Sustainable Composites from Waste Sulfur, Terpenoids, and Pozzolan Cements
Abstract
1. Introduction
2. Materials and Methods
2.1. Differential Scanning Calorimetry
2.2. Compressional Measurements
2.3. Chemical Precursor Sources
2.4. General Synthesis of Terpenoid–Sulfur Composites
2.5. General Procedure for Addition of Fines to Binders
3. Results and Discussion
3.1. Component Properties and Preparation of Composites
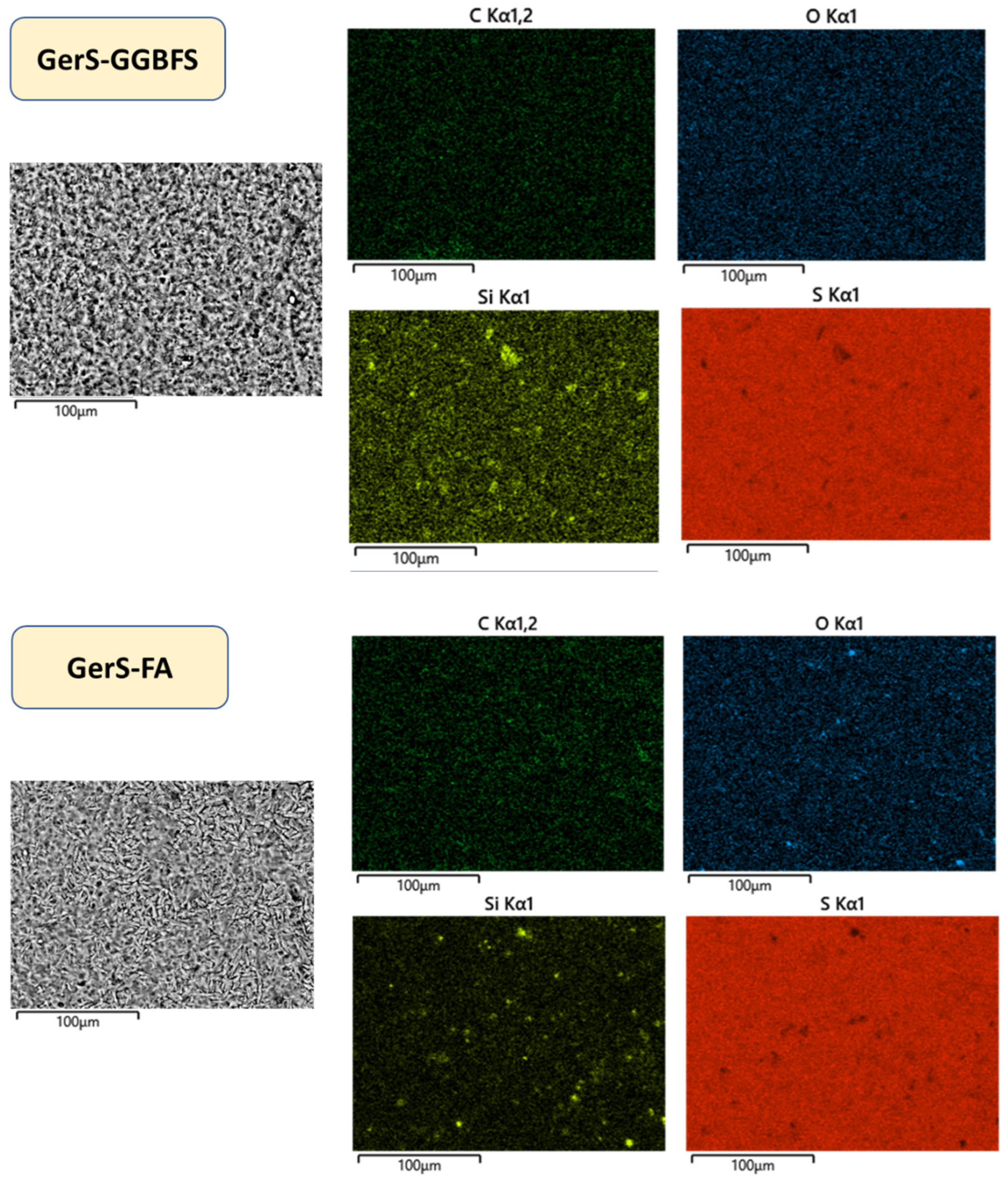
3.2. Physical and Mechanical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Jeong, J.; Choi, J. Adverse outcome pathways potentially related to hazard identification of microplastics based on toxicity mechanisms. Chemosphere 2019, 231, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in marine environment review of methods for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Lauer, M.K.; Smith, R.C. Recent advances in starch-based films towards food packaging applications: Physicochemical, mechanical, and functional properties. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3031–3083. [Google Scholar] [CrossRef] [PubMed]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, R.C.; Tennyson, A.G. Sulfur-Containing Polymers Prepared from Fatty Acid-Derived Monomers: Application of Atom-Economical Thiol-ene/Thiol-yne Click Reactions and Inverse Vulcanization Strategies. Sus. Chem. 2020, 1, 209–237. [Google Scholar] [CrossRef]
- Maladeniya, C.P.; Karunarathna, M.S.; Lauer, M.K.; Lopez, C.V.; Thiounn, T.; Smith, R.C. A Role for Terpenoid Cyclization in the Atom Economical Polymerization of Terpenoids with Sulfur to Yield Durable Composites. Mater. Adv. 2020, 1, 1665–1674. [Google Scholar] [CrossRef]
- Maladeniya, C.P.; Smith, R.C. Influence of Component Ratio on Thermal and Mechanical Properties of Terpenoid-Sulfur Composites. J. Compos. Sci. 2021, 5, 257. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Green Synthesis of Thermoplastic Composites from a Terpenoid-Cellulose Ester. Acs Appl. Polym. Mater. 2020, 2, 3761–3765. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Thermomorphological and mechanical properties of vulcanized octenyl succinate/terpenoid-derivatized corn starch composites. Mater. Adv. 2022, 3, 4186–4193. [Google Scholar] [CrossRef]
- Kristufek, S.L.; Wacker, K.T.; Timothy Tsao, Y.-Y.; Su, L.; Wooley, K.L. Monomer design strategies to create natural product-based polymer materials. Nat. Prod. Rep. 2017, 34, 433–459. [Google Scholar] [CrossRef]
- Bauman, N.; Ajjawi, I. Algal Microorganisms Engineered for Increased Productivity and Biomass. U.S. Patent 10,683,514, 16 June 2017. [Google Scholar]
- Kempinski, C.; Jiang, Z.; Bell, S.; Chappell, J. Metabolic Engineering of Higher Plants and Algae for Isoprenoid Production. Adv. Biochem. Eng./Biotechnol. 2015, 148, 161–199. [Google Scholar] [CrossRef] [PubMed]
