Influence of Alternating Current Density on the Mechanical Behavior and Microstructure of PEO-Coated 7075 Aluminum Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Procedure
2.3. Coating Characterization
2.4. Corrosion Performance
2.5. Mechanical Properties
3. Results
3.1. Voltage
3.2. Characterization of the Coatings
3.3. Corrosion Behavior
3.4. Tribological Performance
4. Conclusions
- The cell voltage during the PEO coating process slightly increased upon increasing the fixed current in the same silicate electrolyte. Furthermore, the obtained microstructures of the coatings showed increased thickness and roughness, resulting in the formation of a pancake structure on the surface, similar to that of the uncoated substrate. These findings demonstrate that plasma sparks on the substrate. According to SEM images, the thickness of the coating increased as the current density increased. Observation of cross-sectional SEM images of the structures indicated that the inner layers of the coatings became more compact with relatively fine porosity.
- The corrosion resistance of the PEO coatings formed under current densities of 13.5 A/dm2 and 17.8 A/dm2 were significantly improved compared to the bare Al alloy. The most effective anticorrosion PEO coating was that formed under a current density of 17.8 A/dm2.
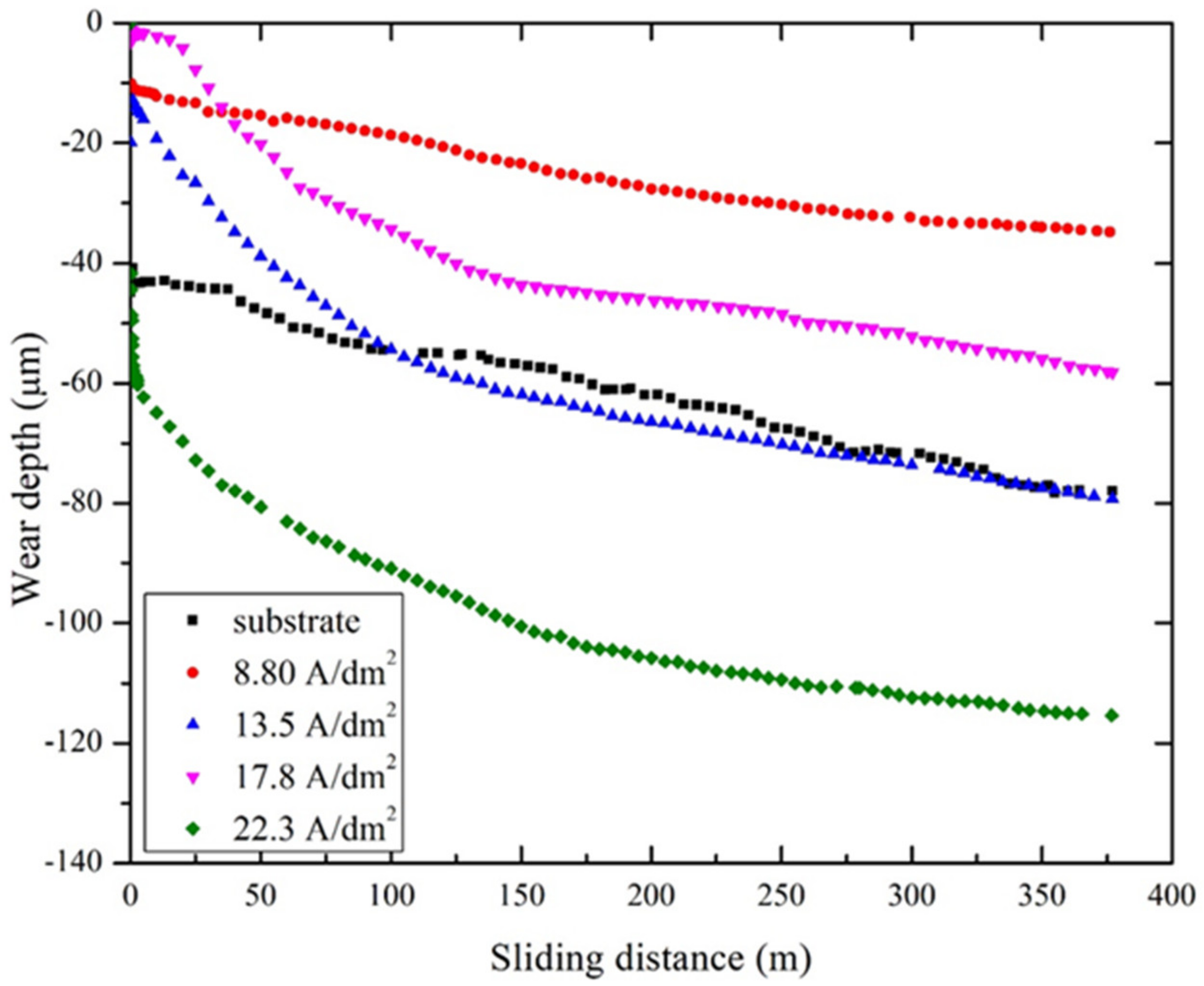
- The wear depths of the PEO coatings formed under current densities of 8.80 A/dm2 and 17.8 A/dm2 were low, which resulted in them exhibiting high wear resistance. However, the wear depth was at a maximum in the coating formed under a current density of 22.3 A/dm2, which was attributed to the highly amorphous nature of the top layer of the coating. These findings indicate that the PEO coatings can effectively protect the Al alloy surface from wear and tear, making them suitable for applications that require high wear resistance.
- The results of the PEO coating process showed that all of the coatings had good hardness values compared to the Al alloy substrate. This indicates that the PEO coating process was successful in enhancing the hardness properties of the 7075 Al alloy, making it more durable and resistant to wear and tear.The high hardness values of the PEO coatings can be attributed to the unique microstructure of the coating, which is composed of fine, homogenous, and well-distributed ceramic particles. These ceramic particles improve the hardness of the coating by increasing its resistance to indentation and wear.
- Among all the PEO coatings, the coating formed under a current density of 17.8 A/dm2 showed the best anti-corrosion and mechanical properties. Overall, this coating is a promising candidate for various industrial applications that require high wear resistance, corrosion resistance, and mechanical properties.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dey, S.; Gunjan, M.K.; Chattoraj, I. Effect of temper on the distribution of pits in AA7075 alloys. Corros. Sci. 2008, 50, 2895–2901. [Google Scholar] [CrossRef]
