Investigation of the Effect of Preparation Parameters on the Structural and Mechanical Properties of Gelatin/Elastin/Sodium Hyaluronate Scaffolds Fabricated by the Combined Foaming and Freeze-Drying Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparation
2.3. Characterization
2.4. Statistical Analysis
3. Results and Discussion
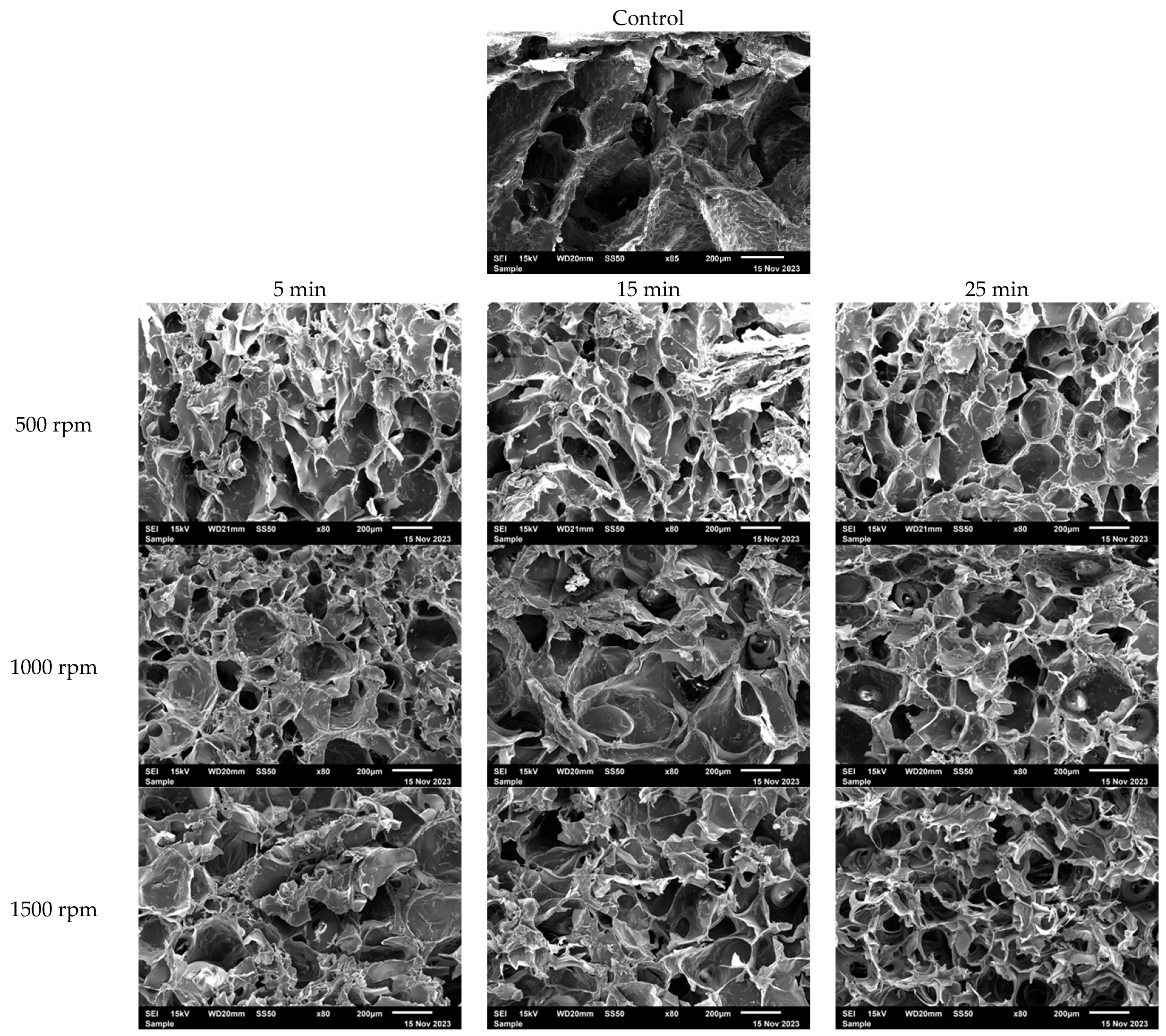
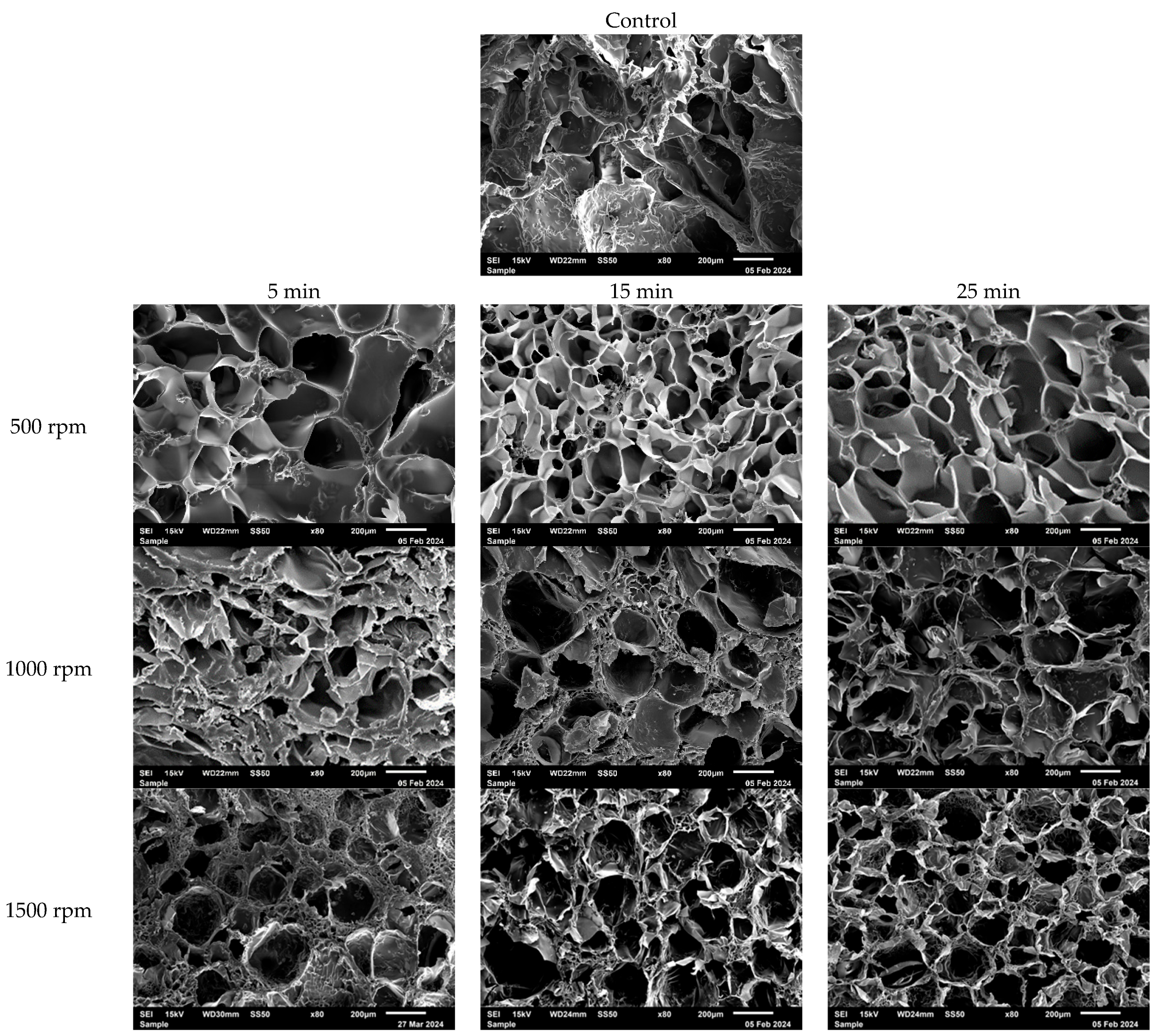
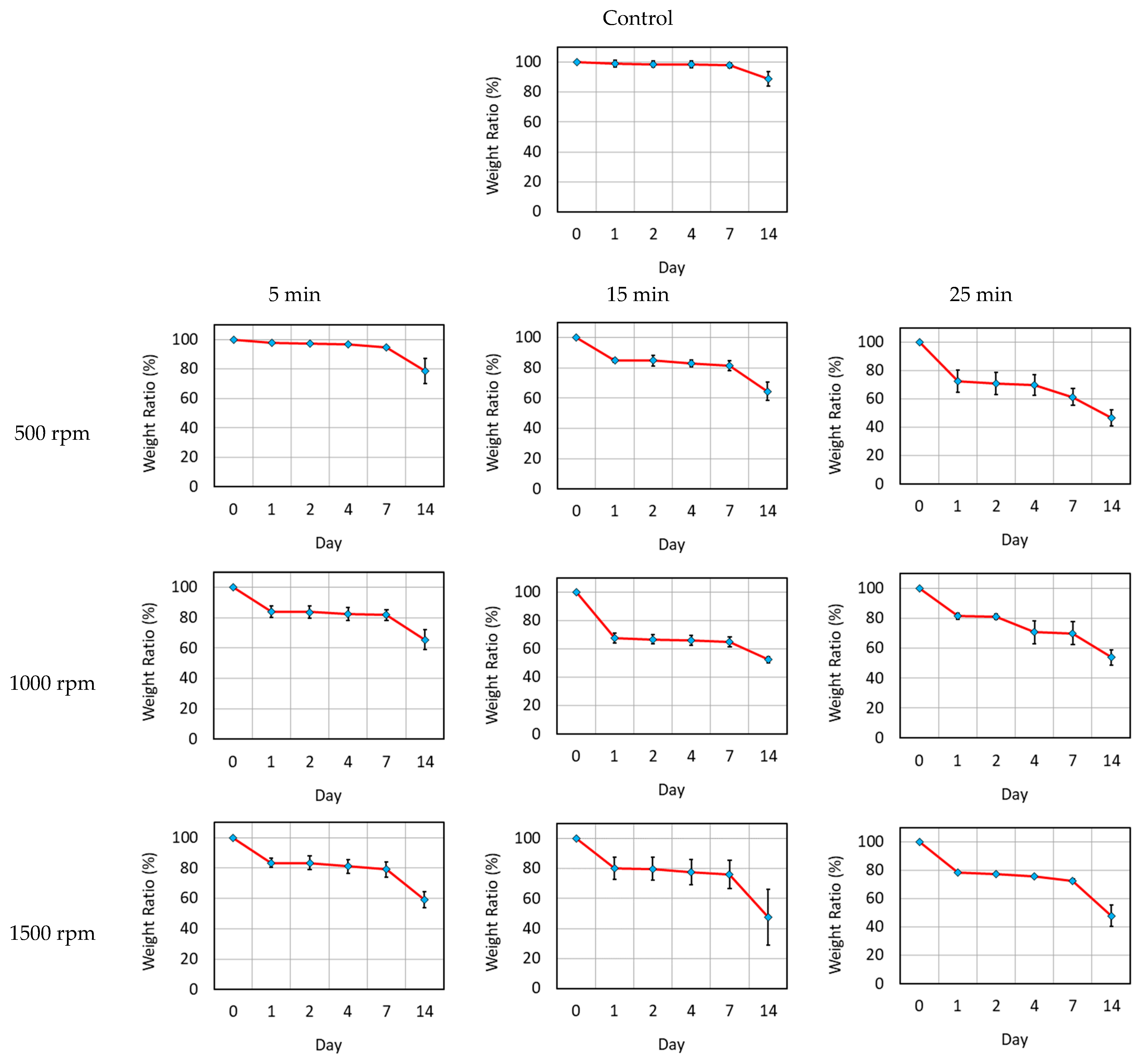
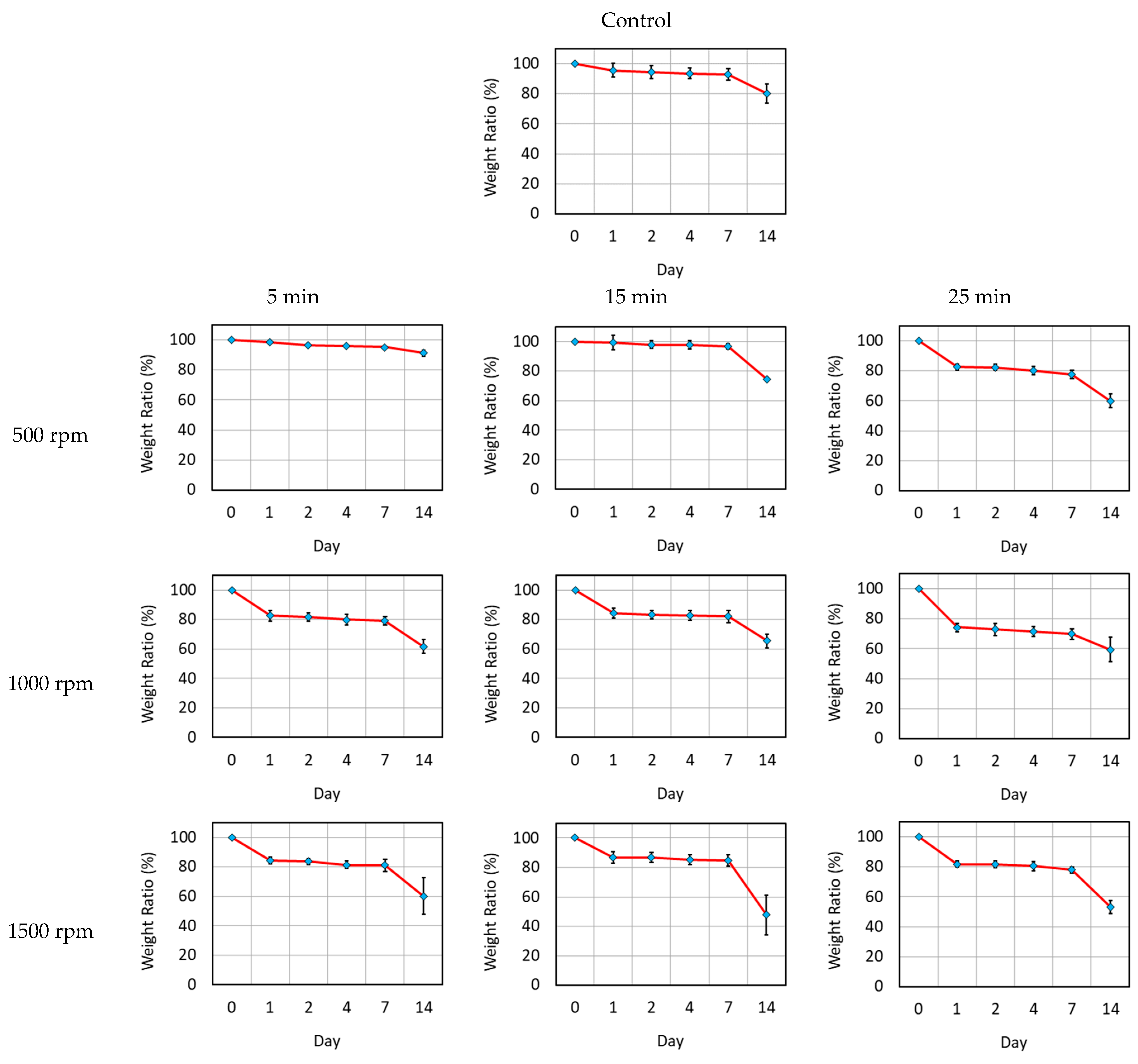
3.1. Characterization
3.2. Mathematical Estimators for Scaffolds’ Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| No. | Group Name | Agitation Speed (rpm) | Agitation Duration Time (min) | Chilling Temperature (°C) |
|---|---|---|---|---|
| 1 | F0 (Control) | 0 | 0 | −20 |
| 2 | F1 | 500 | 5 | −20 |
| 3 | F2 | 1000 | 5 | −20 |
| 4 | F3 | 1500 | 5 | −20 |
| 5 | F4 | 500 | 15 | −20 |
| 6 | F5 | 1000 | 15 | −20 |
| 7 | F6 | 1500 | 15 | −20 |
| 8 | F7 | 500 | 25 | −20 |
| 9 | F8 | 1000 | 25 | −20 |
| 10 | F9 | 1500 | 25 | −20 |
| 11 | F10 | 500 | 5 | −80 |
| 12 | F11 | 1000 | 5 | −80 |
| 13 | F12 | 1500 | 5 | −80 |
| 14 | F13 | 500 | 15 | −80 |
| 15 | F14 | 1000 | 15 | −80 |
| 16 | F15 | 1500 | 15 | −80 |
| 17 | F16 | 500 | 25 | −80 |
| 18 | F17 | 1000 | 25 | −80 |
| 19 | F18 | 1500 | 25 | −80 |
| 20 | F19 (Control) | 0 | 0 | −80 |
References
- Lu, H.; Hoshiba, T.; Kawazoe, N.; Koda, I.; Song, M.; Chen, G. Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials 2011, 32, 9658–9666. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Lu, Y.; Li, Q.; Zhang, X. Decellularized extracellular matrices for tissue engineering and regeneration. In Biomimicked Biomaterials: Advances in Tissue Engineering and Regenerative Medicine; Springer: Singapore, 2020; pp. 15–31. [Google Scholar]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Causa, F.; Netti, P.A.; Ambrosio, L. A multi-functional scaffold for tissue regeneration: The need to engineer a tissue analogue. Biomaterials 2007, 28, 5093–5099. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Clark, A.; Milbrandt, T.A.; Hilt, J.Z.; Puleo, D.A. Mechanical properties and dual drug delivery application of poly(lactic-co-glycolic acid) scaffolds fabricated with a poly(β-amino ester) porogen. Acta Biomater. 2014, 10, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Ding, J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.; Tamura, H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2009, 28, 142–150. [Google Scholar] [CrossRef]
