Effects of Nanofillers and Synergistic Action of Carbon Black/Nanoclay Hybrid Fillers in Chlorobutyl Rubber
Abstract
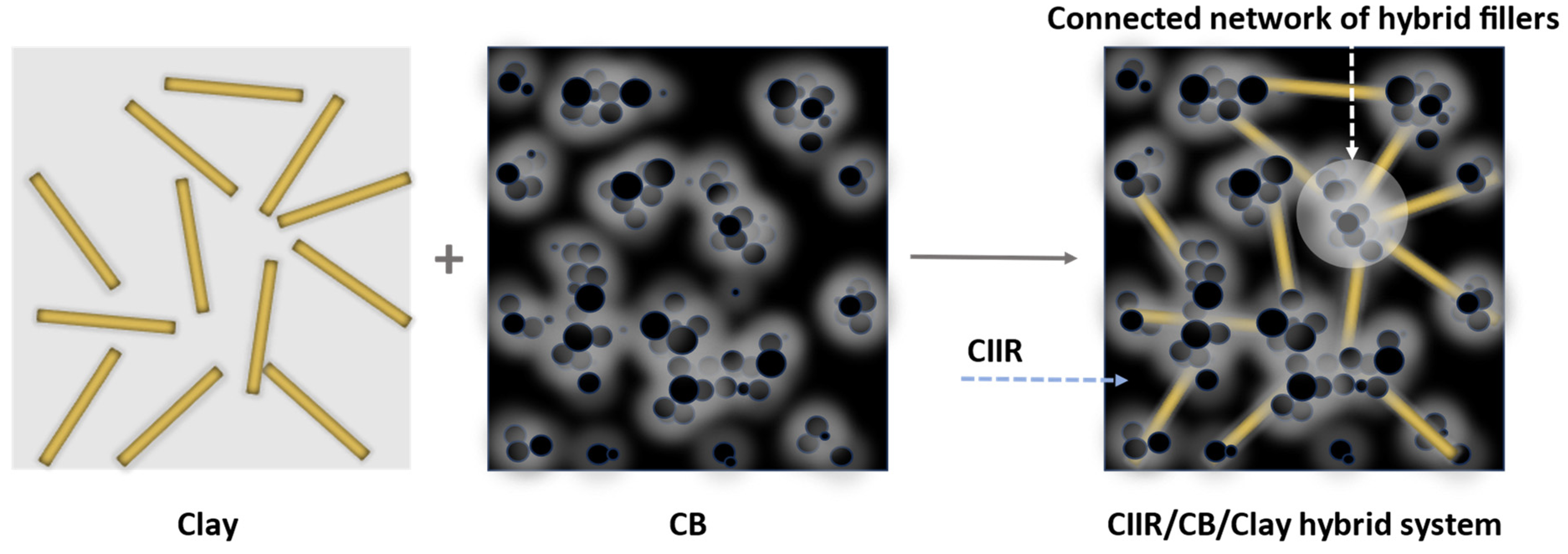
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Graphene Oxide Synthesis
2.3. Preparation of CIIR Nanocomposite
2.4. Protocol
2.5. Characterization
3. Results and Discussion
3.1. Atomic Force Microscopy
3.2. Resistivity Measurement
3.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ritchie, R.O. The Conflicts between Strength and Toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef]
- Suresh, K.I.; Nutenki, R.; Joseph, T.M.; Murali, S. Structural, Molecular and Thermal Properties of Cardanol Based Monomers and Polymers Synthesized via Atom Transfer Radical Polymerization (ATRP). J. Macromol. Sci. Part A 2022, 59, 403–410. [Google Scholar] [CrossRef]
- Joseph, T.M.; Pallikkunnel, M.L.; Mahapatra, D.K.; Kallingal, A.; Thomas, S.; Haponiuk, J.T. Polyurethane−Epoxy Composites: Recent Developments and Future Perspectives. In Polyurethane Chemistry: Renewable Polyols and Isocyanates; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1380, pp. 257–280. ISBN 978-0-8412-9840-8. [Google Scholar]
- Muringayil Joseph, T.; Murali Nair, S.; Kattimuttathu Ittara, S.; Haponiuk, J.T.; Thomas, S. Copolymerization of Styrene and Pentadecylphenylmethacrylate (PDPMA): Synthesis, Characterization, Thermomechanical and Adhesion Properties. Polymers 2020, 12, 97. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kallingal, A.; Suresh, A.M.; Mahapatra, D.K.; Hasanin, M.S.; Haponiuk, J.; Thomas, S. 3D Printing of Polylactic Acid: Recent Advances and Opportunities. Int. J. Adv. Manuf. Technol. 2023, 125, 1015–1035. [Google Scholar] [CrossRef] [PubMed]
- Barreira-Pinto, R.; Carneiro, R.; Miranda, M.; Guedes, R.M. Polymer-Matrix Composites: Characterising the Impact of Environmental Factors on Their Lifetime. Materials 2023, 16, 3913. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Duan, H.; Xing, X. A Review of Composite Materials for Enhancing Support, Flexibility and Strength in Exercise. Alex. Eng. J. 2024, 94, 90–103. [Google Scholar] [CrossRef]
- Reduwan Billah, S.M. Composites and Nanocomposites. In Functional Polymers; Jafar Mazumder, M.A., Sheardown, H., Al-Ahmed, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–67. ISBN 978-3-319-92067-2. [Google Scholar]
- Chowdhury, M.I.S.; Autul, Y.S.; Rahman, S.; Hoque, M.E. 11—Polymer Nanocomposites for Automotive Applications. In Advanced Polymer Nanocomposites; Hoque, M.E., Ramar, K., Sharif, A., Eds.; Woodhead Publishing in Materials; Woodhead Publishing: Sawston, UK, 2022; pp. 267–317. ISBN 978-0-12-824492-0. [Google Scholar]
- Jose Varghese, R.; Vidya, L.; Joseph, T.M.; Gudimalla, A.; Harini Bhuvaneswari, G.; Thomas, S. Potential Applications of XLPE Nanocomposites in the Field of Cable Insulation. In Crosslinkable Polyethylene Based Blends and Nanocomposites; Thomas, J., Thomas, S., Ahmad, Z., Eds.; Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2021; pp. 197–213. ISBN 9789811604850. [Google Scholar]
