rates, efficiency, and outcomes of the pyrolysis process.
Pyrolysis Temperature: The temperature at which the pyrolysis reaction occurs is a critical parameter that influences the product composition and yield. Optimal temperature conditions are essential for achieving the desired outcomes in terms of product quality and quantity [82]. The pyrolysis temperature range for microwave-assisted pyrolysis varies depending on the material being processed and the desired products. In general, the pyrolysis temperature range for microwave-assisted pyrolysis is between 400 and 500 °C for biomass (Table 2). This temperature range is lower than that of conventional pyrolysis, which typically occurs between 400 and 900 °C. The lower temperature range for microwave-assisted pyrolysis is due to the unique heating mechanism of microwaves, which allows for rapid and uniform heating of the material, reducing the need for high temperatures to achieve complete pyrolysis [83].
Residence Time: The duration for which the material is exposed to the pyrolysis conditions, known as residence time, is another important factor. It affects the extent of conversion and the distribution of products obtained from the process [69]. According to the search results, the residence time for microwave-assisted pyrolysis can vary depending on the specific conditions and the desired products. For example, a study using microwave-assisted pyrolysis of biomass found that a residence time of 10 min at a temperature of 400 °C and a microwave power of 350 W resulted in a maximum biochar yield of 86.0 wt% [84]. Another study on microwave pyrolysis of sludge found that the residence time can be optimized to promote the formation or fracture of certain compounds. For example, a residence time of 20 s at a temperature of 550 °C resulted in the highest yield of light alkanes and aromatic hydrocarbons [85].
Microwave Exposure Time: The duration of exposure to microwave energy during pyrolysis is of significant importance. Insufficient exposure time may lead to incomplete conversion, while excessive exposure can alter the product distribution [86].
Microwave Absorbers: The addition of microwave absorbers can enhance the efficiency of microwave-assisted pyrolysis by improving the absorption of electromagnetic waves and facilitating heat transfer to the material being processed. This can lead to better conversion rates and product quality [69]. The types of microwave absorbers used in microwave-assisted pyrolysis include carbon-based materials, such as activated carbon, graphite, SiC, metals and their derivatives, aluminosilicate molecular sieves, and other materials. These absorbers are added to the pyrolysis process to enhance the microwave absorption and improve the heating efficiency.
For example, in the microwave pyrolysis of sludge, microwave absorbers are used to promote the heat transfer and mass transfer of volatile products. The use of microwave absorbers in sludge pyrolysis can effectively promote the heat transfer and mass transfer of volatile products, leading to higher pyrolysis efficiency compared to conventional pyrolysis [85].
In the microwave-assisted pyrolysis of waste plastics, the distribution of pyrolysis products is affected by several factors, including the properties of the plastics, the microwave treatment parameters, the microwave absorber, co-pyrolysis, the catalysts, and the reactor design. The microwave absorber is a crucial factor that affects the efficiency of microwave-assisted pyrolysis of plastics. The absorber can enhance the microwave absorption and improve the heating efficiency, leading to higher yields of high-value-added liquid oil, gas, and solid carbon [87].
In the microwave-assisted pyrolysis of biomass, the use of microwave absorbers can improve the selectivity of the process. If the residence time of the primary product inside the biomass is short, then the selectivity of the process could be improved almost 100 times. This is due to the selective activation of various components of the biomass at different temperatures, making it possible to increase the selectivity of the bio-oil and collect different fractions at different temperatures [84].
2.3. Microwave-Assisted Hydrothermal Treatments (MAHT)
Microwave-assisted hydrothermal treatment refers to a method that combines the benefits of microwave heating with the sustainable features of hydrothermal processes for the conversion of biomass into biofuels, chemicals, and biomaterials. This technology utilizes microwave energy to rapidly and uniformly heat the reaction mixture, enhancing the efficiency and selectivity of the conversion process. By applying microwave-assisted hydrothermal treatments, various reaction products such as hydrochar, bio-crude (bio-oil), and valuable chemicals can be obtained from biomass. The method involves controlling reaction conditions like temperature, time, catalyst type, biomass loading, and microwave power to influence the yields and properties of the final products. Microwave-assisted hydrothermal treatments have emerged as a cutting-edge technology with the potential to revolutionize biomass valorization for energy, pharmaceutical, and chemistry applications [88]. The ranges of hydrothermal treatments are classified into carbonization (180–250 °C, 2–10 Mpa), liquefaction (200–450 °C, 5–25 Mpa), and gasification (500–1400 °C, up to 3.3 MPa) [89].
This method combines the advantages of microwave heating, such as rapid and uniform heating, with the benefits of hydrothermal synthesis, which include the ability to produce high-quality nanoparticles with a narrow particle size distribution and uniform morphology [90]. The microwave-assisted hydrothermal method is different from traditional hydrothermal synthesis methods in that it uses microwave energy for heating, rather than conventional conduction methods [88].
The microwave-assisted hydrothermal method can be divided into low-temperature hydrothermal and supercritical hydrothermal methods, depending on the equipment used and the reaction conditions. The low-temperature hydrothermal method is typically carried out at temperatures below 200 °C, while the supercritical hydrothermal method is carried out at temperatures and pressures above the critical point of water [88,91]. The method can also be combined with other action fields, such as direct current electric and magnetic and microwave fields, to enhance the reaction. The use of microwaves in hydrothermal treatments has several advantages, including faster reaction rates, higher yields, and more uniform heating compared to traditional hydrothermal methods [89]. The high dielectric constant and ionic product of water under hydrothermal conditions make it an excellent reaction medium for the conversion of biomass, while the high density and elevated dissociation constant of water under hydrothermal conditions also accelerate ionic reactions.
Table 3 shows different conditions of MAHT; in order to transform biomass waste into value-added products, different microwave conditions are needed in order to transform different types of biomasses.

Table 3.
Microwave-assisted hydrothermal treatment reported in the literature.
The amount of hydrochar obtained from biomass through microwave-assisted hydrothermal carbonization (MA-HTC) is influenced by several factors, including reaction temperature, time, catalyst type and quantity, and solid-to-liquid ratio. Other factors, such as biomass type, microwave power, and particle size, also have an impact, albeit to a lesser extent. Here is a summary of the effects of these variables:
Reaction temperature: Higher temperatures lead to higher yields and better fuel properties, as they promote the conversion of biomass into hydrochar. Higher temperatures within the range of 200–400 °C, as highlighted in the sources, play a crucial role in the conversion of biomass into hydrochar. Increasing the temperature has been shown to be the most significant factor in increasing carbon content and decreasing oxygen content in the hydrochar, ultimately affecting the higher heating value (HHV) of the material. This demonstrates that the reaction temperature in MAHT processes directly impacts the composition and properties of the hydrochar, influencing its potential as a solid biofuel or other applications [75];
Time: The influence of reaction time in microwave-assisted hydrothermal processes is significant in determining the yield and properties of the resulting products. In the case of microwave-assisted hydrothermal carbonization (MA-HTC), the reaction time is a key factor in the conversion of biomass into hydrochar.
For instance, in the microwave-assisted hydrothermal carbonization of banana peels, the effect of process conditions on the physico–chemical properties of the hydrochar was investigated [98]. In another study, the potential of low-temperature MA-HTC for the reduction in emission precursors in short-rotation coppice willow wood was investigated [99]. In the case of microwave-assisted hydrothermal synthesis, the reaction time plays a crucial role in the formation and growth of the desired products. For example, in the synthesis of perovskites using microwave-assisted hydrothermal synthesis, the reaction time was found to have a pronounced effect on the physico–chemical and catalytic properties of the resulting catalysts [100];
Catalyst (type and amount): The use of catalysts in these processes can result in synergetic chemical activation to the hydrochar adsorbents, enhancing the overall efficiency of the process [101]. The type of catalyst used can greatly affect the adsorption properties of the hydrochar, as demonstrated in a study on the microwave-assisted hydrothermal carbonization of furfural residue [102]. The study found that the use of an alkali-enhanced (0.05 N KOH) hydrochar resulted in improved adsorption properties compared to the acid-enhanced hydrochar (0.05 N HCl) and the commercially activated carbon (CAC). It is worth noting (as presented in Table 3) that catalysts act differently for each biomass and each desired value-added product.
The amount of catalyst used can also have a significant impact on the process. For example, in the synthesis of perovskites using microwave-assisted hydrothermal synthesis, the amount of catalyst used was found to have a pronounced effect on the physico–chemical and catalytic properties of the resulting catalysts [100]. The study found that the microwave-synthesized MWhyd-La 0.8 Ag 0.2 MnO 3 þd catalyst demonstrated excellent conversion of methane, while conventionally prepared catalysts deactivated quickly in the presence of hydrogen sulfide [95];
Biomass type: Different biomass types have different compositions and properties, which can influence the yield and fuel properties of the hydrochar. The type of biomass used in microwave-assisted hydrothermal processes can significantly influence the process’s efficiency and the properties of the resulting products. Biomass is a complex material made up of various components, including cellulose, hemicellulose, lignin, and extractives, each with different chemical and physical properties.
Microwave-assisted hydrothermal carbonization (MA-HTC) of different biomass types has been studied, and the process characteristics of MA-HTC of cellulose have been investigated [103]. The study found that a first-order kinetics model based on carbon content could be used to describe the MA-HTC of cellulose;
Microwave power: The microwave power can affect the heating rate and the uniformity of the heat distribution, which in turn can affect the yield and fuel properties of the hydrochar. The influence of microwave power in microwave-assisted hydrothermal processes is crucial for optimizing the efficiency and selectivity of reactions. The range of microwave power used in these processes typically varies depending on the specific application and desired outcomes. The range of microwave power used in these processes can vary depending on the specific application and the materials being processed. For example, in the synthesis of functional nanomaterials, the microwave power may range from 100 to 1000 W [90], while in the production of solid biofuels, the range may be between 500 and 1500 W [104]. The choice of microwave power is critical as it directly influences the heating rate, reaction kinetics, and overall efficiency of the process;
Particle size: The particle size of the biomass can affect the heat transfer and the reaction kinetics, which can influence the yield and fuel properties of the hydrochar. The particle size of the biomass can affect the heat transfer and the reaction kinetics, which can influence the yield and fuel properties of the hydrochar in microwave-assisted hydrothermal processes. This is because the heat transfer in microwave-assisted hydrothermal processes occurs from the inside to the outside, via direct coupling of electromagnetic waves with the dipoles present in the reaction mixture. Correspondingly, the core of the sample generally remains at a higher temperature than the surface during the heating process [105]. A smaller particle size can lead to a higher surface area-to-volume ratio, which can improve heat transfer and increase the reaction rate. This can lead to a higher yield of hydrochar with better fuel properties [106].
