Carbon Nanotubes (CNTs): A Potential Nanomaterial for Water Purification
Abstract
:1. Introduction
2. Carbon Nanotube Based Water Purification Technology
2.1. CNTs for Adsorption
2.2. CNTs in the Membrane and Filtration
Types of Nanotube Membranes and Their Fabrication
2.3. CNTs as the Catalyst
2.3.1. Photocatalysis
2.3.2. Catalytic Wet Air Oxidation (CWAO)
2.4. Carbon Nanotube Materials for Antimicrobial and Antibiofouling
2.5. Desalination
2.6. CNTs for Sensing and Monitoring
2.7. CNT Electrodes as a Microbial Fuel Cell (MFC)
3. Toxic Potential of CNTs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baker, S.; Satish, S. Endophytes: Toward a Vision in Synthesis of Nanoparticle for Future Therapeutic Agents. Int. J. Bio-Inorg. Hybrid Nanomater. 2012, 1, 67–77. [Google Scholar]
- Feynman, R. There’s plenty of room at the bottom. In Feynman and Computation; Taylor & Francis: Abingdon, UK, 2018; pp. 63–76. ISBN 9780429969003. [Google Scholar]
- Yadav, K.K.; Singh, J.K.; Gupta, N.; Kumar, V. A review of nanobioremediation technologies for environmental cleanup: A novel biological approach. J. Mater. Environ. Sci. 2017, 8, 740–757. [Google Scholar]
- Garg, S.; Bhatia, R.; Attri, P. Black but gold: Carbon nanomaterials for waste water purification. In Nanomaterials for Water Remediation; De Gruyter: Berlin, Germany, 2020; pp. 42–92. [Google Scholar]
- Mishra, A.K. Smart Ceramics: Preparation, Properties, and Applications; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351671644. [Google Scholar]
- Arora, B.; Choi, E.H.; Shiratani, M.; Attri, P. Cellulose: A Smart Material for Water Purification. In Smart Materials for Waste Water Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 335–346. [Google Scholar]
- Attri, P.; Tochikubo, F.; Park, J.H.; Choi, E.H.; Koga, K.; Shiratani, M. Impact of Gamma rays and DBD plasma treatments on wastewater treatment. Sci. Rep. 2018, 8, 2926. [Google Scholar] [CrossRef] [Green Version]
- Attri, P.; Yusupov, M.; Park, J.H.; Lingamdinne, L.P.; Koduru, J.R.; Shiratani, M.; Choi, E.H.; Bogaerts, A. Mechanism and comparison of needle-type non-thermal direct and indirect atmospheric pressure plasma jets on the degradation of dyes. Sci. Rep. 2016, 6, 34419. [Google Scholar] [CrossRef]
- Das, R.; Ali, M.E.; Hamid, S.B.A.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon nanotube membranes for water purification: A bright future in water desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Al-Abri, M.; Al-Ghafri, B.; Bora, T.; Dobretsov, S.; Dutta, J.; Castelletto, S.; Rosa, L.; Boretti, A. Chlorination disadvantages and alternative routes for biofouling control in reverse osmosis desalination. NPJ Clean Water 2019, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Lingamdinne, L.P.; Chang, Y.Y.; Yang, J.K.; Singh, J.; Choi, E.H.; Shiratani, M.; Koduru, J.R.; Attri, P. Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals. Chem. Eng. J. 2017, 307, 74–84. [Google Scholar] [CrossRef]
- Attri, P.; Arora, B.; Bhatia, R.; Venkatesu, P.; Choi, E.H. Plasma Technology: A New Remediation for Water Purification with or without Nanoparticles. In Application of Nanotechnology in Water Research; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 63–77. ISBN 9781118939314. [Google Scholar]
- Ghasemzadeh, G.; Momenpour, M.; Omidi, F.; Hosseini, M.R.; Ahani, M.; Barzegari, A. Applications of nanomaterials in water treatment and environmental remediation. Front. Environ. Sci. Eng. 2014, 8, 471–482. [Google Scholar] [CrossRef]
- Baby, R.; Saifullah, B.; Hussein, M.Z. Carbon Nanomaterials for the Treatment of Heavy Metal-Contaminated Water and Environmental Remediation. Nanoscale Res. Lett. 2019, 14, 341. [Google Scholar] [CrossRef] [Green Version]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically functionalized carbon nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Vecitis, C.D.; Schulze, A.; Cao, B.; Ismail, A.F.; Lu, X.; Chen, J.; Ramakrishna, S. Recent advances in nanomaterials for water protection and monitoring. Chem. Soc. Rev. 2017, 46, 6946–7020. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, M.; Zhang, S.; Pan, B. Application potential of carbon nanotubes in water treatment: A review. J. Environ. Sci. 2013, 25, 1263–1280. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.H.; Wepasnick, K.; Smith, B.A.; Bangash, F.K.; Fairbrother, D.H.; Ball, W.P. Sorption of aqueous Zn[II] and Cd[II] by multiwall carbon nanotubes: The relative roles of oxygen-containing functional groups and graphenic carbon. Langmuir 2010, 26, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Ding, J.; Luan, Z.; Di, Z.; Zhu, Y.; Xu, C.; Wu, D.; Wei, B. Competitive adsorption of Pb2+, Cu2+ and Cd 2+ ions from aqueous solutions by multiwalled carbon nanotubes. Carbon 2003, 41, 2787–2792. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Ahmadi, M.; Bagheri, H. Removal of some cationic dyes from aqueous solutions using magnetic-modified multi-walled carbon nanotubes. J. Hazard. Mater. 2011, 196, 109–114. [Google Scholar] [CrossRef]
- Yang, C.M.; Park, J.S.; An, K.H.; Lim, S.C.; Seo, K.; Kim, B.; Park, K.A.; Han, S.; Park, C.Y.; Lee, Y.H. Selective removal of metallic single-walled carbon nanotubes with small diameters by using nitric and sulfuric acids. J. Phys. Chem. B 2005, 109, 19242–19248. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Hamid, S.B.A.; Das, R.; Hasan, M.R.; Zain, S.M.; Khalid, K.; Uddin, M.N. Preparation of carbonaceous adsorbents from lignocellulosic biomass and their use in removal of contaminants from aqueous solution. BioResources 2013, 8, 6523–6555. [Google Scholar] [CrossRef]
- Das, R.; Abd Hamid, S.B.; Ali, M.E.; Ismail, A.F.; Annuar, M.S.M.; Ramakrishna, S. Multifunctional carbon nanotubes in water treatment: The present, past and future. Desalination 2014, 354, 160–179. [Google Scholar] [CrossRef]
- Gotovac, S.; Honda, H.; Hattori, Y.; Takahashi, K.; Kanoh, H.; Kaneko, K. Effect of nanoscale curvature of single-walled carbon nanotubes on adsorption of polycyclic aromatic hydrocarbons. Nano Lett. 2007, 7, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shao, T.; Bekaroglu, S.S.K.; Karanfil, T. The impacts of aggregation and surface chemistry of carbon nanotubes on the adsorption of synthetic organic compounds. Environ. Sci. Technol. 2009, 43, 5719–5725. [Google Scholar] [CrossRef]
- Yang, K.; Wang, X.; Zhu, L.; Xing, B. Competitive sorption of pyrene, phenanthrene, and naphthalene on multiwalled carbon nanotubes. Environ. Sci. Technol. 2006, 40, 5804–5810. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B. Desorption of polycyclic aromatic hydrocarbons from carbon nanomaterials in water. Environ. Pollut. 2007, 145, 529–537. [Google Scholar] [CrossRef]
- Gotovac, S.; Hattori, Y.; Noguchi, D.; Miyamoto, J.I.; Kanamaru, M.; Utsumi, S.; Kanoh, H.; Kaneko, K. Phenanthrene adsorption from solution on single wall carbon nanotubes. J. Phys. Chem. B 2006, 110, 16219–16224. [Google Scholar] [CrossRef]
- Bhushan, B. (Ed.) Springer Handbook of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2004; ISBN 978-3-540-01218-4. [Google Scholar]
- Sun, F.; Gao, J.; Zhu, Y.; Chen, G.; Wu, S.; Qin, Y. Adsorption of SO2 by typical carbonaceous material: A comparative study of carbon nanotubes and activated carbons. Adsorption 2013, 19, 959–966. [Google Scholar] [CrossRef]
- Yang, Q.H.; Hou, P.X.; Bai, S.; Wang, M.Z.; Cheng, H.M. Adsorption and capillarity of nitrogen in aggregated multi-walled carbon nanotubes. Chem. Phys. Lett. 2001, 345, 18–24. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, S.A.U.; Luan, H.Y.; Farid, M.U.; Huang, H. Challenges and opportunities in functional carbon nanotubes for membrane-based water treatment and desalination. Sci. Total Environ. 2019, 646, 1126–1139. [Google Scholar] [CrossRef]
- Peng, X.; Li, Y.; Luan, Z.; Di, Z.; Wang, H.; Tian, B.; Jia, Z. Adsorption of 1,2-dichlorobenzene from water to carbon nanotubes. Chem. Phys. Lett. 2003, 376, 154–158. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Adsorption of phenolic compounds by carbon nanotubes: Role of aromaticity and substitution of hydroxyl groups. Environ. Sci. Technol. 2008, 42, 7254–7259. [Google Scholar] [CrossRef]
- Sitko, R.; Zawisza, B.; Malicka, E. Modification of carbon nanotubes for preconcentration, separation and determination of trace-metal ions. TrAC Trends Anal. Chem. 2012, 37, 22–31. [Google Scholar] [CrossRef]