- Heaps, N.; Molina, D.; Behnke, C. Terpenes and terpenoids in biofuel production in genetically engineered prokaryotes and eukaryotes. U.S. Patent 2010-US26445, 2010104763, 20100305, 2010. [Google Scholar]
- Arendt, P.; Pollier, J.; Callewaert, N.; Goossens, A. Synthetic biology for production of natural and new-to-nature terpenoids in photosynthetic organisms. Plant J. 2016, 87, 16–37. [Google Scholar] [CrossRef]
- Putignani, L.; Massa, O.; Alisi, A. Engineered Escherichia coli as new source of flavonoids and terpenoids. Food Res. Int. 2013, 54, 1084–1095. [Google Scholar] [CrossRef]
- Sun, C.; Theodoropoulos, C.; Scrutton Nigel, S. Techno-economic assessment of microbial limonene production. Bioresour. Technol. 2020, 300, 122666. [Google Scholar] [CrossRef]
- Wu, W.; Maravelias, C.T. Synthesis and techno-economic assessment of microbial-based processes for terpenes production. Biotechnol. Biofuels 2018, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Wilbon, P.A.; Chu, F.; Tang, C. Progress in Renewable Polymers from Natural Terpenes, Terpenoids, and Rosin. Macromol. Rapid Commun. 2013, 34, 8–37. [Google Scholar] [CrossRef]
- Matsumura, A.; Yang, F.; Goto, H. Synthesis of a Terpene-Based New Chiral Inducer and Preparation of an Asymmetric Polymer. Polymers 2015, 7, 147–155. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Qi, P.; Rostagno, M.; Feteha, A.; Miller, S.A. The quest for high glass transition temperature bioplastics. J. Mater. Chem. A Mater. Energy Sustain. 2018, 6, 9298–9331. [Google Scholar] [CrossRef]
- Della Monica, F.; Kleij, A.W. From terpenes to sustainable and functional polymers. Polym. Chem. 2020, 11, 5109–5127. [Google Scholar] [CrossRef]
- Kamigaito, M.; Satoh, K. Sustainable Vinyl Polymers via Controlled Polymerization of Terpenes. Sustain. Polym. Biomass 2017, 55–90. [Google Scholar] [CrossRef]
- Bruneau, C.; Fischmeister, C. Alkene metathesis for transformations of renewables. Top. Organomet. Chem. 2019, 63, 77–102. [Google Scholar] [CrossRef]
- Song, S.H. Influence of eco-friendly processing aids on silica-based rubber composites. Appl. Sci. 2020, 10, 7244. [Google Scholar] [CrossRef]
- Manoharan, P.; Naskar, K. Eco-friendly composites derived from naturally occurring molecules in promoting dispersion of nanosized silica particulates. Polym. Compos. 2019, 40, 871–883. [Google Scholar] [CrossRef]
- Wu, G.-M.; Kong, Z.-W.; Chen, J.; Huo, S.-P.; Liu, G.-F. Preparation and properties of waterborne polyurethane/epoxy resin composite coating from anionic terpene-based polyol dispersion. Prog. Org. Coat. 2014, 77, 315–321. [Google Scholar] [CrossRef]