- Muringayil Joseph, T.; Mariya, H.J.; Haponiuk, J.T.; Thomas, S.; Esmaeili, A.; Sajadi, S.M. Electromagnetic Interference Shielding Effectiveness of Natural and Chlorobutyl Rubber Blend Nanocomposite. J. Compos. Sci. 2022, 6, 240. [Google Scholar] [CrossRef]
- Yuvaraj, G.; Ramesh, M.; Rajeshkumar, L. Carbon and Cellulose-Based Nanoparticle-Reinforced Polymer Nanocomposites: A Critical Review. Nanomaterials 2023, 13, 1803. [Google Scholar] [CrossRef]
- Raman, N.; Sudharsan, S.; Pothiraj, K. Synthesis and Structural Reactivity of Inorganic–Organic Hybrid Nanocomposites—A Review. J. Saudi Chem. Soc. 2012, 16, 339–352. [Google Scholar] [CrossRef]
- Jaafar, M. Development of Hybrid Fillers/Polymer Nanocomposites for Electronic Applications. In Hybrid Nanomaterials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 349–369. ISBN 978-1-119-16038-0. [Google Scholar]
- Okada, A.; Usuki, A. The Chemistry of Polymer-Clay Hybrids. Mater. Sci. Eng. C 1995, 3, 109–115. [Google Scholar] [CrossRef]
- Usuki, A.; Kojima, Y.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Synthesis of Nylon 6-Clay Hybrid. J. Mater. Res. 1993, 8, 1179–1184. [Google Scholar] [CrossRef]
- Ke, K.; Yue, L.; Shao, H.; Yang, M.-B.; Yang, W.; Manas-Zloczower, I. Boosting Electrical and Piezoresistive Properties of Polymer Nanocomposites via Hybrid Carbon Fillers: A Review. Carbon 2021, 173, 1020–1040. [Google Scholar] [CrossRef]
- Dassan, E.G.B.; Rahman, A.A.A.; Abidin, M.S.Z.; Akil, H.M. Carbon Nanotube–Reinforced Polymer Composite for Electromagnetic Interference Application: A Review. Nanotechnol. Rev. 2020, 9, 768–788. [Google Scholar] [CrossRef]
- Syduzzaman, M.; Chowdhury, K.P.; Fahmi, F.F.; Rumi, S.S.; Hassan, A. Effects of Carbon-Based Nanofillers on Mechanical, Electrical, and Thermal Properties of Bast Fiber Reinforced Polymer Composites. J. Thermoplast. Compos. Mater. 2023, 08927057231216740. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Gong, C.; Liu, B.; Wei, G. Production, Structural Design, Functional Control, and Broad Applications of Carbon Nanofiber-Based Nanomaterials: A Comprehensive Review. Chem. Eng. J. 2020, 402, 126189. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.-W.; Voon, C.H. Synthesis of Graphene Oxide Using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Singh, D.P.; Herrera, C.E.; Singh, B.; Singh, S.; Singh, R.K.; Kumar, R. Graphene Oxide: An Efficient Material and Recent Approach for Biotechnological and Biomedical Applications. Mater. Sci. Eng. C 2018, 86, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and Graphene Oxide as Nanomaterials for Medicine and Biology Application. J. Nanostructure Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef]
- Devi, N.; Kumar, R.; Singh, S.; Singh, R.K. Recent Development of Graphene-Based Composite for Multifunctional Applications: Energy, Environmental and Biomedical Sciences. Crit. Rev. Solid State Mater. Sci. 2024, 49, 72–140. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-Dimensional Printing of High-Content Graphene Scaffolds for Electronic and Biomedical Applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef] [PubMed]
- Khedekar, V.V.; Mohammed Zaeem, S.; Das, S. Graphene-Metal Oxide Nanocomposites for Supercapacitors: A Perspective Review. Adv. Mater. Lett. 2018, 9, 2–19. [Google Scholar] [CrossRef]
