Electrochemical Deposition and Properties of Ni Coatings with Nitrogen-Modified Graphene Oxide
Abstract
1. Introduction
2. Materials and Methods
- (a)
- Modification of GO
- (b) Electrodeposition of Ni coatings
- (c) Study of structure and properties
3. Results and Discussion
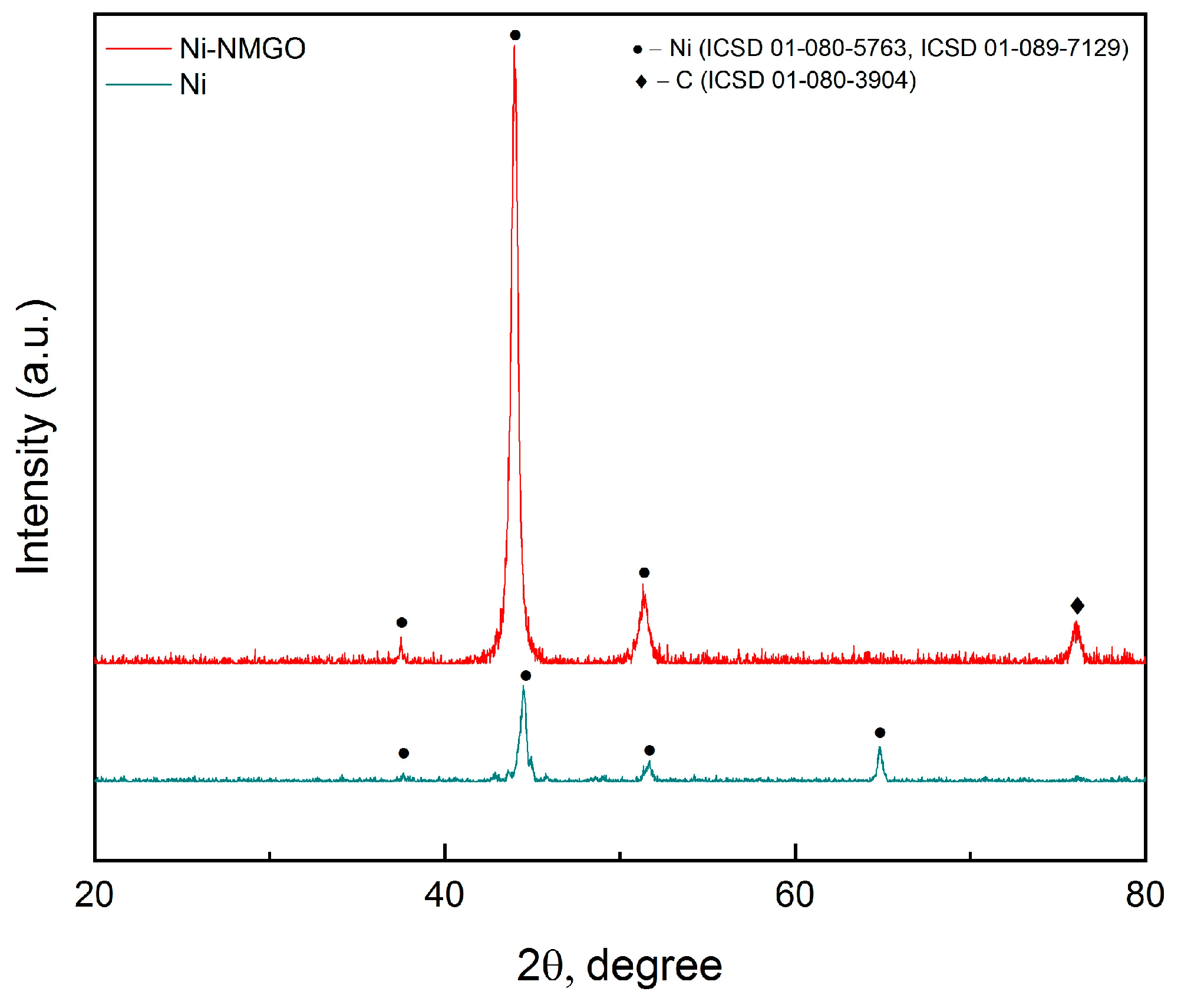
3.1. Structural Studies of NMGO
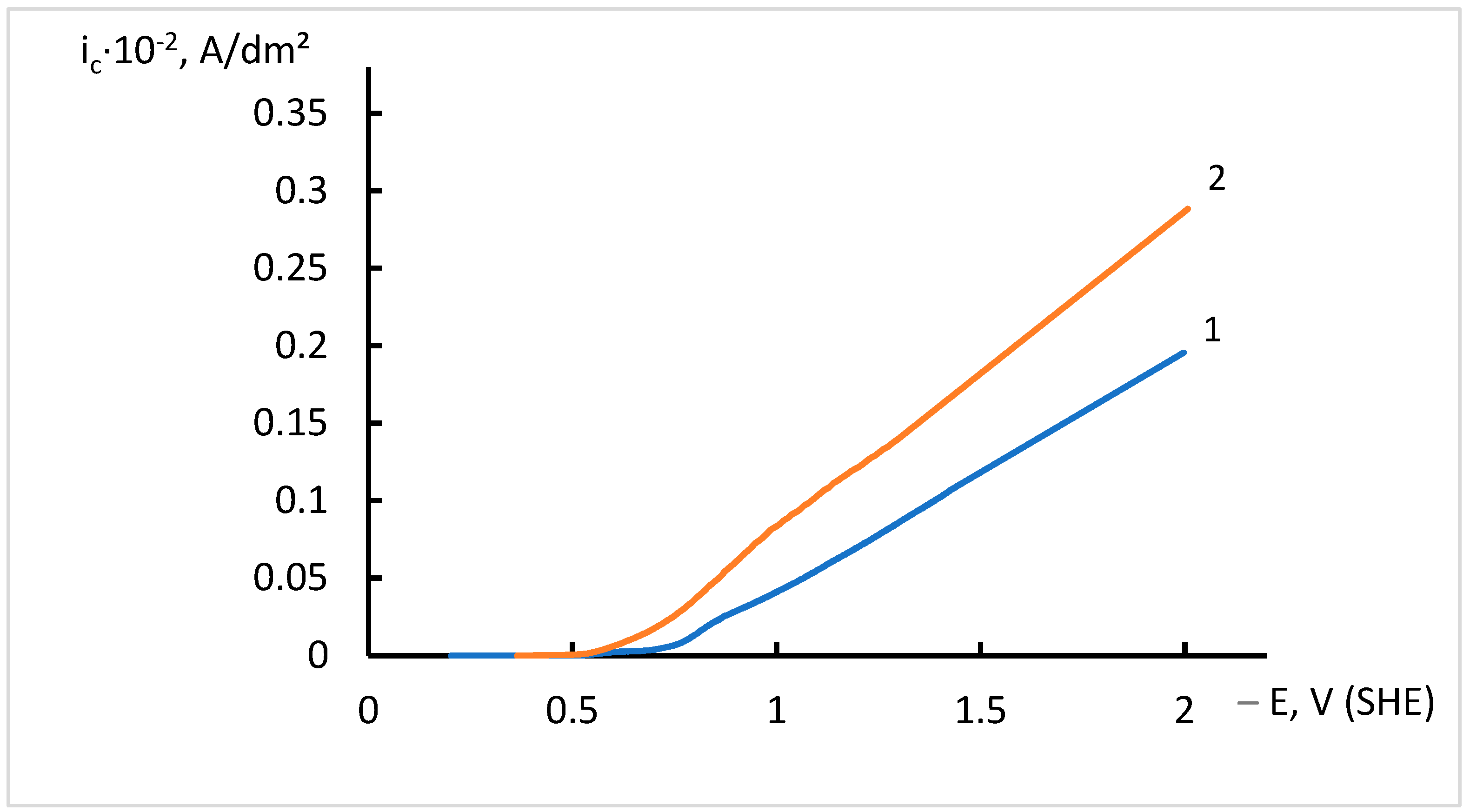
3.2. Electrodeposition of Ni Coatings
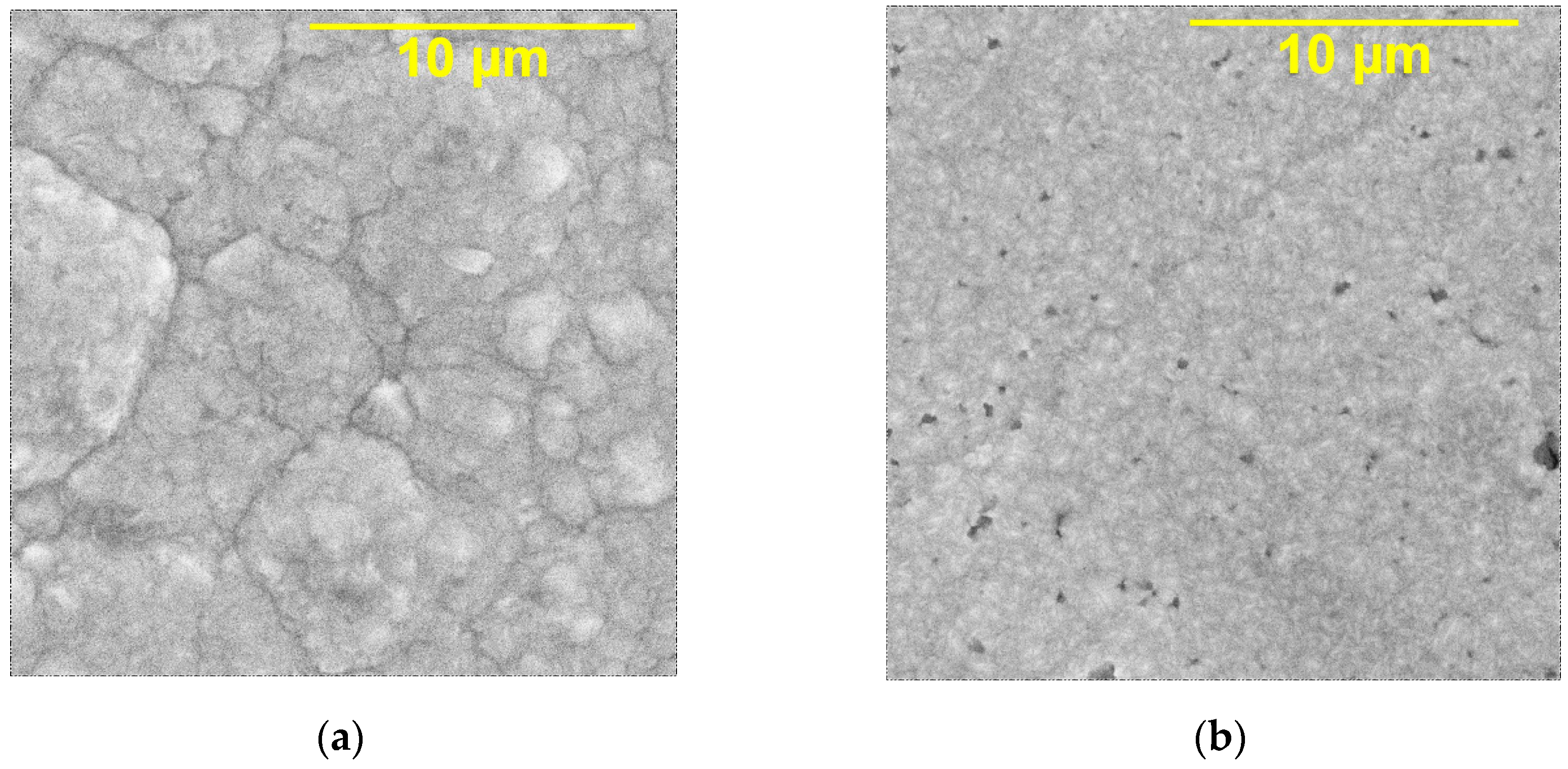
3.3. Microstructural Studies of Ni Coatings
3.4. Microhardness
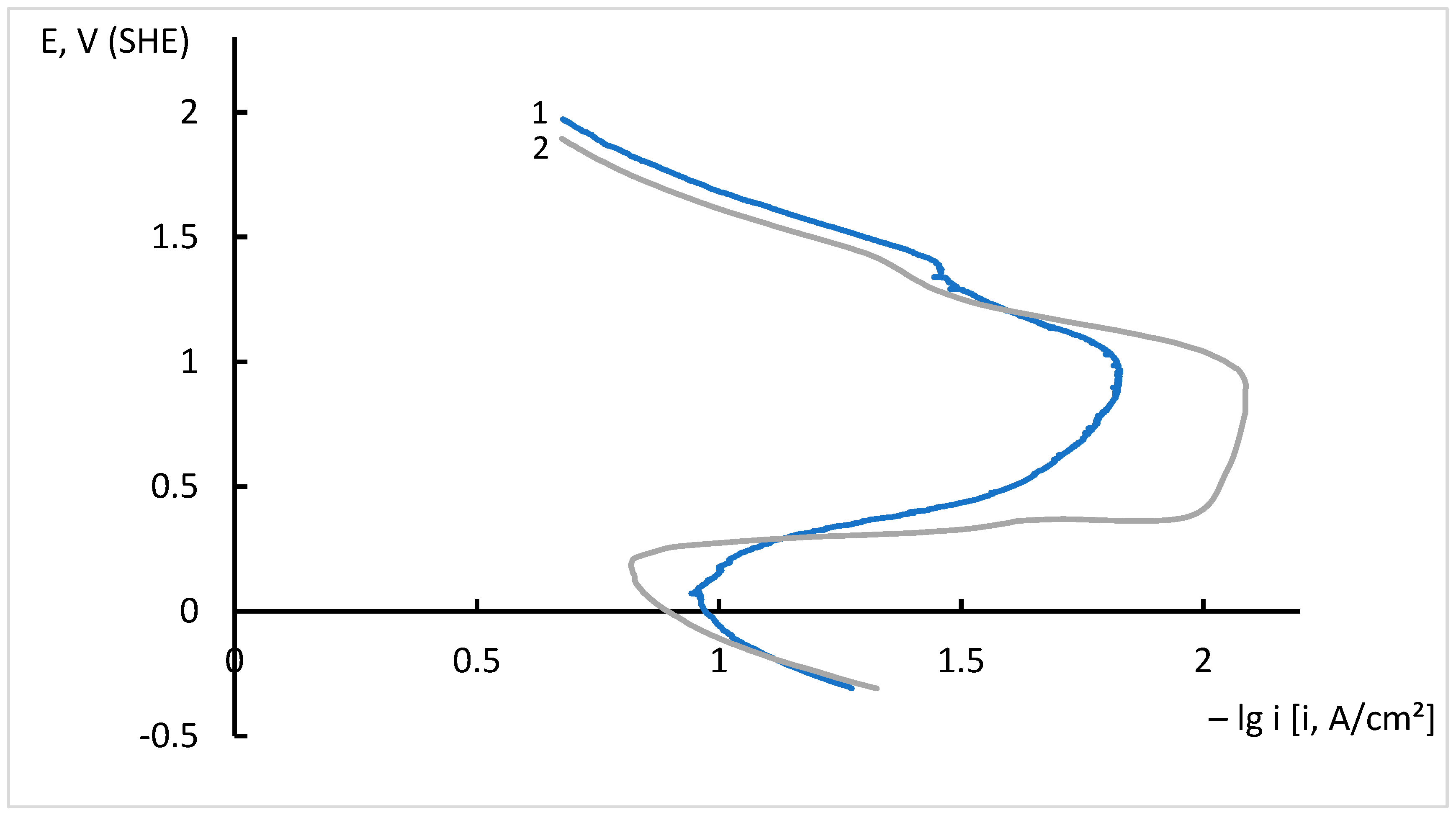
3.5. Corrosion Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Orinakova, R.; Turonova, A.; Kladekova, D.; Galova, M.; Smith, R.M. Recent developments in the electrodeposition of nickel and some nickel-based alloys. J. Appl. Electrochem. 2006, 36, 957–972. [Google Scholar] [CrossRef]
- Low, C.T.J.; Wills, R.G.A.; Walsh, F.C. Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surf. Coat. Technol. 2006, 201, 371–383. [Google Scholar] [CrossRef]
- Tseluikin, V.N. Composite coatings modified with nanoparticles: Structure and properties. Nanotechnol. Russ. 2014, 9, 1–14. [Google Scholar] [CrossRef]
- Walsh, F.C. A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: An established and diversifying technology. Trans. IMF 2014, 92, 83–98. [Google Scholar] [CrossRef]
- Giannopoulos, F.; Chronopoulou, N.; Bai, J.; Zhao, H.; Pantelis, D.; Pavlatou, E.A.I.; Karatonis, A. Nickel/MWCNT-Al2O3 electrochemical co-deposition: Structural properties and mechanistics aspects. Electrochim. Acta 2016, 207, 76–86. [Google Scholar] [CrossRef]
