A Preliminary Experimental Study on Biodegradation of 3D-Printed Samples from Biomass–Fungi Composite Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Raw Materials
2.1.1. Fungal Growth in Rye Berries
2.1.2. Inoculating Biomass in Bags
2.2. Preparation of Sodium Alginate Solution and Calcium Chloride Crosslinking Solution
2.3. Preparation of Mixtures for 3D Printing
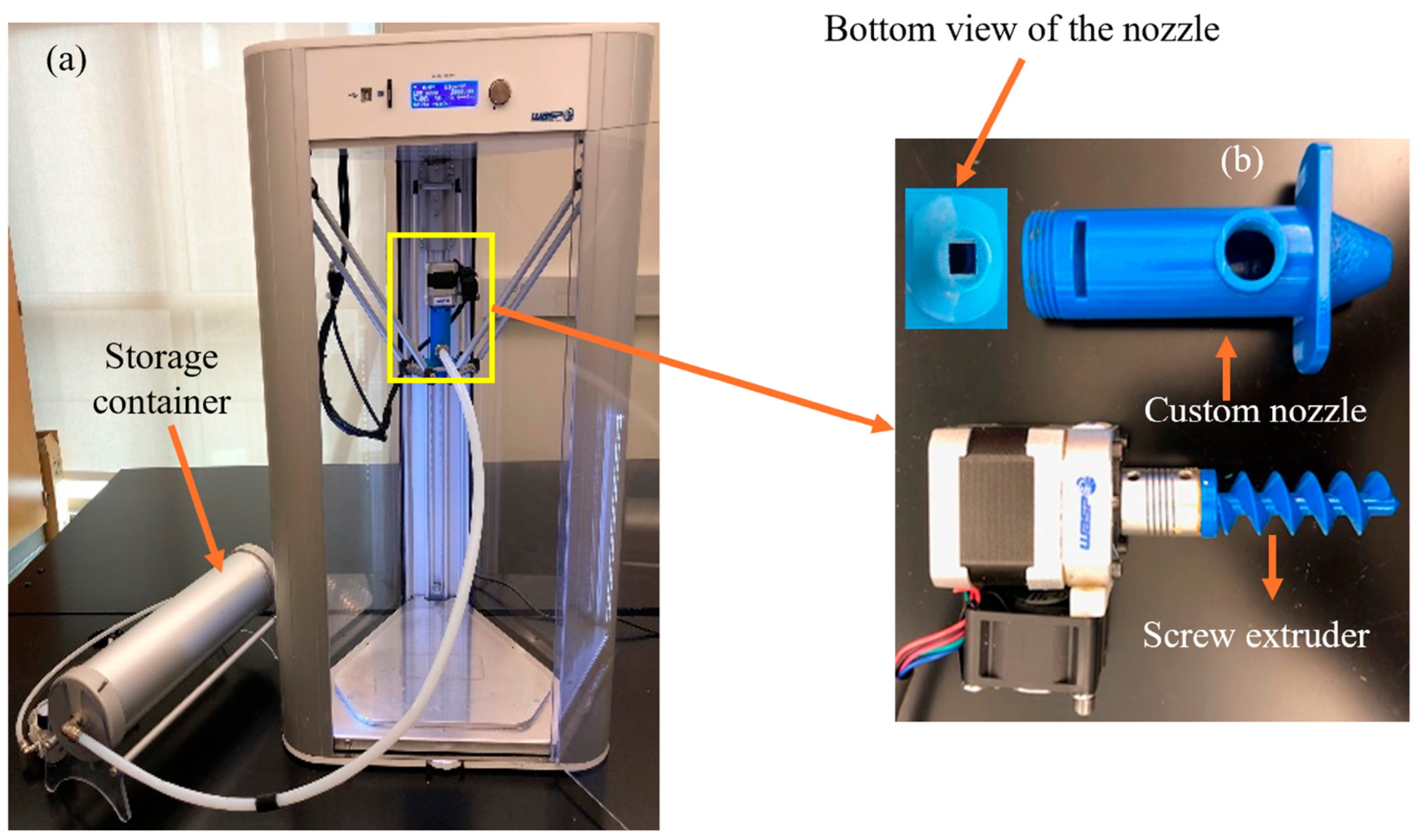
2.4. Preparation of Samples by Extrusion Based 3D Printing
2.5. Soil Burial Test
2.6. Weight Measurement
2.7. Pictures Taken Using iPhone
2.8. Micrographs Taken Using Scanning Electron Microscopy (SEM)
2.9. Observations Using Fourier Transform Infrared Spectroscopy (FTIR)
3. Results and Discussions
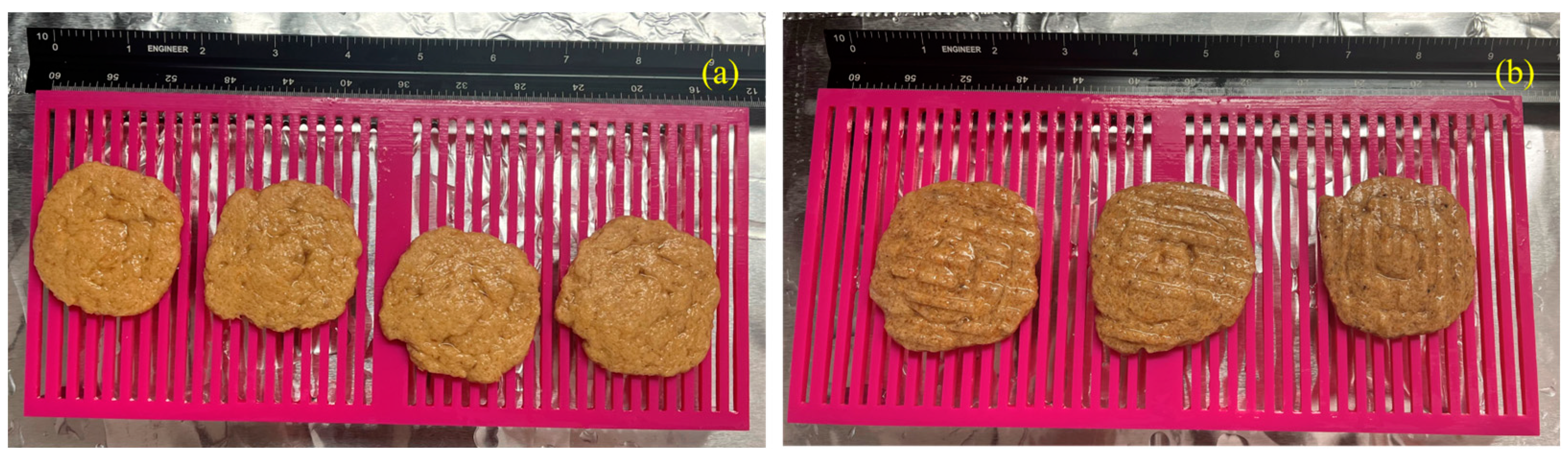
3.1. Visual Observations of Samples
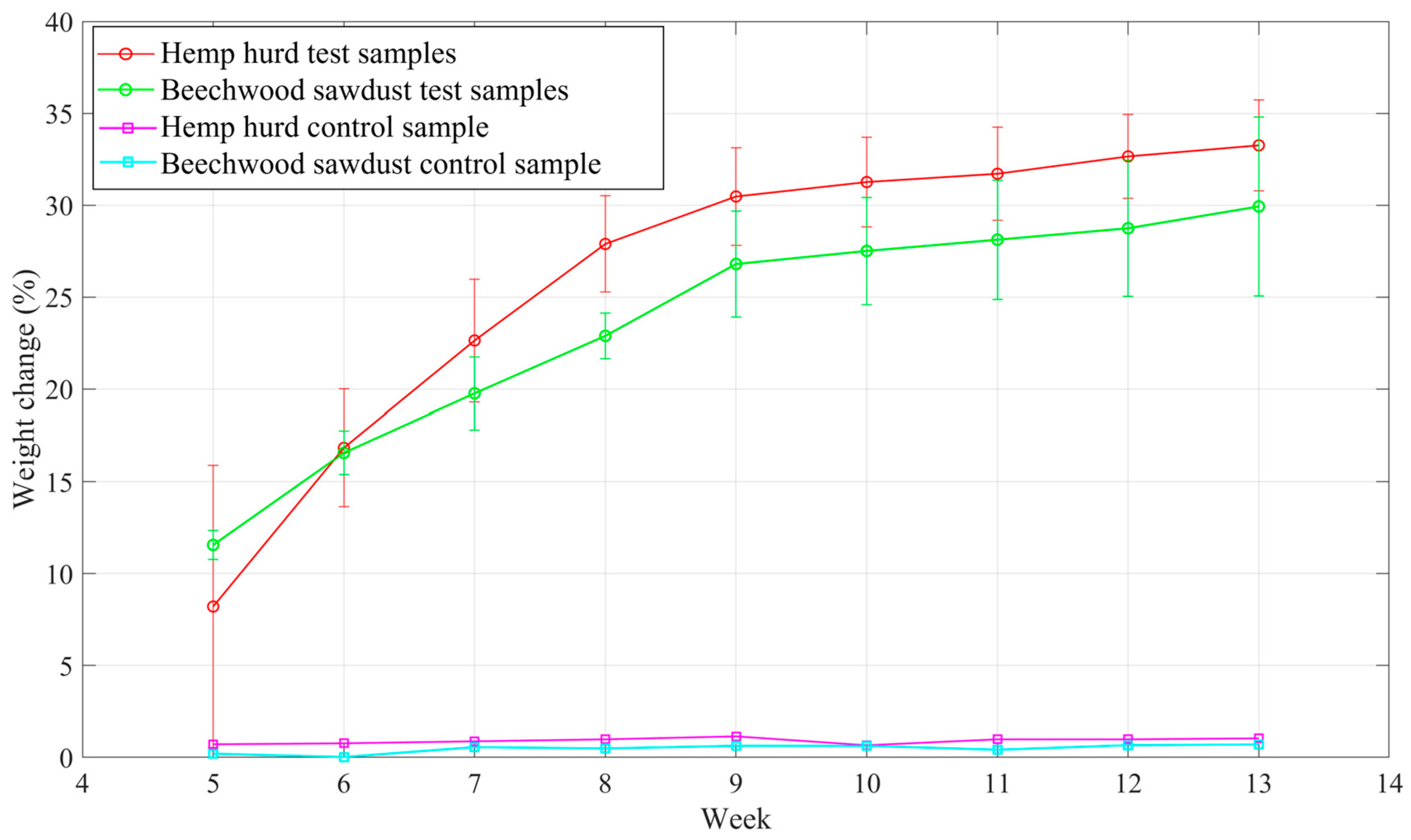
3.2. Weight Change
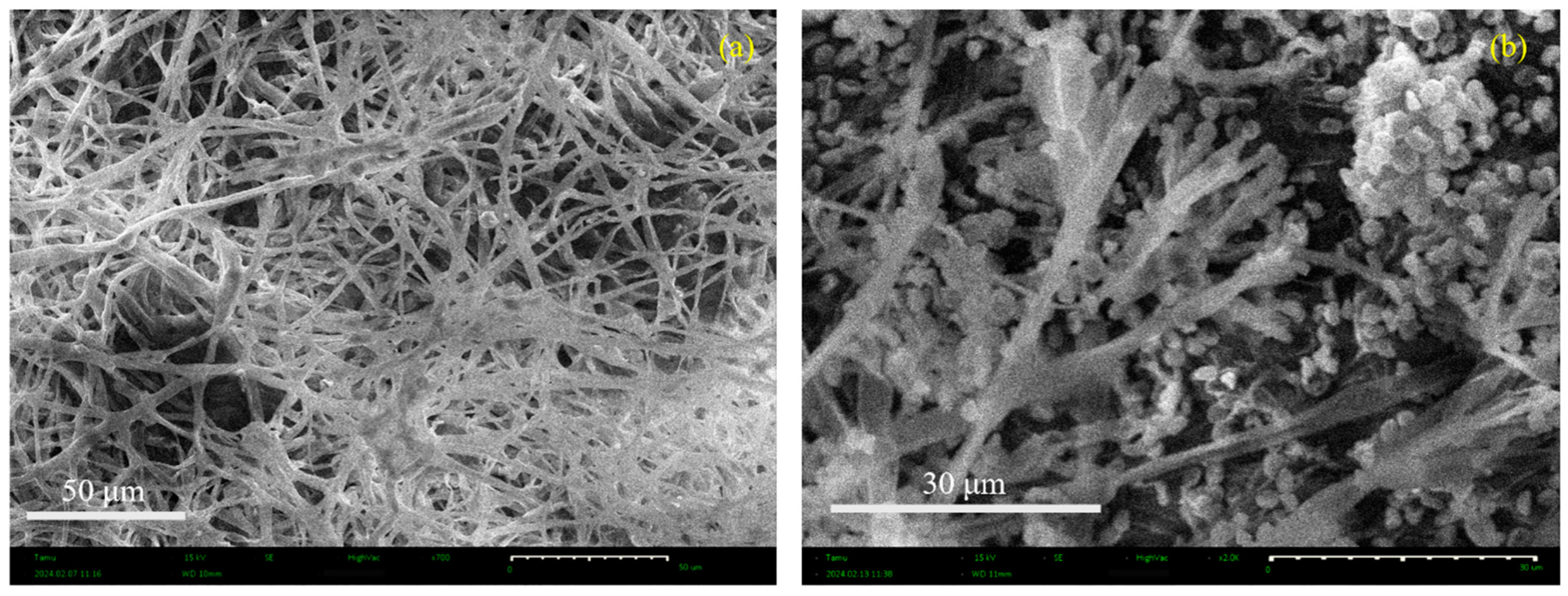
3.3. SEM Micrographs
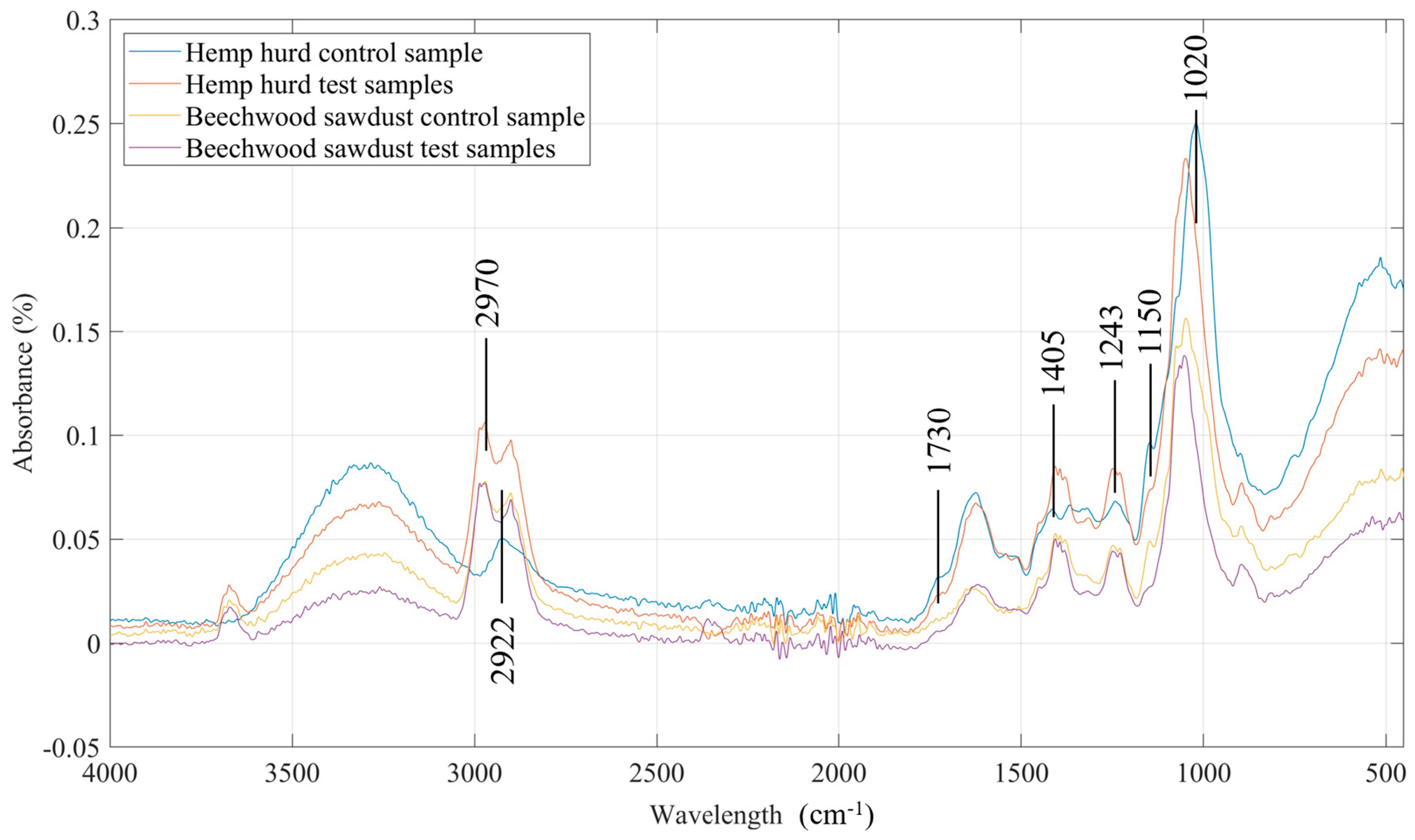
3.4. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akter, M.; Uddin, M.H.; Tania, I.S. Biocomposites based on natural fibers and polymers: A review on properties and potential applications. J. Reinf. Plast. Compos. 2022, 41, 705–742. [Google Scholar] [CrossRef]
