Interfacial Stability of Additively Manufactured Alloy 625–GRCop-42 Bimetallic Structures
Abstract
:1. Introduction
2. Materials and Methods
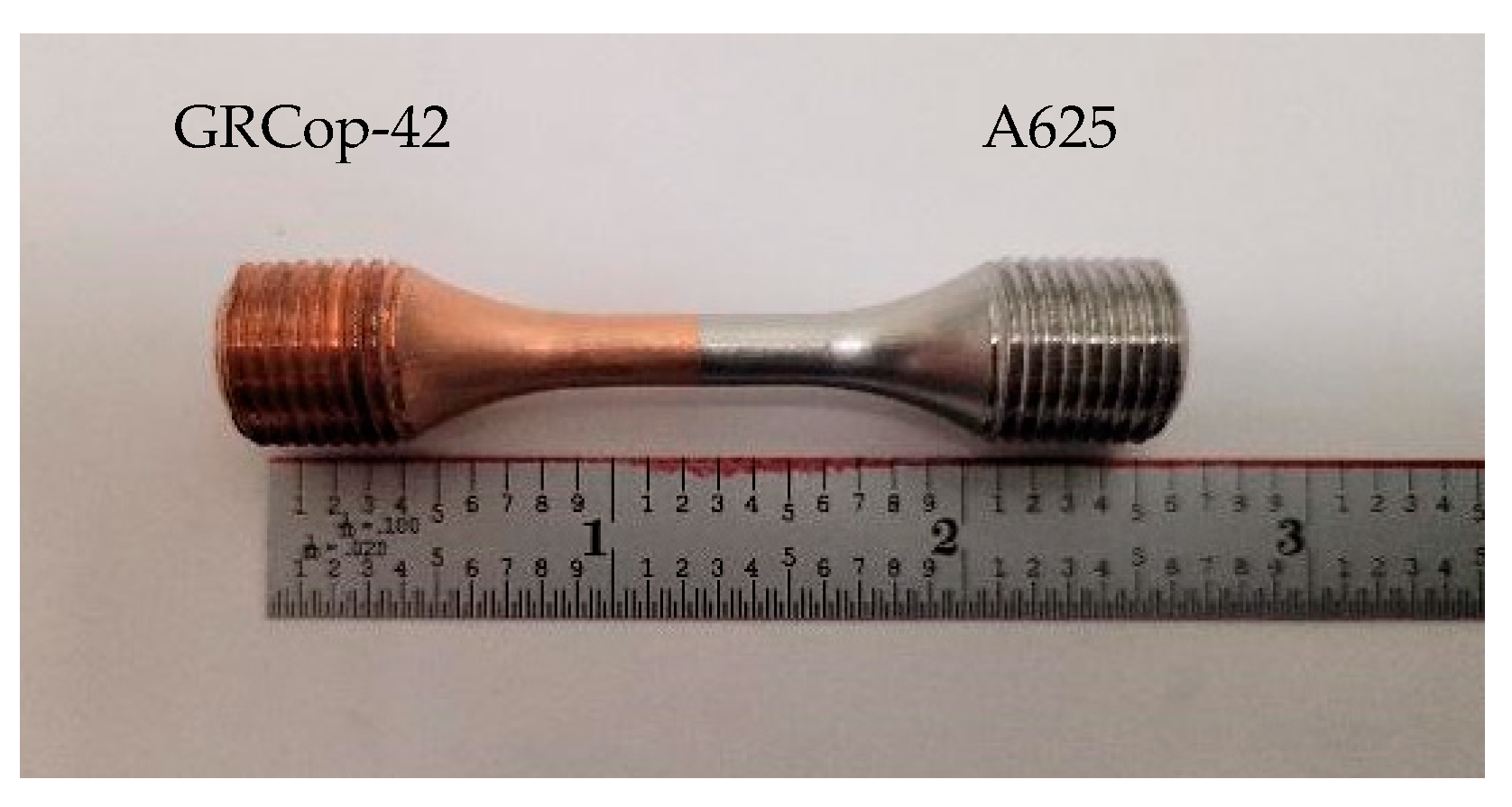
2.1. As-Built Samples
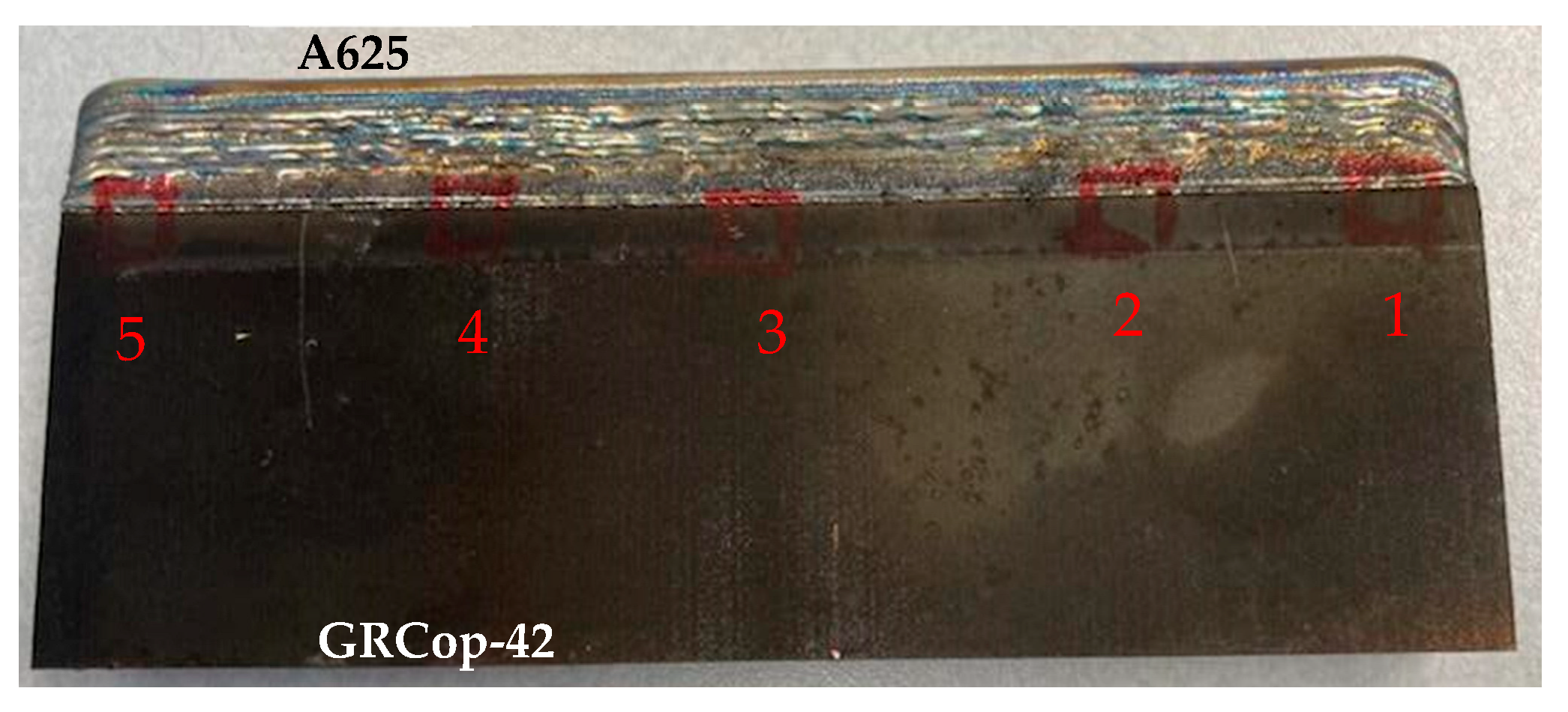
2.1.1. Sample Preparation
2.1.2. Optical Microscopy
2.1.3. Electron Microprobe
2.2. Thermally Exposed Samples
2.2.1. Sample Preparation
2.2.2. Optical Microscopy
2.2.3. Electron Probe Microanalysis
2.3. Mechanical Testing
3. Results
3.1. As-Built Samples
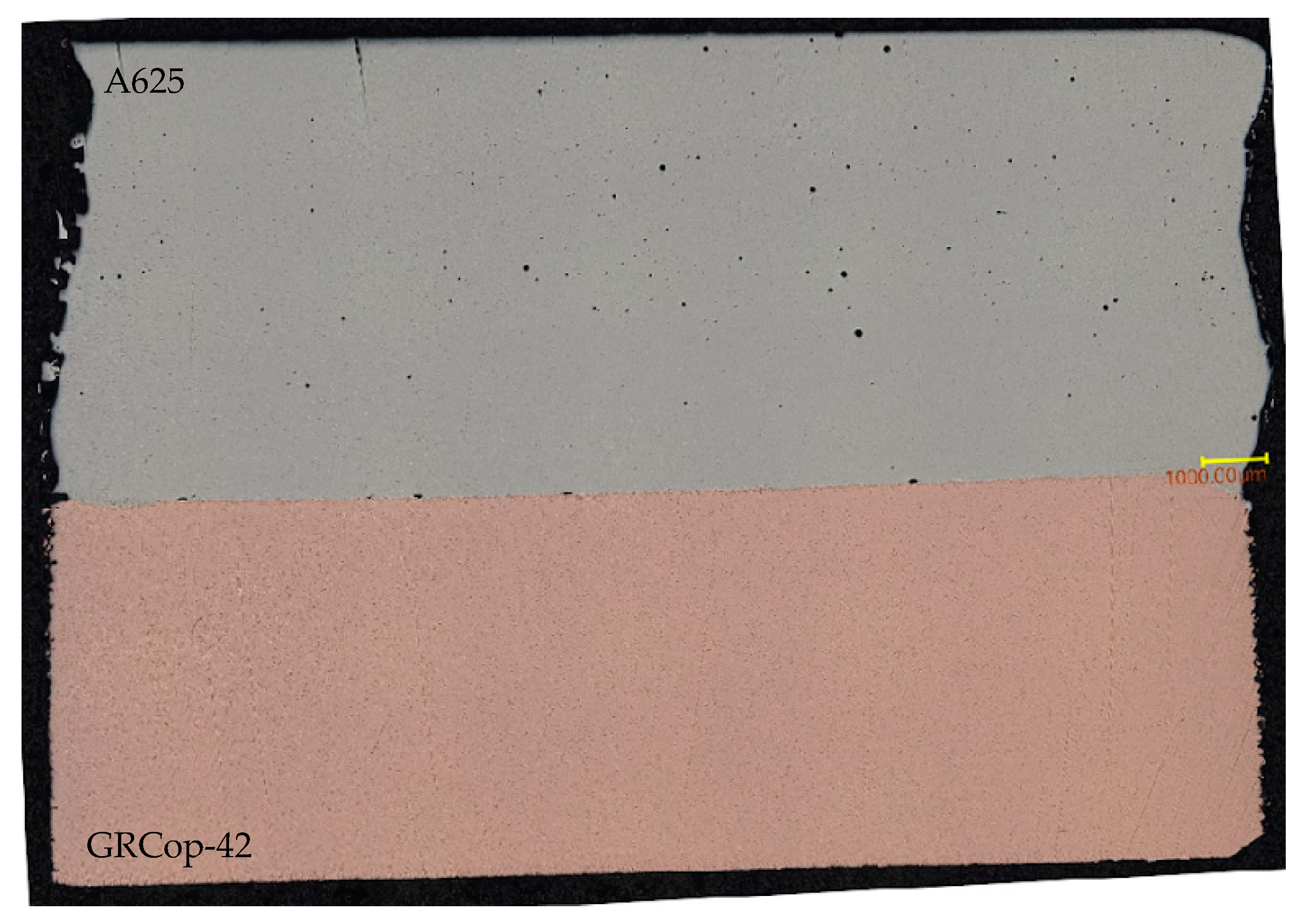
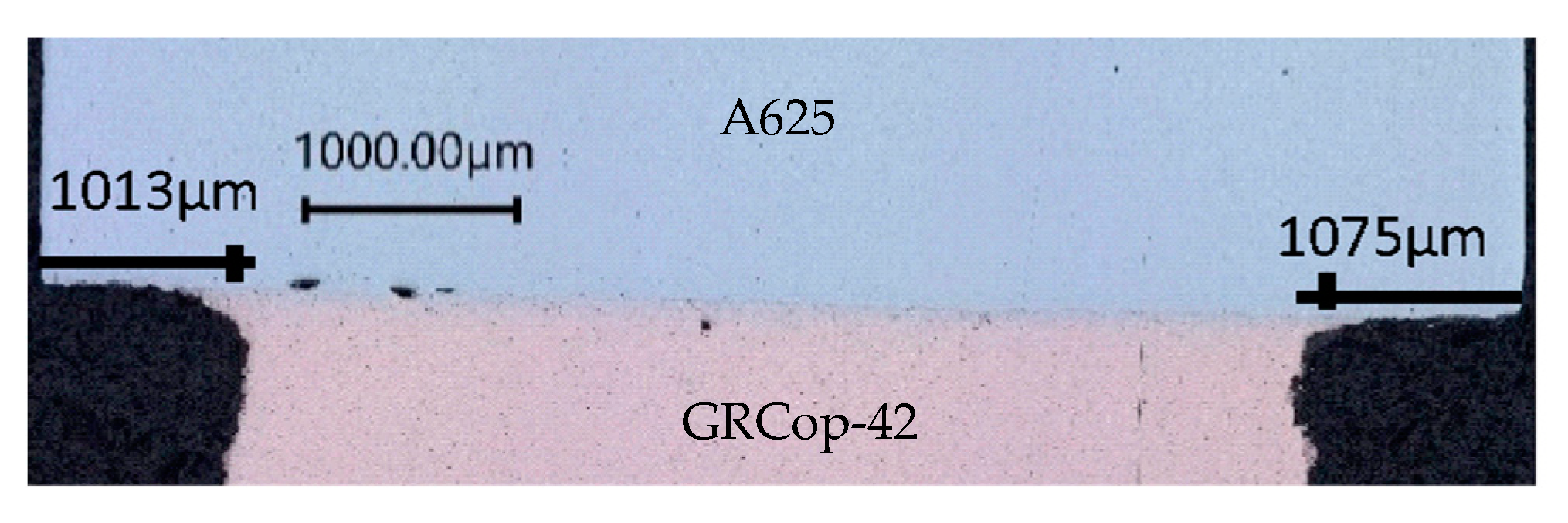
3.1.1. Optical Microscopy/Porosity
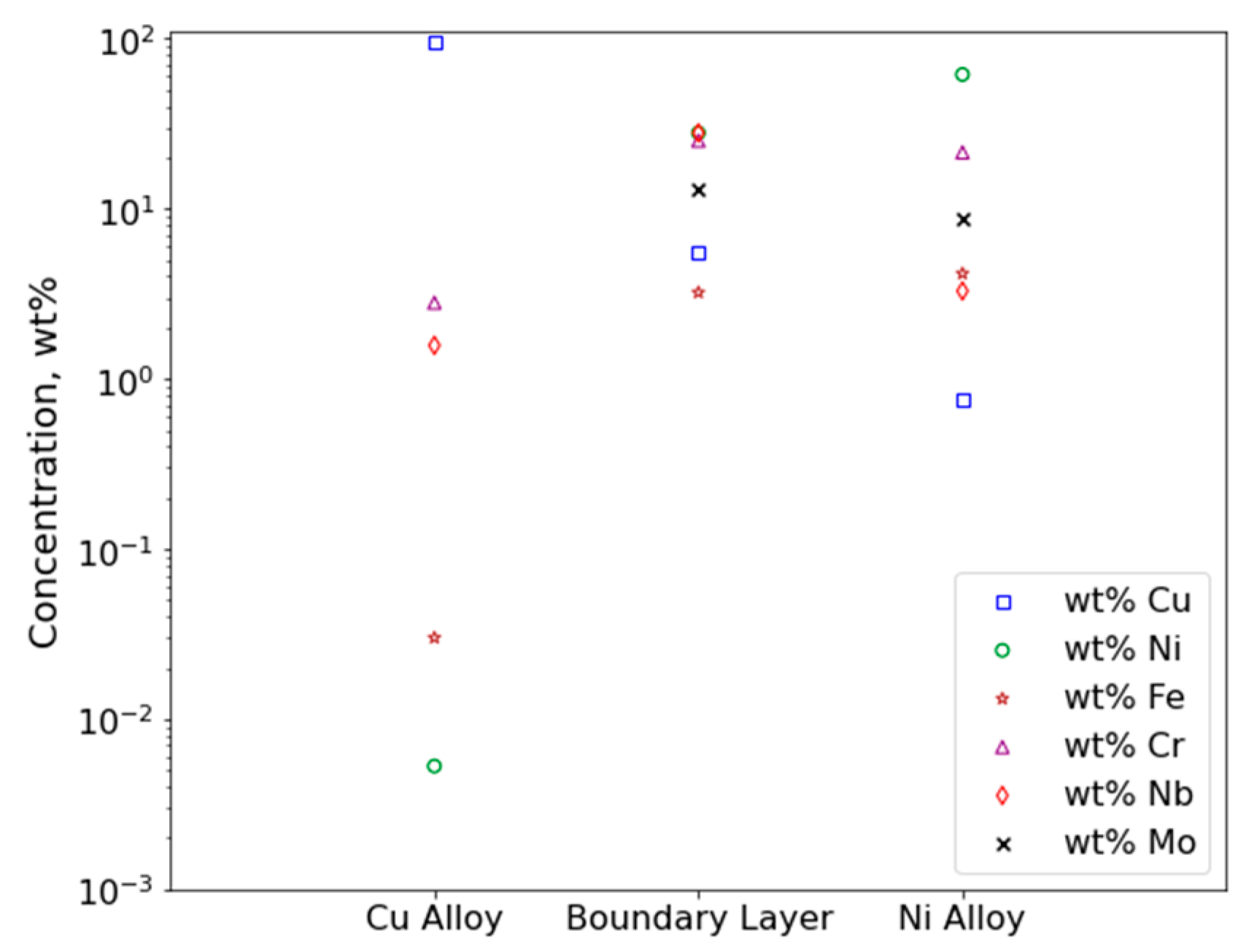
3.1.2. Electron Probe Microanalysis
3.2. Thermally Exposed Samples
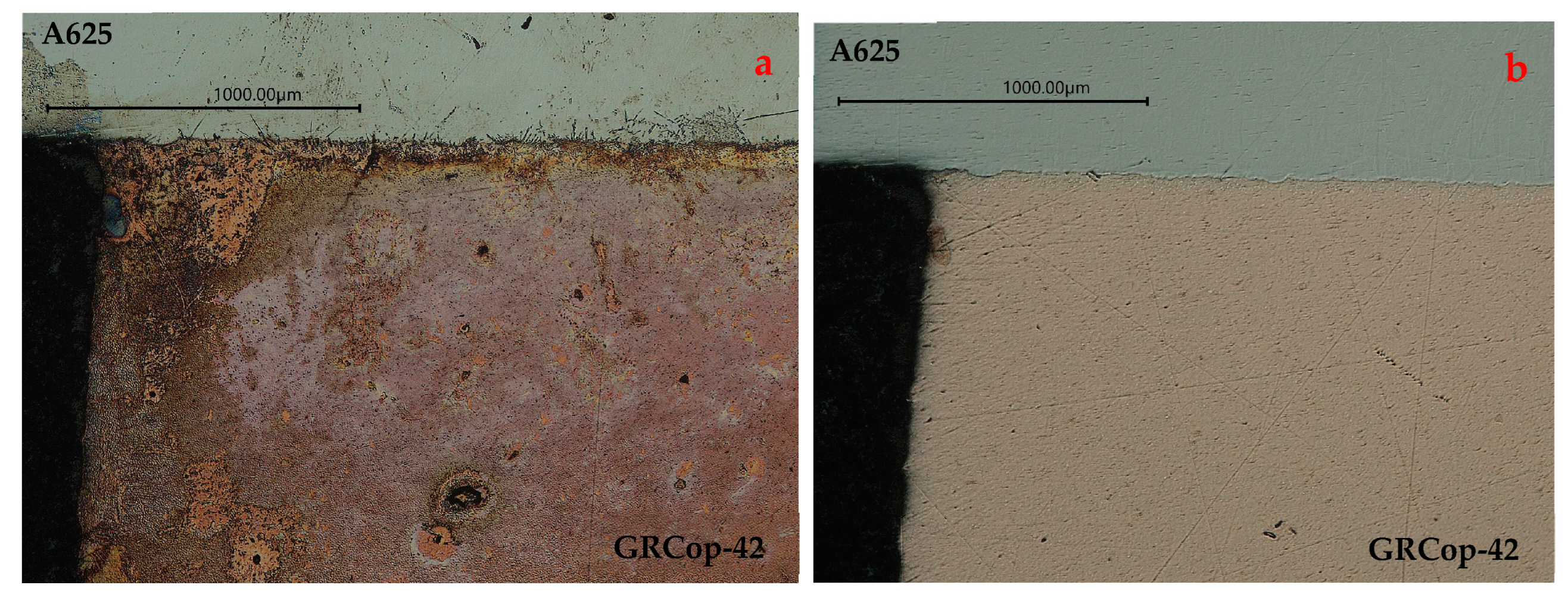
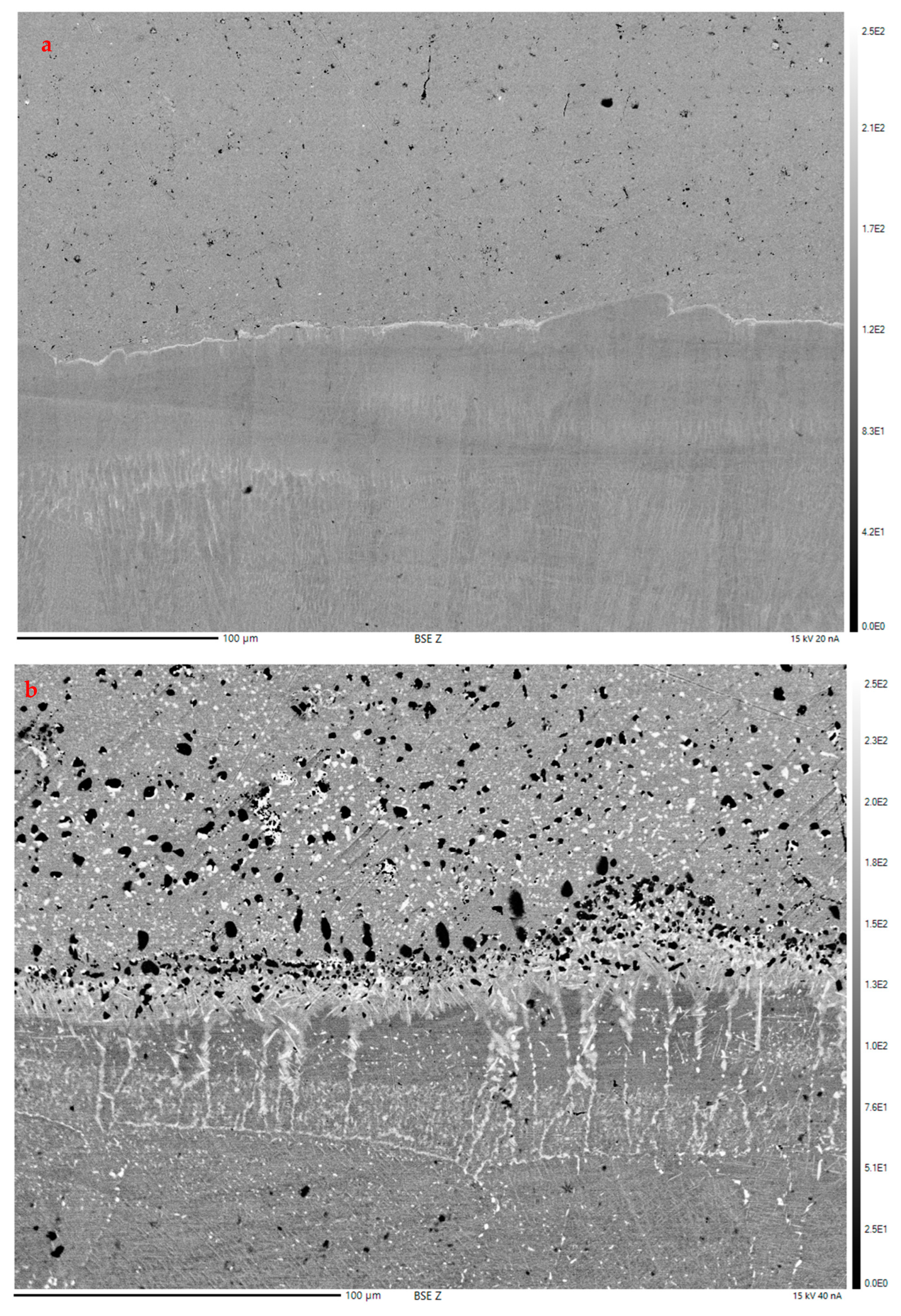
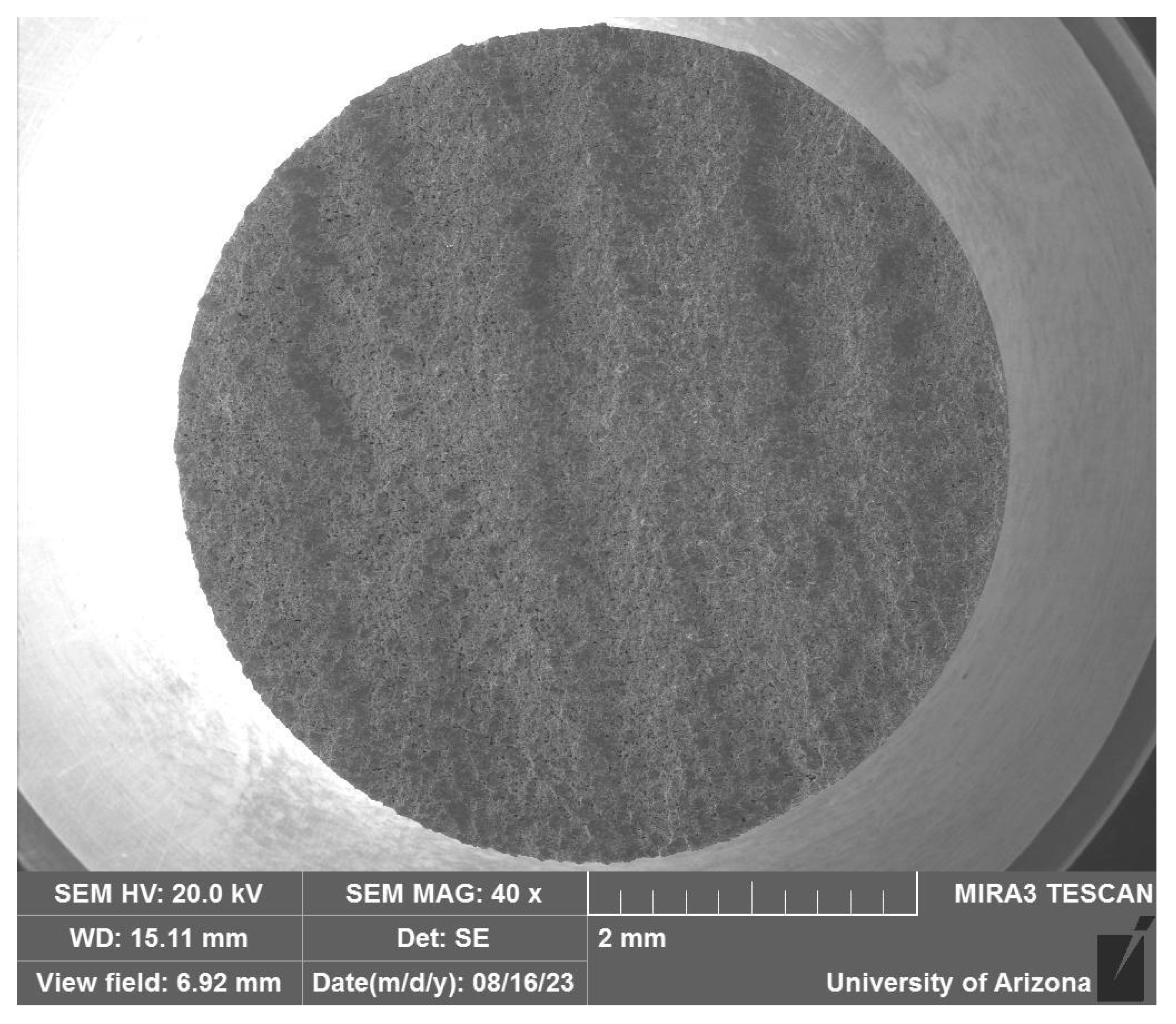