- Jyotheender, K.S.; Gupta, A.; Srivastava, C. Grain boundary engineering in Ni-carbon nanotube composite coatings and its effect on the corrosion behaviour of the coatings. Materialia 2020, 9, 100617. [Google Scholar] [CrossRef]
- Yang, P.; Wang, N.; Zhang, J.; Lei, Y.; Shu, B. Investigation of the microstructure and tribological properties of CNTs/Ni composites prepared by electrodeposition. Mater. Res. Express 2022, 9, 036404. [Google Scholar] [CrossRef]
- Antihovich, I.V.; Ablazhey, N.M.; Chernik, A.A.; Zharsky, I.M. Electrodeposition of nickel and composite nickel-fullerenol coatings from low-temperature sulphate-chloride-isobutyrate electrolyte. Procedia Chem. 2014, 10, 373–377. [Google Scholar] [CrossRef]
- Tseluikin, V.N. Electrodeposition and properties of composite coatings modified by fullerene C60. Prot. Met. Phys. Chem. Surf. 2017, 53, 433–436. [Google Scholar] [CrossRef]
- Chayeuski, V.V.; Zhylinski, V.V.; Rudak, P.V.; Rusalsky, D.P.; Visniakov, N.; Cernasejus, O. Characteristics of ZrC/Ni-UDD coatings for a tungsten carbide cutting tool. Appl. Surf. Sci. 2018, 446, 18–26. [Google Scholar] [CrossRef]
- Makarova, I.; Dobryden, I.; Kharitonov, D.; Kasach, A.; Ryl, J.; Repo, E.; Vuorinen, E. Nickel-nanodiamond coatings electrodeposited from tartrate electrolyte at ambient temperature. Surf. Coat. Technol. 2019, 380, 125063. [Google Scholar] [CrossRef]