- Harding, K.; Gounden, T.; Pretorius, S. “Biodegradable” plastics: A myth of marketing? Procedia Manuf. 2017, 7, 106–110. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Grimm, D.; Wosten, H.A.B. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef] [PubMed]
- Holt, G.A.; McIntyre, G.; Flagg, D.; Bayer, E.; Wanjura, J.D.; Pelletier, M.G. Fungal Mycelium and Cotton Plant Materials in the Manufacture of Biodegradable Molded Packaging Material: Evaluation Study of Select Blends of Cotton Byproducts. J. Biobased Mater. Bioenergy 2012, 6, 431–439. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Rahman, A.M.; Wei, X.; Pei, Z.; Truong, D.; Lucht, M.; Zou, N. 3D Printing of Biomass–Fungi Composite Material: Effects of Mixture Composition on Print Quality. J. Manuf. Mater. Process. 2021, 5, 112. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Jiang, L.; Walczyk, D.; McIntyre, G.; Bucinell, R.; Tudryn, G. Manufacturing of biocomposite sandwich structures using mycelium-bound cores and preforms. J. Manuf. Process. 2017, 28, 50–59. [Google Scholar] [CrossRef]
- Ghazvinian, A.; Farrokhsiar, P.; Vieira, F.; Pecchia, J.; Gursoy, B. Mycelium-based bio-composites for architecture: Assessing the effects of cultivation factors on compressive strength. Mater. Res. Innov. 2019, 2, 505–514. [Google Scholar] [CrossRef]