- Bouchama, L.; Azzouz, N.; Boukmouche, N. Enhancing aluminum corrosion resistance by two-step anodizing process. Surf. Coat. Technol. 2013, 235, 676–684. [Google Scholar] [CrossRef]
- Yang, D.; Od, D.G.; Ramu, A.; Choi, D. Fabrication of enhanced corrosion protection of PEO/PFOTES nanocomposite film coatings on aluminum alloy deposited by plasma electrolytic oxidation. Mater. Lett. 2022, 315, 131898. [Google Scholar] [CrossRef]
- Groot, C.; Peekema, R.M. The corrosion of aluminum and its alloys. In Hanford Atomic Products Operation; US Atomic Energy Commission: Washington, DC, USA, 1955; Volume 36692. [Google Scholar]
- Barati, N.; Yerokhin, A.; Golestanifard, F.; Rastegari, S.; Meletis, E.I. Alumina-zirconia coatings produced by Plasma Electrolytic Oxidation on Al alloy for corrosion resistance improvement. J. Alloys Compd. 2017, 724, 435–442. [Google Scholar] [CrossRef]
- Abreu, C.M.; Cristóbal, M.J.; Figueroa, R.; Pena, G. Wear and corrosion performance of two different tempers (T6 and T73) of AA7075 aluminium alloy after nitrogen implantation. Appl. Surf. Sci. 2015, 327, 51–61. [Google Scholar] [CrossRef]
- Wei, R.P.; Liao, C.; Gao, M. A transmission electron microscopy study of constituent-particle-induced corrosion in 7075-T6 and 2024-T3 aluminum alloys. Metall. Mater. Trans. A 1998, 29, 1153–1160. [Google Scholar] [CrossRef]
- Gencer, Y.; Gulec, A. The effect of Zn on the microarc oxidation coating behavior of synthetic Al–Zn binary alloys. J. Alloys Compd. 2012, 525, 159–165. [Google Scholar] [CrossRef]
- Ghazali, M.J.; Rainforth, W.M.; Jones, H. The wear of wrought aluminium alloys under dry sliding conditions. Tribol. Int. 2007, 40, 160–169. [Google Scholar] [CrossRef]
- Telmenbayar, L.; Ramu, A.G.; Yang, D.; Song, M.; Erdenebat, T.; Choi, D. Corrosion resistance of the anodization/glycidoxypropyltrimethoxysilane composite coating on 6061 aluminum alloy. Surf. Coat. Technol. 2020, 403, 126433. [Google Scholar] [CrossRef]
- Tang, J.; Han, Z.; Zuo, Y.; Tang, Y. A corrosion resistant cerium oxide based coating on aluminum alloy 2024 prepared by brush plating. Appl. Surf. Sci. 2011, 257.7, 2806–2812. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Choi, D. Investigation of ZrO2 nanoparticles concentration and processing time effect on the localized PEO coatings formed on AZ91 alloy. J. Magnes. Alloy. 2019, 7, 555–565. [Google Scholar] [CrossRef]
- Ur Rehman, Z.; Heun Koo, B.; Choi, D. Influence of complex SiF62- Ions on the PEO coatings formed on Mg-Al6-Zn1 alloy for enhanced wear and corrosion protection. Coatings 2020, 10, 94. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Huang, Y.; Ovri, H.; Zheludkevich, M.L.; Kainer, K.U. Plasma electrolytic oxidation coatings on Mg alloy with addition of SiO2 particles. Electrochim. Acta 2016, 187, 20–33. [Google Scholar] [CrossRef]