- Osanloo, M.; Noori, F.; Varaa, N.; Tavassoli, A.; Goodarzi, A.; Moghaddam, M.T.; Ebrahimi, L.; Abpeikar, Z.; Farmani, A.R.; Safaei, M.; et al. The wound healing effect of polycaprolactone-chitosan scaffold coated with a gel containing Zataria multiflora Boiss. volatile oil nanoemulsions. BMC Complement. Med. Ther. 2024, 24, 56. [Google Scholar] [CrossRef] [PubMed]
- Pahlevanzadeh, F.; Emadi, R.; Kharaziha, M.; Poursamar, S.; Nejatidanesh, F.; Emadi, H.; Aslani, R.; Moroni, L.; Setayeshmehr, M. Amorphous magnesium phosphate-graphene oxide nano particles laden 3D-printed chitosan scaffolds with enhanced osteogenic potential and antibacterial properties. Biomater. Adv. 2024, 158, 213760. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, N.; Frampton, J.P.; Zaree, S.R.A.; Jahanpanah, M.; Heydari, P.; Zamanian, A. The corrosion and biological behavior of 3D-printed polycaprolactone/chitosan scaffolds as protective coating for Mg alloy implants. Surf. Coatings Technol. 2024, 477, 130368. [Google Scholar] [CrossRef]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolym. Orig. Res. Biomol. 2008, 89, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Dewey, M.J.; Collins, A.J.; Tiffany, A.; Barnhouse, V.R.; Lu, C.; Kolliopoulos, V.; Mutreja, I.; Hickok, N.J.; Harley, B.A. Evaluation of bacterial attachment on mineralized collagen scaffolds and addition of manuka honey to increase mesenchymal stem cell osteogenesis. Biomaterials 2023, 294, 122015. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Nguyen, R.Y.; Pizzurro, G.A.; Zhang, X.; Gong, X.; Martinez, A.R.; Mak, M. Self-assembly of mesoscale collagen architectures and applications in 3D cell migration. Acta Biomater. 2023, 155, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Kolliopoulos, V.; Harley, B.A. Mineralized collagen scaffolds for regenerative engineering applications. Curr. Opin. Biotechnol. 2024, 86, 103080. [Google Scholar] [CrossRef]