- Lopez, C.V.; Smith, A.D.; Smith, R.C. High strength composites from low-value animal coproducts and industrial waste sulfur. Rsc Adv. 2022, 12, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.V.; Karunarathna, M.S.; Lauer, M.K.; Maladeniya, C.P.; Thiounn, T.; Ackley, E.D.; Smith, R.C. High Strength, Acid-Resistant Composites from Canola, Sunflower, or Linseed Oils: Influence of Triglyceride Unsaturation on Material Properties. J. Poly. Sci. 2020, 58, 2259–2266. [Google Scholar] [CrossRef]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A.; et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef]
- Zhang, Y.; Glass, R.S.; Char, K.; Pyun, J. Recent advances in the polymerization of elemental sulphur, inverse vulcanization and methods to obtain functional Chalcogenide Hybrid Inorganic/Organic Polymers (CHIPs). Polym. Chem. 2019, 10, 4078–4105. [Google Scholar] [CrossRef]
- Kleine, T.S.; Glass, R.S.; Lichtenberger, D.L.; MacKay, M.E.; Char, K.; Norwood, R.A.; Pyun, J. 100th Anniversary of Macromolecular Science Viewpoint: High Refractive Index Polymers from Elemental Sulfur for Infrared Thermal Imaging and Optics. Acs Macro Lett. 2020, 9, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Zhao, W.; Zhang, B.; Jiang, L.; Petcher, S.; Smith, J.A.; Parker, D.J.; Cooper, A.I.; Lei, J.; Hasell, T. Inverse vulcanized polymers with shape memory, enhanced mechanical properties, and vitrimer behavior. Angew. Chem. Int. Ed. 2020, 59, 13371–13378. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Kucera, R.L.; Chalker, J.M. Green chemistry and polymers made from sulfur. Green Chem. 2017, 19, 2748–2761. [Google Scholar] [CrossRef]
- Chalker, J.M.; Worthington, M.J.H.; Lundquist, N.A.; Esdaile, L.J. Synthesis and Applications of Polymers Made by Inverse Vulcanization. Top. Curr. Chem. 2019, 377, 125–151. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, R.C.; Tennyson, A.G. Carbon-Negative Polymer Cements by Copolymerization of Waste Sulfur, Oleic Acid, and Pozzolan Cements. Sustain. Chem. Pharm. 2020, 16, 100249. [Google Scholar] [CrossRef]
- Thiounn, T.; Karunarathna, M.S.; Slann, L.M.; Lauer, M.K.; Smith, R.C. Sequential Crosslinking for Mechanical Property Development in High Sulfur Content Composites. J. Polym. Sci. 2020, 58, 2943–2950. [Google Scholar] [CrossRef]
- Lauer, M.K.; Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Robust, remeltable and remarkably simple to prepare biomass-sulfur composites. Mater. Adv. 2020, 1, 2271–2278. [Google Scholar] [CrossRef]
- Lauer, M.K.; Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Recyclable, Sustainable, and Stronger than Portland Cement: A Composite from Unseparated Biomass and Fossil Fuel Waste. Mater. Adv. 2020, 1, 590–594. [Google Scholar] [CrossRef]
- Lauer, M.K.; Estrada-Mendoza, T.A.; McMillen, C.D.; Chumanov, G.; Tennyson, A.G.; Smith, R.C. Durable Cellulose-Sulfur Composites Derived from Agricultural and Petrochemical Waste. Adv. Sustain. Syst. 2019, 3, 1900062. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, H.; Yan, P.; Petcher, S.; Hasell, T. Inverse vulcanization below the melting point of sulfur. Mater. Chem. Front. 2020, 4, 669–675. [Google Scholar] [CrossRef]