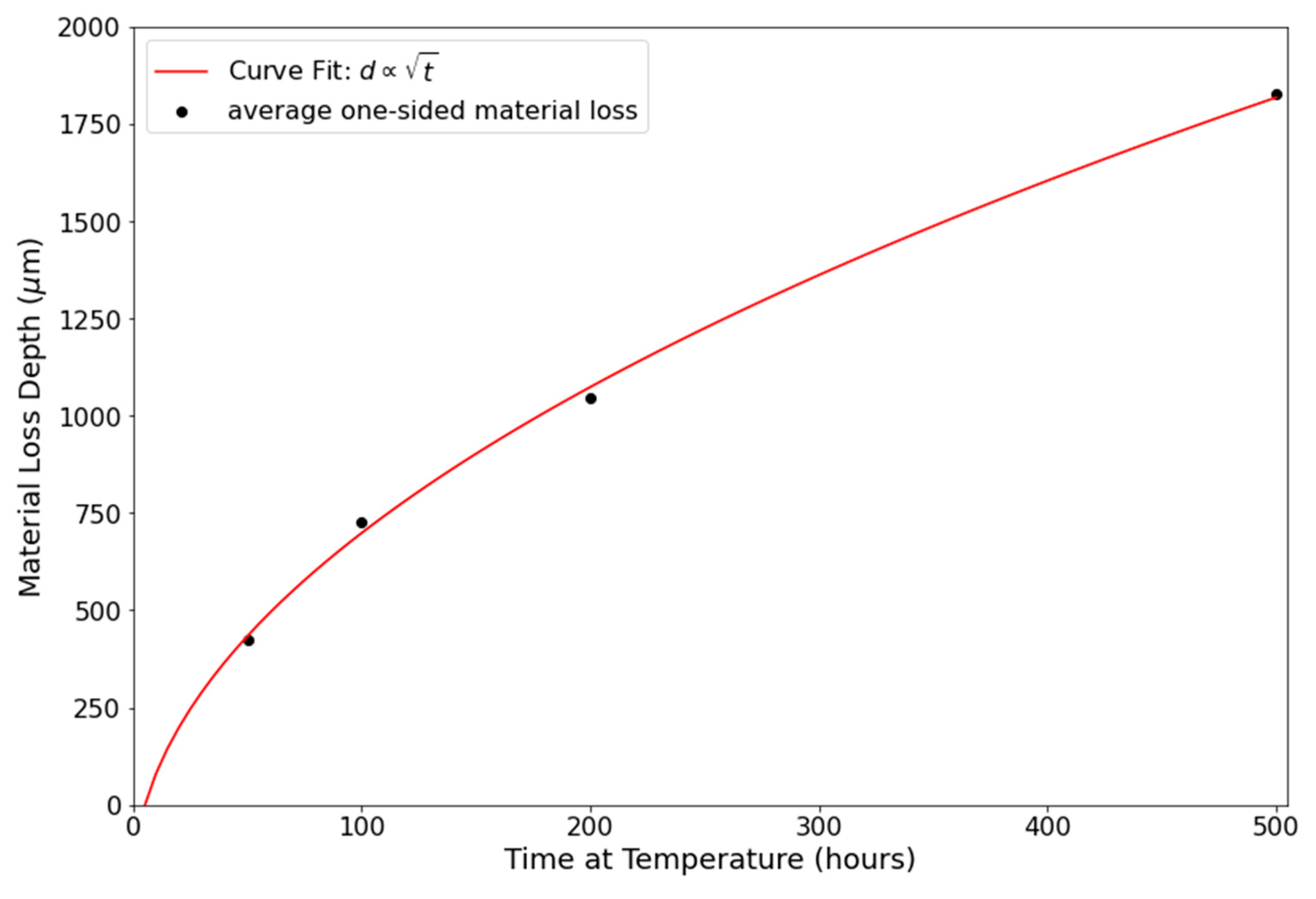
3.2.1. Optical Microscopy/Copper Alloy Oxidation
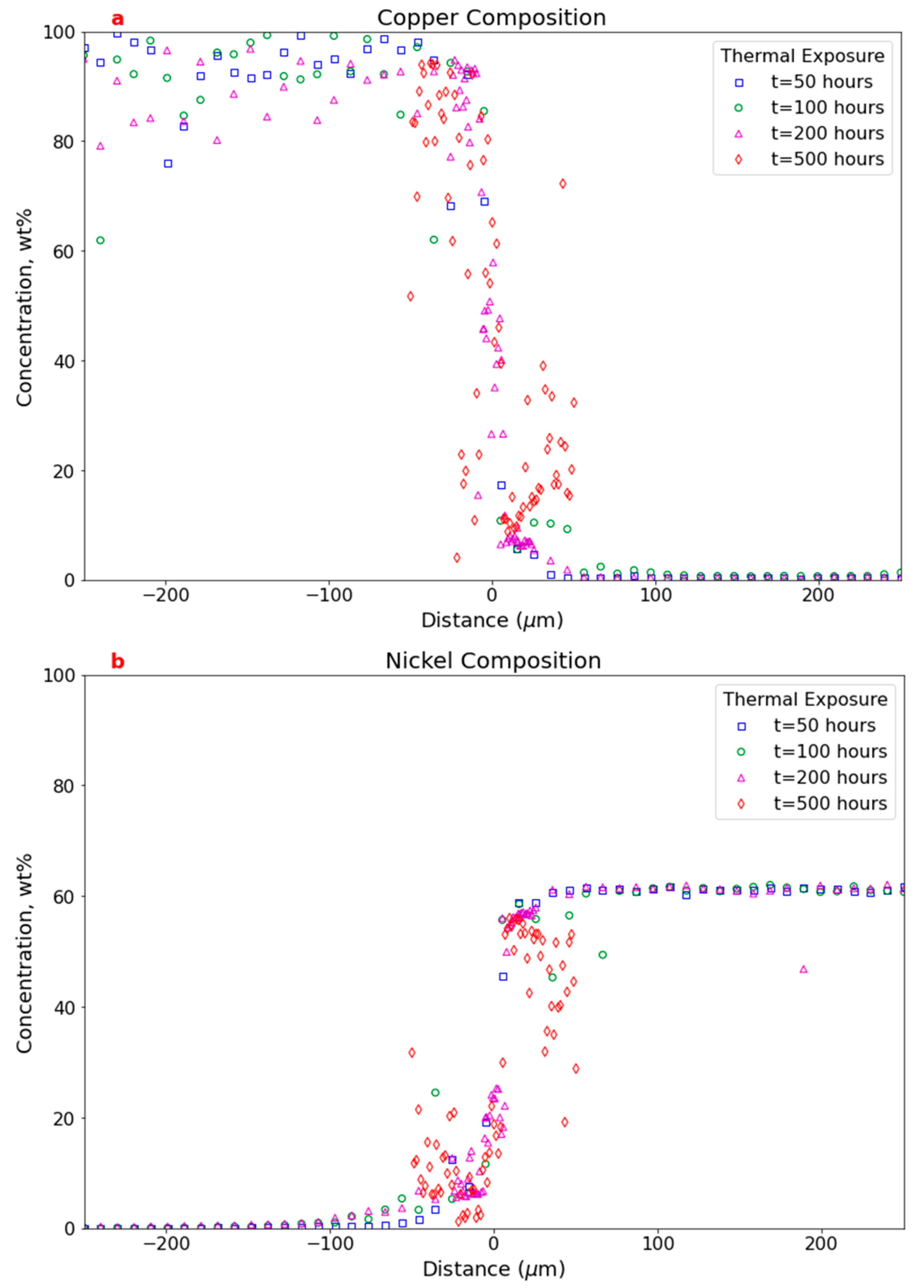
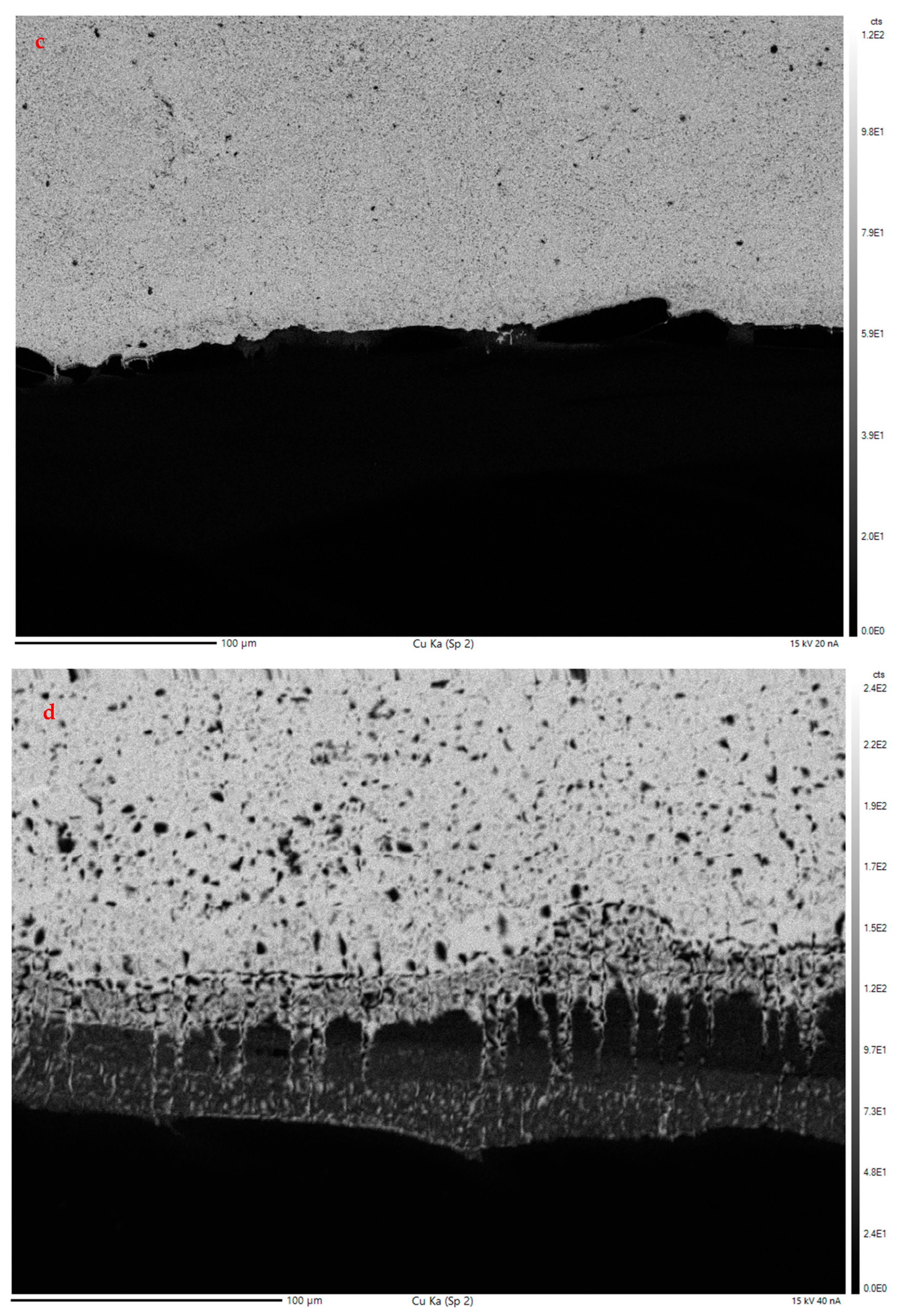
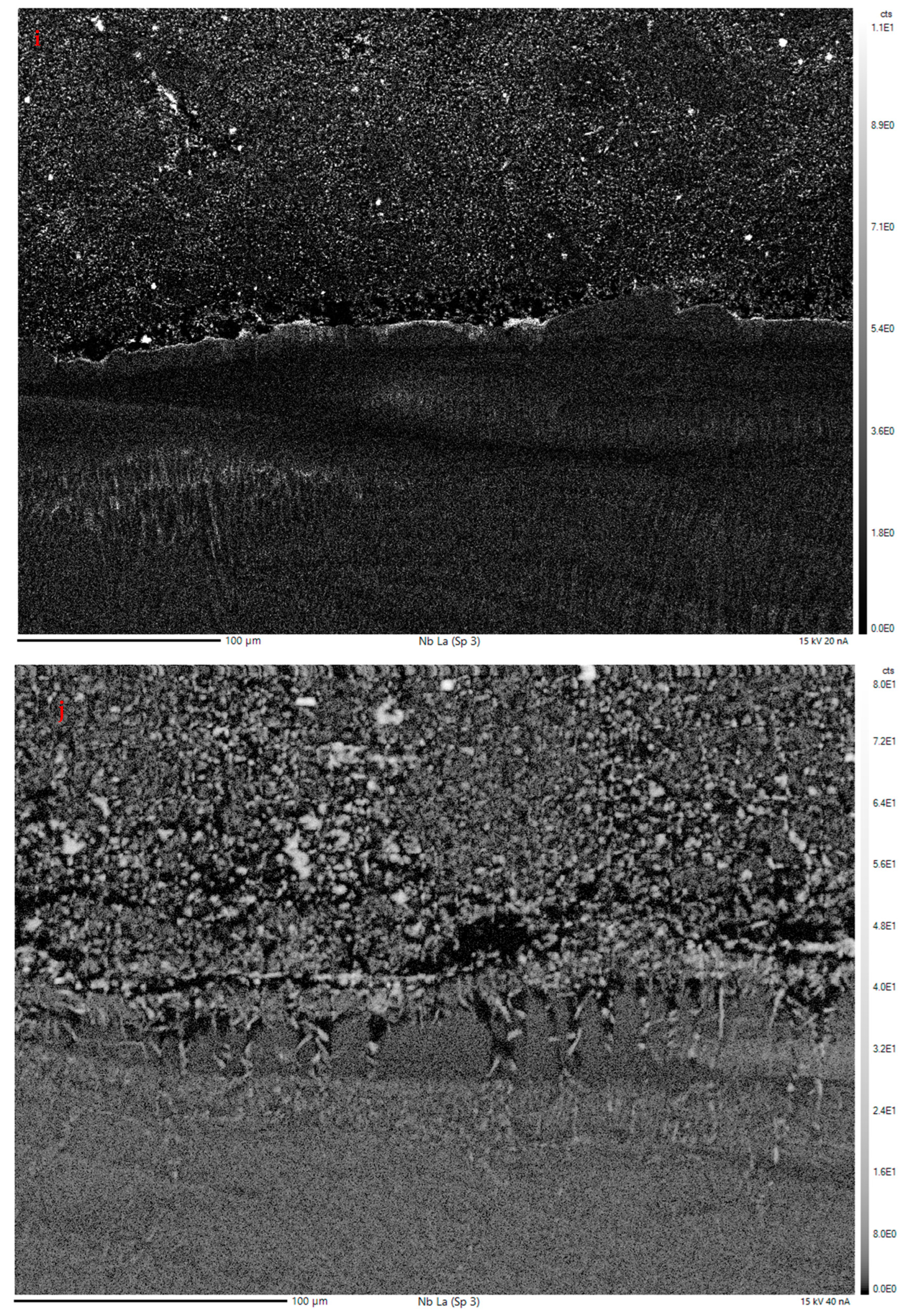
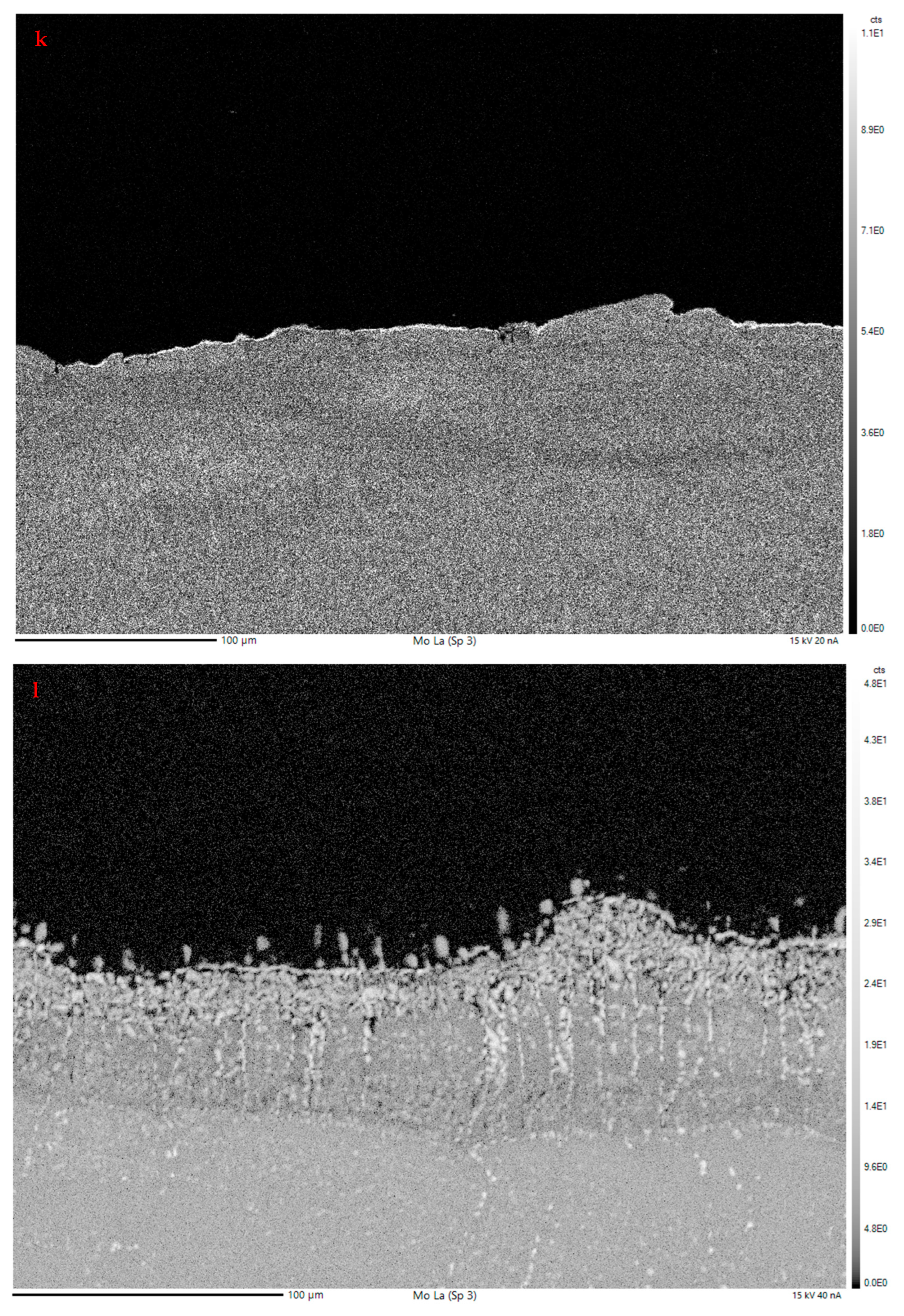
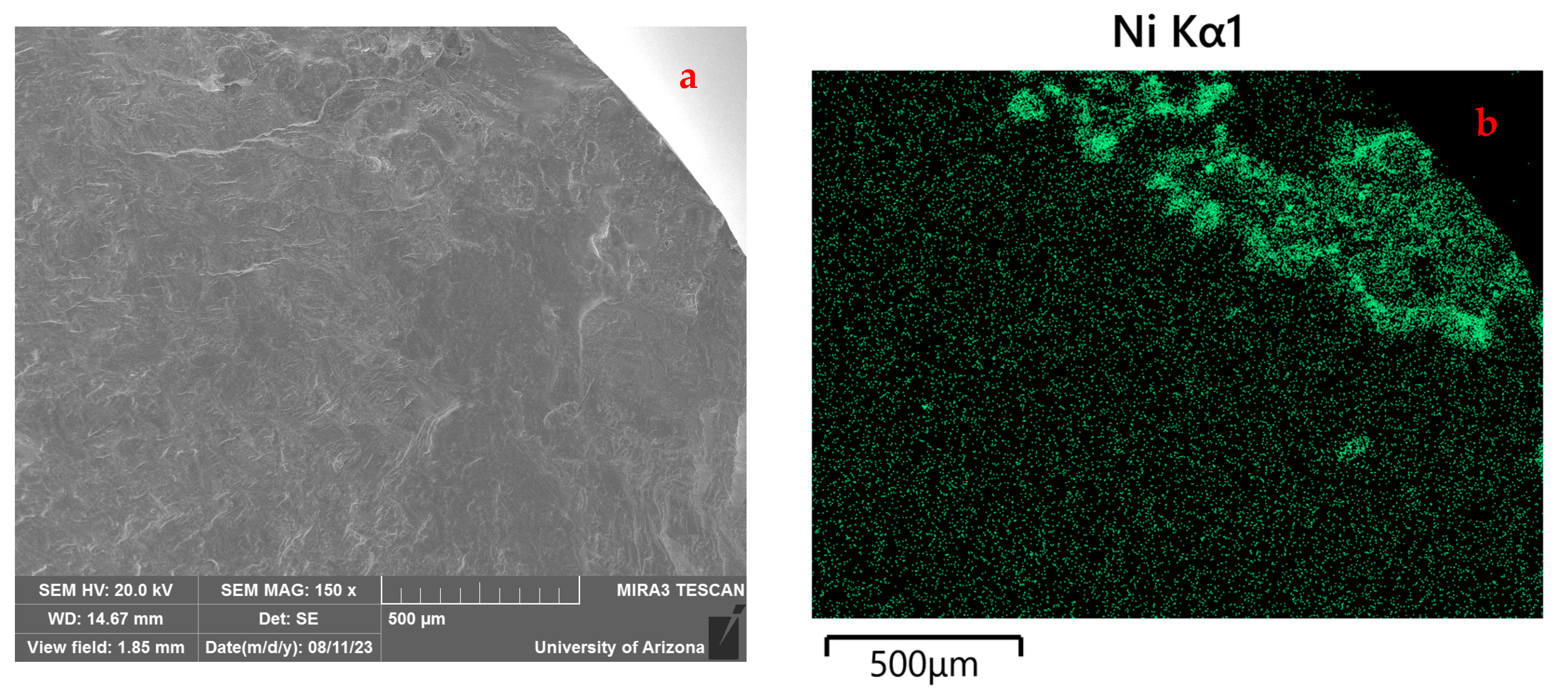
3.2.2. Electron Probe Microanalysis
3.3. Comparison Between As-Built and Thermally Exposed Samples
3.4. Mechanical Testing
3.4.1. Tensile Testing
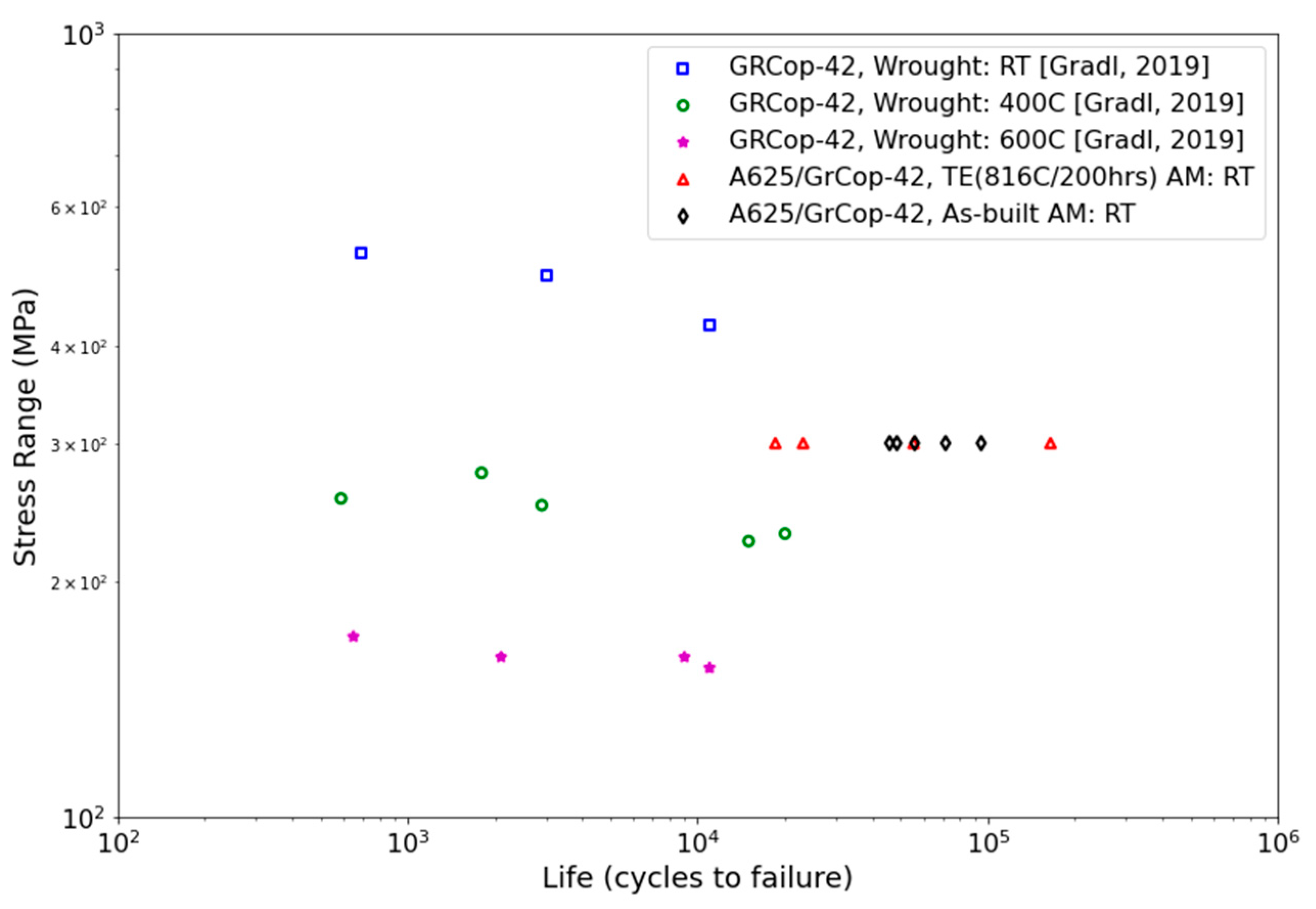
3.4.2. Fatigue Testing
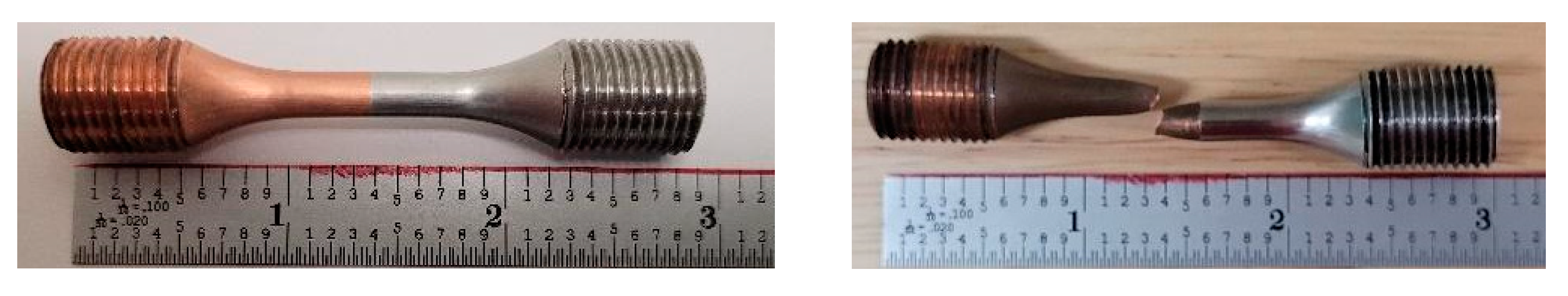
3.4.3. Fatigue Fracture Analysis
4. Discussion
4.1. As-Built Samples
4.1.1. Optical Microscopy/Porosity
4.1.2. Electron Probe Microanalysis
4.2. Thermally Exposed Samples
Optical Microscopy/Copper Alloy Oxidation
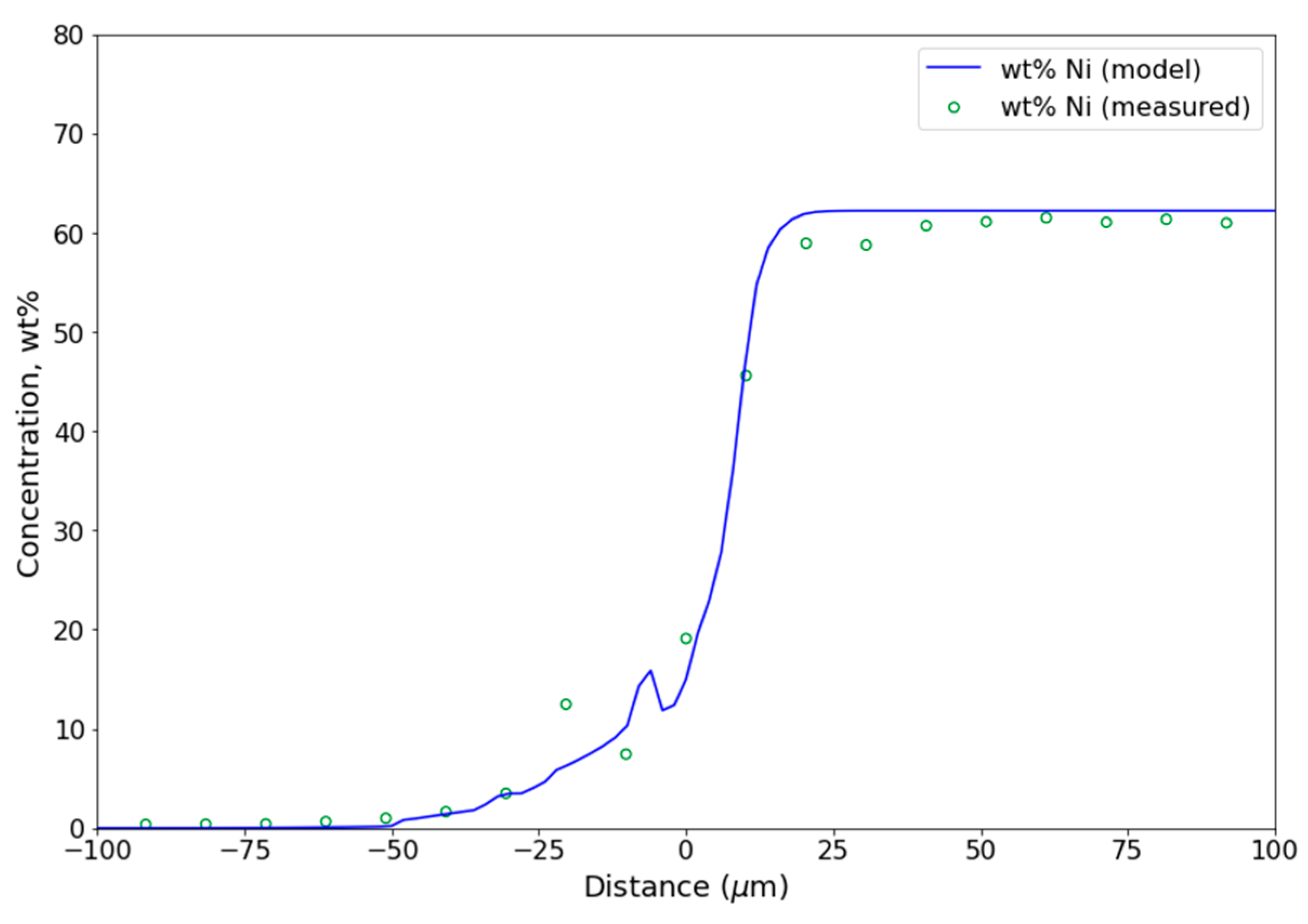
4.3. Diffusion Modeling and Computational Methods