- McBee, R.M.; Lucht, M.; Mukhitov, N.; Richardson, M.; Srinivasan, T.; Meng, D.; Chen, H.; Kaufman, A.; Reitman, M.; Munck, C.; et al. Engineering living and regenerative fungal-bacterial biocomposite structures. Nat. Mater. 2022, 21, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Tudryn, G.; Bucinell, R.; Schadler, L.; Picu, R.C. Morphology and mechanics of fungal mycelium. Sci. Rep. 2017, 7, 13070. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Vasselli, J.; Lucht, M.; Pei, Z.; Shaw, B.; Grasley, Z.; Wei, X.; Zou, N. 3D Printing of Biomass-Fungi Composite Material: A Preliminary Study. Manuf. Lett. 2020, 24, 96–99. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Kang, W.; Liu, X.; Wang, Q. Current advances and future perspectives of additive manufacturing for functional polymeric materials and devices. SusMat 2021, 1, 127–147. [Google Scholar] [CrossRef]
- Attias, N.; Danai, O.; Abitbol, T.; Tarazi, E.; Ezov, N.; Pereman, I.; Grobman, Y.J. Mycelium bio-composites in industrial design and architecture: Comparative review and experimental analysis. J. Clean. Prod. 2020, 246, 119037. [Google Scholar] [CrossRef]