2.4. Microwave-Assisted Acid Hydrolysis (MAAH)
Microwave-assisted hydrolysis (acid) is a technique that uses microwave radiation to heat and catalyze the hydrolysis of biomass, such as cellulose, in the presence of an acid catalyst. This technique has been shown to be more efficient than conventional hydrothermal processing, which typically yields platy, needles, or irregular agglomerated particles. In contrast, microwave-hydrothermal processing yields solids with agglomerate-free narrow particle size distribution, which is expected to have better sintering properties.
One example of microwave-assisted acid hydrolysis is the use of a biomass char sulfonic acid (BC-SO3H) catalyst to hydrolyze cellulose in water under microwave irradiation. The BC-SO3H catalysts prepared from biomass char have been found to be effective in catalyzing the hydrolysis of cellulose, resulting in high yields of glucose and other sugars [107].
Another example of microwave-assisted acid hydrolysis is the hydrolysis of saponins, which are natural compounds found in plants. The hydrolysis of saponins can be performed chemically using acid hydrolysis, and microwave-assisted acid hydrolysis has been shown to be an efficient and green method for the hydrolysis of saponins. The optimal conditions for microwave-assisted acid hydrolysis of saponins, such as temperature, time, concentration of solvent, and ratio of solvent to solid, have been studied and optimized for the production of specific compounds, such as diosgenin from yam [108].
The MAAH process is typically carried out in a suitable solvent, such as water, under controlled temperature and pressure conditions. The microwave irradiation provides efficient and uniform heating, promoting the breakdown of chemical bonds within the protein. The acid catalyst used in MAAH can be sulfuric acid or hydrochloric acid, and the concentration of the acid can vary depending on the specific application [109].
Table 4 presents different processes that have been performed using MAAH in order to valorize and transform different types of feedstocks.

Table 4.
Microwave-assisted acid hydrolysis reported in the literature.
Similar to other types of microwave-assisted processes, the parameters that influence microwave-assisted acid hydrolysis (MAAH) include the type and amount of acid, temperature, reaction time, and microwave power. These factors can affect the yield and quality of the hydrolyzed products.
The type and amount of acid used in MAAH can significantly affect the hydrolysis process. For instance, phosphoric acid has been found to have the highest ability to hydrolyze proteins compared to other acids such as hydrochloric acid, sulfuric acid, and citric acid [110]. The concentration of the acid also plays a crucial role in the hydrolysis process. For example, a 4 M phosphoric acid concentration with a ratio of 1:6 (enzyme and substrate) has been found to provide the highest degree of hydrolysis (DH) of 9.5% [110].
The temperature and reaction time are also critical factors in MAAH. Higher temperatures and longer reaction times can lead to more extensive hydrolysis, but they can also cause degradation of the products. For instance, microwave-assisted acidic hydrolysis of phytate from rye bran is conducted at temperatures up to 200 °C and heating times of up to 15 min [114].
The microwave power used in MAAH can also affect the hydrolysis process. For example, a high microwave power level and 10 min irradiation time have been found to result in the highest glucose concentration in the hydrolysis of peanut shells using dilute sulfuric acid [115]. This can be seen in results in previous research such as the study conducted by Tong et al. (2021), where the galactose yield increased from 0 to over 40% at 110 °C and with H2SO4 (0.1–0.3 M), leading to its production at 15 min (40% yield), when, without MAAH, it was not produced until after 50 min (35% yield) of reaction time [115].
2.5. Microwave-Assisted Organosolv (MAO)
Microwave-assisted organosolv is a method of biomass pretreatment that combines microwave irradiation with organosolv solvents to enhance the breakdown of lignocellulosic biomass into its constituent parts, such as cellulose, hemicellulose, and lignin. The use of microwave irradiation allows for faster and more energy-efficient heating compared to conventional methods, which can result in higher yields and better quality of the resulting products.
Microwave-assisted organosolv is a process that leverages microwave irradiation and organic solvents to pretreat lignocellulosic biomass efficiently. The process involves several key steps that are crucial for its successful implementation, as supported by the provided sources.
Preparation of Reaction Mixture: The first step in microwave-assisted organosolv involves preparing a reaction mixture containing the lignocellulosic biomass, an organic solvent (e.g., ethanol), and, optionally, a catalyst such as sulfuric acid [116].
Lignocellulosic biomass is a type of biomass that is mainly composed of cellulose, hemicellulose, and lignin. Examples of lignocellulosic biomass include agricultural residues such as corn stover, wheat straw, and sugarcane bagasse, as well as forestry residues such as wood chips and sawdust [117]. Organic solvents are commonly used in the solvolysis of lignocellulosic biomass to enhance the breakdown of the biomass. Examples of organic solvents used in organosolv pretreatment include ethanol, methanol, and acetone [118]. Catalysts such as sulfuric acid, hydrochloric acid, and acetic acid are often used in microwave-assisted organosolv pretreatment to enhance the breakdown of lignocellulosic biomass. These catalysts can help to lower the reaction temperature and time required for the pretreatment process, leading to higher yields of cellulose and hemicellulose [119]. This can be observed in valorization processes of pistachio shells (PS) and cherry tree pruning (CTP), where the use of MAO allowed for a selective increase in cellulose with lower times (20 min) and less energy (165 °C), going from 31% to 91% cellulose for PS and from 38% to 81% cellulose content for CTP [120].
In the microwave-assisted organosolv pretreatment process, the lignocellulosic biomass is first mixed with the organic solvent and catalyst in a reaction vessel. The mixture is then subjected to microwave irradiation, which causes the temperature of the solvent to increase rapidly, leading to the breakdown of the biomass. The microwave irradiation also causes the dipoles in the solvent molecules to rotate and collide, generating heat and further enhancing the breakdown of the biomass [121].
The choice of an organic solvent and catalyst can significantly affect the efficiency of the microwave-assisted organosolv pretreatment process. For example, a study found that the use of ethanol as a solvent in microwave-assisted organosolv pretreatment resulted in higher yields of lignin. Another study found that the use of sulfuric acid as a catalyst in microwave-assisted organosolv pretreatment resulted in higher yields of cellulose and hemicellulose compared to the use of hydrochloric acid or acetic acid.
Microwave Heating: The prepared reaction mixture is then subjected to microwave irradiation in a specialized reactor. This microwave energy rapidly and uniformly heats the mixture, facilitating the breakdown of lignin and hemicellulose components in the biomass [116].
Microwave heating in organosolv-assisted processes plays a crucial role in the efficient breakdown of lignin and hemicellulose components in biomass. The ranges of microwave power used in these processes can vary depending on the specific application and equipment [122]. Laboratory microwave ovens typically operate in the range of 300 to 1200 watts, while industrial microwave systems can have power levels ranging from a few kilowatts to tens of kilowatts. The efficient energy transfer enabled via microwave irradiation facilitates the rapid extraction of lignin in a relatively short time, ensuring a low level of process severity [123]. Studies have shown that microwave-assisted organosolv processes can achieve high cellulose recovery rates (around 80%) and lignin purity (approximately 70%), highlighting the effectiveness of this method for biomass fractionation [116].
Temperature and Pressure Control: Throughout the microwave-assisted heating process, precise control of temperature and pressure is essential to optimize the organosolv reaction. Adjusting the microwave power based on temperature feedback ensures the desired reaction conditions are maintained. Temperature and pressure control are crucial factors in microwave-assisted organosolv processes, as they can significantly impact the efficiency and quality of the resulting lignin and cellulose fractions.
The temperature range used in microwave-assisted organosolv processes typically varies from 100 to 200 °C, depending on the specific biomass being processed and the desired product yields. For example, a study conducting microwave-assisted organosolv extraction of lignin from spruce wood used a temperature range of 140–180 °C for 5–20 min, achieving high cellulose recovery rates (around 80%) and lignin purity (approximately 70%) [123].
Pressure control is also important in microwave-assisted organosolv processes, as high pressures can facilitate the breakdown of lignin and hemicellulose components in the biomass. A study using microwave-assisted organosolv pretreatment of a sawmill mixed feedstock for bioethanol production in a wood biorefinery used a pressure range of 1.5–2.0 MPa [116].
The use of microwave irradiation in organosolv processes can significantly reduce processing time and energy consumption compared to traditional heating methods. For example, a study using microwave-assisted organosolv extraction of coconut shell lignin via microwave-assisted organosolv extraction achieved high lignin yields and purities under milder conditions and lower pressure (atmospheric) compared to traditional heating methods [124].
Fractionation and Filtration: Following the reaction, the solid residue (crude cellulose) is separated from the liquid phase. The solid residue undergoes washing and drying to obtain purified cellulose, while the liquid phase is processed to collect organosolv lignin through filtration and subsequent drying [116].
An example of the application of microwave-assisted organosolv is demonstrated in a study optimizing the pretreatment of cherry tree pruning and pistachio for enzymatic hydrolysis, a crucial step in bioethanol production. This research work successfully applied microwave-assisted organosolv pretreatment to enhance the efficiency of cellulose hydrolysis and delignification, showcasing the potential of this method for sustainable biofuel production [125].
Table 5 shows different organosolv processes reported in the literature and the different conditions used.

Table 5.
Microwave-assisted organosolv reports in the literature.
The parameters of microwave-assisted organosolv include temperature and pressure control. The temperature range typically used in microwave-assisted organosolv processes is from 100 to 200 °C, while the pressure range can vary depending on the specific application and equipment used. For example, a study using microwave-assisted organosolv extraction of coconut shell lignin used a temperature range of 140–180 °C and a pressure range of 1.5–2.0 MPa [124]. Another study using microwave-assisted organosolv delignification of sugar cane bagasse used a temperature range of 180–200 °C and a pressure range of 1.2–1.5 MPa [124].