- Ray, S. Applications of Graphene and Graphene-Oxide Based Nanomaterials; William Andrew: Norwich, NY, USA, 2015; ISBN 978-0-323-37522-1. [Google Scholar]
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of Graphene in Energy Storage Device—A Review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Kumar, H.; Sharma, R.; Yadav, A.; Kumari, R. Recent Advancement Made in the Field of Reduced Graphene Oxide-Based Nanocomposites Used in the Energy Storage Devices: A Review. J. Energy Storage 2021, 33, 102032. [Google Scholar] [CrossRef]
- Mohammad, A.; Simon, G.P. Rubber-Clay Nanocomposites. In Polymer Nanocomposites; Woodhead Publishing Limited: Sawston, UK, 2006; pp. 297–325. [Google Scholar]
- Robertson, C.G.; Hardman, N.J. Nature of Carbon Black Reinforcement of Rubber: Perspective on the Original Polymer Nanocomposite. Polymers 2021, 13, 538. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Huang, W.; Wang, J.; Wang, X.; Liu, W.; Zhu, Y. Surface Treatment Effects on the Mechanical Properties of Silica Carbon Black Reinforced Natural Rubber/Butadiene Rubber Composites. Polymers 2019, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Fowler, G.D.; Zhao, M. The Past, Present and Future of Carbon Black as a Rubber Reinforcing Filler—A Review. J. Clean. Prod. 2020, 247, 119115. [Google Scholar] [CrossRef]
- Sankaran, K.; Manoharan, P.; Chattopadhyay, S.; Nair, S.; Govindan, U.; Arayambath, S.; Nando, G.B. Effect of Hybridization of Organoclay with Carbon Black on the Transport, Mechanical, and Adhesion Properties of Nanocomposites Based on Bromobutyl/Epoxidized Natural Rubber Blends. RSC Adv. 2016, 6, 33723–33732. [Google Scholar] [CrossRef]
- Castaño-Rivera, P.; Calle-Holguín, I.; Castaño, J.; Cabrera-Barjas, G.; Galvez-Garrido, K.; Troncoso-Ortega, E. Enhancement of Chloroprene/Natural/Butadiene Rubber Nanocomposite Properties Using Organoclays and Their Combination with Carbon Black as Fillers. Polymers 2021, 13, 1085. [Google Scholar] [CrossRef]
- He, S.J.; Wang, Y.Q.; Lin, J.; Zhang, L.Q. Combined Effect of Nano-Clay and Carbon Black on Mechanical Properties and Aging Resistance of Styrene Butadiene Rubber Nanocomposites. Adv. Mater. Res. 2012, 393–395, 28–31. [Google Scholar] [CrossRef]
- He, S.-J.; Wang, Y.-Q.; Wu, Y.-P.; Wu, X.-H.; Lu, Y.-L.; Zhang, L.-Q. Preparation, Structure, Performance, Industrialisation and Application of Advanced Rubber/Clay Nanocomposites Based on Latex Compounding Method. Plast. Rubber Compos. 2010, 39, 33–42. [Google Scholar] [CrossRef]
- Vasilenko, I.V.; Frolov, A.N.; Kostjuk, S.V. Cationic Polymerization of Isobutylene Using AlCl3OBu2 as a Coinitiator: Synthesis of Highly Reactive Polyisobutylene. Macromolecules 2010, 43, 5503–5507. [Google Scholar] [CrossRef]
- Princi, E. Rubber: Science and Technology; Walter de Gruyter GmbH & Co. KG.: Berlin, Germany, 2019; ISBN 978-3-11-064032-8. [Google Scholar]
- Tsai, C.-Y.; Lin, S.-Y.; Tsai, H.-C. Butyl Rubber Nanocomposites with Monolayer MoS2 Additives: Structural Characteristics, Enhanced Mechanical, and Gas Barrier Properties. Polymers 2018, 10, 238. [Google Scholar] [CrossRef]