- Modanloo, B.; Ghazvinian, A.; Matini, M.; Andaroodi, E. Tilted Arch; Implementation of Additive Manufacturing and Bio-Welding of Mycelium-Based Composites. Biomimetics 2021, 6, 68. [Google Scholar] [CrossRef]
- Sydor, M.; Bonenberg, A.; Doczekalska, B.; Cofta, G. Mycelium-Based Composites in Art, Architecture, and Interior Design: A Review. Polymers 2021, 14, 145. [Google Scholar] [CrossRef]
- Karimi, A.; Rahmatabadi, D.; Baghani, M. Various FDM mechanisms used in the fabrication of continuous-fiber reinforced composites: A review. Polymers 2024, 16, 831. [Google Scholar] [CrossRef]
- Rahmatabadi, D.; Khajepour, M.; Bayati, A.; Mirasadi, K.; Yousefi, M.A.; Shegeft, A.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Bodaghi, M. Advancing sustainable shape memory polymers through 4D printing of polylactic acid-polybutylene adipate terephthalate blends. Eur. Polym. J. 2024, 216, 113289. [Google Scholar] [CrossRef]
- Rahman, A.M.; Bhardwaj, A.; Pei, Z.; Ufodike, C.; Castell-Perez, E. The 3D Printing of Biomass–Fungi Composites: Effects of Waiting Time after Mixture Preparation on Mechanical Properties, Rheological Properties, Minimum Extrusion Pressure, and Print Quality of the Prepared Mixture. J. Compos. Sci. 2022, 6, 237. [Google Scholar] [CrossRef]