The microwave power used in microwave-assisted organosolv processes can also vary depending on the specific application and equipment used. For example, a study using microwave-assisted organosolv extraction of coconut shell lignin used a microwave power of 700 W [124], while another study using microwave-assisted organosolv delignification of sugar cane bagasse used a microwave power of 900 W [128].
The duration of microwave-assisted organosolv processes can also vary depending on the specific application and equipment used. For example, a study using microwave-assisted organosolv extraction of coconut shell lignin used a duration of 15–30 min, while another study using microwave-assisted organosolv delignification of sugar cane bagasse used a duration of 60–90 min [128].
3. Previous Designs for Microwave-Assisted Processes
Microwave design plays a pivotal role in the application of microwave-assisted processes, revolutionizing various industries by harnessing the power of electromagnetic waves for efficient and precise heating applications. The intricate design of microwaves not only influences the uniformity and speed of heating but also impacts the overall effectiveness and control of these processes. By optimizing the design of microwave systems, engineers and researchers can enhance the performance, reliability, and versatility of microwave-assisted technologies across diverse fields such as chemistry, materials science, food processing, and pharmaceuticals.
The significance of microwave design lies in its ability to tailor electromagnetic fields to interact with specific materials, enabling targeted and rapid heating that can lead to improved reaction kinetics, reduced energy consumption, and enhanced product quality. Through careful consideration of factors like cavity geometry, waveguide configuration, power distribution, and frequency modulation, designers can fine-tune microwave systems to meet the unique requirements of different applications. This precision in design not only maximizes the efficiency of heating processes but also opens doors to innovative advancements in areas like synthesis, extraction, drying, and sterilization, showcasing the indispensable role of microwave design in driving progress and innovation in microwave-assisted technologies.
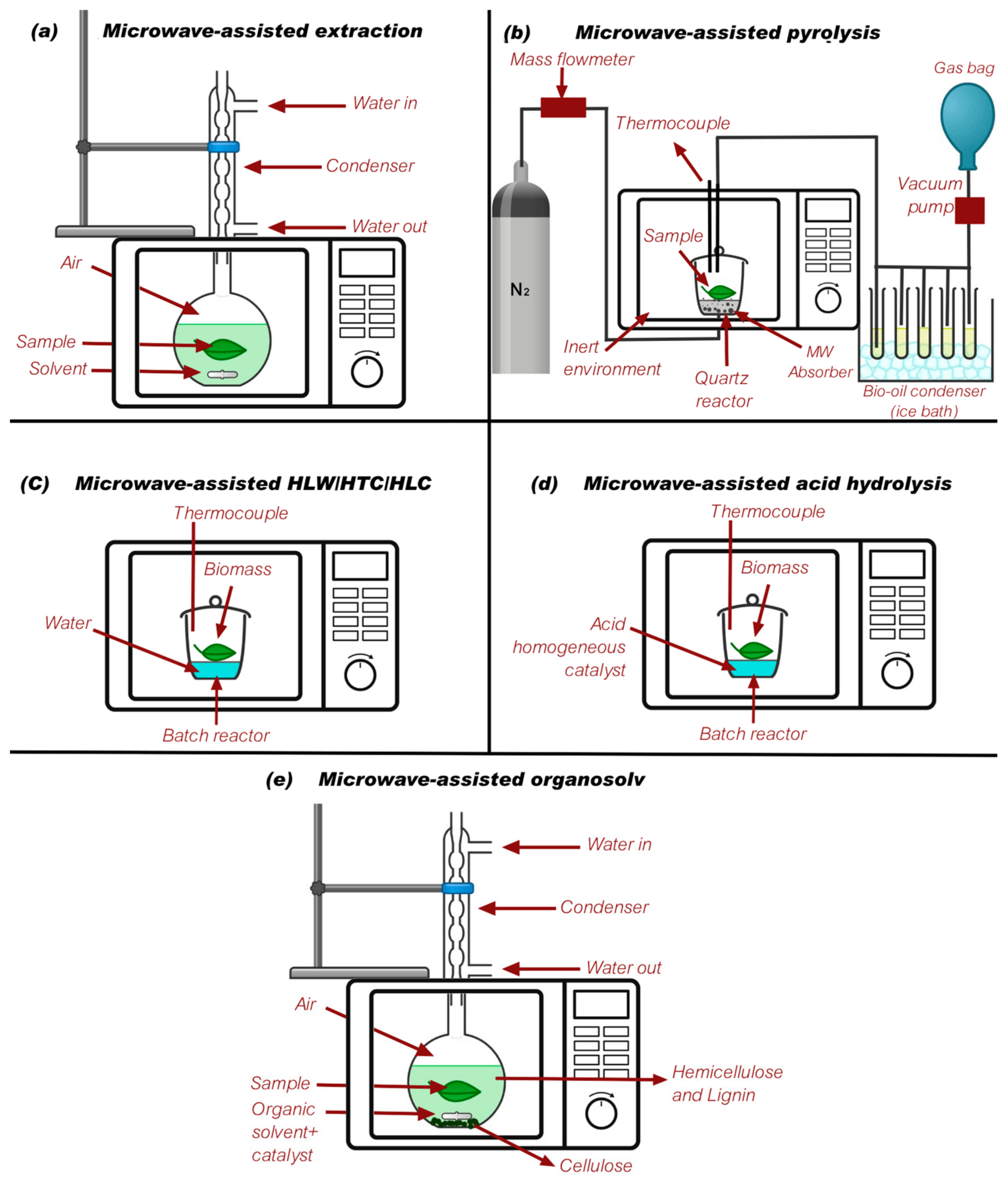
Different microwave-assisted processes need modifications in the microwave design in order to have an optimal yield, extraction, and reaction. Figure 3 shows different designs reported in the literature for microwave-assisted processes. This, added to the microwave conditions previously reported, allows for reactions with biomass, plastics, and other feedstocks to take place efficiently.
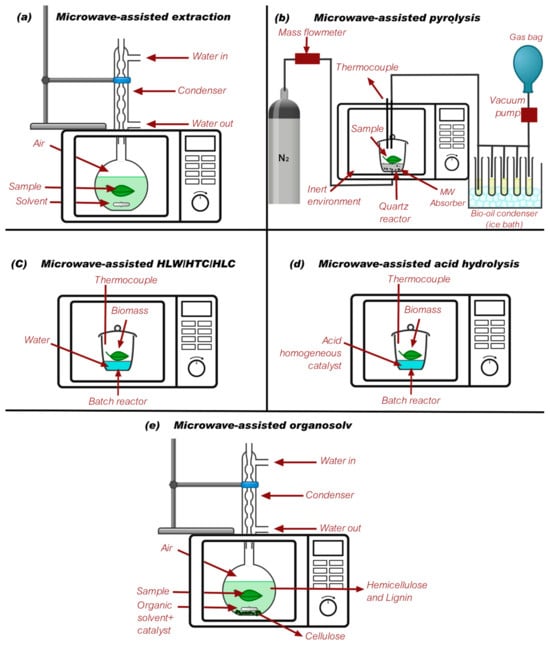
Figure 3.
Different designs reported for microwave-assisted processes. (a) [129], (b) [130], (c) [131], (d) [132], and (e) [127].
When designing a microwave system for microwave-assisted processes, it is crucial to consider the characteristics of the sample being processed and the desired products to ensure optimal performance and efficiency. The properties of the sample, such as its size, shape, composition, and dielectric properties, directly influence how it interacts with the microwave energy. Understanding these characteristics is essential for designing a system that can deliver uniform heating, precise control over temperature profiles, and efficient energy transfer to the sample. For instance, a sample with high dielectric loss will absorb microwave energy more readily, requiring careful power control to prevent overheating and ensure even heating throughout the sample.
Furthermore, the desired products play a significant role in determining the specific requirements for the microwave system. Different products may have unique heating requirements, such as specific temperature ranges, heating times, or energy levels, that must be met to achieve the desired outcomes. For example, in the synthesis of nanomaterials, precise temperature control is crucial to ensure the formation of uniform particles with the desired size and shape. By considering the desired products, engineers can tailor the microwave system to meet the unique needs of the process, ensuring consistent results, high product quality, and overall process optimization.
In addition to optimizing performance and efficiency, considering the sample’s characteristics and desired products also contributes to the safety and reliability of the microwave-assisted process. By understanding the sample’s properties, engineers can design a system that minimizes the risk of overheating, thermal runaway, or other hazards that could compromise the safety of the process or the quality of the products. Moreover, by considering the desired products, engineers can ensure that the microwave system is capable of consistently meeting the process requirements, reducing the risk of variability or inconsistency in the final product. In summary, keeping the sample’s characteristics and desired products in mind during the design phase of a microwave system for microwave-assisted processes is essential for achieving optimal performance, efficiency, safety, and reliability.
4. Added-Value Products from Biomass via Microwave-Assisted Processes
Biomass is usable in microwave-assisted processes due to its unique properties and the interaction between the microwave energy and the biomass components. Biomass materials, such as lignocellulosic biomass, contain polar molecules and ions that can interact with microwave radiation, resulting in rapid and uniform heating. This interaction is based on dielectric heating, which involves the rotation of polar molecules and the oscillation of ions in the presence of an alternating electromagnetic field. This process generates heat, which facilitates the conversion of biomass into value-added products.
For instance, in microwave-assisted biodiesel production, biomass-derived catalysts, such as lignin-rich biomass, can be used to enhance the esterification process and achieve high yields of biodiesel [133]. Additionally, microwave-assisted pyrolysis offers several advantages over conventional pyrolysis, including fast heating rates, high pyrolysis temperatures, uniform heating, and the ability to achieve more selective pyrolysis, resulting in higher yields of bio-oil (a complex mixture of organic compounds including aliphatic and aromatic hydrocarbons, oxygen-containing compounds, and nitrogen-containing compounds), bio-gas (consists mainly of methane, hydrogen, and carbon dioxide), fuels (bioalcohol, biofuels, and value-added chemicals, offering sustainable alternatives to traditional fossil fuels) and biochar from waste materials [134].
Furthermore, microwave pretreatment of biomass is a promising technology for the conversion of lignocellulosic materials into renewable biofuels. This method enhances the efficiency of biomass conversion processes, making it a valuable approach for producing added-value products from biomass [135]. Overall, the unique properties of biomass and the interaction between biomass and microwave radiation make it a suitable feedstock for microwave-assisted processes, enabling the production of a range of value-added products.