- Algul, H.; Tokur, M.; Ozcan, S.; Uysal, M.; Cetinkaya, T.; Akbulut, H.; Alp, A. The effect of graphene content and sliding speed on the wearmechanism of nickel–graphene nanocomposites. Appl. Surf. Sci. 2015, 359, 340–348. [Google Scholar] [CrossRef]
- Yasin, G.; Arif, M.; Nizam, N.M.; Shakeel, M.; Khan, M.A.; Khan, W.Q.; Hassan, T.M.; Abbas, Z.; Farahbakhsh, I.; Zuo, Y. Effect of surfactant concentration in electrolyte on the fabrication and properties of nickel-graphene nanocomposite coating synthesized by electrochemical co-deposition. RSC Adv. 2018, 8, 20039–20047. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Pumera, M. The structural stability of graphene anticorrosion coating materials is compromised at low potentials. Chem.-A Eur. J. 2015, 21, 7896–7901. [Google Scholar] [CrossRef] [PubMed]
- Jyotheender, K.S.; Srivastava, C. Ni-graphene oxide composite coatings: Optimum graphene oxide for enhanced corrosion resistance. Compos. Part B 2019, 175, 107145. [Google Scholar] [CrossRef]
- Tseluikin, V.; Dzhumieva, A.; Tikhonov, D.; Yakovlev, A.; Strilets, A.; Tribis, A.; Lopukhova, M. Pulsed electrodeposition and properties of nickel-based composite coatings modified with graphene oxide. Coatings 2022, 12, 656. [Google Scholar] [CrossRef]
- Tseluikin, V.; Dzhumieva, A.; Yakovlev, A.; Tikhonov, D.; Tribis, A.; Strilets, A.; Lopukhova, M. Electrodeposition and Properties of Composite Ni Coatings Modified with Multilayer Graphene Oxide. Micromachines 2023, 14, 1747. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, S.; Kariper, İ.A. Graphene and graphene oxide based aerogels: Synthesis, characteristics and supercapacitor applications. J. Energy Storage 2020, 27, 101038. [Google Scholar] [CrossRef]