4.4. Comparison Between As-Built and Thermally Exposed Samples
EPMA Mapping
4.5. Mechanical Testing
4.5.1. Tensile Testing
4.5.2. Fatigue Testing
4.5.3. Fatigue Fracture Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofmann, D.C.; Roberts, S.; Otis, R.; Kolodziejska, J.; Dillon, R.P.; Suh, J.-O.; Shapiro, A.A.; Liu, Z.-K.; Borgonia, J.-P. Developing Gradient Metal Alloys through Radial Deposition Additive Manufacturing. Sci. Rep. 2014, 4, 5357. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, H.; Bose, S.; Bandyopadhyay, A. Laser processed calcium phosphate reinforced CoCrMo for load-bearing applications: Processing and wear induced damage evaluation. Acta Biomater. 2018, 66, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Sing, S.; Tey, C.; Tan, J.; Huang, S.; Yeong, W.Y. 3D printing of metals in rapid prototyping of biomaterials: Techniques in additive manufacturing. In Rapid Prototyping of Biomaterials; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Chapter 2; pp. 17–40. [Google Scholar]
- Zheng, R.; Cui, J.; Yang, Y.; Li, S.; Misra, R.; Kondoh, K.; Zhu, Q.; Lu, Y.; Li, X. Enhanced densification of copper during laser powder bed fusion through powder surface alloying. J. Mech. Work. Technol. 2022, 305, 117575. [Google Scholar] [CrossRef]
- Ellis, D.L. Conductivity of GRCop-42 Alloy Enhanced; NASA Research & Technology: Washington, DC, USA, 2005. [Google Scholar]
- Gradl, P.R.; Protz, C.S.; Cooper, K.; Ellis, D.; Evans, L.J.; Garcia, C. GRCop-42 Development and Hot-fire Testing Using Additive Manufacturing Powder Bed Fusion for Channel Cooled Combustion Chambers. In Proceedings of the 55th American Institute