- Rahman, A.M.; Bhardwaj, A.; Vasselli, J.G.; Pei, Z.; Shaw, B.D. Three-Dimensional Printing of Biomass–Fungi Biocomposite Materials: The Effects of Mixing and Printing Parameters on Fungal Growth. J. Manuf. Mater. Process. 2023, 8, 2. [Google Scholar] [CrossRef]
- Malik, S.; Hagopian, J.; Mohite, S.; Lintong, C.; Stoffels, L.; Giannakopoulos, S.; Beckett, R.; Leung, C.; Ruiz, J.; Cruz, M.; et al. Robotic Extrusion of Algae-Laden Hydrogels for Large-Scale Applications. Glob. Chall. 2020, 4, 1900064. [Google Scholar] [CrossRef] [PubMed]
- Elsacker, E.; Peeters, E.; De Laet, L. Large-scale robotic extrusion-based additive manufacturing with living mycelium materials. Sustain. Futures 2022, 4, 100085. [Google Scholar] [CrossRef]
- Soh, E.; Teoh, J.H.; Leong, B.; Xing, T.; Le Ferrand, H. 3D printing of mycelium engineered living materials using a waste-based ink and non-sterile conditions. Mater. Des. 2023, 236, 112481. [Google Scholar] [CrossRef]
- Mohseni, A.; Vieira, F.R.; Pecchia, J.A.; Gursoy, B. Three-Dimensional Printing of Living Mycelium-Based Composites: Material Compositions, Workflows, and Ways to Mitigate Contamination. Biomimetics 2023, 8, 257. [Google Scholar] [CrossRef]