- Arvas, M.B.; Gürsu, H.; Gencten, M.; Sahin, Y. Preparation of different heteroatom doped graphene oxide based electrodes by electrochemical method and their supercapacitor applications. J. Energy Storage 2021, 35, 102328. [Google Scholar] [CrossRef]
- Yadav, R.; Subhash, A.; Chemmenchery, N.; Kandasubramanian, B. Graphene and graphene oxide for fuel cell technology. Ind. Eng. Chem. Res. 2018, 57, 9333–9350. [Google Scholar] [CrossRef]
- Tian, Y.; Yu, Z.; Cao, L.; Zhang, X.L.; Sun, C.; Wang, D.W. Graphene oxide: An emerging electromaterial for energy storage and conversion. J. Energy Chem. 2021, 55, 323–344. [Google Scholar] [CrossRef]
- Joshi, D.J.; Koduru, J.R.; Malek, N.I.; Hussain, C.M.; Kailasa, S.K. Surface modifications and analytical applications of graphene oxide: A review. TrAC Trends Anal. Chem. 2021, 144, 116448. [Google Scholar] [CrossRef]
- Gholampour, A.; Valizadeh Kiamahalleh, M.; Tran, D.N.; Ozbakkaloglu, T.; Losic, D. From graphene oxide to reduced graphene oxide: Impact on the physiochemical and mechanical properties of graphene–cement composites. ACS Appl. Mater. Interfaces 2017, 9, 43275–43286. [Google Scholar] [CrossRef] [PubMed]
- Carboni, N.; Mazzapioda, L.; Caprì, A.; Gatto, I.; Carbone, A.; Baglio, V.; Navarra, M.A. Composite Anion Exchange Membranes based on Graphene Oxide for Water Electrolyzer Applications. Electrochim. Acta 2024, 486, 144090. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef]