- Ray, P.Z.; Shipley, H.J. Inorganic nano-adsorbents for the removal of heavy metals and arsenic: A review. RSC Adv. 2015, 5, 29885–29907. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef] [Green Version]
- Adeleye, A.S.; Keller, A.A. Long-term colloidal stability and metal leaching of single wall carbon nanotubes: Effect of temperature and extracellular polymeric substances. Water Res. 2014, 49, 236–250. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Wang, S.; Wei, J.; Zhang, X.; Xu, C.; Luan, Z.; Wu, D.; Wei, B. Lead adsorption on carbon nanotubes. Chem. Phys. Lett. 2002, 357, 263–266. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Saleh, T.A. Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res. 2011, 45, 2207–2212. [Google Scholar] [CrossRef]
- Di, Z.C.; Ding, J.; Peng, X.J.; Li, Y.H.; Luan, Z.K.; Liang, J. Chromium adsorption by aligned carbon nanotubes supported ceria nanoparticles. Chemosphere 2006, 62, 861–865. [Google Scholar] [CrossRef]
- Peng, X.; Luan, Z.; Ding, J.; Di, Z.; Li, Y.; Tian, B. Ceria nanoparticles supported on carbon nanotubes for the removal of arsenate from water. Mater. Lett. 2005, 59, 399–403. [Google Scholar] [CrossRef]
- Yu, F.; Wu, Y.; Li, X.; Ma, J. Kinetic and thermodynamic studies of toluene, ethylbenzene, and m -xylene adsorption from aqueous solutions onto KOH-activated multiwalled carbon nanotubes. J. Agric. Food Chem. 2012, 60, 12245–12253. [Google Scholar] [CrossRef]
- Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Rao, G.P.; Lu, C.; Su, F. Sorption of divalent metal ions from aqueous solution by carbon nanotubes: A review. Sep. Purif. Technol. 2007, 58, 224–231. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S. Removal of heavy metals from wastewater using carbon nanotubes. Sep. Purif. Rev. 2014, 43, 311–338. [Google Scholar] [CrossRef]
- Kandah, M.I.; Meunier, J.L. Removal of nickel ions from water by multi-walled carbon nanotubes. J. Hazard. Mater. 2007, 146, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Xing, B. Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ. Sci. Technol. 2008, 42, 9005–9013. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.; Gallego, M.; Valcárcel, M. Speciation of organometallic compounds in environmetal samples by gas chromatography after flow preconcentration on fullerenes and nanotubes. Anal. Chem. 2005, 77, 5389–5395. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Carbon nanotubes as superior sorbent for dioxin removal. J. Am. Chem. Soc. 2001, 123, 2058–2059. [Google Scholar] [CrossRef]
- Cai, Y.Q.; Cai, Y.E.; Mou, S.F.; Lu, Y.Q. Multi-walled carbon nanotubes as a solid-phase extraction adsorbent for the determination of chlorophenols in environmental water samples. J. Chromatogr. A 2005, 1081, 245–247. [Google Scholar] [CrossRef]
- Zhou, Q.; Xiao, J.; Wang, W. Comparison of multiwalled carbon nanotubes and a conventional absorbent on the enrichment of sulfonylurea herbicides in water samples. Anal. Sci. 2007, 23, 189–192. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Xiao, J.; Wang, W.; Liu, G.; Shi, Q.; Wang, J. Determination of atrazine and simazine in environmental water samples using multiwalled carbon nanotubes as the adsorbents for preconcentration prior to high performance liquid chromatography with diode array detector. Talanta 2006, 68, 1309–1315. [Google Scholar] [CrossRef]
- Zhou, Q.; Xiao, J.; Wang, W. Using multi-walled carbon nanotubes as solid phase extraction adsorbents to determine dichlorodiphenyltrichloroethane and its metabolites at trace level in water samples by high performance liquid chromatography with UV detection. J. Chromatogr. A 2006, 1125, 152–158. [Google Scholar] [CrossRef]
- Chi, W.; Shi, H.; Shi, W.; Guo, Y.; Guo, T. 4-Nitrophenol surface molecularly imprinted polymers based on multiwalled carbon nanotubes for the elimination of paraoxon pollution. J. Hazard. Mater. 2012, 227–228, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Chiu, H.; Bai, H. Comparisons of adsorbent cost for the removal of zinc (II) from aqueous solution by carbon nanotubes and activated carbon. J. Nanosci. Nanotechnol. 2007, 7, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Chiu, H. Adsorption of zinc(II) from water with purified carbon nanotubes. Chem. Eng. Sci. 2006, 61, 1138–1145. [Google Scholar] [CrossRef]