- Tonkin, S.J.; Gibson, C.T.; Campbell, J.A.; Lewis, D.A.; Karton, A.; Hasell, T.; Chalker, J.M. Chemically induced repair, adhesion, and recycling of polymers made by inverse vulcanization. Chem. Sci. 2020, 11, 5537–5546. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Smith, J.A.; Petcher, S.; Zhang, B.; Parker, D.J.; Griffin, J.M.; Hasell, T. Catalytic inverse vulcanization. Nat. Commun. 2019, 10, 10035–10044. [Google Scholar] [CrossRef] [PubMed]
- Tikoalu, A.D.; Lundquist, N.A.; Chalker, J.M. Mercury Sorbents Made By Inverse Vulcanization of Sustainable Triglycerides: The Plant Oil Structure Influences the Rate of Mercury Removal from Water. Adv. Sustain. Syst. 2020, 4, 1900111. [Google Scholar] [CrossRef]
- Lundquist, N.A.; Tikoalu, A.D.; Worthington, M.J.H.; Shapter, R.; Tonkin, S.J.; Stojcevski, F.; Mann, M.; Gibson, C.T.; Gascooke, J.R.; Karton, A.; et al. Reactive Compression Molding Post-Inverse Vulcanization: A Method to Assemble, Recycle, and Repurpose Sulfur Polymers and Composites. Chem. A Eur. J. 2020, 26, 10035–10044. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Green, S.J.; Petcher, S.; Parker, D.J.; Zhang, B.; Worthington, M.J.H.; Wu, X.; Kelly, C.A.; Baker, T.; Gibson, C.T.; et al. Crosslinker Copolymerization for Property Control in Inverse Vulcanization. Chem. A Eur. J. 2019, 25, 10433–10440. [Google Scholar] [CrossRef] [PubMed]
- Scheiger, J.M.; Direksilp, C.; Falkenstein, P.; Welle, A.; Koenig, M.; Heissler, S.; Matysik, J.; Levkin, P.A.; Theato, P. Inverse Vulcanization of Styrylethyltrimethoxysilane-Coated Surfaces, Particles, and Crosslinked Materials. Angew. Chem. Int. Ed. 2020, 59, 18639–18645. [Google Scholar] [CrossRef]
- Duarte, M.E.; Huber, B.; Theato, P.; Mutlu, H. The unrevealed potential of elemental sulfur for the synthesis of high sulfur content bio-based aliphatic polyesters. Polym. Chem. 2020, 11, 241–248. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Hoefling, A.; Yee, M.; Nguyen, G.T.H.; Theato, P.; Lee, Y.J.; Song, S.-W. Enabling High-Rate and Safe Lithium Ion-Sulfur Batteries by Effective Combination of Sulfur-Copolymer Cathode and Hard-Carbon Anode. ChemSusChem 2019, 12, 480–486. [Google Scholar] [CrossRef]
- Mutlu, H.; Theato, P.; Ceper Ezgi, B.; Ozmen Mehmet, M.; Li, X.; Yang, J.; Dong, W.; Theato, P.; Yang, J. Sulfur Chemistry in Polymer and Materials Science. Macromol. Rapid Commun. 2019, 40, e1800650. [Google Scholar] [CrossRef]
- Hoefling, A.; Nguyen, D.T.; Partovi-Azar, P.; Sebastiani, D.; Theato, P.; Song, S.-W.; Lee, Y.J. Mechanism for the Stable Performance of Sulfur-Copolymer Cathode in Lithium-Sulfur Battery Studied by Solid-State NMR Spectroscopy. Chem. Mater. 2018, 30, 2915–2923. [Google Scholar] [CrossRef]