- Lin, N.; Taghizadehmakoei, A.; Polovina, L.; McLean, I.; Santana-Martinez, J.C.; Naese, C.; Moraes, C.; Hallam, S.J.; Dahmen, J. 3D Bioprinting of Food Grade Hydrogel Infused with Living Pleurotus ostreatus Mycelium in Non-sterile Conditions. ACS Appl. Bio Mater. 2024, 7, 2982–2992. [Google Scholar] [CrossRef]
- Zimele, Z.; Irbe, I.; Grinins, J.; Bikovens, O.; Verovkins, A.; Bajare, D. Novel Mycelium-Based Biocomposites (MBB) as Building Materials. J. Renew. Mater. 2020, 8, 1067–1076. [Google Scholar] [CrossRef]
- Ly, L.; Jitjak, W. Biocomposites from agricultural wastes and mycelia of a local mushroom, Lentinus squarrosulus (Mont.) Singer. Open Agric. 2022, 7, 634–643. [Google Scholar] [CrossRef]
- Van Wylick, A.; Elsacker, E.; Yap, L.L.; Peeters, E.; De Laet, L. Mycelium composites and their biodegradability: An exploration on the disintegration of mycelium-based materials in soil. Constr. Technol. Archit. 2022, 1, 652–659. [Google Scholar]
- Vasselli, J.G.; Hancock, H.; Bedsole, C.O.; Kainer, E.; Chappell, T.M.; Shaw, B.D. The conidial coin toss: A polarized conidial adhesive in Colletotrichum graminicola. Fungal Genet. Biol. 2022, 163, 103747. [Google Scholar] [CrossRef]
- LaForest, J. Acid Potato Dextrose Agar. Available online: https://wiki.bugwood.org/Acid_potato_dextrose_agar_(half_strength) (accessed on 7 July 2024).