Coffee waste, comprising various components like silverskin, parchment, husk, mucilage, and spent coffee grounds (SCG), presents a valuable resource for valorization. With SCG alone constituting 65% of the waste, the diverse composition of coffee residues offers a rich source of organic material for sustainable processes. For instance, the lignin, cellulose, and hemicellulose content in different coffee residues, as detailed in Table 6 below, showcases the potential for extracting valuable compounds and bio-based products through biorefinery approaches. Coffee cherry, pulp, husk, and SCG (spent coffee grounds) each contain varying proportions of these components, highlighting the versatility of coffee waste for bioconversion processes.

Table 6.
Structural components of coffee waste [8,136].
The significant presence of cellulose, hemicellulose, and lignin in coffee residues underscores their potential for biorefinery applications. These components can be harnessed for the production of biofuels, chemicals, and biomaterials through processes like microwave-assisted extraction and pyrolysis. By utilizing the diverse composition of coffee waste in biorefineries, it is possible to extract valuable compounds such as antioxidants, polyphenols, and caffeine, contributing to the circular economy and sustainable resource management. The rich organic content of coffee waste, coupled with its lignocellulosic structure, offers a promising avenue for transforming this waste stream into high-value products, aligning with the principles of green chemistry and bioeconomy initiatives.
Table 7 showcases various valorization processes applied to coffee waste using microwave-assisted techniques. By carefully adjusting temperature, pressure, energy, time, solvent, catalyst, and other conditions, a comprehensive valorization of the waste can be achieved. The table highlights the potential of microwave-assisted methods in extracting valuable compounds and producing high-value products from coffee waste, thereby contributing to the development of a circular bioeconomy and sustainable resource management.

Table 7.
Microwave-assisted processes applied to coffee waste.
As seen in Table 7, coffee wastes offer significant potential for valorization, with the appropriate conditions in microwave-assisted processes enabling the extraction and production of a wide array of value-added products. Initially, polyphenols, colorants, caffeine, and other organics can be extracted under specific conditions. Subsequently, a microwave-assisted hydrolysis process can be employed to obtain carbohydrates, which can be further transformed into organic solvents. Additionally, microwave-assisted organosolv techniques can be applied to remove lignin and cellulose, which can be utilized in various other processes. Finally, microwave-assisted hydrothermal treatment or microwave-assisted pyrolysis can be used to produce biochar, bio-oils, and biogas, further maximizing the value derived from coffee waste.
Designing a versatile microwave system capable of accommodating a range of microwave-assisted processes for valorizing different components of coffee waste is highly beneficial and worthwhile. Such a system would enable the extraction of maximum value from coffee waste by offering flexibility in temperature control, reactor configurations, pressure conditions, gas atmospheres, flow rates, stirring mechanisms, energy input, and the ability to handle various phases (liquid, gas, and solid). This versatility allows for tailored processing conditions to target specific compounds or products within the diverse composition of coffee waste, optimizing the extraction of valuable bioactive compounds, antioxidants, biofuels, and other high-value products. By incorporating a range of parameters and capabilities, the designed microwave system can facilitate efficient and sustainable valorization processes, contributing to resource efficiency, waste reduction, and the development of innovative solutions for coffee waste management within a circular bioeconomy framework.
5. Strengths and Weaknesses of Microwave-Assisted Processes
Microwave-assisted processes offer several advantages, including high efficiency and productivity, uniform distribution, rapid volumetric heating, high reaction rates, and product and process quality. These benefits can lead to significant reductions in synthesis times, often saving over 90% compared to conventional methods, improved product quality due to uniform size distribution, and the possibility of scaling into pilot type and commercial installations [144]. Microwave-assisted extraction, in particular, has several advantages over other extraction processes, such as a low cost of production, a shorter time period of processing, and less solvent usage [145].
However, there are also some limitations to consider with microwave-assisted processes. The choice of materials used in the process can impact the efficiency of microwave heating, and careful control of synthesis parameters is necessary to optimize the final properties of the products. Additionally, microwave irradiation has disadvantages such as not being feasible for reaction monitoring [146]. Microwave-assisted extraction, ultrasound-assisted extraction, and the supercritical CO2 method have the disadvantages of high cost, complex operating procedures, and limited applications [147].
Table 8 presents a comparison of advantages and disadvantages of microwave-assisted processes, where, in most cases, the biggest disadvantage to overcome is the lack of scaling technologies and applicability in the industry. As for advantages, an overall increase in the efficiency and selectivity and a decrease in reaction times can be achieved.

Table 8.
Advantages and disadvantages of microwave-assisted processes.
6. Future Directions
The future of microwave-assisted processes, including microwave-assisted extraction (MAE), microwave-assisted pyrolysis, microwave-assisted hydrothermal treatment, microwave-assisted acid hydrolysis, and microwave-assisted organosolv, is expected to focus on enhancing scalability, sustainability, and industrial applicability. One key direction is the development of large-scale microwave-assisted systems that can efficiently handle high-volume extraction and treatment processes [159]. Researchers are working on designing and building industrial-scale microwave reactors that can maintain consistent and controlled conditions, ensuring high-quality products and reducing energy consumption.
Another future direction is the integration of microwave-assisted processes with other emerging technologies, such as supercritical fluid extraction (SFE), ultrasonic extraction, and enzyme-assisted extraction. By combining these techniques, researchers can create hybrid processes that offer enhanced efficiency, selectivity, and sustainability. This integration has the potential to significantly improve the scalability and industrial applicability of microwave-assisted processes, enabling their widespread adoption in various industries [160].
Furthermore, the future of microwave-assisted processes will likely involve the exploration of new solvent systems and advanced materials that can further enhance extraction efficiency and sustainability. Researchers are investigating the use of green solvents, ionic liquids, and deep eutectic solvents, which can offer improved solvent properties, reduced environmental impact, and better extraction yields [161] Additionally, the development of novel materials, such as microwave-absorbing materials and catalysts, can help optimize microwave conditions, enhance reaction rates, and improve overall process efficiency [137].
Lastly, future research in microwave-assisted processes will focus on the optimization of process parameters for specific applications, ensuring the scalability and industrial applicability of these techniques. By fine-tuning microwave power levels, irradiation times, and temperature control, researchers can create tailored processes that meet the unique requirements of various industries [89]. This focus on application-specific optimization will drive the widespread adoption of microwave-assisted processes, enabling sustainable and efficient extraction, treatment, and processing of various materials.
7. Conclusions
This review article delved into the realm of microwave-assisted processes, particularly focusing on microwave-assisted extraction (MAE), microwave-assisted pyrolysis, microwave-assisted hydrothermal treatment, microwave-assisted acid hydrolysis, and microwave-assisted organosolv. Through a comprehensive analysis of these techniques, this review underscores the versatility and efficiency of microwave-assisted methods in biomass valorization and product generation. It highlights the potential of these processes to revolutionize the conversion of biomass into valuable end-products, offering sustainable and environmentally friendly alternatives to traditional extraction and treatment methods.
Moreover, this review shed light on how different microwave-assisted processes can influence the products obtained from the same biomass, using coffee as an illustrative example. By employing either MAE, MAP, MAHT, MAAH, or MAO on coffee biomass, researchers can tailor the processing steps to yield a diverse range of products. For instance, MAE may focus on extracting bioactive compounds, MAP on bio-oil production, MAHT on bio-crude generation, MAAH on sugar production, and MAO on compound extraction using organic solvents. This example showcases the versatility and adaptability of microwave-assisted processes in extracting a spectrum of valuable products from the same biomass source, emphasizing the potential for customized and efficient biomass utilization strategies.