- Hoefling, A.; Nguyen, D.T.; Lee, Y.J.; Song, S.-W.; Theato, P. A sulfur-eugenol allyl ether copolymer: A material synthesized via inverse vulcanization from renewable resources and its application in Li-S batteries. Mater. Chem. Front. 2017, 1, 1818–1822. [Google Scholar] [CrossRef]
- Chen, Z.; Droste, J.; Zhai, G.; Zhu, J.; Yang, J.; Hansen, M.R.; Zhuang, X. Sulfur-anchored azulene as a cathode material for Li-S batteries. Chem. Commun. 2019, 55, 9047–9050. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Li, Y.; Feng, W. Recent Advances in Applying Vulcanization/Inverse Vulcanization Methods to Achieve High-Performance Sulfur-Containing Polymer Cathode Materials for Li-S Batteries. Small Methods 2018, 2, 1800156. [Google Scholar] [CrossRef]
- Lopez, C.V.; Maladeniya, C.P.; Smith, R.C. Lithium-Sulfur Batteries: Advances and Trends. Electrochem 2020, 1, 226–259. [Google Scholar] [CrossRef]
- Griebel, J.J.; Namnabat, S.; Kim, E.T.; Himmelhuber, R.; Moronta, D.H.; Chung, W.J.; Simmonds, A.G.; Kim, K.-J.; van der Laan, J.; Nguyen, N.A.; et al. New Infrared Transmitting Material via Inverse Vulcanization of Elemental Sulfur to Prepare High Refractive Index Polymers. Adv. Mater. 2014, 26, 3014–3018. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-K.; Lai, Y.-S.; Liu, Y.-L. Cross-Linkable and Self-Foaming Polysulfide Materials for Repairable and Mercury Capture Applications. Acs Sustain. Chem. Eng. 2019, 7, 4515–4522. [Google Scholar] [CrossRef]
- Abraham, A.M.; Kumar, S.V.; Alhassan, S.M. Porous sulphur copolymer for gas-phase mercury removal and thermal insulation. Chem. Eng. J. 2018, 332, 1–7. [Google Scholar] [CrossRef]
- Parker, D.J.; Jones, H.A.; Petcher, S.; Cervini, L.; Griffin, J.M.; Akhtar, R.; Hasell, T. Low cost and renewable sulfur-polymers by inverse vulcanization, and their potential for mercury capture. J. Mater. Chem. A Mater. Energy Sustain. 2017, 5, 11682–11692. [Google Scholar] [CrossRef]
- Akay, S.; Kayan, B.; Kalderis, D.; Arslan, M.; Yagci, Y.; Kiskan, B. Poly(benzoxazine-co-sulfur): An efficient sorbent for mercury removal from aqueous solution. J. Appl. Polym. Sci. 2017, 134, 45306. [Google Scholar] [CrossRef]
- Hasell, T.; Parker, D.J.; Jones, H.A.; McAllister, T.; Howdle, S.M. Porous inverse vulcanized polymers for mercury capture. Chem. Commun. 2016, 52, 5383–5386. [Google Scholar] [CrossRef]
- Valle, S.F.; Giroto, A.S.; Klaic, R.; Guimaraes, G.G.F.; Ribeiro, C. Sulfur fertilizer based on inverse vulcanization process with soybean oil. Polym. Degrad. Stab. 2019, 162, 102–105. [Google Scholar] [CrossRef]
- Mann, M.; Kruger, J.E.; Andari, F.; McErlean, J.; Gascooke, J.R.; Smith, J.A.; Worthington, M.J.H.; McKinley, C.C.C.; Campbell, J.A.; Lewis, D.A.; et al. Sulfur polymer composites as controlled-release fertilizers. Org. Biomol. Chem. 2019, 17, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Thiounn, T.; Lauer, M.K.; Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Copolymerization of a Bisphenol a Derivative and Elemental Sulfur by the RASP Process. Sus. Chem. 2020, 1, 183–197. [Google Scholar] [CrossRef]