- Rahman, A.M.; Bedsole, C.O.; Akib, Y.M.; Hamilton, J.; Rahman, T.T.; Shaw, B.D.; Pei, Z. Effects of Sodium Alginate and Calcium Chloride on Fungal Growth and Viability in Biomass-Fungi Composite Materials Used for 3D Printing. Biomimetics 2024, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Abdullayev, A.; Bekheet, M.F.; Schmidt, B.; Regler, I.; Pohl, C.; Vakifahmetoglu, C.; Czasny, M.; Kamm, P.H.; Meyer, V.; et al. Extrusion-based additive manufacturing of fungal-based composite materials using the tinder fungus Fomes fomentarius. Fungal Biol. Biotechnol. 2021, 8, 21. [Google Scholar] [CrossRef]
- Liu, S.; Bastola, A.K.; Li, L. A 3D Printable and Mechanically Robust Hydrogel Based on Alginate and Graphene Oxide. ACS Appl. Mater. Interfaces 2017, 9, 41473–41481. [Google Scholar] [CrossRef]
- Sanhawong, W.; Banhalee, P.; Boonsang, S.; Kaewpirom, S. Effect of concentrated natural rubber latex on the properties and degradation behavior of cotton-fiber-reinforced cassava starch biofoam. Ind. Crops Prod. 2017, 108, 756–766. [Google Scholar] [CrossRef]
- Rocha, D.N.; Carvalho, E.D.; Relvas, J.B.; Oliveira, M.J.; Pêgo, A.P. Mechanotransduction: Exploring new therapeutic avenues in central nervous system pathology. Front. Neurosci. 2022, 16, 861613. [Google Scholar] [CrossRef] [PubMed]
- Mazibuko, M.; Ndumo, J.; Low, M.; Ming, D.; Harding, K. Investigating the natural degradation of textiles under controllable and uncontrollable environmental conditions. Procedia Manuf. 2019, 35, 719–724. [Google Scholar] [CrossRef]
- Mattos, B.D.; de Cademartori, P.H.G.; Lourençon, T.V.; Gatto, D.A.; Magalhães, W.L.E. Biodeterioration of wood from two fast-growing eucalypts exposed to field test. Int. Biodeterior. Biodegrad. 2014, 93, 210–215. [Google Scholar] [CrossRef]
- Kumar, S.; Kruth, J.P. Composites by rapid prototyping technology. Mater. Des. 2010, 31, 850–856. [Google Scholar] [CrossRef]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced Materials From Fungal Mycelium: Fabrication and Tuning of Physical Properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef]
- Rigobello, A.; Ayres, P. Compressive behaviour of anisotropic mycelium-based composites. Sci. Rep. 2022, 12, 6846. [Google Scholar] [CrossRef]
- Mohebby, B. Attenuated total reflection infrared spectroscopy of white-rot decayed beech wood. Int. Biodeterior. Biodegrad. 2005, 55, 247–251. [Google Scholar] [CrossRef]
- Stevulova, N.; Cigasova, J.; Estokova, A.; Terpakova, E.; Geffert, A.; Kacik, F.; Singovszka, E.; Holub, M. Properties Characterization of Chemically Modified Hemp Hurds. Materials 2014, 7, 8131–8150. [Google Scholar] [CrossRef]
- ISO 20200:2015; ISO: Global Standards for Trusted Goods and Services. ISO: Geneva, Switzerland. Available online: https://www.iso.org/standard/63367.html (accessed on 7 June 2024).