- Sharma, R.K.; Mohanty, S.; Gupta, V. Advances in Butyl Rubber Synthesis via Cationic Polymerization: An Overview. Polym. Int. 2021, 70, 1165–1175. [Google Scholar] [CrossRef]
- Smith, M.; Berlioz, S.; Chailan, J.F. Radiochemical Ageing of Butyl Rubbers for Space Applications. Polym. Degrad. Stab. 2013, 98, 682–690. [Google Scholar] [CrossRef]
- Cao, R.; Zhao, X.; Zhao, X.; Wu, X.; Li, X.; Zhang, L. Bromination Modification of Butyl Rubber and Its Structure, Properties, and Application. Ind. Eng. Chem. Res. 2019, 58, 16645–16653. [Google Scholar] [CrossRef]
- Akiba, M.; Hashim, A.S. Vulcanization and Crosslinking in Elastomers. Prog. Polym. Sci. 1997, 22, 475–521. [Google Scholar] [CrossRef]
- Ciesielski, A. An Introduction to Rubber Technology; Smithers Rapra Publishing: Shrewsbury, UK, 1999; ISBN 978-1-85957-150-7. [Google Scholar]
- Rodgers, B.; Waddell, W. 9—The Science of Rubber Compounding. In Science and Technology of Rubber, 3rd ed.; Mark, J.E., Erman, B., Eirich, F.R., Eds.; Academic Press: Burlington, MA, USA, 2005; pp. 401–454. ISBN 978-0-12-464786-2. [Google Scholar]
- Vairon, J.-P.; Spassky, N. Industrial Cationic Polymerizations: An Overview. In Cationic Polymerizations; CRC Press: Boca Raton, FL, USA, 1996; ISBN 978-0-429-16842-0. [Google Scholar]
- Sukhareva, K.V.; Sukharev, N.R.; Levina, I.I.; Offor, P.O.; Popov, A.A. Solvent Swelling-Induced Halogenation of Butyl Rubber Using Polychlorinated N-Alkanes: Structure and Properties. Polymers 2023, 15, 4137. [Google Scholar] [CrossRef] [PubMed]
- De, S.K.; White, J.R. Rubber Technologist’s Handbook; Smithers Rapra Publishing: Shrewsbury, UK, 2001; ISBN 978-1-85957-262-7. [Google Scholar]
- Ashok, N.; Balachandran, M. Effect of Nanoclay and Nanosilica on Carbon Black Reinforced EPDM/CIIR Blends for Nuclear Applications. Mater. Res. Express 2020, 6, 125364. [Google Scholar] [CrossRef]
- Neelesh, A.; Vidhyashree, S.; Meera, B. The Influence of MWCNT and Hybrid (MWCNT/Nanoclay) Fillers on Performance of EPDM-CIIR Blends in Nuclear Applications: Mechanical, Hydrocarbon Transport, and Gamma-Radiation Aging Characteristics. J. Appl. Polym. Sci. 2020, 137, 49271. [Google Scholar] [CrossRef]
- Su, C.; He, P.; Yan, R.; Zhao, C.; Zhang, C. Study of the Orientation-Controlled Damping Temperature Based on Selective Distribution of Oligo-Phenol in Acrylate Rubber/Chlorinated Butyl Rubber Blends. Polym. Compos. 2012, 33, 860–865. [Google Scholar] [CrossRef]
- Keloth Paduvilan, J.; Velayudhan, P.; Amanulla, A.; Joseph Maria, H.; Saiter-Fourcin, A.; Thomas, S. Assessment of Graphene Oxide and Nanoclay Based Hybrid Filler in Chlorobutyl-Natural Rubber Blend for Advanced Gas Barrier Applications. Nanomaterials 2021, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, A.K.; Geethamma, V.G.; Chandra, A.K.; Mohammed, P.K.; Thomas, S. Rheological Behaviour of Clay Incorporated Natural Rubber and Chlorobutyl Rubber Nanocomposites. RSC Adv. 2014, 4, 58047–58058. [Google Scholar] [CrossRef]
- Saritha, A.; Joseph, K.; Thomas, S.; Muraleekrishnan, R. Chlorobutyl Rubber Nanocomposites as Effective Gas and VOC Barrier Materials. Compos. Part Appl. Sci. Manuf. 2012, 43, 864–870. [Google Scholar] [CrossRef]