- Megha, K.B.; Syama, S.; Sangeetha, V.P.; Vandana, U.; Oyane, A.; Mohanan, P.V. Development of a 3D multifunctional collagen scaffold impregnated with peptide LL-37 for vascularised bone tissue regeneration. Int. J. Pharm. 2024, 652, 123797. [Google Scholar] [CrossRef] [PubMed]
- Echave, M.C.; Burgo, L.S.; Pedraz, J.L.; Orive, G. Gelatin as biomaterial for tissue engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef] [PubMed]
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Progress in gelatin as biomaterial for tissue engineering. Pharmaceutics 2022, 14, 1177. [Google Scholar] [CrossRef] [PubMed]
- Daamen, W.F.; Veerkamp, J.H.; Van Hest, J.C.M.; Van Kuppevelt, T.H. Elastin as a biomaterial for tissue engineering. Biomaterials 2007, 28, 4378–4398. [Google Scholar] [CrossRef] [PubMed]
- Audelo, M.L.D.P.; Mendoza-Muñoz, N.; Escutia-Guadarrama, L.; Giraldo-Gomez, D.; González-Torres, M.; Florán, B.; Cortes, H.; Leyva-Gómez, G. Recent advances in elastin-based biomaterial. J. Pharm. Pharm. Sci. 2020, 23, 314–332. [Google Scholar] [CrossRef] [PubMed]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Guo, Z.-X.; Zhang, Z.; Yan, J.-F.; Xu, H.-Q.; Wang, S.-Y.; Ye, T.; Han, X.-X.; Wang, W.-R.; Wang, Y.; Gao, J.-L.; et al. A biomaterial-based therapy using a sodium hyaluronate/bioglass composite hydrogel for the treatment of oral submucous fibrosis. Acta Biomater. 2023, 157, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, L.; Cui, Z.; Ye, H. A tri-component knee plug for the 3rd generation of autologous chondrocyte implantation. Sci. Rep. 2020, 10, 17048. [Google Scholar] [CrossRef] [PubMed]
- Vahdatinia, F.; Hooshyarfard, A.; Jamshidi, S.; Shojaei, S.; Patel, K.; Moeinifard, E.; Haddadi, R.; Farhadian, M.; Gholami, L.; Tayebi, L. 3D-printed soft membrane for periodontal guided tissue regeneration. Materials 2023, 16, 1364. [Google Scholar] [CrossRef]