- Aiduang, W.; Jatuwong, K.; Jinanukul, P.; Suwannarach, N.; Kumla, J.; Thamjaree, W.; Teeraphantuvat, T.; Waroonkun, T.; Oranratmanee, R.; Lumyong, S. Sustainable Innovation: Fabrication and Characterization of Mycelium-Based Green Composites for Modern Interior Materials Using Agro-Industrial Wastes and Different Species of Fungi. Polymers 2024, 16, 550. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, A.; Price, M.; Lilley, C.; Guzniczak, E.; Archer, I. A Review of Standards for Biodegradable Plastics; Industrial Biotechnology Innovation Center: Birmingham, UK, 2018. [Google Scholar]
- Nashiruddin, N.I.; Chua, K.S.; Mansor, A.F.; Rahman, R.A.; Lai, J.C.; Wan Azelee, N.I.; El Enshasy, H.A. Effect of growth factors on the production of mycelium-based biofoam. Clean. Technol. Environ. Policy 2021, 24, 351–361. [Google Scholar] [CrossRef]
- Rajeshkumar, L.; Kumar, P.S.; Ramesh, M.; Sanjay, M.R.; Siengchin, S. Assessment of biodegradation of lignocellulosic fiber-based composites—A systematic review. Int. J. Biol. Macromol. 2023, 253, 127237. [Google Scholar] [CrossRef] [PubMed]
- Volk, T.J. Fungi. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Elsevier: New York, NY, USA, 2001; pp. 141–163. [Google Scholar] [CrossRef]
- Tian, B.-Y.; Huang, Q.-G.; Xu, Y.; Wang, C.-X.; Lv, R.-R.; Huang, J.-Z. Microbial community structure and diversity in a native forest wood-decomposed hollow-stump ecosystem. World J. Microbiol. Biotechnol. 2009, 26, 233–240. [Google Scholar] [CrossRef]
- Griffiths, P.R. Fourier transform infrared spectrometry. Science 1983, 222, 297–302. [Google Scholar] [CrossRef]
- Aravindhan, R.; Madhan, B.; Rao, J.R.; Nair, B.U.; Ramasami, T. Bioaccumulation of chromium from tannery wastewater: An approach for chrome recovery and reuse. Env. Sci. Technol. 2004, 38, 300–306. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akib, Y.M.; Bedsole, C.O.; Rahman, A.M.; Hamilton, J.; Khan, F.; Pei, Z.; Shaw, B.D.; Ufodike, C.O. A Preliminary Experimental Study on Biodegradation of 3D-Printed Samples from Biomass–Fungi Composite Materials. J. Compos. Sci. 2024, 8, 412. https://doi.org/10.3390/jcs8100412
Akib YM, Bedsole CO, Rahman AM, Hamilton J, Khan F, Pei Z, Shaw BD, Ufodike CO. A Preliminary Experimental Study on Biodegradation of 3D-Printed Samples from Biomass–Fungi Composite Materials. Journal of Composites Science. 2024; 8(10):412. https://doi.org/10.3390/jcs8100412
Chicago/Turabian StyleAkib, Yeasir Mohammad, Caleb Oliver Bedsole, Al Mazedur Rahman, Jillian Hamilton, Fahim Khan, Zhijian Pei, Brian D. Shaw, and Chukwuzubelu Okenwa Ufodike. 2024. "A Preliminary Experimental Study on Biodegradation of 3D-Printed Samples from Biomass–Fungi Composite Materials" Journal of Composites Science 8, no. 10: 412. https://doi.org/10.3390/jcs8100412
APA StyleAkib, Y. M., Bedsole, C. O., Rahman, A. M., Hamilton, J., Khan, F., Pei, Z., Shaw, B. D., & Ufodike, C. O. (2024). A Preliminary Experimental Study on Biodegradation of 3D-Printed Samples from Biomass–Fungi Composite Materials. Journal of Composites Science, 8(10), 412. https://doi.org/10.3390/jcs8100412