Author Contributions
Conceptualization, A.S.L.P.; methodology, A.S.L.P.; formal analysis, A.S.L.P. and J.J.L.C.; investigation, A.S.L.P. and C.A.G.F.; resources, C.A.G.F.; data curation, A.S.L.P.; writing—original draft preparation, A.S.L.P.; writing—review and editing, A.S.L.P. and C.A.G.F.; visualization, A.S.L.P. and J.J.L.C.; supervision, C.A.G.F.; project administration, C.A.G.F. and J.J.L.C.; funding acquisition, A.S.L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MINCIENCIAS, grant number Contrato de financiamiento de recuperación contingente No. 80740-101-2022. “Implementación de una biorrefinería hidrotermal para la producción de productos químicos de alto valor agregado, mediante el uso de biomasas residuales de procesos agroindustriales, en alianza intersectorial (academia-industria)”. Código 1101-914-91642.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank the Universidad Nacional de Colombia and the Departamento de Química-Facultad de Ciencias for their support and the possibility of using equipment and techniques that allowed the development of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Omer, A.M. Biomass energy resources utilisation and waste management. Agric. Sci. 2012, 3, 124–145. [Google Scholar] [CrossRef]
- Moustakas, K.; Loizidou, M. Waste and biomass management and valorization. Environ. Sci. Pollut. Res. 2021, 28, 24224–24229. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Chen, A.; Bai, M.; Peng, L.; Shao, J.; Yuan, J.; Shang, C.; Zhang, J.; Huang, H.; Peng, C. Valorization of heavy metal contaminated biomass: Recycling and expanding to functional materials. J. Clean. Prod. 2022, 366, 132771. [Google Scholar] [CrossRef]
- Kumar, K.; Kumar, R.; Kaushal, S.; Thakur, N.; Umar, A.; Akbar, S.; Ibrahim, A.A.; Baskoutas, S. Biomass waste-derived carbon materials for sustainable remediation of polluted environment: A comprehensive review. Chemosphere 2023, 345, 140419. [Google Scholar] [CrossRef]
- Tišma, M.; Bucić-Kojić, A.; Planinić, M. Bio-based Products from Lignocellulosic Waste Biomass. Chem. Biochem. Eng. Q. 2021, 35, 139–156. [Google Scholar] [CrossRef]
- Wang, G.; Dai, Y.; Yang, H.; Xiong, Q.; Wang, K.; Zhou, J.; Li, Y.; Wang, S. A Review of Recent Advances in Biomass Pyrolysis. Energy Fuels 2020, 34, 15557–15578. [Google Scholar] [CrossRef]
- Pereira, E.G.; da Silva, J.N.; de Oliveira, J.L.; Machado, C.S. Sustainable energy: A review of gasification technologies. Renew. Sustain. Energy Rev. 2012, 16, 4753–4762. [Google Scholar] [CrossRef]
- Lozano-Pérez, A.S.; Guerrero-Fajardo, C.A. Liquid Hot Water (LHW) and Hydrothermal Carbonization (HTC) of Coffee Berry Waste: Kinetics, Catalysis, and Optimization for the Synthesis of Platform Chemicals. Sustainability 2024, 16, 2854. [Google Scholar] [CrossRef]
- Galvis-Sandoval, D.E.; Lozano-Pérez, A.S.; Guerrero-Fajardo, C.A. Hydrothermal Valorization via Liquid Hot Water and Hydrothermal Carbonization of Pea Pod Waste: Characterization of the Biochar and Quantification of Platform Molecules. Appl. Sci. 2024, 14, 2329. [Google Scholar] [CrossRef]
- Palvasha, B.A.; Ahmad, S.; Abbasi, B.B.K.; Nazir, M.S.; Abdullah, M.A. Bioconversion of Straw Biomass into Bioproducts; Springer: Cham, Switzerland, 2021; pp. 369–383. [Google Scholar] [CrossRef]
- Lyu, Q.; Song, L.; Tong, Y.W.; Wang, W.; Zhou, J.; Yan, Z. Editorial: Highly efficient bioconversion of biomass waste: From theory to industry. Front. Bioeng. Biotechnol. 2023, 11, 1147993. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Sziple, F.; Vlazan, P.; Sfarloaga, P.; Grozesku, I.; Daniel, V. Biomass Extraction Methods. In Biomass Now—Sustainable Growth and Use; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Rasheed, T.; Anwar, M.T.; Ahmad, N.; Sher, F.; Khan, S.U.-D.; Ahmad, A.; Khan, R.; Wazeer, I. Valorisation and emerging perspective of biomass based waste-to-energy technologies and their socio-environmental impact: A review. J. Environ. Manag. 2021, 287, 112257. [Google Scholar] [CrossRef]
- Kapoore, R.; Butler, T.; Pandhal, J.; Vaidyanathan, S. Microwave-Assisted Extraction for Microalgae: From Biofuels to Biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Nomanbhay, S.; Salman, B.; Hussain, R.; Ong, M.Y. Microwave pyrolysis of lignocellulosic biomass—A contribution to power Africa. Energy Sustain. Soc. 2017, 7, 23. [Google Scholar] [CrossRef]
- Allende, S.; Brodie, G.; Jacob, M.V. Breakdown of biomass for energy applications using microwave pyrolysis: A technological review. Environ. Res. 2023, 226, 115619. [Google Scholar] [CrossRef]
- Berko, E.; Adomako, D. FOSC 613 Industrial Food Processing Technologies. In Microwave Processing; 2016.
- Das, A.; Banik, B. Microwaves in Chemistry Applications: Fundamentals, Methods and Future Trends; Elsevier Science: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Gaba, M.; Dhingra, N. Microwave Chemistry: General Features and Applications. Indian J. Pharm. Educ. Res. 2010, 45, 175–183. [Google Scholar]
- Wang, C.; Yao, W.; Zhu, H.; Yang, Y.; Yan, L. Uniform and highly efficient microwave heating based on dual-port phase-difference-shifting method. Int. J. RF Microw. Comput.-Aided Eng. 2021, 31, e22784. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Li, D. A new concept to improve microwave heating uniformity through data-driven process modelling. In Proceedings of the 17th International Conference on Microwave and High Frequency Heating, Valencia, Spain, 9–12 September 2019; Universitat Politècnica de València: Valencia, Spain, 2019. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L. Microwave dielectric heating in modern organic synthesis and drug discovery. In Microwave Heating; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, W. Microwave-assisted Inorganic Syntheses. In Modern Inorganic Synthetic Chemistry; Elsevier: Amsterdam, The Netherlands, 2011; pp. 173–195. [Google Scholar] [CrossRef]
- Harrison, A.; Whittaker, A.G. Microwave Heating. In Comprehensive Coordination Chemistry II; Elsevier: Amsterdam, The Netherlands, 2003; pp. 741–745. [Google Scholar] [CrossRef]
- Církva, V. Microwaves in Photochemistry and Photocatalysis. In Microwaves in Organic Synthesis; Wiley: Hoboken, NJ, USA, 2012; pp. 563–605. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef]
- Uddin, M.S.; Ferdosh, S.; Akanda, J.H.; Ghafoor, K.; Rukshana, A.H.; Ali, E.; Kamaruzzaman, B.Y.; Fauzi, M.B.; Hadijah, S.; Shaarani, S.; et al. Techniques for the extraction of phytosterols and their benefits in human health: A review. Sep. Sci. Technol. 2018, 53, 2206–2223. [Google Scholar] [CrossRef]
- Delazar, A.; Nahar, L.; Hamedeyazdan, S.; Sarker, S.D. Microwave-Assisted Extraction in Natural Products Isolation; Humana Press: Totowa, NJ, USA, 2012; pp. 89–115. [Google Scholar] [CrossRef]
- Nour, A.H.; Oluwaseun, A.R.; Nour, A.H.; Omer, M.S.; Ahmed, N. Microwave-Assisted Extraction of Bioactive Compounds (Review). In Microwave Heating—Electromagnetic Fields Causing Thermal and Non-Thermal Effects; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction of essential oil from aromatic herbs: Comparison with conventional hydro-distillation. J. Chromatogr. A 2004, 1043, 323–327. [Google Scholar] [CrossRef]
- Rosa, R.; Ferrari, E.; Veronesi, P. From Field to Shelf: How Microwave-Assisted Extraction Techniques Foster an Integrated Green Approach. In Emerging Microwave Technologies in Industrial, Agricultural, Medical and Food Processing; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Ridlo, M.; Kumalaningsih, S.; Pranowo, D. Process of microwave assisted extraction (MAE) for Rhodomyrtus tomentosa fruit and its bioactive compounds. IOP Conf. Ser. Earth Environ. Sci. 2020, 475, 012038. [Google Scholar] [CrossRef]
- Prommuak, C.; Pavasant, P.; Quitain, A.T.; Goto, M.; Shotipruk, A. Microalgal Lipid Extraction and Evaluation of Single-Step Biodiesel Production. Eng. J. 2012, 16, 157–166. [Google Scholar] [CrossRef]
- Ma, G.; Hu, W.; Pei, H.; Jiang, L.; Song, M.; Mu, R. In situ heterogeneous transesterification of microalgae using combined ultrasound and microwave irradiation. Energy Convers. Manag. 2015, 90, 41–46. [Google Scholar] [CrossRef]
- Passos, F.; Carretero, J.; Ferrer, I. Comparing pretreatment methods for improving microalgae anaerobic digestion: Thermal, hydrothermal, microwave and ultrasound. Chem. Eng. J. 2015, 279, 667–672. [Google Scholar] [CrossRef]
- Ruen-ngam, D.; Shotipruk, A.; Pavasant, P. Comparison of Extraction Methods for Recovery of Astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2010, 46, 64–70. [Google Scholar] [CrossRef]
- Košťálová, Z.; Aguedo, M.; Hromádková, Z. Microwave-assisted extraction of pectin from unutilized pumpkin biomass. Chem. Eng. Process. Process Intensif. 2016, 102, 9–15. [Google Scholar] [CrossRef]
- Sebastian, J.; Rouissi, T.; Brar, S.K.; Hegde, K.; Verma, M. Microwave-assisted extraction of chitosan from Rhizopus oryzae NRRL 1526 biomass. Carbohydr. Polym. 2019, 219, 431–440. [Google Scholar] [CrossRef]