- Venugopal, A.; Srinath, J.; Krishna, L.R.; Narayanan, P.R.; Sharma, S.C.; Venkitakrishnan, P.V. Corrosion and nanomechanical behaviors of plasma electrolytic oxidation coated AA7020-T6 aluminum alloy. Mater. Sci. Eng. 2016, 13, 39–46. [Google Scholar] [CrossRef]
- Barati, N.; Meletis, E.I.; Fard, F.G.; Yerokhin, A.; Rastegari, S.; Faghihi-Sani, M.A. Al2O3-ZrO2 nanostructured coatings using DC plasma electrolytic oxidation to improve tribological properties of Al substrates. Appl. Surf. Sci. 2015, 30, 927–934. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Gashti, S.O.; Molaie, M. Effects of Disodium Phosphate Concentration (Na 2 HPO 4- 2H 2 O) on Microstructure and Corrosion Resistance of Plasma Electrolytic Oxidation (PEO) Coatings on 2024 Al Alloy. J. Mater. Eng. Perform. 2018, 27, 825–834. [Google Scholar] [CrossRef]
- Aliramezani, R.; Raeissi, K.; Santamaria, M.; Hakimizad, A. Characterization and properties of PEO coatings on 7075 Al alloy grown in alkaline silicate electrolyte containing KMnO4 additive. Surf. Coat. Technol. 2017, 329, 250–261. [Google Scholar] [CrossRef]
- Arrabal, R.; Mohedano, M.; Matykina, E.; Pardo, A.; Mingo, B.; Merino, M.C. Characterization and wear behaviour of PEO coatings on 6082-T6 aluminium alloy with incorporated α-Al2O3 particles. Surf. Coat. Technol. 2015, 269, 64–73. [Google Scholar] [CrossRef]
- Nie, X.; Meletis, E.I.; Jiang, J.C.; Leyland, A.; Yerokhin, A.L.; Matthews, A. Abrasive wear/corrosion properties and TEM analysis of Al2O3 coatings fabricated using plasma electrolysis. Surf. Coat. Technol. 2002, 149, 245–251. [Google Scholar] [CrossRef]
- Wei, T.; Yan, F.; Tian, J. Characterization and wear-and corrosion-resistance of microarc oxidation ceramic coatings on aluminum alloy. J. Alloys Compd. 2005, 389, 169–176. [Google Scholar] [CrossRef]
- Krishna, L.R.; Purnima, A.S.; Sundararajan, G. A comparative study of tribological behavior of microarc oxidation and hard-anodized coatings. Wear 2006, 261, 1095–1101. [Google Scholar] [CrossRef]
- Lampke, T.; Meyer, D.; Alisch, G.; Nickel, D.; Scharf, I. Alumina coatings obtained by thermal spraying and plasma anodizing–A comparison. Surf. Coat. Technol. 2011, 206, 2012–2016. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Snizhko, L.O.; Gurevina, N.L.; Leyland, A.; Pilkington, A.; Matthews, A. Spatial characteristics of discharge phenomena in plasma electrolytic oxidation of aluminium alloy. Surf. Coat. Technol. 2004, 177, 779–783. [Google Scholar] [CrossRef]