- Lu, C.; Chiu, H.; Liu, C. Removal of zinc(II) from aqueous solution by purified carbon nanotubes: Kinetics and equilibrium studies. Ind. Eng. Chem. Res. 2006, 45, 2850–2855. [Google Scholar] [CrossRef]
- Chen, C.L.; Wang, X.K.; Nagatsu, M. Europium adsorption on multiwall carbon nanotube/iron oxide magnetic composite in the presence of polyacrylic acid. Environ. Sci. Technol. 2009, 43, 2362–2367. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, L.; Wu, W.; Lu, G.; Xu, F.; Tong, Y.; Liu, W.; Du, J. Enhanced adsorption of malachite green onto carbon nanotube/polyaniline composites. J. Appl. Polym. Sci. 2013, 127, 2475–2482. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Jiang, R.; Xiao, L.; Zeng, G.M. Preparation, characterization, adsorption kinetics and thermodynamics of novel magnetic chitosan enwrapping nanosized γ-Fe2O3 and multi-walled carbon nanotubes with enhanced adsorption properties for methyl orange. Bioresour. Technol. 2010, 101, 5063–5069. [Google Scholar] [CrossRef]
- Maggini, L.; Raquez, J.M.; Marega, R.; Jensen Ahrens, J.; Pineux, F.; Meyer, F.; Dubois, P.; Bonifazi, D. Magnetic poly(vinylpyridine)-coated carbon nanotubes: An efficient supramolecular tool for wastewater purification. ChemSusChem 2013, 6, 367–373. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Quan, X.; Zhang, Y. Enhanced adsorption of PFOA and PFOS on multiwalled carbon nanotubes under electrochemical assistance. Environ. Sci. Technol. 2011, 45, 8498–8505. [Google Scholar] [CrossRef]
- Ursino, C.; Castro-Muñoz, R.; Drioli, E.; Gzara, L.; Albeirutty, M.H.; Figoli, A. Progress of nanocomposite membranes for water treatment. Membranes 2018, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Kurwadkar, S.; Hoang, T.V.; Malwade, K.; Kanel, S.R.; Harper, W.F.; Struckhoff, G. Application of carbon nanotubes for removal of emerging contaminants of concern in engineered water and wastewater treatment systems. Nanotechnol. Environ. Eng. 2019, 4, 12. [Google Scholar] [CrossRef]
- Ahn, C.H.; Baek, Y.; Lee, C.; Kim, S.O.; Kim, S.; Lee, S.; Kim, S.-H.; Bae, S.S.; Park, J.; Yoon, J. Carbon nanotube-based membranes: fabrication and application to desalination. J. Ind. Eng. Chem. 2012, 18, 1551–1559. [Google Scholar]
- Hinds, B.J.; Chopra, N.; Rantell, T.; Andrews, R.; Gavalas, V.; Bachas, L.G. Aligned multiwalled carbon nanotube membranes. Science 2004, 303, 62–65. [Google Scholar]
- Mattia, D.; Lee, K.P.; Calabrò, F. Water permeation in carbon nanotube membranes. Curr. Opin. Chem. Eng. 2014, 4, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.J.; Park, H.D. The most densified vertically-aligned carbon nanotube membranes and their normalized water permeability and high pressure durability. J. Memb. Sci. 2016, 501, 144–151. [Google Scholar] [CrossRef]
- Lee, B.; Baek, Y.; Lee, M.; Jeong, D.H.; Lee, H.H.; Yoon, J.; Kim, Y.H. A carbon nanotube wall membrane for water treatment. Nat. Commun. 2015, 6, 7109. [Google Scholar] [CrossRef]
- Trivedi, S.; Alameh, K. Effect of vertically aligned carbon nanotube density on the water flux and salt rejection in desalination membranes. Springerplus 2016, 5, 1158. [Google Scholar] [CrossRef] [Green Version]
- Jafari, A.; Mahvi, A.H.; Nasseri, S.; Rashidi, A.; Nabizadeh, R.; Rezaee, R. Ultrafiltration of natural organic matter from water by vertically aligned carbon nanotube membrane. J. Environ. Heal. Sci. Eng. 2015, 13, 51. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Jegal, J.; Kim, W.N. Fabrication and characterization of multi-walled carbon nanotubes/polymer blend membranes. J. Memb. Sci. 2006, 284, 406–415. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Novel ultrafiltration membranes prepared from a multi-walled carbon nanotubes/polymer composite. J. Memb. Sci. 2010, 362, 374–383. [Google Scholar] [CrossRef]
- Brunet, L.; Lyon, D.Y.; Zodrow, K.; Rouch, J.-C.; Caussat, B.; Serp, P.; Remigy, J.-C.; Wiesner, M.R.; Alvarez, P.J.J. Properties of Membranes Containing Semi-dispersed Carbon Nanotubes. Environ. Eng. Sci. 2008, 25, 565–576. [Google Scholar] [CrossRef]