- Tayebi, L.; Rasoulianboroujeni, M.; Moharamzadeh, K.; Almela, T.K.D.; Cui, Z.; Ye, H. 3D-printed membrane for guided tissue regeneration. Mater. Sci. Eng. C 2018, 84, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Rasoulianboroujeni, M.; Ghasemi, H.; Keshel, S.H.; Nozarian, Z.; Hashemian, M.N.; Zarei-Ghanavati, M.; Latifi, G.; Ghaffari, R.; Cui, Z.; et al. 3D-Printed membrane as an alternative to amniotic membrane for ocular surface/conjunctival defect reconstruction: An in vitro & in vivo study. Biomaterials 2018, 174, 95–112. [Google Scholar] [PubMed]
- Shokri, A.; Ramezani, K.; Jamalpour, M.R.; Mohammadi, C.; Vahdatinia, F.; Irani, A.D.; Sharifi, E.; Haddadi, R.; Jamshidi, S.; Amirabad, L.M. In vivo efficacy of 3D-printed elastin–gelatin–hyaluronic acid scaffolds for regeneration of nasal septal cartilage defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 614–624. [Google Scholar] [CrossRef]
- Keleshteri, A.R.; Moztarzadeh, F.; Farokhi, M.; Mehrizi, A.A.; Basiri, H.; Mohseni, S.S. Preparation of microfluidic-based pectin microparticles loaded carbon dots conjugated with BMP-2 embedded in gelatin-elastin-hyaluronic acid hydrogel scaffold for bone tissue engineering application. Int. J. Biol. Macromol. 2021, 184, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Namini, M.S.; Bayat, N.; Tajerian, R.; Ebrahimi-Barough, S.; Azami, M.; Irani, S.; Jangjoo, S.; Shirian, S.; Ai, J. A comparison study on the behavior of human endometrial stem cell-derived osteoblast cells on PLGA/HA nanocomposite scaffolds fabricated by electrospinning and freeze-drying methods. J. Orthop. Surg. Res. 2018, 13, 63. [Google Scholar] [CrossRef]
- Thomas, A.; Agarwal, A.K.; Kashyap, Y.S.; Kumar, I.P.; Bera, J. Quantifying pore characteristics in polymer glass–ceramics composite scaffolds using micro-tomography. J. Mater. Res. 2024, 39, 1258–1272. [Google Scholar] [CrossRef]
- Diaz, F.; Zimmermann, L.; Dale, T.P.; Forsyth, N.R.; Boccaccini, A.R. Tuning the properties of all natural polymeric scaffolds for tendon repair with cellulose microfibers. Carbohydr. Polym. Technol. Appl. 2024, 7, 100447. [Google Scholar] [CrossRef]
- Hajiabbas, M.; Alemzadeh, I.; Vossoughi, M. A porous hydrogel-electrospun composite scaffold made of oxidized alginate/gelatin/silk fibroin for tissue engineering application. Carbohydr. Polym. 2020, 245, 116465. [Google Scholar] [CrossRef]