- De Sousa e Silva, A.; de Magalhães, W.T.; Moreira, L.M.; Rocha, M.V.P.; Bastos, A.K.P. Microwave-assisted extraction of polysaccharides from Arthrospira (Spirulina) platensis using the concept of green chemistry. Algal Res. 2018, 35, 178–184. [Google Scholar] [CrossRef]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Palma, M.; Carrera, C. Optimization of a Microwave Assisted Extraction Method for Maximum Flavonols and Antioxidant Activity of Onion Extracts. Antioxidants 2022, 11, 2393. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Zaini, M.A.A. Dielectric properties in microwave-assisted solvent extraction—Present trends and future outlook. Asia-Pac. J. Chem. Eng. 2018, 13, e2230. [Google Scholar] [CrossRef]
- Pan, X.; Niu, G.; Liu, H. Comparison of microwave-assisted extraction and conventional extraction techniques for the extraction of tanshinones from Salvia miltiorrhiza bunge. Biochem. Eng. J. 2002, 12, 71–77. [Google Scholar] [CrossRef]
- Abed, K.A. Effect of Microwave Power on the Yield of Essential Oil from Lavender. J. Alasmarya Univ. Basic Appl. Sci. 2020, 5, 35–53. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Zhang, M.; Zhou, H.-L. Microwave-Assisted Extraction, Purification, Partial Characterization, and Bioactivity of Polysaccharides from Panax ginseng. Molecules 2019, 24, 1605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-F.; Yang, X.-H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Sen, K.; Chouhan, K.S.; Tandey, R.; Mehta, R.; Mandal, V. Impact of microwaves on the extraction yield of phenolics, flavonoids, and triterpenoids from centella leaves: An approach toward digitized robust botanical extraction. Pharmacogn. Mag. 2019, 15, 267. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Cui, B.; Xu, X.-R.; Song, Y.; Ai, X.-X.; Li, H.-B. Microwave-Assisted Extraction of Oxymatrine from Sophora flavescens. Molecules 2011, 16, 7391–7400. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.C.J.; Nillian, E. Microwave-assisted extraction of bioactive compounds from Sarawak Liberica sp. coffee pulp: Statistical optimization and comparison with conventional methods. Food Sci. Nutr. 2023, 11, 5364–5378. [Google Scholar] [CrossRef] [PubMed]
- Mandal, V.; Dewanjee, S.; Mandal, S.C. Role of Modifier in Microwave Assisted Extraction of Oleanolic Acid from Gymnema sylvestre: Application of Green Extraction Technology for Botanicals. Nat. Prod. Commun. 2009, 4, 1934578X0900400. [Google Scholar] [CrossRef]
- Destandau, E.; Michel, T.; Elfakir, C. CHAPTER 4. Microwave-Assisted Extraction; Royal Society of Chemistry: London, UK, 2013; pp. 113–156. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, B.; Chen, Y.; Xie, M.; Duan, H.; Li, Y.; Duan, G. Nitrogen-protected microwave-assisted extraction of ascorbic acid from fruit and vegetables. J. Sep. Sci. 2009, 32, 4227–4233. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.-H.; Wang, J.-X.; Wang, G.; Wang, J.-Y.; Li, G.-K. Evaluation of vacuum microwave-assisted extraction technique for the extraction of antioxidants from plant samples. J. Chromatogr. A 2009, 1216, 8867–8873. [Google Scholar] [CrossRef]
- Xiao, X.; Song, W.; Wang, J.; Li, G. Microwave-assisted extraction performed in low temperature and in vacuo for the extraction of labile compounds in food samples. Anal. Chim. Acta 2012, 712, 85–93. [Google Scholar] [CrossRef]
- Luque-García, J.L.; Luque de Castro, M.D. Focused microwave-assisted Soxhlet extraction: Devices and applications. Talanta 2004, 64, 571–577. [Google Scholar] [CrossRef]
- Virot, M.; Tomao, V.; Colnagui, G.; Visinoni, F.; Chemat, F. New microwave-integrated Soxhlet extraction. J. Chromatogr. A 2007, 1174, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gu, X.; Huang, S.; Li, J.; Wang, X.; Tang, J. Optimization of ultrasonic/microwave assisted extraction (UMAE) of polysaccharides from Inonotus obliquus and evaluation of its anti-tumor activities. Int. J. Biol. Macromol. 2010, 46, 429–435. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Lianfu, Z.; Zelong, L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason. Sonochem. 2008, 15, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, E.E.; Jaramillo, B.E.; Martı, J.R. Analysis of volatile secondary metabolites from Colombian Xylopia aromatica (Lamarck) by different extraction and headspace methods and gas chromatography. J. Chromatogr. A 2004, 1025, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Golmakani, M.-T.; Rezaei, K. Comparison of microwave-assisted hydrodistillation withthe traditional hydrodistillation method in the extractionof essential oils from Thymus vulgaris L. Food Chem. 2008, 109, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Farhat, A.; Ginies, C.; Romdhane, M.; Chemat, F. Eco-friendly and cleaner process for isolation of essential oil using microwave energy. J. Chromatogr. A 2009, 1216, 5077–5085. [Google Scholar] [CrossRef] [PubMed]
- Sahraoui, N.; Vian, M.A.; Bornard, I.; Boutekedjiret, C.; Chemat, F. Improved microwave steam distillation apparatus for isolation of essential oils. J. Chromatogr. A 2008, 1210, 229–233. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. An original solvent free microwave extraction of essential oils from spices. Flavour. Fragr. J. 2004, 19, 134–138. [Google Scholar] [CrossRef]
- López-Hortas, L.; Falqué, E.; Domínguez, H.; Torres, M.D. Microwave Hydrodiffusion and Gravity (MHG) Extraction from Different Raw Materials with Cosmetic Applications. Molecules 2019, 25, 92. [Google Scholar] [CrossRef]
- Camponogara, J.A.; Farias, C.A.A.; Moraes, D.P.; Bettio, L.; Citadin, I.; Mallman, C.A.; Schmiele, M.; Ballus, C.A.; Barin, J.S.; Barcia, M.T. Optimization of Microwave Hydrodiffusion and Gravity (MHG) for Pre-treatment of Dehydration and Obtaining a Jaboticaba Extract. Food Bioprocess Technol. 2023, 17, 1479–1491. [Google Scholar] [CrossRef]
- Chambaud, M.; Colas, C.; Destandau, E. Water-Based Microwave-Assisted Extraction of Pigments from Madder Optimized by a Box–Behnken Design. Separations 2023, 10, 433. [Google Scholar] [CrossRef]
- Flórez, N.; Conde, E.; Domínguez, H. Microwave assisted water extraction of plant compounds. J. Chem. Technol. Biotechnol. 2015, 90, 590–607. [Google Scholar] [CrossRef]
- Al Mamoori, F.; Al Janabi, R. Recent Advances in Microwave-Assisted Extraction (MAE) of Medicinal Plants: A Review. Int. Res. J. Pharm. 2018, 9, 22–29. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Kamal, S.M.M.; Awang Biak, D.R.; Zubaidi, S.L. Microwave-Assisted Pyrolysis of Biomass Waste: A Mini Review. Processes 2020, 8, 1190. [Google Scholar] [CrossRef]
- Cardoso-Ugarte, G.A.; Juárez-Becerra, G.P.; SosaMorales, M.E.; López-Malo, A. Microwave-assisted Extraction of Essential Oils from Herbs. J. Microw. Power Electromagn. Energy 2013, 47, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kurian, V.; Gill, M.; Dhakal, B.; Kumar, A. Recent trends in the pyrolysis and gasification of lignocellulosic biomass. In Biofuels and Bioenergy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 511–552. [Google Scholar] [CrossRef]
- Prakash, A.; Singh, R.; Balagurumurthy, B.; Bhaskar, T.; Arora, A.K.; Puri, S.K. Thermochemical Valorization of Lignin. In Recent Advances in Thermo-Chemical Conversion of Biomass; Elsevier: Amsterdam, The Netherlands, 2015; pp. 455–478. [Google Scholar] [CrossRef]
- Idris, S.S.; Rahman, N.A.; Ismail, K.; Mohammed Yunus, M.F.; Mohd Hakimi, N.I.N. Microwave-Assisted Pyrolysis of Oil Palm Biomass: Multi-Optimisation of Solid Char Yield and Its Calorific Value Using Response Surface Methodology. Front. Chem. Eng. 2022, 4, 864589. [Google Scholar] [CrossRef]
- Arshad, H.; Sulaiman, S.A.; Hussain, Z.; Naz, Y.; Basrawi, F. Microwave assisted pyrolysis of plastic waste for production of fuels: A review. MATEC Web Conf. 2017, 131, 02005. [Google Scholar] [CrossRef]
- Zulkornain, M.F.; Shamsuddin, A.H.; Normanbhay, S.; Saad, J.M.; Zhang, Y.S.; Samsuri, S.; Ghani, W.A.W.A.K. Microwave-assisted Hydrothermal Carbonization for Solid Biofuel Application: A Brief Review. Carbon Capture Sci. Technol. 2021, 1, 100014. [Google Scholar] [CrossRef]
- Huang, Y.F.; Kuan, W.H.; Lo, S.L.; Lin, C.F. Hydrogen-rich fuel gas from rice straw via microwave-induced pyrolysis. Bioresour. Technol. 2010, 101, 1968–1973. [Google Scholar] [CrossRef]
- Tian, Y.; Zuo, W.; Ren, Z.; Chen, D. Estimation of a novel method to produce bio-oil from sewage sludge by microwave pyrolysis with the consideration of efficiency and safety. Bioresour. Technol. 2011, 102, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Aishwarya, K.N.; Sindhu, N. Microwave Assisted Pyrolysis of Plastic Waste. Procedia Technol. 2016, 25, 990–997. [Google Scholar] [CrossRef]
- Irfan, M.; Saleem, R.; Shoukat, B.; Hussain, H.; Shukrullah, S.; Naz, M.Y.; Rahman, S.; Ghanim, A.A.J.; Nawalany, G.; Jakubowski, T. Production of combustible fuels and carbon nanotubes from plastic wastes using an in-situ catalytic microwave pyrolysis process. Sci. Rep. 2023, 13, 9057. [Google Scholar] [CrossRef]
- Ahmed, A. Microwave Assisted Pyrolysis of Moringa Seed and Karanja for Bio-Oil Production. Int. J. Renew. Energy Resour. 2023, 13, 14–24. [Google Scholar] [CrossRef]
- Fodah, A.E.M.; Ghosal, M.K.; Behera, D. Studies on Microwave-Assisted Pyrolysis of Rice Straw Using Solar Photovoltaic Power. Bioenergy Res. 2021, 14, 190–208. [Google Scholar] [CrossRef]