of Aeronautics and Astronautics Joint Propulsion Conference, Indianapolis, IN, USA, 19–22 August 2019. AIAA-2019-4228. [Google Scholar]
- Onuike, B.; Bandyopadhyay, A. Bond Strength Measurement for Additively Manufactured Inconel 718-GRCop84 Copper Alloy Bimetallic Joints. Addit. Manuf. 2019, 27, 576–585. [Google Scholar] [CrossRef]
- Scannapieco, D.S.; Lewandowski, J.J.; Rogers, R.B.; Ellis, D.L. In-Situ Alloying of GRCop-42 via Additive Manufacturing: Precipitate Analysis. In Scientific and Technical Information Program; NASA: Washington, DC, USA, 2020. [Google Scholar]
- Ellis, D.L. GRCop-84: A High-Temperature Copper Alloy for High-Heat-Flux Applications. In Scientific and Technical Information Program; NASA: Washington, DC, USA, 2005. [Google Scholar]
- Thomas-Ogbuji, L.U. Oxidation Behavior of GRCop-84 Copper Alloy Assessed; NASA: Washington, DC, USA, 2005. [Google Scholar]
- Souther, M. Microstructural Transformation of Cold-Sprayed GRCop-42 for Rocket Engine Combustion Chamber Liners. Bachelor’s Thesis, California Polytechnic University, San Luis Obispo, CA, USA, 2019. [Google Scholar]
- Minneci, R.P.; Lass, E.A.; Bunn, J.R.; Choo, H.; Rawn, C.J. Copper-based alloys for structural high-heat-flux applications: A review of development, properties, and performance of Cu-rich Cu-Cr-Nb alloys. Int. Mater. Rev. 2020, 66, 394–425. [Google Scholar] [CrossRef]
- Gradl, P.R.; Protz, C.S.; Zagorski, K.; Doshi, V.; McCallum, H. Additive Manufacturing and Hot-fire Testing of Bimetallic GRCop-84 and C-18150 Channel-Cooled Combustion Chambers using Powder Bed Fusion and Inconel 625 Hybrid Directed Energy Deposition. In Proceedings of the 55th American Institue of Aeronautics and Astronautics Joint Propulsion Conference, Indianapolis, IN, USA, 19–22 August 2019. AIAA-2019-4390. [Google Scholar]
- Zhang, C.; Shirzadi, A.A. Diffusion bonding of copper alloy to nickel-based superalloy: Effect of heat treatment on the microstructure and mechanical properties of the joints. Sci. Technol. Weld. Join. 2021, 26, 213–219. [Google Scholar] [CrossRef]
- ASTM B446-19; Standard Specification for Nickel-Chromium-Molybdenum-Columbium Alloy (UNS N06625), Nickel-Chromium-Molybdenum-Silicon Alloy (UNS N06219), and Nickel-Chromium-Molybdenum-Tungsten Alloy (UNS N06650) Rod and Bar. ASTM International: West Conshohocken, PA, USA, 2019.
- AMS5599E; Nickel Alloy, Corrosion and Heat-Resistant, Sheet, Strip, and Plate 62Ni-21.5Cr-9.0Mo-3.7Cb(Nb) Annealed. SAE International: Warrendale, PA, USA, 2016.