- Sreehari, H.; Aparna, A.; Jayan, J.S.; Sethulekshmi, A.S.; Gopika, V.; Anjali, K.P.; Parvathy, N.; Saritha, A. Evaluation of Solvent Transport and Cure Characteristics of Chlorobutyl Rubber Graphene Oxide Nanocomposites. Mater. Today Proc. 2022, 49, 1431–1435. [Google Scholar] [CrossRef]
- Zhang, F.; He, G.; Xu, K.; Wu, H.; Guo, S.; Zhang, C. Damping Mechanism and Different Modes of Molecular Motion through the Glass Transition of Chlorinated Butyl Rubber and Petroleum Resin Blends. J. Appl. Polym. Sci. 2014, 131, 40464. [Google Scholar] [CrossRef]
- Kraus, G. Degree of Cure in Filler-Reinforced Vulcanizates by the Swelling Method. Rubber Chem. Technol. 1957, 30, 928–951. [Google Scholar] [CrossRef]
- Picard, E.; Espuche, E.; Fulchiron, R. Effect of an Organo-Modified Montmorillonite on PLA Crystallization and Gas Barrier Properties. Appl. Clay Sci. 2011, 53, 58–65. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Avérous, L. Nano-Biocomposites: Biodegradable Polyester/Nanoclay Systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Capezza, A.; Andersson, R.L.; Ström, V.; Wu, Q.; Sacchi, B.; Farris, S.; Hedenqvist, M.S.; Olsson, R.T. Preparation and Comparison of Reduced Graphene Oxide and Carbon Nanotubes as Fillers in Conductive Natural Rubber for Flexible Electronics. ACS Omega 2019, 4, 3458–3468. [Google Scholar] [CrossRef] [PubMed]
- Dal Lago, E.; Cagnin, E.; Boaretti, C.; Roso, M.; Lorenzetti, A.; Modesti, M. Influence of Different Carbon-Based Fillers on Electrical and Mechanical Properties of a PC/ABS Blend. Polymers 2020, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhao, H.; Li, G.; Dai, K.; Zheng, G.; Liu, C.; Shen, C. Synergistic Effect of Carbon Fibers on the Conductive Properties of a Segregated Carbon Black/Polypropylene Composite. Mater. Lett. 2014, 129, 72–75. [Google Scholar] [CrossRef]
- Clingerman, M.L.; Weber, E.H.; King, J.A.; Schulz, K.H. Synergistic Effect of Carbon Fillers in Electrically Conductive Nylon 6,6 and Polycarbonate Based Resins. Polym. Compos. 2002, 23, 911–924. [Google Scholar] [CrossRef]
- Vipulanandan, C.; Mohammed, A. Effect of Nanoclay on the Electrical Resistivity and Rheological Properties of Smart and Sensing Bentonite Drilling Muds. J. Pet. Sci. Eng. 2015, 130, 86–95. [Google Scholar] [CrossRef]
- Rajini, N.; Jappes, J.T.W.; Rajakarunakaran, S.; Poornanand, P. Electrical Properties of Montmorillonite Nanoclay Reinforced Unsaturated Polyester Nanocomposite. In Proceedings of the IEEE-International Conference On Advances In Engineering, Science And Management (ICAESM-2012), Nagapattinam, India, 30–31 March 2012. [Google Scholar]
- Rashmi; Renukappa, N.M.; Chikkakuntappa, R.; Kunigal, N.S. Montmorillonite Nanoclay Filler Effects on Electrical Conductivity, Thermal and Mechanical Properties of Epoxy-Based Nanocomposites. Polym. Eng. Sci. 2011, 51, 1827–1836. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A Review and Analysis of Electrical Percolation in Carbon Nanotube Polymer Composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Mott, S.N.F.; Davis, E.A. Electronic Processes in Non-Crystalline Materials; OUP Oxford: Oxford, UK, 2012; ISBN 978-0-19-964533-6. [Google Scholar]