- Gupta, A.; Srivastava, C. Enhanced corrosion resistance by SnCu-graphene oxide composite coatings. Thin Solid Films 2019, 669, 85–95. [Google Scholar] [CrossRef]
- Lai, R.; Shi, P.; Yi, Z.; Li, H.; Yi, Y. Triple-band surface plasmon resonance metamaterial absorber based on open-ended prohibited sign type monolayer graphene. Micromachines 2023, 14, 953. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cai, P.; Wen, Q.; Chen, H.; Tang, Y.; Yi, Z.; Wei, K.; Li, G.; Tang, B.; Yi, Y. Graphene multi-frequency broadband and ultra-broadband terahertz absorber based on surface plasmon resonance. Electronics 2023, 12, 2655. [Google Scholar] [CrossRef]
- Xhanari, K.; Grah, N.; Finšgar, M.; Fuchs-Godec, R.; Maver, U. Corrosion inhibition and surface analysis of amines on mild steel in chloride medium. Chem. Pap. 2017, 71, 81–89. [Google Scholar] [CrossRef]
- Muhsin, M.S.; Shihab, M.S. Synthesis New Triethylammonium salts as corrosion inhibitors for mild steel in 1 M H2SO4. Al-Nahrain J. Sci. 2023, 26, 6–18. [Google Scholar] [CrossRef]
- Shang, W.; He, C.; Wen, Y.; Wang, Y.; Zhang, Z. Performance evaluation of triethanolamine as corrosion inhibitor for magnesium alloy in 3.5 wt% NaCl solution. RSC Adv. 2016, 6, 113967–113980. [Google Scholar] [CrossRef]
- Ban, C.G.; Zhang, K.; Huang, L.; Yuan, Y. Triethanolamine modified graphene oxide for epoxy resin-based coatings: Corrosion inhibition on metal substrates. Surf. Interface Anal. 2022, 54, 1151–1162. [Google Scholar] [CrossRef]