- Majeed, S.; Fierro, D.; Buhr, K.; Wind, J.; Du, B.; Boschetti-de-Fierro, A.; Abetz, V. Multi-walled carbon nanotubes (MWCNTs) mixed polyacrylonitrile (PAN) ultrafiltration membranes. J. Memb. Sci. 2012, 403–404, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, F.; Santos, C.M.; Mangadlao, J.; Advincula, R.; Rodrigues, D.F. Antimicrobial PVK: SWNT nanocomposite coated membrane for water purification: Performance and toxicity testing. Water Res. 2013, 47, 3966–3975. [Google Scholar] [CrossRef]
- Mehwish, N.; Kausar, A.; Siddiq, M. High-performance polyvinylidene fluoride/poly(styrene–butadiene–styrene)/functionalized MWCNTs-SCN-Ag nanocomposite membranes. Iran. Polym. J. 2015, 24, 549–559. [Google Scholar] [CrossRef]
- Shawky, H.A.; Chae, S.R.; Lin, S.; Wiesner, M.R. Synthesis and characterization of a carbon nanotube/polymer nanocomposite membrane for water treatment. Desalination 2011, 272, 46–50. [Google Scholar] [CrossRef]
- Zhao, H.; Qiu, S.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Improving the performance of polyamide reverse osmosis membrane by incorporation of modified multi-walled carbon nanotubes. J. Memb. Sci. 2014, 450, 249–256. [Google Scholar] [CrossRef]
- Zarrabi, H.; Yekavalangi, M.E.; Vatanpour, V.; Shockravi, A.; Safarpour, M. Improvement in desalination performance of thin film nanocomposite nanofiltration membrane using amine-functionalized multiwalled carbon nanotube. Desalination 2016, 394, 83–90. [Google Scholar] [CrossRef]
- Lee, J.; Ye, Y.; Ward, A.J.; Zhou, C.; Chen, V.; Minett, A.I.; Lee, S.; Liu, Z.; Chae, S.R.; Shi, J. High flux and high selectivity carbon nanotube composite membranes for natural organic matter removal. Sep. Purif. Technol. 2016, 163, 109–119. [Google Scholar] [CrossRef]
- Ho, K.C.; Teow, Y.H.; Ang, W.L.; Mohammad, A.W. Novel GO/OMWCNTs mixed-matrix membrane with enhanced antifouling property for palm oil mill effluent treatment. Sep. Purif. Technol. 2017, 177, 337–349. [Google Scholar] [CrossRef]
- Liu, L.; Son, M.; Chakraborty, S.; Bhattacharjee, C.; Choi, H. Fabrication of ultra-thin polyelectrolyte/carbon nanotube membrane by spray-assisted layer-by-layer technique: Characterization and its anti-protein fouling properties for water treatment. Desalin. Water Treat. 2013, 51, 6194–6200. [Google Scholar] [CrossRef]
- Ihsanullah. Carbon nanotube membranes for water purification: Developments, challenges, and prospects for the future. Sep. Purif. Technol. 2019, 209, 307–337. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, G.Z.; Qiu, S.; Cheng, L.H.; Chen, H.L. Preparation of high-flux thin film nanocomposite reverse osmosis membranes by incorporating functionalized multi-walled carbon nanotubes. Desalin. Water Treat. 2011, 34, 19–24. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, K.; Baek, Y.; Kim, D.G.; Shim, J.; Yoon, J.; Lee, J.C. High-performance reverse osmosis CNT/polyamide nanocomposite membrane by controlled interfacial interactions. ACS Appl. Mater. Interfaces 2014, 6, 2819–2829. [Google Scholar] [CrossRef] [PubMed]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Di Paola, A.; García-López, E.; Marcì, G.; Palmisano, L. A survey of photocatalytic materials for environmental remediation. J. Hazard. Mater. 2012, 211–212, 3–29. [Google Scholar] [CrossRef]
- Saleh, A.T. The Role of Carbon Nanotubes in Enhancement of Photocatalysis. In Syntheses and Applications of Carbon Nanotubes and Their Composites; InTech: London, UK, 2013. [Google Scholar]
- Yao, Y.; Li, G.; Ciston, S.; Lueptow, R.M.; Gray, K.A. Photoreactive TiO2/carbon nanotube composites: Synthesis and reactivity. Environ. Sci. Technol. 2008, 42, 4952–4957. [Google Scholar] [CrossRef]
- Duong, T.T.; Nguyen, Q.D.; Hong, S.K.; Kim, D.; Yoon, S.G.; Pham, T.H. Enhanced photoelectrochemical activity of the TiO 2/ITO nanocomposites grown onto single-walled carbon nanotubes at a low temperature by nanocluster deposition. Adv. Mater. 2011, 23, 5557–5562. [Google Scholar] [CrossRef]
- Martínez, C.; Canle L, M.; Fernández, M.I.; Santaballa, J.A.; Faria, J. Kinetics and mechanism of aqueous degradation of carbamazepine by heterogeneous photocatalysis using nanocrystalline TiO2, ZnO and multi-walled carbon nanotubes-anatase composites. Appl. Catal. B Environ. 2011, 102, 563–571. [Google Scholar] [CrossRef]