- Yang, Y.; Sun, H.; Zhu, B.; Wang, Z.; Wei, J.; Xiong, R.; Shi, J.; Liu, Z.; Lei, Q. Enhanced Dielectric Performance of Three Phase Percolative Composites Based on Thermoplastic-Ceramic Composites and Surface Modified Carbon Nanotube. Appl. Phys. Lett. 2015, 106, 012902. [Google Scholar] [CrossRef]
- Vorobiev, A.; Dennison, A.; Chernyshov, D.; Skrypnychuk, V.; Barbero, D.; Talyzin, A.V. Graphene Oxide Hydration and Solvation: An in Situ Neutron Reflectivity Study. Nanoscale 2014, 6, 12151–12156. [Google Scholar] [CrossRef]
- Rezania, B.; Severin, N.; Talyzin, A.V.; Rabe, J.P. Hydration of Bilayered Graphene Oxide. Nano Lett. 2014, 14, 3993–3998. [Google Scholar] [CrossRef]
- Pei, S.; Zhao, J.; Du, J.; Ren, W.; Cheng, H.-M. Direct Reduction of Graphene Oxide Films into Highly Conductive and Flexible Graphene Films by Hydrohalic Acids. Carbon 2010, 48, 4466–4474. [Google Scholar] [CrossRef]
- Vallés, C.; David Núñez, J.; Benito, A.M.; Maser, W.K. Flexible Conductive Graphene Paper Obtained by Direct and Gentle Annealing of Graphene Oxide Paper. Carbon 2012, 50, 835–844. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, X.; Zhu, J.; Zeng, B.; Wu, Z.; Li, X. The Effect of Ambient Humidity on the Electrical Properties of Graphene Oxide Films. Nanoscale Res. Lett. 2012, 7, 363. [Google Scholar] [CrossRef] [PubMed]




| Experiment | Mass of CIIR (Phr) | Mass of Nanoclay (Phr) | Mass of CB (Phr) | Mass of GO (Phr) |
|---|---|---|---|---|
| CIIR | 100 | 0 | 0 | 0 |
| CIIR + CB | 100 | 0 | 1 | 0 |
| CIIR + clay | 100 | 1 | 0 | 0 |
| CIIR + GO | 100 | 0 | 0 | 1 |
| CIIR + clay + CB | 100 | 0.5 | 0.5 | 0 |
| CIIR | CIIR + Clay | |
|---|---|---|
| Ra (average surface roughness) | 10.88 nm | 48.90 nm |
| RMS (root mean square) | 16.88 nm | 75.25 nm |
| Sample | CIIR pure | CIIR + GO | CIIR + CB | CIIR + Clay | CIIR + Clay + CB |
|---|---|---|---|---|---|
| Resistivity (Ω-cm) | 5.76 × 1015 | 4.05 × 1015 | 6.55 × 1015 | 1.20 × 1014 | 3.69 × 1012 |
| (MPa) | CIIR Pure | CIIR/GO | CIIR/CB | CIIR/Clay | CIIR/CB/Clay |
|---|---|---|---|---|---|
| Stress at 100% strain | 0.27 | 0.36 | 0.36 | 0.44 | 0.45 |
| Stress at 200% strain | 0.35 | 0.47 | 0.45 | 0.53 | 0.53 |
| Stress at 300% strain | 0.36 | 0.47 | 0.44 | 0.53 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, T.M.; Maria, H.J.; Thomas, M.G.; Haponiuk, J.T.; Thomas, S. Effects of Nanofillers and Synergistic Action of Carbon Black/Nanoclay Hybrid Fillers in Chlorobutyl Rubber. J. Compos. Sci. 2024, 8, 209. https://doi.org/10.3390/jcs8060209
Joseph TM, Maria HJ, Thomas MG, Haponiuk JT, Thomas S. Effects of Nanofillers and Synergistic Action of Carbon Black/Nanoclay Hybrid Fillers in Chlorobutyl Rubber. Journal of Composites Science. 2024; 8(6):209. https://doi.org/10.3390/jcs8060209
Chicago/Turabian StyleJoseph, Tomy Muringayil, Hanna J. Maria, Martin George Thomas, Józef T. Haponiuk, and Sabu Thomas. 2024. "Effects of Nanofillers and Synergistic Action of Carbon Black/Nanoclay Hybrid Fillers in Chlorobutyl Rubber" Journal of Composites Science 8, no. 6: 209. https://doi.org/10.3390/jcs8060209