- Thiounn, T.; Tennyson, A.G.; Smith, R.C. Durable, acid-resistant copolymers from industrial by-product sulfur and microbially-produced tyrosine. Rsc Adv. 2019, 9, 31460–31465. [Google Scholar] [CrossRef] [PubMed]
- Thiounn, T.; Lauer, M.K.; Bedford, M.S.; Smith, R.C.; Tennyson, A.G. Thermally-healable network solids of sulfur-crosslinked poly(4-allyloxystyrene). Rsc Adv. 2018, 8, 39074–39082. [Google Scholar] [CrossRef]
- Smith, A.D.; McMillin, C.D.; Smith, R.C.; Tennyson, A.G. Copolymers by Inverse Vulcanization of Sulfur with Pure or Technical Grade Unsaturated Fatty Acids. J. Poly. Sci. 2020, 58, 438–445. [Google Scholar] [CrossRef]
- Smith, A.D.; Thiounn, T.; Lyles, E.W.; Kibler, E.K.; Smith, R.C.; Tennyson, A.G. Combining agriculture and energy industry waste products to yield recyclable, thermally healable copolymers of elemental sulfur and oleic acid. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1704–1710. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Facile new approach to high sulfur-content materials and preparation of sulfur-lignin copolymers. J. Mater. Chem. A Mater. Energy Sustain. 2020, 8, 548–553. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Smith, R.C. Valorization of Lignin as a Sustainable Component of Structural Materials and Composites: Advances from 2011 to 2019. Sustainability 2020, 12, 734–748. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Copolymerization of an aryl halide and elemental sulfur as a route to high sulfur content materials. Polym. Chem. 2020, 11, 1621–1628. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Smith, R.C. Facile route to an organosulfur composite from biomass-derived guaiacol and waste sulfur. J. Mater. Chem. A 2020, 8, 20318–20322. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Thiounn, T.; Smith, R.C.; Tennyson, A.G. Valorization of waste to yield recyclable composites of elemental sulfur and lignin. J. Mater. Chem. A Mater. Energy Sustain. 2019, 7, 15683–15690. [Google Scholar] [CrossRef]
- Vedernikov, A.; Minchenkov, K.; Gusev, S.; Sulimov, A.; Zhou, P.; Li, C.; Xian, G.; Akhatov, I.; Safonov, A. Effects of the Pre-Consolidated Materials Manufacturing Method on the Mechanical Properties of Pultruded Thermoplastic Composites. Polymers 2022, 14, 2246. [Google Scholar] [CrossRef]
- Minchenkov, K.; Vedernikov, A.; Kuzminova, Y.; Gusev, S.; Sulimov, A.; Gulyaev, A.; Kreslavskaya, A.; Prosyanoy, I.; Xian, G.; Akhatov, I.; et al. Effects of the quality of pre-consolidated materials on the mechanical properties and morphology of thermoplastic pultruded flat laminates. Compos. Commun. 2022, 35, 101281. [Google Scholar] [CrossRef]
- Vedernikov, A.; Tucci, F.; Carlone, P.; Gusev, S.; Konev, S.; Firsov, D.; Akhatov, I.; Safonov, A. Effects of pulling speed on structural performance of L-shaped pultruded profiles. Compos. Struct. 2021, 255, 112967. [Google Scholar] [CrossRef]