- Parfenov, E.V.; Yerokhin, A.L.; Matthews, A. Frequency response studies for the plasma electrolytic oxidation process. Surf. Coat. Technol. 2007, 201, 8661–8670. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E. Investigation of the growth processes of coatings formed by AC plasma electrolytic oxidation of aluminium. Electrochim. Acta 2009, 54, 6767–6778. [Google Scholar] [CrossRef]
- Wang, D.D.; Liu, X.T.; Wu, Y.K.; Han, H.P.; Yang, Z.; Su, Y.; Shen, D.J. Evolution process of the plasma electrolytic oxidation (PEO) coating formed on aluminum in an alkaline sodium hexametaphosphate ((NaPO3) 6) electrolyte. J. Alloys Compd. 2019, 798, 129–143. [Google Scholar] [CrossRef]
- Guo-Hua, L.; Wei-Chao, G.; Huan, C.; Li, L.; Er-Wu, N.; Si-Ze, Y. Microstructure and corrosion performance of oxide coatings on aluminium by plasma electrolytic oxidation in silicate and phosphate electrolytes. Chin. Phys. Lett. 2006, 23, 3331. [Google Scholar] [CrossRef]
- G99-05 (Reapproved); Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus. ASTM International: West Conshohocken, PA, USA, 2010.
- Sah, S.P.; Tsuji, E.; Aoki, Y.; Habazaki, H. Cathodic pulse breakdown of anodic films on aluminium in alkaline silicate electrolyte-understanding the role of cathodic half-cycle in AC plasma electrolytic oxidation. Corros. Sci. 2012, 55, 90–96. [Google Scholar] [CrossRef]
- Sundararajan, G.; Krishna, L.R. Mechanisms underlying the formation of thick alumina coatings through the MAO coating technology. Surf. Coat. Technol. 2003, 167, 269–277. [Google Scholar] [CrossRef]
- Li, Q.B.; Liu, C.C.; Yang, W.B.; Liang, J. Growth mechanism and adhesion of PEO coatings on 2024Al alloy. Surf. Eng. 2017, 33, 760–766. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. J. Phys. D Appl. Phys. 2010, 43, 10. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdenebat, T.O.; Telmenbayar, L.; Yang, D.; Song, M.; Ramu, A.G.; Choi, D. Influence of Alternating Current Density on the Mechanical Behavior and Microstructure of PEO-Coated 7075 Aluminum Alloy. J. Compos. Sci. 2023, 7, 50. https://doi.org/10.3390/jcs7020050
Erdenebat TO, Telmenbayar L, Yang D, Song M, Ramu AG, Choi D. Influence of Alternating Current Density on the Mechanical Behavior and Microstructure of PEO-Coated 7075 Aluminum Alloy. Journal of Composites Science. 2023; 7(2):50. https://doi.org/10.3390/jcs7020050
Chicago/Turabian StyleErdenebat, Tumur Ochir, Lkhagvaa Telmenbayar, Daejeong Yang, Minjung Song, Adam Gopal Ramu, and Dongjin Choi. 2023. "Influence of Alternating Current Density on the Mechanical Behavior and Microstructure of PEO-Coated 7075 Aluminum Alloy" Journal of Composites Science 7, no. 2: 50. https://doi.org/10.3390/jcs7020050
APA StyleErdenebat, T. O., Telmenbayar, L., Yang, D., Song, M., Ramu, A. G., & Choi, D. (2023). Influence of Alternating Current Density on the Mechanical Behavior and Microstructure of PEO-Coated 7075 Aluminum Alloy. Journal of Composites Science, 7(2), 50. https://doi.org/10.3390/jcs7020050