- Silva, C.G.; Faria, J.L. Photocatalytic oxidation of benzene derivatives in aqueous suspensions: Synergic effect induced by the introduction of carbon nanotubes in a TiO2 matrix. Appl. Catal. B Environ. 2010, 101, 81–89. [Google Scholar] [CrossRef]
- Li, Z.; Gao, B.; Chen, G.Z.; Mokaya, R.; Sotiropoulos, S.; Li Puma, G. Carbon nanotube/titanium dioxide (CNT/TiO2) core-shell nanocomposites with tailored shell thickness, CNT content and photocatalytic/photoelectrocatalytic properties. Appl. Catal. B Environ. 2011, 110, 50–57. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.L.; Jiang, W.F.; Li, Z.Q. Photocatalytic degradation of 2,4-dinitrophenol (DNP) by multi-walled carbon nanotubes (MWCNTs)/TiO2 composite in aqueous solution under solar irradiation. Water Res. 2009, 43, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Xiong, Z.; Zhao, X.S. Pillaring chemically exfoliated graphene oxide with carbon nanotubes for photocatalytic degradation of dyes under visible light irradiation. ACS Nano 2010, 4, 7030–7036. [Google Scholar] [CrossRef]
- Li, S.; Yu, X.; Zhang, G.; Ma, Y.; Yao, J.; Keita, B.; Louis, N.; Zhao, H. Green chemical decoration of multiwalled carbon nanotubes with polyoxometalate-encapsulated gold nanoparticles: Visible light photocatalytic activities. J. Mater. Chem. 2011, 21, 2282–2287. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, H.; Yao, P.; Kang, S.Z.; Mu, J. Effect of multi-walled carbon nanotubes loaded with Ag nanoparticles on the photocatalytic degradation of rhodamine B under visible light irradiation. Appl. Surf. Sci. 2011, 257, 3620–3626. [Google Scholar] [CrossRef]
- Qu, J.; Cong, Q.; Luo, C.; Yuan, X. Adsorption and photocatalytic degradation of bisphenol A by low-cost carbon nanotubes synthesized using fallen leaves of poplar. RSC Adv. 2013, 3, 961–965. [Google Scholar] [CrossRef]
- Garcia, J.; Gomes, H.T.; Serp, P.; Kalck, P.; Figueiredo, J.L.; Faria, J.L. Platinum catalysts supported on MWNT for catalytic wet air oxidation of nitrogen containing compounds. Catal. Today 2005, 102, 101–109. [Google Scholar] [CrossRef]
- Gomes, H.T.; Samant, P.V.; Serp, P.; Kalck, P.; Figueiredo, J.L.; Faria, J.L. Carbon nanotubes and xerogels as supports of well-dispersed Pt catalysts for environmental applications. Appl. Catal. B Environ. 2004, 54, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Taboada, C.D.; Batista, J.; Pintar, A.; Levec, J. Preparation, characterization and catalytic properties of carbon nanofiber-supported Pt, Pd, Ru monometallic particles in aqueous-phase reactions. Appl. Catal. B Environ. 2009, 89, 375–382. [Google Scholar] [CrossRef]
- Garcia, J.; Gomes, H.T.; Serp, P.; Kalck, P.; Figueiredo, J.L.; Faria, J.L. Carbon nanotube supported ruthenium catalysts for the treatment of high strength wastewater with aniline using wet air oxidation. Carbon 2006, 44, 2384–2391. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, W.; Li, X.; Wang, J.; Zhou, Y. Multi-walled carbon nanotubes (MWNTs) as an efficient catalyst for catalytic wet air oxidation of phenol. Catal. Commun. 2007, 8, 2059–2063. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Zhu, W.; Wang, J.; Descorme, C. Catalytic activity, stability and structure of multi-walled carbon nanotubes in the wet air oxidation of phenol. Carbon 2008, 46, 445–452. [Google Scholar] [CrossRef]
- Mestl, G.; Maksimova, N.I.; Keller, N.; Roddatis, V.V.; Schlögl, R. Carbon Nanofilaments in Heterogeneous Catalysis: An Industrial Application for New Carbon Materials? Angew. Chemie Int. Ed. 2001, 40, 2066–2068. [Google Scholar] [CrossRef]
- Kim, K.H.; Ihm, S.K. Heterogeneous catalytic wet air oxidation of refractory organic pollutants in industrial wastewaters: A review. J. Hazard. Mater. 2011, 186, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, M.; May, S.; Mebberson, N.; Pendleton, P.; Vasilev, K.; Plush, S.; Hayball, J. Activated Carbon, Carbon Nanotubes and Graphene: Materials and Composites for Advanced Water Purification. C J. Carbon Res. 2017, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.J.; Ashcroft, J.M.; Chen, D.; Min, G.; Kim, C.H.; Murkhejee, B.; Larabell, C.; Keasling, J.D.; Chen, F.F. Charge-associated effects of fullerene derivatives on microbial structural integrity and central metabolism. Nano Lett. 2007, 7, 754–760. [Google Scholar] [CrossRef]