- Vedernikov, A.; Gemi, L.; Madenci, E.; Onuralp Özkılıç, Y.; Yazman, Ş.; Gusev, S.; Sulimov, A.; Bondareva, J.; Evlashin, S.; Konev, S.; et al. Effects of high pulling speeds on mechanical properties and morphology of pultruded GFRP composite flat laminates. Compos. Struct. 2022, 301, 116216. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Shearer, C.J.; Esdaile, L.J.; Campbell, J.A.; Gibson, C.T.; Legg, S.K.; Yin, Y.; Lundquist, N.A.; Gascooke, J.R.; Albuquerque, I.S.; et al. Sustainable Polysulfides for Oil Spill Remediation: Repurposing Industrial Waste for Environmental Benefit. Adv. Sustain. Syst. 2018, 2, 1800024. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Kucera, R.L.; Albuquerque, I.S.; Gibson, C.T.; Sibley, A.; Slattery, A.D.; Campbell, J.A.; Alboaiji, S.F.K.; Muller, K.A.; Young, J.; et al. Laying Waste to Mercury: Inexpensive Sorbents Made from Sulfur and Recycled Cooking Oils. Chem. A Eur. J. 2017, 23, 16106. [Google Scholar] [CrossRef]
- Orme, K.; Fistrovich, A.H.; Jenkins, C.L. Tailoring Polysulfide Properties through Variations of Inverse Vulcanization. Macromolecules 2020, 53, 9353–9361. [Google Scholar] [CrossRef]
- Herrera, C.; Ysinga, K.J.; Jenkins, C.L. Polysulfides Synthesized from Renewable Garlic Components and Repurposed Sulfur Form Environmentally Friendly Adhesives. Acs Appl. Mater. Interfaces 2019, 11, 35312–35318. [Google Scholar] [CrossRef]
- Westerman, C.R.; Jenkins, C.L. Dynamic Sulfur Bonds Initiate Polymerization of Vinyl and Allyl Ethers at Mild Temperatures. Macromolecules 2018, 51, 7233–7238. [Google Scholar] [CrossRef]
- Lauer, M.K.; Sanders, Z.E.; Smith, A.D.; Smith, R.C. Morphological and mechanical characterization of high-strength sulfur composites prepared with variably-sized lignocellulose particles. Mater. Adv. 2021, 2, 7413–7422. [Google Scholar] [CrossRef]
- Holm, T. Lightweight Concrete and Aggregates; ASTM 169C, Chapter 48; ASTM International: West Conshohocken, PA, USA, 2006. [Google Scholar]
- Akers, D.J.; Gruber, R.D.; Ramme, B.W.; Boyle, M.J.; Grygar, J.G.; Rowe, S.K.; Sheetz, S.R.; Snow, P.G.; Speck, J.F.; Sypher, W.X. Guide for Structural Lightweight Aggregate Concrete; American Concrete Institute: Farmington Hills, MI, USA, 2003. [Google Scholar]
- Moudio, A.M.N.; Tchakouté, H.K.; Ngnintedem, D.L.V.; Andreola, F.; Kamseu, E.; Nanseu-Njiki, C.P.; Leonelli, C.; Rüscher, C.H. Influence of the synthetic calcium aluminate hydrate and the mixture of calcium aluminate and silicate hydrates on the compressive strengths and the microstructure of metakaolin-based geopolymer cements. Mater. Chem. Phys. 2021, 264, 124459. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Inverse vulcanization of octenyl succinate-modified corn starch as a route to biopolymer-sulfur composites. Mater. Adv. 2021, 2, 2391–2397. [Google Scholar] [CrossRef]