- Quillope, J.C.C.; Carpio, R.B.; Gatdula, K.M.; Detras, M.C.M.; Doliente, S.S. Optimization of process parameters of self-purging microwave pyrolysis of corn cob for biochar production. Heliyon 2021, 7, e08417. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhang, Y.; Zhu, H.; Zhao, X.; Zhu, L.; Liu, W.; Sun, M.; Miao, G.; Li, S.; Kong, L.Z. Microwave-assisted low-temperature biomass pyrolysis: From mechanistic insights to pilot scale. Green Chem. 2021, 23, 821–827. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Liu, S.; Fan, L.; Zhou, N.; Min, M.; Cheng, Y.; Peng, P.; Anderson, E.; Wang, Y.; et al. Microwave-Assisted Pyrolysis of Biomass for Bio-Oil Production. In Pyrolysis; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Shao, Z. Microwave pyrolysis of sludge: A review. Sustain. Environ. Res. 2022, 32, 23. [Google Scholar] [CrossRef]
- Alam, S.S.; Khan, A.H. Microwave-assisted pyrolysis for waste plastic recycling: A review on critical parameters, benefits, challenges, and scalability perspectives. Int. J. Environ. Sci. Technol. 2024, 21, 5311–5330. [Google Scholar] [CrossRef]
- Yang, C.; Shang, H.; Li, J.; Fan, X.; Sun, J.; Duan, A. A Review on the Microwave-Assisted Pyrolysis of Waste Plastics. Processes 2023, 11, 1487. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.-J. Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Remón, J.; Matharu, A.S. Microwave-assisted hydrothermal treatments for biomass valorisation: A critical review. Green Chem. 2021, 23, 3502–3525. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Wang, B.; Ma, M.-G.; Lin, K.-L. The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials. Mater. Today Chem. 2016, 1–2, 63–83. [Google Scholar] [CrossRef]
- Deng, C.; Kang, X.; Lin, R.; Murphy, J.D. Microwave assisted low-temperature hydrothermal treatment of solid anaerobic digestate for optimising hydrochar and energy recovery. Chem. Eng. J. 2020, 395, 124999. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, K.; Yuan, Q. Comparative study of microwave and conventional hydrothermal treatment of chicken carcasses: Bio-oil yields and properties. Energy 2020, 200, 117539. [Google Scholar] [CrossRef]
- Sininart, C.; Chakrit, T. Optimization of soluble sugar production from pineapple peel by microwave-assisted water pretreatment. Songklanakarin J. Sci. Technol. 2019, 41, 237–245. [Google Scholar]
- Li, Y.; Li, B.; Du, F.; Wang, Y.; Pan, L.; Chen, D. Microwave-assisted hydrothermal liquefaction of lignin for the preparation of phenolic formaldehyde adhesive. J. Appl. Polym. Sci. 2017, 134, 44510. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.-Y.; Li, C.; Shupe, T.F.; Hu, T.; Qi, J.; De Hoop, C.F. Characterization of Microwave Liquefied Bamboo Residue and Its Potential Use in the Generation of Nanofibrillated Cellulosic Fiber. ACS Sustain. Chem. Eng. 2016, 4, 3477–3485. [Google Scholar] [CrossRef]
- Torrado, I.; Neves, B.G.; da Conceição Fernandes, M.; Carvalheiro, F.; Pereira, H.; Duarte, L.C. Microwave-assisted hydrothermal processing of pine nut shells for oligosaccharide production. Biomass Convers. Biorefin. 2024. [Google Scholar] [CrossRef]
- Nassar, A.E.; El-Aswar, E.I.; Rizk, S.A.; Gaber, S.E.-S.; Jahin, H.S. Microwave-assisted hydrothermal preparation of magnetic hydrochar for the removal of organophosphorus insecticides from aqueous solutions. Sep. Purif. Technol. 2023, 306, 122569. [Google Scholar] [CrossRef]
- Knappe, V.; Paczkowski, S.; Tejada, J.; Diaz Robles, L.A.; Gonzales, A.; Pelz, S. Low temperature microwave assisted hydrothermal carbonization (MAHC) reduces combustion emission precursors in short rotation coppice willow wood. J. Anal. Appl. Pyrolysis 2018, 134, 162–166. [Google Scholar] [CrossRef]
- Ifrah, S.; Kaddouri, A.; Gelin, P.; Leonard, D. Conventional hydrothermal process versus microwave-assisted hydrothermal synthesis of La1−xAgxMnO3+δ (x = 0, 0.2) perovskites used in methane combustion. Comptes Rendus Chim. 2007, 10, 1216–1226. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, L.; Mo, H.; Chen, Y.; Hu, X. Microwave-assisted catalytic hydrothermal carbonization of Laminaria japonica for hydrochars catalyzed and activated by potassium compounds. Bioresour. Technol. 2021, 341, 125835. [Google Scholar] [CrossRef] [PubMed]
- Khushk, S.; Zhang, L.; Li, A.; Irfan, M.; Zhang, X. Microwave-Assisted Hydrothermal Carbonization of Furfural Residue for Adsorption of Cr(VI): Adsorption and Kinetic Study. Pol. J. Environ. Stud. 2020, 29, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; An, Y.; Borrion, A.; He, W.; Wang, N.; Chen, Y.; Li, G. Process characteristics for microwave assisted hydrothermal carbonization of cellulose. Bioresour. Technol. 2018, 259, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Lorente, A.; Remon, J.; Salgado, M.; Huertas-Alonso, A.J.; Sanchez-Verdu, P.; Moreno, A.; Clark, J.H. Sustainable Production of Solid Biofuels and Biomaterials by Microwave-Assisted, Hydrothermal Carbonization (MA-HTC) of Brewers’ Spent Grain (BSG). ACS Sustain. Chem. Eng. 2020, 8, 18982–18991. [Google Scholar] [CrossRef]
- Wang, Z.; Xiang, H.; Zou, J.; Mai, Z.; Cao, Z.; Li, M.; Fan, B.; Shao, G.; Wang, H.; Xu, H.; et al. Effect of process factors of microwave hydrothermal method on the preparation of micron-sized spherical α-Al2O3 particles. Inorg. Chem. Commun. 2021, 133, 108938. [Google Scholar] [CrossRef]
- Álvarez-Chávez, B.J.; Godbout, S.; Raghavan, V. Optimization of microwave-assisted hydrothermal pretreatment and its effect on pyrolytic oil quality obtained by an auger reactor. Biofuel Res. J. 2021, 8, 1316–1329. [Google Scholar] [CrossRef]
- Wu, Y.; Fu, Z.; Yin, D.; Xu, Q.; Liu, F.; Lu, C.; Mao, L. Microwave-assisted hydrolysis of crystalline cellulose catalyzed by biomass char sulfonic acids. Green Chem. 2010, 12, 696. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Cantero-Bahillo, E.; Fernández-Felipe, M.T.; Martin, D. Microwave-Assisted Acid Hydrolysis vs. Conventional Hydrolysis to Produce Sapogenin-Rich Products from Fenugreek Extracts. Foods 2022, 11, 1934. [Google Scholar] [CrossRef]
- Jill, J. Microwave-Assisted Acid Hydrolysis. In Microwave-Assisted Proteomics; The Royal Society of Chemistry: London, UK, 2009; pp. 56–70. [Google Scholar] [CrossRef]
- Seo, M.-Y.; Kim, J.-H.; Park, S.-H.; Kim, T.-H.; Lee, J.-H.; Kim, J.-K. Microwave-assisted Weak Acid Hydrolysis of Proteins. Mass Spectrom. Lett. 2012, 3, 47–49. [Google Scholar] [CrossRef]
- Colleary, C.; Little, N.C.; Cleland, T.P. Microwave-assisted acid hydrolysis for whole-bone proteomics and paleoproteomics. Rapid Commun. Mass Spectrom. 2020, 34, e8568. [Google Scholar] [CrossRef]
- Tanaka, Y.; Okamoto, K.; Matsushima, A.; Ota, T.; Matsumoto, Y.; Akasaki, T. Microwave-assisted acid hydrolysis of konjac products for determining the konjac powder content. Anal. Sci. 2013, 29, 1049–1053. [Google Scholar] [CrossRef]
- Sunarti, T.C.; Yanti, S.D.; Ruriani, E. Two-steps microwave-assisted treatment on acid hydrolysis of sago pith for bioethanol production. IOP Conf. Ser. Earth Environ. Sci. 2017, 65, 012052. [Google Scholar] [CrossRef]
- Mayer, N.; Dirauf, M.P.; Kaltschmitt, M. Microwave-assisted acidic hydrolysis of phytate from rye bran—Experimental procedure and model prediction. LWT 2023, 189, 115499. [Google Scholar] [CrossRef]
- Noguerra, J. Parametric and Kinetic Studies of Microwave-Assisted Dilute Sulfuric Acid Hydrolysis of Peanut (Arachis hypogaea L.) Shells. Bachelor’s Thesis, University of the Philippines, Quezon City, Philippines, 2018. [Google Scholar]
- Tong, K.T.X.; Tan, I.S.; Foo, H.C.Y.; Tiong, A.C.Y.; Lam, M.K.; Lee, K.T. Third-generation L-Lactic acid production by the microwave-assisted hydrolysis of red macroalgae Eucheuma denticulatum extract. Bioresour. Technol. 2021, 342, 125880. [Google Scholar] [CrossRef]
- Alio, M.A.; Tugui, O.-C.; Vial, C.; Pons, A. Microwave-assisted Organosolv pretreatment of a sawmill mixed feedstock for bioethanol production in a wood biorefinery. Bioresour. Technol. 2019, 276, 170–176. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Rodríguez, A.; Espinosa, E.; Domínguez-Robles, J.; Sánchez, R.; Bascón, I.; Rosal, A. Different Solvents for Organosolv Pulping. In Pulp and Paper Processing; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Jablonowski, N.D.; Pauly, M.; Dama, M. Microwave Assisted Pretreatment of Szarvasi (Agropyron elongatum) Biomass to Enhance Enzymatic Saccharification and Direct Glucose Production. Front. Plant Sci. 2022, 12, 767254. [Google Scholar] [CrossRef] [PubMed]
- Corsi, L.; Mateo, S.; Spaccini, F.; Buratti, C.; Moya, A.J. Optimization of Microwave Assisted Organosolv Pretreatment for Enzymatic Hydrolysis of Cherry Tree Pruning and Pistachio Shells. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Merino-Pérez, O.; Martínez-Palou, R.; Labidi, J.; Luque, R. Microwave-Assisted Pretreatment of Lignocellulosic Biomass to Produce Biofuels and Value-Added Products. In Production of Biofuels and Chemicals with Microwave. Biofuels and Biorefineries; Springer: Berlin/Heidelberg, Germany, 2015; pp. 197–224. [Google Scholar] [CrossRef]
- Yang, X.; Cui, C.; Zheng, A.; Zhao, Z.; Wang, C.; Xia, S.; Huang, Z.; Wei, G.; Li, H. Ultrasonic and microwave assisted organosolv pretreatment of pine wood for producing pyrolytic sugars and phenols. Ind. Crops Prod. 2020, 157, 112921. [Google Scholar] [CrossRef]
- Yao, J.; Karlsson, M.; Lawoko, M.; Odelius, K.; Hakkarainen, M. Microwave-assisted organosolv extraction for more native-like lignin and its application as a property-enhancing filler in a light processable biobased resin. RSC Sustain. 2023, 1, 1211–1222. [Google Scholar] [CrossRef]