- Kang, S.; Mauter, M.S.; Elimelech, M. Microbial cytotoxicity of carbon-based nanomaterials: Implications for river water and wastewater effluent. Environ. Sci. Technol. 2009, 43, 2648–2653. [Google Scholar] [CrossRef]
- Brady-Estévez, A.S.; Kang, S.; Elimelech, M. A single-walled-carbon-nanotube filter for removal of viral and bacterial pathogens. Small 2008, 4, 481–484. [Google Scholar] [CrossRef]
- Bui, N.; Meshot, E.R.; Kim, S.; Peña, J.; Gibson, P.W.; Wu, K.J.; Fornasiero, F. Ultrabreathable and Protective Membranes with Sub-5 nm Carbon Nanotube Pores. Adv. Mater. 2016, 28, 5871–5877. [Google Scholar] [CrossRef]
- Baek, Y.; Kim, C.; Seo, D.K.; Kim, T.; Lee, J.S.; Kim, Y.H.; Ahn, K.H.; Bae, S.S.; Lee, S.C.; Lim, J.; et al. High performance and antifouling vertically aligned carbon nanotube membrane for water purification. J. Memb. Sci. 2014, 460, 171–177. [Google Scholar] [CrossRef]
- Dong, X.; Yang, L. Dual functional nisin-multi-walled carbon nanotubes coated filters for bacterial capture and inactivation. J. Biol. Eng. 2015, 9, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, J.K.; Park, H.G.; Wang, Y.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Sanip, S.M.; Ismail, A.F.; Goh, P.S.; Soga, T.; Tanemura, M.; Yasuhiko, H. Gas separation properties of functionalized carbon nanotubes mixed matrix membranes. Sep. Purif. Technol. 2011, 78, 208–213. [Google Scholar] [CrossRef]
- Das, R.; Leo, B.F.; Murphy, F. The Toxic Truth About Carbon Nanotubes in Water Purification: A Perspective View. Nanoscale Res. Lett. 2018, 13, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumée, L.F.; Sears, K.; Schütz, J.; Finn, N.; Huynh, C.; Hawkins, S.; Duke, M.; Gray, S. Characterization and evaluation of carbon nanotube Bucky-Paper membranes for direct contact membrane distillation. J. Memb. Sci. 2010, 351, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Kar, S.; Bindal, R.C.; Tewari, P.K. Carbon nanotube membranes for desalination and water purification: Challenges and opportunities. Nano Today 2012, 7, 385–389. [Google Scholar] [CrossRef]
- US7993524B2—Membranes with Embedded Nanotubes for Selective Permeability—Google Patents. Available online: https://patents.google.com/patent/US7993524B2/en (accessed on 23 July 2020).
- Yang, H.Y.; Han, Z.J.; Yu, S.F.; Pey, K.L.; Ostrikov, K.; Karnik, R. Carbon nanotube membranes with ultrahigh specific adsorption capacity for water desalination and purification. Nat. Commun. 2013, 4, 2220. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Funke, H.H.; Falconer, J.L.; Noble, R.D. High density, vertically-aligned carbon nanotube membranes. Nano Lett. 2009, 9, 225–229. [Google Scholar] [CrossRef]
- Gong, X.; Li, J.; Xu, K.; Wang, J.; Yang, H. A controllable molecular sieve for Na+ and K+ ions. J. Am. Chem. Soc. 2010, 132, 1873–1877. [Google Scholar] [CrossRef]
- Majumder, M.; Zhan, X.; Andrews, R.; Hinds, B.J. Voltage gated carbon nanotube membranes. Langmuir 2007, 23, 8624–8631. [Google Scholar] [CrossRef]
- Fornasiero, F.; Hyung, G.P.; Holt, J.K.; Stadermann, M.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Ion exclusion by sub-2-nm carbon nanotube pores. Proc. Natl. Acad. Sci. USA. 2008, 105, 17250–17255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, M.; Choi, H.; Liu, L.; Celik, E.; Park, H.; Choi, H. Efficacy of carbon nanotube positioning in the polyethersulfone support layer on the performance of thin-film composite membrane for desalination. Chem. Eng. J. 2015, 266, 376–384. [Google Scholar] [CrossRef]
- Pérez López, B.; Merkoçi, A. Improvement of the electrochemical detection of catechol by the use of a carbon nanotube based biosensor. Analyst 2009, 134, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Zhang, X.; Teng, Y.; Fu, Y.; Zhang, S.; Wang, T.; Wang, C.; Jin, L.; Zhang, W. Lectin-based electrochemical biosensor constructed by functionalized carbon nanotubes for the competitive assay of glycan expression on living cancer cells. Chem. Sci. 2011, 2, 2353–2360. [Google Scholar]