- Yakovlev, A.V.; Yakovleva, E.V.; Vikulova, M.A.; Frolov, I.N.; Rakhmetulina, L.A.; Tseluikin, V.N.; Krasnov, V.V.; Mostovoy, A.S. Synthesis of multilayer graphene oxide in electrochemical graphite dispersion in H2SO4. Russ. J. Appl. Chem. 2020, 93, 219–224. [Google Scholar] [CrossRef]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik, M.; Gryglewicz, G. Characterization of graphite oxide and reduced graphene oxide obtained from different graphite precursors and oxidized by different methods using Raman Spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, N.; Fang, F. Fabrication of high-performance nickel/graphene oxide composite coatings using ultrasonic-assisted. Ultrason. Sonochem. 2020, 62, 104858. [Google Scholar] [CrossRef] [PubMed]
- Rekha, M.Y.; Srivastava, C. Microstructural evolution and corrosion behavior of Zn-Ni-graphene oxide composite coatings. Metall. Mater. Trans. A 2019, 50, 5896–5913. [Google Scholar] [CrossRef]
- Yang, F.; Kang, H.; Guo, E.; Li, R.; Chen, Z.; Zeng, Y. The role of nickel in mechanical perfopmance and corrosion behaviour of nickel-aluminium bronze in 3.5 wt.% NaCl solution. Corros. Sci. 2018, 139, 333–345. [Google Scholar] [CrossRef]




| Electrolyte Bath | Concentration, g/L | Deposition Parameters |
|---|---|---|
| NiSO4·7H2O | 220 | Temperature t = 45 °C, pH ≈ 4.5 |
| NiCl2·6H2O | 40 | Constant stirring |
| CH3COONa | 30 | Cathode current densities |
| NMGO | 1 | ic = 7, 8, 9, 10 A/dm2 |
| Cathode Current Density ic, A/dm2 | Ni | Ni–GO | Ni–NMGO |
|---|---|---|---|
| 7 | 1938 | 2200 | 2350 |