- Avelino, F.; da Silva, K.T.; de Souza Filho, M.d.S.M.; Mazzetto, S.E.; Lomonaco, D.; Avelino, F.; da Silva, K.T.M.; de Souza Filho, M.d.S.M.; Mazzetto, S.E. Microwave-assisted organosolv extraction of coconut shell lignin by Brønsted and Lewis acids catalysts. J. Clean. Prod. 2018, 189, 785–796. [Google Scholar] [CrossRef]
- Corsi, L.; Mateo, S.; Spaccini, F.; Buratti, C.; Moya, A.J. Optimization of Microwave-Assisted Organosolv Pretreatment for Enzymatic Hydrolysis of Cherry Tree Pruning and Pistachio Shells: A Step to Bioethanol Production. Bioenergy Res. 2023, 17, 294–308. [Google Scholar] [CrossRef]
- Monteil-Rivera, F.; Huang, G.H.; Paquet, L.; Deschamps, S.; Beaulieu, C.; Hawari, J. Microwave-assisted extraction of lignin from triticale straw: Optimization and microwave effects. Bioresour. Technol. 2012, 104, 775–782. [Google Scholar] [CrossRef]
- Yimtrakarn, T.; Kaveevivitchai, W.; Lee, W.-C.; Lerkkasemsan, N. Study of Lignin Extracted from Rubberwood Using Microwave Assisted Technology for Fuel Additive. Polymers 2022, 14, 814. [Google Scholar] [CrossRef]
- Avelino, F.; Marques, F.; Soares, A.K.L.; Silva, K.T.; Leitão, R.C.; Mazzetto, S.E.; Lomonaco, D. Microwave-Assisted Organosolv Delignification: A Potential Eco-Designed Process for Scalable Valorization of Agroindustrial Wastes. Ind. Eng. Chem. Res. 2019, 58, 10698–10706. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; Van Vuong, Q. Optimization of Microwave-Assisted Extraction of Polyphenols from Lemon Myrtle: Comparison of Modern and Conventional Extraction Techniques Based on Bioactivity and Total Polyphenols in Dry Extracts. Processes 2021, 9, 2212. [Google Scholar] [CrossRef]
- Shi, K.; Yan, J.; Menéndez, J.A.; Luo, X.; Yang, G.; Chen, Y.; Lester, E.; Wu, T. Production of H2-Rich Syngas From Lignocellulosic Biomass Using Microwave-Assisted Pyrolysis Coupled with Activated Carbon Enabled Reforming. Front. Chem. 2020, 8, 3. [Google Scholar] [CrossRef]
- Wang, J.-X.; Chen, S.-W.; Lai, F.-Y.; Liu, S.-Y.; Xiong, J.-B.; Zhou, C.-F.; Yi-Yu, Y.; Huang, H.-J. Microwave-assisted hydrothermal carbonization of pig feces for the production of hydrochar. J. Supercrit. Fluids 2020, 162, 104858. [Google Scholar] [CrossRef]
- Chen, L.; Wang, N.; Sun, D.; Li, L. Microwave-assisted acid hydrolysis of proteins combined with peptide fractionation and mass spectrometry analysis for characterizing protein terminal sequences. J. Proteom. 2014, 100, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Yadav, G.; Ahmaruzzaman, M. Microwave-assisted biodiesel production using –SO3H functionalized heterogeneous catalyst derived from a lignin-rich biomass. Sci. Rep. 2023, 13, 9074. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, Y.; Arenillas, A.; Angel, J. Microwave Heating Applied to Pyrolysis. In Advances in Induction and Microwave Heating of Mineral and Organic Materials; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Fia, A.Z.; Amorim, J. Microwave pretreatment of biomass for conversion of lignocellulosic materials into renewable biofuels. J. Energy Inst. 2023, 106, 101146. [Google Scholar] [CrossRef]
- Park, J.; Lee, G.; Jeong, C.; Kim, H.; Kim, C. Determination of Relationship between Higher Heating Value and Atomic Ratio of Hydrogen to Carbon in Spent Coffee Grounds by Hydrothermal Carbonization. Energies 2021, 14, 6551. [Google Scholar] [CrossRef]
- Coelho, J.; Robalo, M.; Boyadzhieva, S.; Stateva, R. Microwave-Assisted Extraction of Phenolic Compounds from Spent Coffee Grounds. Process Optimization Applying Design of Experiments. Molecules 2021, 26, 7320. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, A.; Menéndez, J.; Fernández, Y.; Pis, J.; Nabais, J.V.; Carrott, P.; Carrott, M.R. Conventional and microwave induced pyrolysis of coffee hulls for the production of a hydrogen rich fuel gas. J. Anal. Appl. Pyrolysis 2007, 79, 128–135. [Google Scholar] [CrossRef]
- Divyashri, G.; Murthy, T.P.K.; Ragavan, K.V.; Sumukh, G.M.; Sudha, L.S.; Nishka, S.; Himanshi, G.; Misriya, N.; Sharada, B.; Venkataramanaiah, R.A. Valorization of coffee bean processing waste for the sustainable extraction of biologically active pectin. Heliyon 2023, 9, e20212. [Google Scholar] [CrossRef] [PubMed]
- López-Linares, J.C.; García-Cubero, M.T.; Coca, M.; Lucas, S. A biorefinery approach for the valorization of spent coffee grounds to produce antioxidant compounds and biobutanol. Biomass Bioenergy 2021, 147, 106026. [Google Scholar] [CrossRef]
- Yang, J.; Niu, H.; Corscadden, K.; He, Q.; Zhou, N. MW-assisted hydrothermal liquefaction of spent coffee grounds. Can. J. Chem. Eng. 2022, 100, 1729–1738. [Google Scholar] [CrossRef]
- Lopes, G.R.; Passos, C.P.; Rodrigues, C.; Teixeira, J.A.; Coimbra, M.A. Impact of microwave-assisted extraction on roasted coffee carbohydrates, caffeine, chlorogenic acids and coloured compounds. Food Res. Int. 2020, 129, 108864. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S.; Nunes, A.A.; Alves, C.C.O. Microwave assisted thermal treatment of defective coffee beans press cake for the production of adsorbents. Bioresour. Technol. 2010, 101, 1068–1074. [Google Scholar] [CrossRef]
- Ondruschka, B.; Bonrath, W.; Stuerga, D. Development and Design of Laboratory and Pilot Scale Reactors for Microwave-assisted Chemistry. In Microwaves in Organic Synthesis; Wiley: Hoboken, NJ, USA, 2006; pp. 62–107. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Nobre, B.P.; Ertekin, F.; Hayes, M.; Garciá-Vaquero, M.; Vieira, F.; Koc, M.; Gouveia, L.; Aires-Barros, M.R.; Palavra, A.M.F. Extraction of value-added compounds from microalgae. In Microalgae-Based Biofuels and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2017; pp. 461–483. [Google Scholar] [CrossRef]
- Ambrozic, G.; Crnjak, Z.; Zigon, M. Microwave-Assisted Non-Aqueous Synthesisof ZnO Nanoparticles. Mater. Technol. 2011, 45, 173–177. [Google Scholar]
- Danlami, J.M.; Arsad, A.; Ahmad Zaini, M.A.; Sulaiman, H. A comparative study of various oil extraction techniques from plants. Rev. Chem. Eng. 2014, 30, 605–626. [Google Scholar] [CrossRef]
- Gotama, B.; Rahman, A.K.; Ahmad, A.; Hariyadi, A. Extraction of rice bran oil using microwave-assisted extraction and green solvents. IOP Conf. Ser. Earth Environ. Sci. 2022, 1105, 012052. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Rivas, S.; Torres, M.D.; Domínguez, H. Microwave-Assisted Extraction of Carrageenan from Sarcopeltis skottsbergii. Mar. Drugs 2023, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Dertli, H.; Saloglu, D. A valorization approach of food industry wastewater using microwave-assisted extraction. In Advanced Technologies in Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 155–178. [Google Scholar] [CrossRef]
- López-Salazar, H.; Camacho-Díaz, B.H.; Ocampo, M.L.A.; Jiménez-Aparicio, A.R. Microwave-assisted extraction of functional compounds from plants: A Review. BioResources 2023, 18, 6614–6638. [Google Scholar] [CrossRef]
- Robinson, J.; Binner, E.; Vallejo, D.B.; Perez, N.D.; Al Mughairi, K.; Ryan, J.; Shepherd, B.; Adam, M.; Budarin, V.; Fan, J.; et al. Unravelling the mechanisms of microwave pyrolysis of biomass. Chem. Eng. J. 2022, 430, 132975. [Google Scholar] [CrossRef]
- Bing, W.; Hongbin, Z.; Zeng, D.; Yuefeng, F.; Yu, Q.; Rui, X. Microwave fast pyrolysis of waste tires: Effect of microwave power on product composition and quality. J. Anal. Appl. Pyrolysis 2021, 155, 104979. [Google Scholar] [CrossRef]
- Zhou, N.; Zhou, J.; Dai, L.; Guo, F.; Wang, Y.; Li, H.; Deng, W.; Lei, H.; Chen, P.; Liu, Y.; et al. Syngas production from biomass pyrolysis in a continuous microwave assisted pyrolysis system. Bioresour. Technol. 2020, 314, 123756. [Google Scholar] [CrossRef]
- Ahmad, F.; Idrees, F.; Fazal-e-Aleem. Recent Advancements in Microwave-Assisted Synthesis of NiO Nanostructures and their Supercapacitor Properties: A Comprehensive Review. Curr. Nanomater. 2018, 3, 5–17. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave heating processing as alternative of pretreatment in second-generation biorefinery: An overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef]
- Thangavelu, S.K.; Rajkumar, T.; Pandi, D.K.; Ahmed, A.S.; Ani, F.N. Microwave assisted acid hydrolysis for bioethanol fuel production from sago pith waste. Waste Manag. 2019, 86, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.A.; Murton, K.D.; Smith, D.A.; Dedual, G. A review on organosolv pretreatment of softwood with a focus on enzymatic hydrolysis of cellulose. Biomass Convers. Biorefinery 2022, 12, 5427–5442. [Google Scholar] [CrossRef]
- Goyal, H.; Chen, T.-Y.; Chen, W.; Vlachos, D.G. A review of microwave-assisted process intensified multiphase reactors. Chem. Eng. J. 2022, 430, 133183. [Google Scholar] [CrossRef]
- Radoiu, M.; Mello, A. Technical advances, barriers, and solutions in microwave—Assisted technology for industrial processing. Chem. Eng. Res. Des. 2022, 181, 331–342. [Google Scholar] [CrossRef]
- Sun, J.; Chen, W.; Jia, K.; Li, S.; Jia, P.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y.; Chen, S. Progress on the Microwave-Assisted Recycling of Spent Lithium Battery Graphite. Processes 2023, 11, 1451. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).