- Kalluri, L.; Duan, Y.; Janorkar, A.V. Electrospun polymeric nanofibers for dental applications. J. Appl. Polym. Sci. 2024, 141, e55224. [Google Scholar] [CrossRef]
- Shadmehri, F.D.; Karimi, E.; Saburi, E. Electrospun PCL/fibrin scaffold as a bone implant improved the differentiation of human adipose-derived mesenchymal stem cells into osteo-like cells. Int. J. Polym. Mater. Polym. Biomater. 2022, 73, 71–78. [Google Scholar] [CrossRef]
- Zhu, P.; Yu, Z.; Sun, H.; Zheng, D.; Zheng, Y.; Qian, Y.; Wei, Y.; Lee, J.; Srebnik, S.; Chen, W.; et al. 3D Printed Cellulose Nanofiber Aerogel Scaffold with Hierarchical Porous Structures for Fast Solar-Driven Atmospheric Water Harvesting. Adv. Mater. 2023, 36, e2306653. [Google Scholar] [CrossRef]
- Law, A.C.C.; Wang, R.; Chung, J.; Kucukdeger, E.; Liu, Y.; Barron, T.; Johnson, B.N.; Kong, Z. Process parameter optimization for reproducible fabrication of layer porosity quality of 3D-printed tissue scaffold. J. Intell. Manuf. 2023, 35, 1825–1844. [Google Scholar] [CrossRef]
- Hanif, M.; Zhang, L.; Shah, A.H.; Chen, Z. Mechanical Analysis and Biodegradation of Oxides-Based Magneto-Responsive Shape Memory Polymers for Material Extrusion 3D Printing of Biomedical Scaffolds. Addit. Manuf. 2024, 86, 104174. [Google Scholar] [CrossRef]
- Mattavelli, D.; Verzeletti, V.; Deganello, A.; Fiorentino, A.; Gualtieri, T.; Ferrari, M.; Taboni, S.; Anfuso, W.; Ravanelli, M.; Rampinelli, V.; et al. Computer-aided designed 3D-printed polymeric scaffolds for personalized reconstruction of maxillary and mandibular defects: A proof-of-concept study. Eur. Arch. Oto-Rhino-Laryngol. 2024, 281, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Farrag, A.; Jin, Y.; Zhou, Y. Sodium alginate hydrogel scaffolds with internal channels using 3D-printed polyvinyl alcohol (PVA) sacrificial molds. J. Mater. Sci. 2024, 59, 1593–1607. [Google Scholar] [CrossRef]