| 8 | 2150 | 2520 | 2639 |
| 9 | 2350 | 2938 | 3140 |
| 10 | 2459 | 3076 | 3235 |
| Cathode Current Density ic, A/dm2 | Ni | Ni–GO | Ni–NMGO |
|---|---|---|---|
| 7 | 0.656 | 0.492 | 0.430 |
| 8 | 0.574 | 0.410 | 0.369 |
| 9 | 0.451 | 0.328 | 0.287 |
| 10 | 0.328 | 0.205 | 0.185 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseluikin, V.; Dzhumieva, A.; Tribis, A.; Brudnik, S.; Tikhonov, D.; Yakovlev, A.; Mostovoy, A.; Lopukhova, M. Electrochemical Deposition and Properties of Ni Coatings with Nitrogen-Modified Graphene Oxide. J. Compos. Sci. 2024, 8, 147. https://doi.org/10.3390/jcs8040147
Tseluikin V, Dzhumieva A, Tribis A, Brudnik S, Tikhonov D, Yakovlev A, Mostovoy A, Lopukhova M. Electrochemical Deposition and Properties of Ni Coatings with Nitrogen-Modified Graphene Oxide. Journal of Composites Science. 2024; 8(4):147. https://doi.org/10.3390/jcs8040147
Chicago/Turabian StyleTseluikin, Vitaly, Asel Dzhumieva, Alena Tribis, Sergey Brudnik, Denis Tikhonov, Andrey Yakovlev, Anton Mostovoy, and Marina Lopukhova. 2024. "Electrochemical Deposition and Properties of Ni Coatings with Nitrogen-Modified Graphene Oxide" Journal of Composites Science 8, no. 4: 147. https://doi.org/10.3390/jcs8040147
APA StyleTseluikin, V., Dzhumieva, A., Tribis, A., Brudnik, S., Tikhonov, D., Yakovlev, A., Mostovoy, A., & Lopukhova, M. (2024). Electrochemical Deposition and Properties of Ni Coatings with Nitrogen-Modified Graphene Oxide. Journal of Composites Science, 8(4), 147. https://doi.org/10.3390/jcs8040147