- Miller, S.A.; Young, V.Y.; Martin, C.R. Electroosmotic flow in template-prepared carbon nanotube membranes. J. Am. Chem. Soc. 2001, 123, 12335–12342. [Google Scholar] [CrossRef]
- Srivastava, A.; Srivastava, O.N.; Talapatra, S.; Vajtai, R.; Ajayan, P.M. Carbon nanotube filters. Nat. Mater. 2004, 3, 610–614. [Google Scholar] [CrossRef]
- Chatterjee, A.N.; Cannon, D.M.; Gatimu, E.N.; Sweedler, J.V.; Aluru, N.R.; Bohn, P.W. Modeling and simulation of ionic currents in three-dimensional microfluidic devices with nanofluidic interconnects. J. Nanoparticle Res. 2005, 7, 507–516. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Xiang, C.; Yang, L.; Sun, L.X.; Xu, F.; Cao, Z. A mediatorless microbial fuel cell using polypyrrole coated carbon nanotubes composite as anode material. Int. J. Hydrogen Energy 2008, 33, 4856–4862. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, C.M.; Bao, S.J.; Bao, Q.L. Carbon nanotube/polyaniline composite as anode material for microbial fuel cells. J. Power Sources 2007, 170, 79–84. [Google Scholar] [CrossRef]
- Mink, J.E.; Rojas, J.P.; Logan, B.E.; Hussain, M.M. Vertically Grown Multiwalled Carbon Nanotube Anode and Nickel Silicide Integrated High Performance Microsized (1.25 μL) Microbial Fuel Cell. Nano Lett. 2012, 12, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Hu, L.; Pasta, M.; Wells, G.F.; Kong, D.; Criddle, C.S.; Cui, Y. Three-dimensional carbon nanotube-textile anode for high-performance microbial fuel cells. Nano Lett. 2011, 11, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.W.; Sun, X.F.; Huang, Y.X.; Sheng, G.P.; Wang, S.G.; Yu, H.Q. Carbon nanotube/chitosan nanocomposite as a biocompatible biocathode material to enhance the electricity generation of a microbial fuel cell. Energy Environ. Sci. 2011, 4, 1422–1427. [Google Scholar] [CrossRef]
- Chen, Y.; Lv, Z.; Xu, J.; Peng, D.; Liu, Y.; Chen, J.; Sun, X.; Feng, C.; Wei, C. Stainless steel mesh coated with MnO2/carbon nanotube and polymethylphenyl siloxane as low-cost and high-performance microbial fuel cell cathode materials. J. Power Sources 2012, 201, 136–141. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Li, S.; Sun, J.; Hou, B. Manganese dioxide-coated carbon nanotubes as an improved cathodic catalyst for oxygen reduction in a microbial fuel cell. J. Power Sources 2011, 196, 9284–9289. [Google Scholar] [CrossRef]
- Lam, C.W.; James, J.T.; McCluskey, R.; Arepalli, S.; Hunter, R.L. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 2006, 36, 189–217. [Google Scholar] [CrossRef]
- Francis, A.P.; Devasena, T. Toxicity of carbon nanotubes: A review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef]
- Jafar, A.; Alshatti, Y.; Ahmad, A. Carbon nanotube toxicity: The smallest biggest debate in medical care. Cogent Med. 2016, 3. [Google Scholar] [CrossRef]
- Mehra, N.K.; Jain, A.K.; Lodhi, N.; Raj, R.; Dubey, V.; Mishra, D.; Nahar, M.; Jain, N.K. Challenges in the Use of Carbon Nanotubes for Biomedical Applications. Crit. Rev. Ther. Drug Carr. Syst. 2008, 25, 169–206. [Google Scholar] [CrossRef]
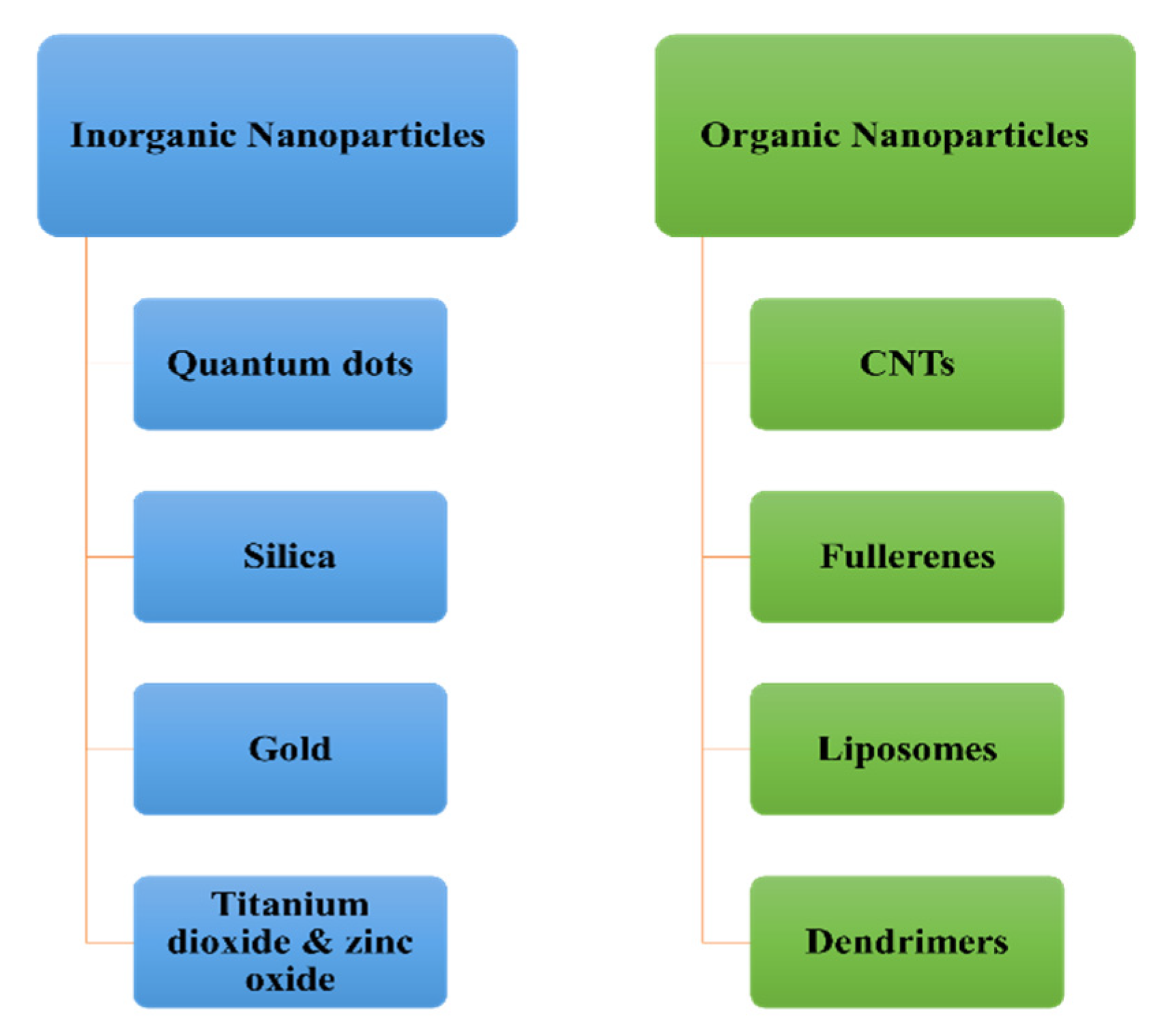


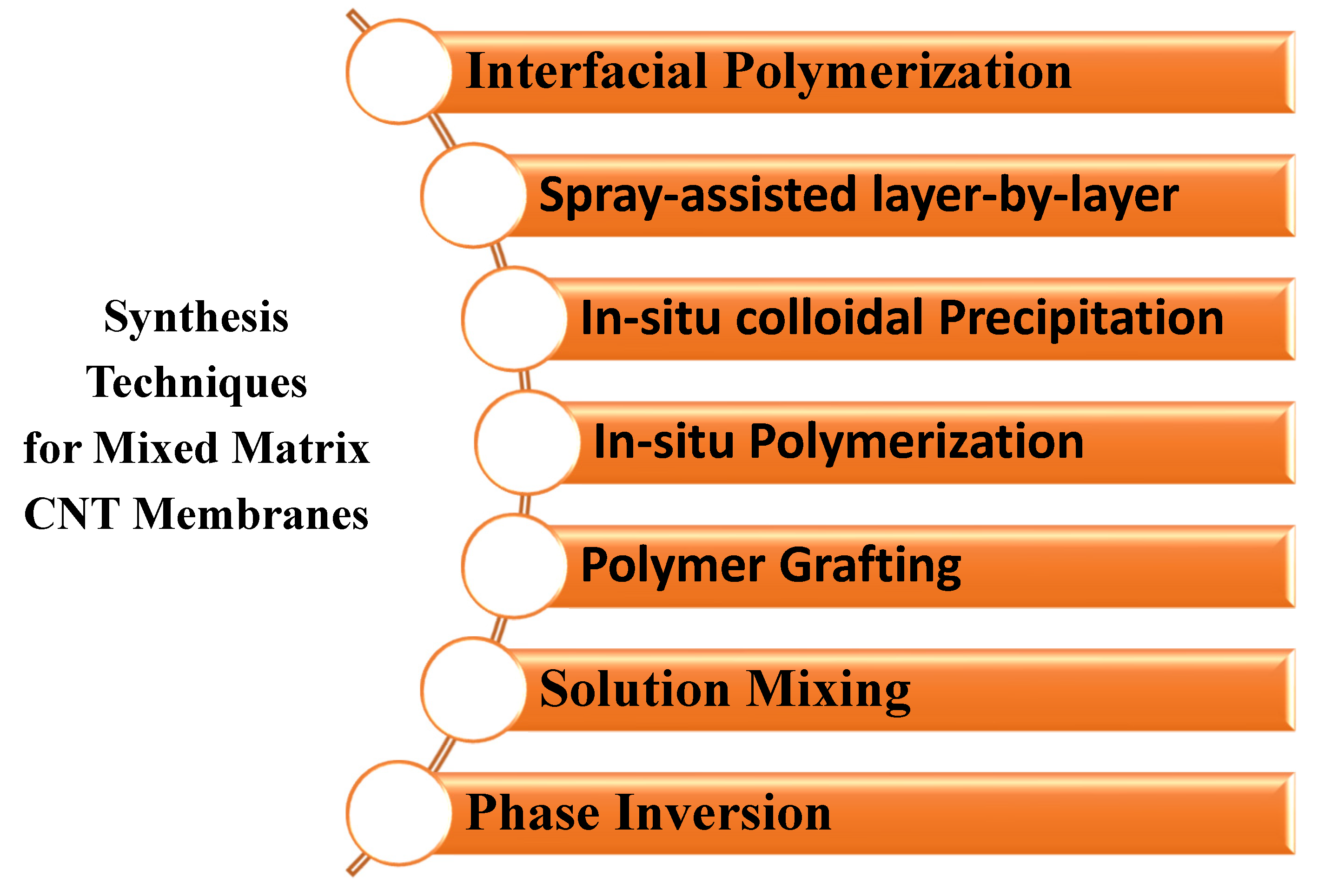
| Vertically Aligned CNT Membrane | Mixed Matrix CNT Membranes |
|---|---|
| Vertical CNT arrangement | Mixed CNT arrangement |
| Compact CNT network | CNT networks loosely fit |
| Water flux rate is high | Water flux rate is moderately fast |
| Complicated fabrication | Simple fabrication |
| Operating system adjustable | Operating system feasible |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arora, B.; Attri, P. Carbon Nanotubes (CNTs): A Potential Nanomaterial for Water Purification. J. Compos. Sci. 2020, 4, 135. https://doi.org/10.3390/jcs4030135
Arora B, Attri P. Carbon Nanotubes (CNTs): A Potential Nanomaterial for Water Purification. Journal of Composites Science. 2020; 4(3):135. https://doi.org/10.3390/jcs4030135
Chicago/Turabian StyleArora, Bharti, and Pankaj Attri. 2020. "Carbon Nanotubes (CNTs): A Potential Nanomaterial for Water Purification" Journal of Composites Science 4, no. 3: 135. https://doi.org/10.3390/jcs4030135
APA StyleArora, B., & Attri, P. (2020). Carbon Nanotubes (CNTs): A Potential Nanomaterial for Water Purification. Journal of Composites Science, 4(3), 135. https://doi.org/10.3390/jcs4030135