- Seyedmahmoud, R.; Rainer, A.; Mozetic, P.; Giannitelli, S.M.; Trombetta, M.; Traversa, E.; Licoccia, S.; Rinaldi, A. A primer of statistical methods for correlating parameters and properties of electrospun poly(l-lactide) scaffolds for tissue engineering-PART 1: Design of experiments. J. Biomed. Mater. Res. Part A 2014, 103, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Szentivanyi, A.; Chakradeo, T.; Zernetsch, H.; Glasmacher, B. Electrospun cellular microenvironments: Understanding controlled release and scaffold structure. Adv. Drug Deliv. Rev. 2010, 63, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Virijević, K.; Grujić, J.; Kokanović, M.; Nikolić, D.; Živanović, M.; Filipović, N. Electrospinning and Electrospun Nanofibrous Materials–Promising Scaffolds in Tissue Engineering. In Proceedings of the International Conference on Medical and Biological Engineering, Mostar, Bosnia and Herzegovina, 21–24 April 2021; Springer: Cham, Switzerland, 2021; pp. 726–733. [Google Scholar]
- Walker, J.M.; Bodamer, E.; Kleinfehn, A.; Luo, Y.; Becker, M.; Dean, D. Design and mechanical characterization of solid and highly porous 3D printed poly(propylene fumarate) scaffolds. Prog. Addit. Manuf. 2017, 2, 99–108. [Google Scholar] [CrossRef]
- Sivashankari, P.R.; Prabaharan, M. Three-dimensional porous scaffolds based on agarose/chitosan/graphene oxide composite for tissue engineering. Int. J. Biol. Macromol. 2020, 146, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Barui, S.; Chatterjee, S.; Mandal, S.; Kumar, A.; Basu, B. Microstructure and compression properties of 3D powder printed Ti-6Al-4V scaffolds with designed porosity: Experimental and computational analysis. Mater. Sci. Eng. C 2017, 70, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Rasoulianboroujeni, M.; Kiaie, N.; Tabatabaei, F.S.; Yadegari, A.; Fahimipour, F.; Khoshroo, K.; Tayebi, L. Dual porosity protein-based scaffolds with enhanced cell infiltration and proliferation. Sci. Rep. 2018, 8, 14889. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y.; Li, X.; Wen, P.; Zhang, Y.; Long, Y.; Wang, X.; Guo, Y.; Xing, F.; Gao, J. Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomater. 2009, 6, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Hajiabbas, M.; Alemzadeh, I.; Vossoughi, M.; Shamloo, A. In-situ crosslinking of electrospun gelatin-carbodiimide nanofibers: Fabrication, characterization, and modeling of solution parameters. Chem. Eng. Commun. 2020, 208, 976–992. [Google Scholar] [CrossRef]
