Electrochemical Enrichment of Biocharcoal Modified on Carbon Electrodes for the Detection of Nitrite and Paraxon Ethyl Pesticide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solutions and Electrodes
2.2. BioC Preparation from Almond Shell
2.3. Electrode Modification and Electrochemical Measurements
2.4. Microscopic and Spectroscopic Characterization
3. Results
3.1. Physical and Electrochemical Characterization
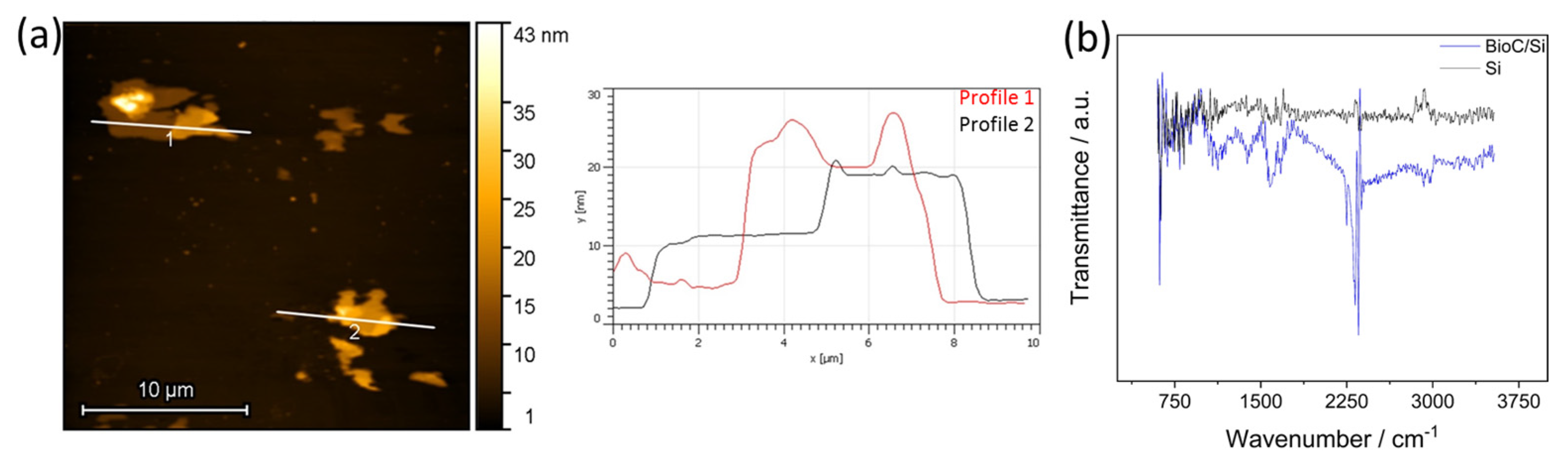