- Eiselstein, H.; Tillack, D. The Invention and Definition of Alloy 625; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 1991. [Google Scholar]
- Gupta, R.; Kumar, V.A.; Gururaja, U.; Shivaram, B.; Prasad, Y.M.; Ramkumar, P.; Chakravarthi, K.; Sarkar, P. Processing and Characterization of Inconel 625 Nickel base Superalloy. In Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2015; Volume 830–831, pp. 38–40. [Google Scholar] [CrossRef]
- Floreen, S.; Fuchs, G.E.; Yang, W.J. The Metallurgy of Alloy 625; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 1994. [Google Scholar]
- Shankar, V.; Valsan, M.; Rao, K.B.S.; Mannan, S.L. Effects of Temperature and Strain Rate on Tensile Properties and Activation Energy for Dynamic Strain Aging in Alloy 625. Met. Mater. Trans. A 2004, 35, 3129–3139. [Google Scholar] [CrossRef]
- Suave, L.M.; Cormier, J.; Villechaise, P.; Soula, A.; Hervier, Z.; Bertheau, D.; Laigo, J. Microstructural evolutions during thermal aging of Alloy 625: Impact of temperature and forming process. Met. Mater. Trans. A 2014, 45, 2963–2982. [Google Scholar] [CrossRef]
- Silva, C.C.; de Miranda, H.C.; Motta, M.F.; Farias, J.P.; Afonso, C.R.M.; Ramirez, A.J. New insight on the solidification path of an alloy 625 weld overlay. J. Mater. Res. Technol. 2013, 2, 228–237. [Google Scholar] [CrossRef]
- Lingenfelter, A. Welding of Inconel Alloy 718: A Historical Overview; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 1989. [Google Scholar]
- Singhal, T.S.; Jain, J.K.; Kumar, M.; Bhojak, V.; Saxena, K.K.; Buddhi, D.; Prakash, C. A comprehensive comparative review: Welding and additive manufacturing. Int. J. Interact. Des. Manuf. 2023, 18, 1829–1843. [Google Scholar] [CrossRef]
- Hu, Y.; Lin, X.; Li, Y.; Zhang, S.; Zhang, Q.; Chen, W.; Li, W.; Huang, W. IInfluence of heat treatments on the microstructure and mechanical properties of Inconel 625 fabricated by directed energy deposition. Mater. Sci. Eng. A 2021, 817, 141309. [Google Scholar] [CrossRef]
- Dubiel, B.; Gola, K.; Staroń, S.; Pasiowiec, H.; Indyka, P.; Gajewska, M.; Zubko, M.; Kalemba-Rec, I.; Moskalewicz, T.; Kąc, S. Effect of high temperature annealing on the microstructure evolution and hardness behavior of the Inconel 625 superalloy additively manufactured by laser powder bed fusion. Arch. Civ. Mech. Eng. 2023, 23, 249. [Google Scholar] [CrossRef]
- Saple, J.; Ahmadi, B.; Shaeri, M.R.; Bigham, S. Experimental study of a 3D-printed wick condenser for enhanced condensation heat transfer. In Proceedings of the 2024 23rd IEEE Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (ITherm), Aurora, CO, USA, 28–31 May 2024; Volume 23. [Google Scholar] [CrossRef]
- Steeves, C.A.; He, M.Y.; Kasen, S.D.; Valdevit, L.; Wadley, H.N.G.; Evans, A.G. Feasibility of Metallic Structural Heat Pipes as Sharp Leading Edges for Hypersonic Vehicles. J. Appl. Mech. 2009, 76, 031014. [Google Scholar] [CrossRef]
- Onuike, B.; Heer, B.; Bandyopadhyay, A. Additive manufacturing of Inconel 718—Copper alloy bimetallic structure using laser engineered net shaping (LENS). Addit. Manuf. 2018, 21, 133–140. [Google Scholar] [CrossRef]
- Pérez-Álvarez, R.; Montoya, A.; López-Puente, J.; Santana, D. Solar pwer tower plants with Bimetallic receiver tubes: A thermomechanical study of two- and three- layer composite tubes configurations. Energy 2023, 283, 129170. [Google Scholar] [CrossRef]
- Llovet, X. Electron Probe Microanalysis. In Encyclopedia of Analytical Science, 3rd ed.; Universitat de Barcelona: Barcelona, Spain; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Sec. Microscopy; Volume 7, pp. 30–38. [Google Scholar]
- Taylor, N.; Heath, J. Electron Probe Microanalysis, 1st ed.; (Microscopy, Spectroscopy and Separations EKB); John Wiley & Sons: Chichester, UK, 2015. [Google Scholar]
- Demeneghi, G.; Barnes, B.; Gradl, P.; Mayeur, J.R.; Hazeli, K. Size effects on microstructure and mechanical properties of additively manufactured copper-chromium-niobium alloy. Mater. Sci. Eng. A 2021, 820, 141511. [Google Scholar] [CrossRef]
- Kim, M.J.; Saldana, C. Thin wall deposition of IN625 using directed energy deposition. In Proceedings of the 48th SME North American Manufacturing Research Conference, Cincinnati, OH, USA, 22–26 June 2020; Available online: www.sciencedirect.com (accessed on 17 January 2024).
- Wang, F.; Del Bosque, H.; Hyder, J.; Corliss, M.; Hung, W.N. Experimental investigation of porosity distribution in selective laser melted Inconel 718. Procedia Manuf. 2020, 48, 807–813. [Google Scholar] [CrossRef]
- Poirier, D.R.; Geiger, G.H. Transport Phenomena in Materials Processing; The Minerals, Metals & Materials Series; Springer Nature: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Taguchi, O.; Iijima, Y.; Hirano, K.-I. Application of Atomic Absorption Analysis to Impurity Diffusion of Copper in Nickel in a Wide Range of Temperature. J. Jpn. Inst. Met. Mater. 1984, 48, 20–24. [Google Scholar] [CrossRef]
- Wu, H.; Mayeshiba, T.; Morgan, D. High-throughput ab-initio dilute solute diffusion database. Sci. Data 2016, 3, 160054. [Google Scholar] [CrossRef]
- INCONEL ® Alloy 625. 2013. Available online: www.specialmetals.com (accessed on 3 October 2022).


















| Element | Minimum | Maximum |
|---|---|---|
| Carbon | - | 0.10 |
| Manganese | - | 0.50 |
| Silicon | - | 0.50 |
| Phosphorus | - | 0.015 |
| Sulfur | - | 0.015 |
| Chromium | 20.00 | 23.00 |
| Molybdenum | 8.00 | 10.00 |
| Niobium | 3.15 | 4.15 |
| Iron | - | 5.00 |
| Cobalt | - | 1.00 |
| Titanium | - | 0.40 |
| Aluminum | - | 0.40 |
| Nickel | Remainder | |
| Exposure Time (816 °C) | ) | ) | ) |
|---|---|---|---|
| 50 h | 383 | 470 | 426.5 |
| 100 h | 764 | 690 | 727 |
| 200 h | 1075 | 1013 | 1044 |
| 500 h | 1710 | 1942 | 1826 |
| Tensile Test Results | Thermal Exposure 816 °C/200 h | As-Built Samples | Literature [12,33] | ||
|---|---|---|---|---|---|
| Sample Number | TE2 | TE5 | SN1 | SN5 | Range As-Built |
| Elastic Modulus (MPa) | 39,682 | 44,144 | 50,887 | 44,932 | 52,000–84,500 |
| Yield Strength (MPa) | 212.87 | 208.00 | 242.81 | 273.92 | 173–305 |
| Max/Ultimate Strength (MPa) | 339.16 | 342.22 | 363.93 | 357.58 | 355–495 |
| Break Location | Interface | Interface | Interface | Copper | Copper only |
| As-Built Samples | ||||
|---|---|---|---|---|
| Sample | SN2 | SN3 | SN4 | SN6 |
| Cycles to Failure | 45,935 | 71,615 | 55,984–95,000 * | 48,663 |
| Fracture Location | Copper | Copper | Copper | Interface/Copper |
| Thermally Exposed (816 °C/200 h) Samples | ||||
|---|---|---|---|---|
| Sample | TE1 | TE3 | TE4 | TE6 |
| Cycles to Failure | 55,507 | 164,580 | 23,111 | 18,519 |
| Fracture Location | Copper | Copper | Copper | Interface |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rieffer, A.; Wessman, A. Interfacial Stability of Additively Manufactured Alloy 625–GRCop-42 Bimetallic Structures. J. Manuf. Mater. Process. 2025, 9, 34. https://doi.org/10.3390/jmmp9020034
Rieffer A, Wessman A. Interfacial Stability of Additively Manufactured Alloy 625–GRCop-42 Bimetallic Structures. Journal of Manufacturing and Materials Processing. 2025; 9(2):34. https://doi.org/10.3390/jmmp9020034
Chicago/Turabian StyleRieffer, Ariel, and Andrew Wessman. 2025. "Interfacial Stability of Additively Manufactured Alloy 625–GRCop-42 Bimetallic Structures" Journal of Manufacturing and Materials Processing 9, no. 2: 34. https://doi.org/10.3390/jmmp9020034
APA StyleRieffer, A., & Wessman, A. (2025). Interfacial Stability of Additively Manufactured Alloy 625–GRCop-42 Bimetallic Structures. Journal of Manufacturing and Materials Processing, 9(2), 34. https://doi.org/10.3390/jmmp9020034