- Zahra, G.; Gymama, S. Storage stability of electrospun pure gelatin stabilized with EDC/Sulfo-NHS. Biopolymers 2018, 109, e23232. [Google Scholar]
- Mohanta, K.; Kumar, A.; Parkash, O.; Kumar, D. Processing and properties of low cost macroporous alumina ceramics with tailored porosity and pore size fabricated using rice husk and sucrose. J. Eur. Ceram. Soc. 2014, 34, 2401–2412. [Google Scholar] [CrossRef]
- Hu, L.; Benitez, R.; Basu, S.; Karaman, I.; Radovic, M. Processing and characterization of porous Ti2AlC with controlled porosity and pore size. Acta Mater. 2012, 60, 6266–6277. [Google Scholar] [CrossRef]
- Hashemi, M.; Yadegari, A.; Yazdanpanah, G.; Omidi, M.; Jabbehdari, S.; Haghiralsadat, F.; Yazdian, F.; Tayebi, L. Normalization of doxorubicin release from graphene oxide: New approach for optimization of effective parameters on drug loading. Biotechnol. Appl. Biochem. 2016, 64, 433–442. [Google Scholar] [CrossRef]
- Perez, R.A.; Mestres, G. Role of pore size and morphology in musculo-skeletal tissue regeneration. Mater. Sci. Eng. C 2016, 61, 922–939. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kang, S.G.; Kim, E.S.; Cho, S.H.; Lee, J.H. Fabrication and characterization of hydrophilic poly (lactic-co-glycolic acid)/poly (vinyl alcohol) blend cell scaffolds by melt-molding particulate-leaching method. Biomaterials 2003, 24, 4011–4021. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.K.; Stevens, R.; Pearson, R.G.; Davies, M.C.; Tendler, S.J.B.; Roberts, C.J.; Williams, P.M.; Shakesheff, K.M. Interactions of 3T3 fibroblasts and endothelial cells with defined pore features. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2002, 61, 212–217. [Google Scholar] [CrossRef]
- Whang, K.; Healy, K.; Elenz, D.; Nam, E.; Tsai, D.; Thomas, C.; Nuber, G.; Glorieux, F.; Travers, R.; Sprague, S. Engineering bone regeneration with bioabsorbable scaffolds with novel microarchitecture. Tissue Eng. 1999, 5, 35–51. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Wu, L.; Ding, J. Effects of porosity and pore size on in vitro degradation of three-dimensional porous poly (D,L-lactide-co-glycolide) scaffolds for tissue engineering. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2005, 75, 767–777. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Y.; Zhang, Y.; Ye, Z.; Tan, W.; Lang, M. Effect of porosity on long-term degradation of poly (ε-caprolactone) scaffolds and their cellular response. Polym. Degrad. Stab. 2012, 98, 209–218. [Google Scholar] [CrossRef]





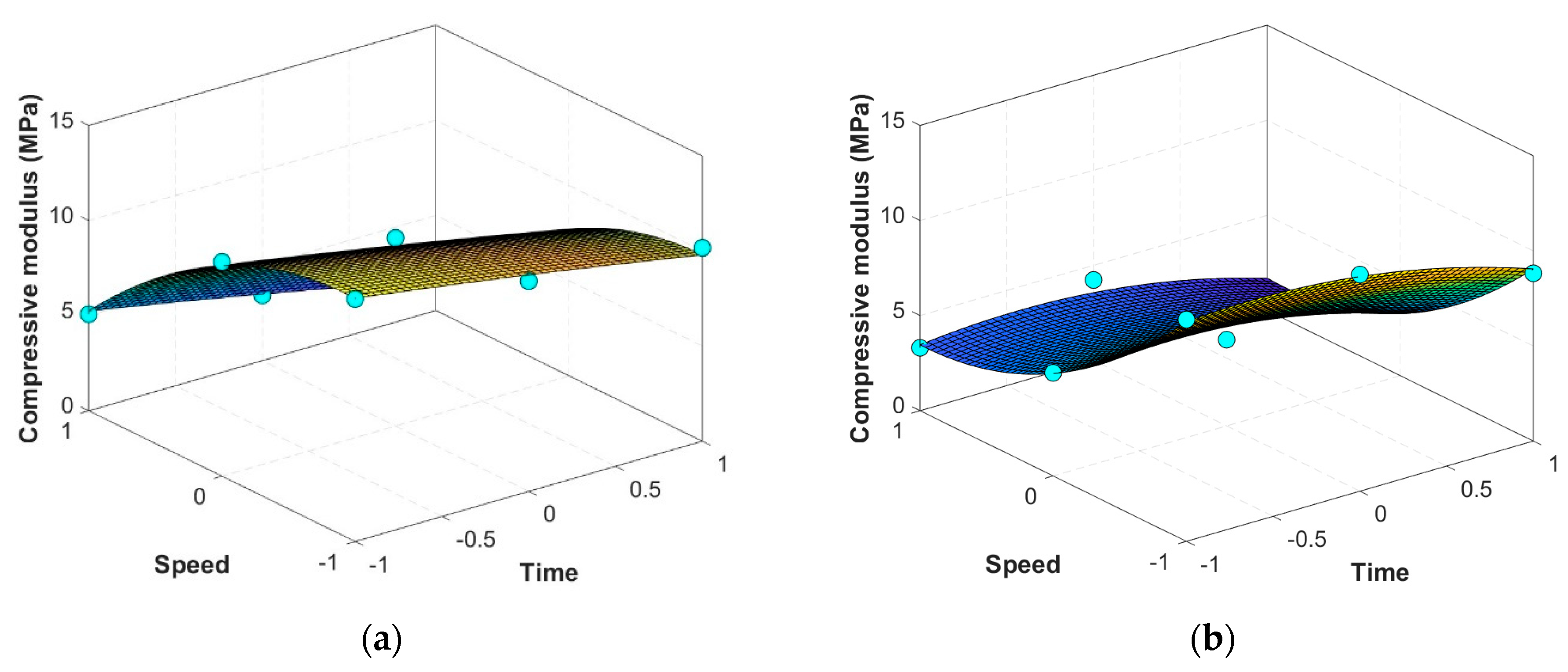
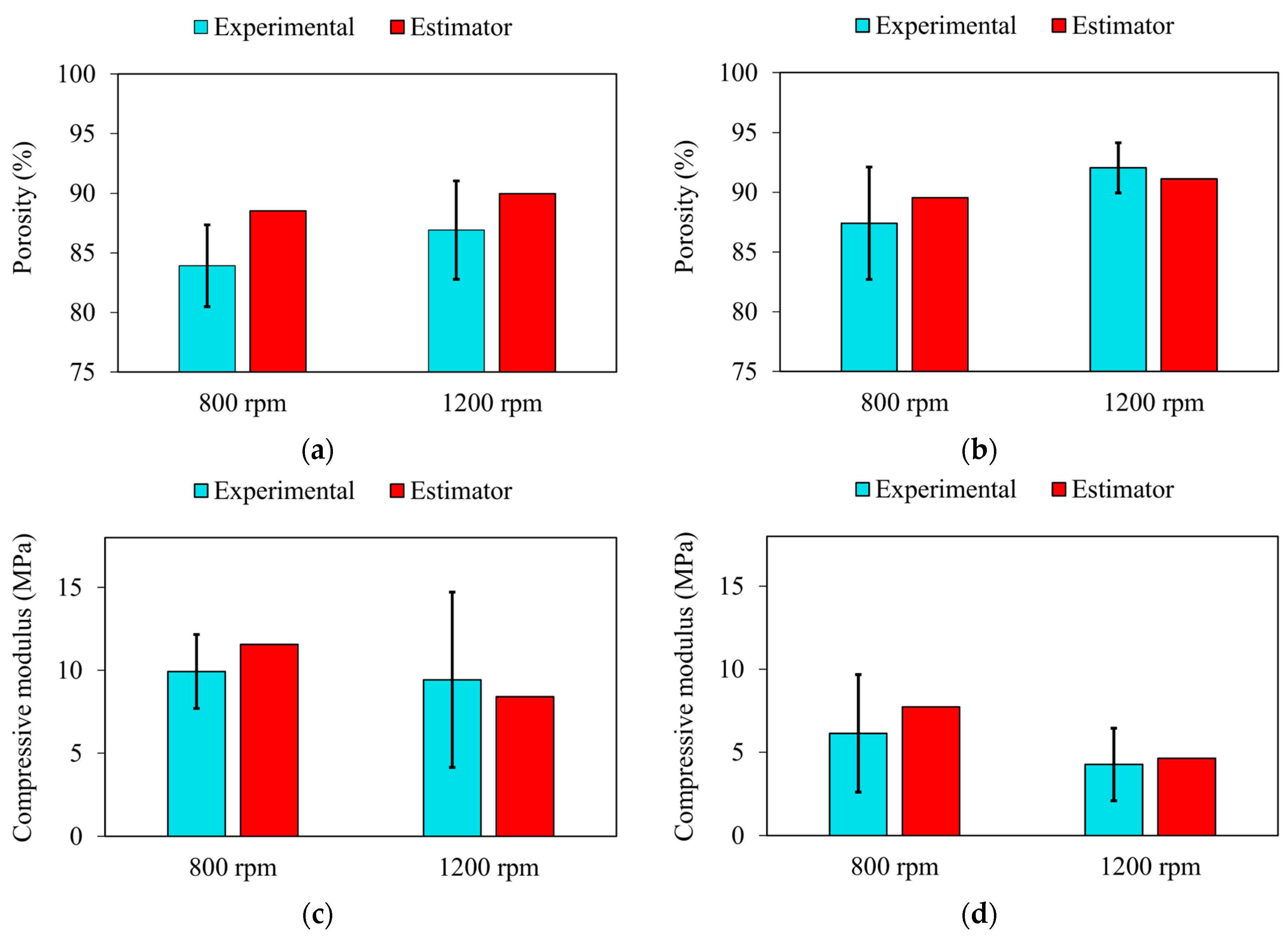
| 9.47972 | −1.66895 | −3.94140 | −0.09458 | −2.03813 | −0.17377 | |
| (0.000183) | (0.005192) | (0.000416) | (0.82530) (ns) | (0.013917) | (0.576049) (ns) | |
| 5.3079 | −1.0655 | −3.8576 | −0.9869 | 2.1152 | 0.1813 | |
| (0.00305) | (0.04791) | (0.00133) | (0.18174) (ns) | (0.03400) | (0.68320) (ns) | |
| 89.67964 | 0.86910 | 1.82147 | 0.11133 | 1.19023 | 0.05743 | |
| (3.16e−07) | (0.04304) | (0.00578) | (0.81865) (ns) | (0.07544) (ns) | (0.86687) (ns) | |
| 90.96153 | 1.27855 | 1.96650 | 0.06855 | 0.14360 | 0.19497 | |
| (7.92e−07) | (0.0365) | (0.0115) | (0.9181) (ns) | (0.8300) (ns) | (0.6836) (ns) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qamash, M.; Imani, S.M.; Omidi, M.; Glancy, C.; Tayebi, L. Investigation of the Effect of Preparation Parameters on the Structural and Mechanical Properties of Gelatin/Elastin/Sodium Hyaluronate Scaffolds Fabricated by the Combined Foaming and Freeze-Drying Techniques. J. Compos. Sci. 2024, 8, 408. https://doi.org/10.3390/jcs8100408
Qamash M, Imani SM, Omidi M, Glancy C, Tayebi L. Investigation of the Effect of Preparation Parameters on the Structural and Mechanical Properties of Gelatin/Elastin/Sodium Hyaluronate Scaffolds Fabricated by the Combined Foaming and Freeze-Drying Techniques. Journal of Composites Science. 2024; 8(10):408. https://doi.org/10.3390/jcs8100408
Chicago/Turabian StyleQamash, Mansour, S. Misagh Imani, Meisam Omidi, Ciara Glancy, and Lobat Tayebi. 2024. "Investigation of the Effect of Preparation Parameters on the Structural and Mechanical Properties of Gelatin/Elastin/Sodium Hyaluronate Scaffolds Fabricated by the Combined Foaming and Freeze-Drying Techniques" Journal of Composites Science 8, no. 10: 408. https://doi.org/10.3390/jcs8100408