| Materials | Tg/°C | Tm/°C | ΔHm [a] J/g | ΔHcc [b] J/g | Percentage Crystallinity [c] |
|---|---|---|---|---|---|
| S8 | NA | 119 | 45 | NA | 100 |
| CitS | NA | 114 | ND | ND | ND |
| GerS | −37 | 116 | 25 | 20 | 23 |
| Materials | Fineness Modulus | Primary Constituents |
|---|---|---|
| Silica Fume | 3.78 | SiO2 |
| Fly Ash | 2.53 | SiO2, CaO, Al2O3, Fe3O2 |
| Ground Granulated Blast-Furnace Slag | 3.35 | 2CaO·SiO2, CaAl2Si2O8 |
| Metakaolin | 4.63 | Al2Si2O7 |
| Compressional Strength [c] | |||||
|---|---|---|---|---|---|
| Materials | Density [a] (kg/m3) | Water Uptake [b] (wt%) | As-Prepared (MPa) | After Acid (MPa) | Retained Strength (% of As-Prepared) |
| CitS | 1800 | 0.1 ± 0.1 | 18.8 ± 2.3 | 15.8 ± 0.5 | 85 |
| CitS-MK | 1800 | 0.2 ± 0.01 | 20.4 ± 2.2 | 19.5 ± 0.4 | 95 |
| CitS-SF | 1800 | 0.7 ± 0.3 | 23.2 ± 3.2 | 18.7 ± 1.3 | 80 |
| CitS-GGBFS | 1800 | 0.2 ± 0.1 | 17.0 ± 0.4 | 16.7 ± 0.6 | 98 |
| CitS-FA | 1800 | 0.2 ± 0.3 | 15.8 ± 2.1 | 17.1 ± 1.2 | 108 |
| GerS | 1800 | 0.2 ± 0.2 | 11.7 ± 1.5 | 9.4 ± 2.1 | 80 |
| GerS-MK | 1800 | 0.1 ± 0.1 | 19.8 ± 2.3 | 23.2 ± 3.6 | 116 |
| GerS-SF | 1900 | 0.2 ± 0.2 | 16.4 ± 0.9 | 14.2 ± 1.3 | 87 |
| GerS-GGBFS | 1900 | 0.2 ± 0.1 | 19.4 ± 1.6 | 18.0 ± 3.1 | 93 |
| GerS-FA | 1900 | 0.2 ± 0.2 | 16.5 ± 0.7 | 16.5 ± 0.7 | 100 |
| OPC [d] | 1500 | Up to 28% | 17 | decomposed | 0 |
| ZOS90 | 1700 | 0.0 | 19.4 ± 1.8 | ND | ND |
| FAOS | 1700 | 0.0 | 20.6 ± 5.7 | ND | ND |
| GGBFSOS | 1700 | 0.0 | 8.50 ± 0.1 | ND | ND |
| MKOS | 1700 | 0.0 | 9.1 ± 1.2 | ND | ND |
| PCOS | 1600 | 0.1 | 22.0 ± 0.1 | ND | ND |
| SFOS | 1600 | 0.0 | 12.4 ± 4.4 | ND | ND |
| GCN0 | ND | ND | 49.50 | ND | ND |
| GCN5 | ND | ND | 57.17 | ND | ND |
| GCN10 | ND | ND | 63.59 | ND | ND |
| GCN15 | ND | ND | 38.79 | ND | ND |
| GCN20 | ND | ND | 35.05 | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tisdale, K.A.; Maladeniya, C.P.; Lopez, C.V.; Tennyson, A.G.; Smith, R.C. Sustainable Composites from Waste Sulfur, Terpenoids, and Pozzolan Cements. J. Compos. Sci. 2023, 7, 35. https://doi.org/10.3390/jcs7010035
Tisdale KA, Maladeniya CP, Lopez CV, Tennyson AG, Smith RC. Sustainable Composites from Waste Sulfur, Terpenoids, and Pozzolan Cements. Journal of Composites Science. 2023; 7(1):35. https://doi.org/10.3390/jcs7010035
Chicago/Turabian StyleTisdale, Katelyn A., Charini P. Maladeniya, Claudia V. Lopez, Andrew G. Tennyson, and Rhett C. Smith. 2023. "Sustainable Composites from Waste Sulfur, Terpenoids, and Pozzolan Cements" Journal of Composites Science 7, no. 1: 35. https://doi.org/10.3390/jcs7010035
APA StyleTisdale, K. A., Maladeniya, C. P., Lopez, C. V., Tennyson, A. G., & Smith, R. C. (2023). Sustainable Composites from Waste Sulfur, Terpenoids, and Pozzolan Cements. Journal of Composites Science, 7(1), 35. https://doi.org/10.3390/jcs7010035