3.1.1. AS-BioC on Silicon Substrate
3.1.2. Morphological and Structural Characterization of AS-BioC Modified SPCE Electrodes
3.1.3. Electrochemical Characterization of AS-BioC Modified SPCE Electrodes
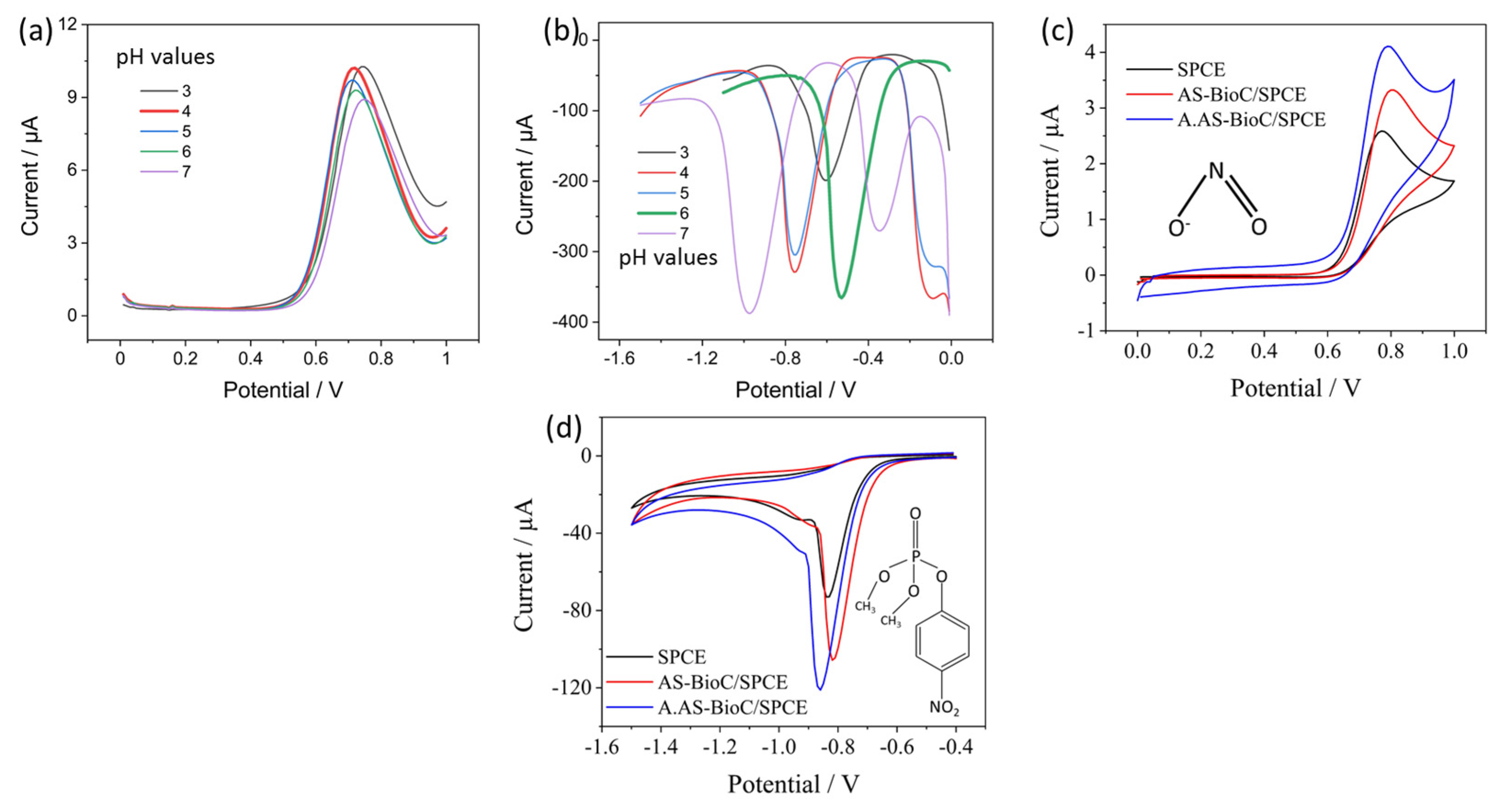
3.2. Optimization of pH and Electrochemical Response of SPCE, AS-BioC/SPCE and AS-A. BioC/SPCE
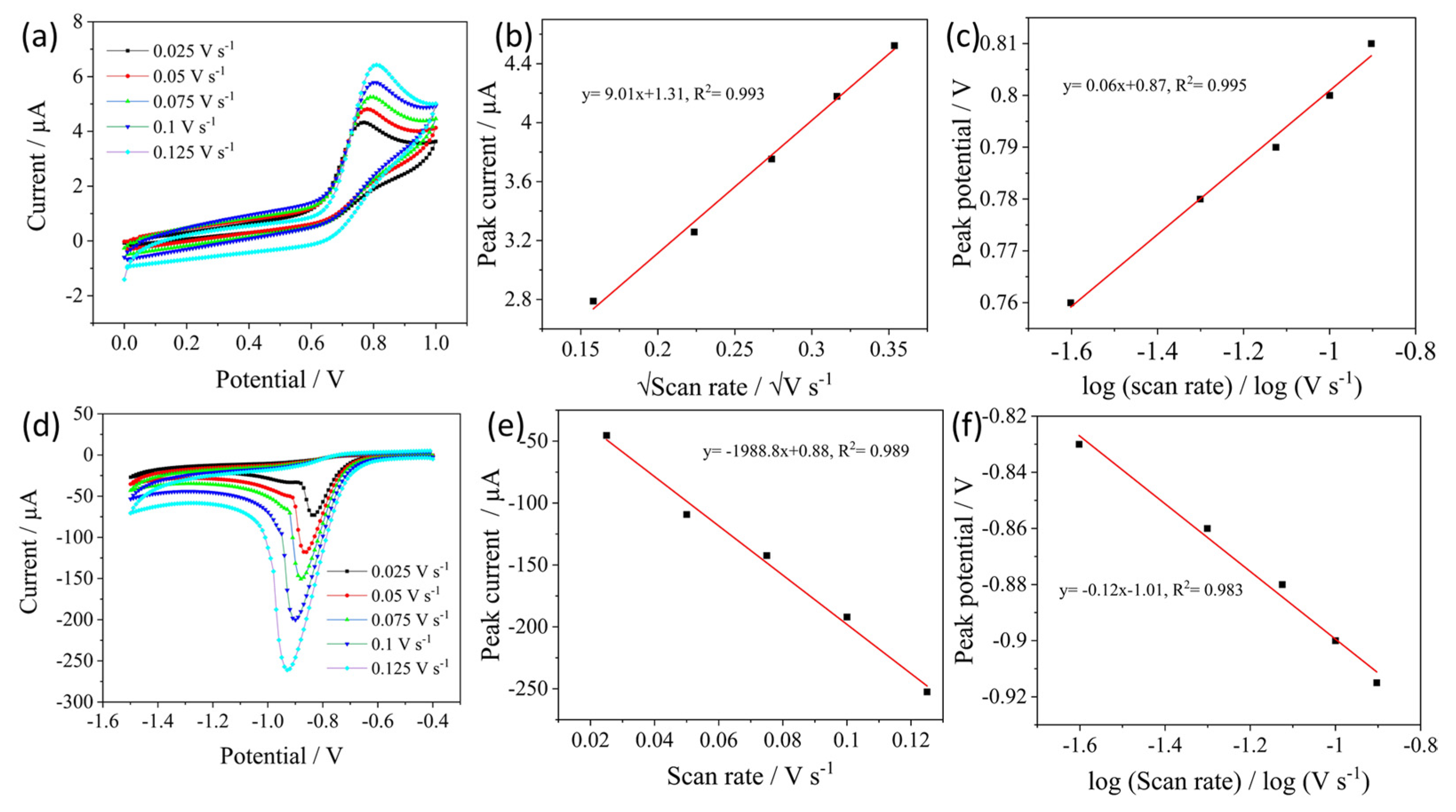
3.3. Catalytic Activity of AS-A. BioC/SPCE towards Nitrite and Paraxon Ethyl
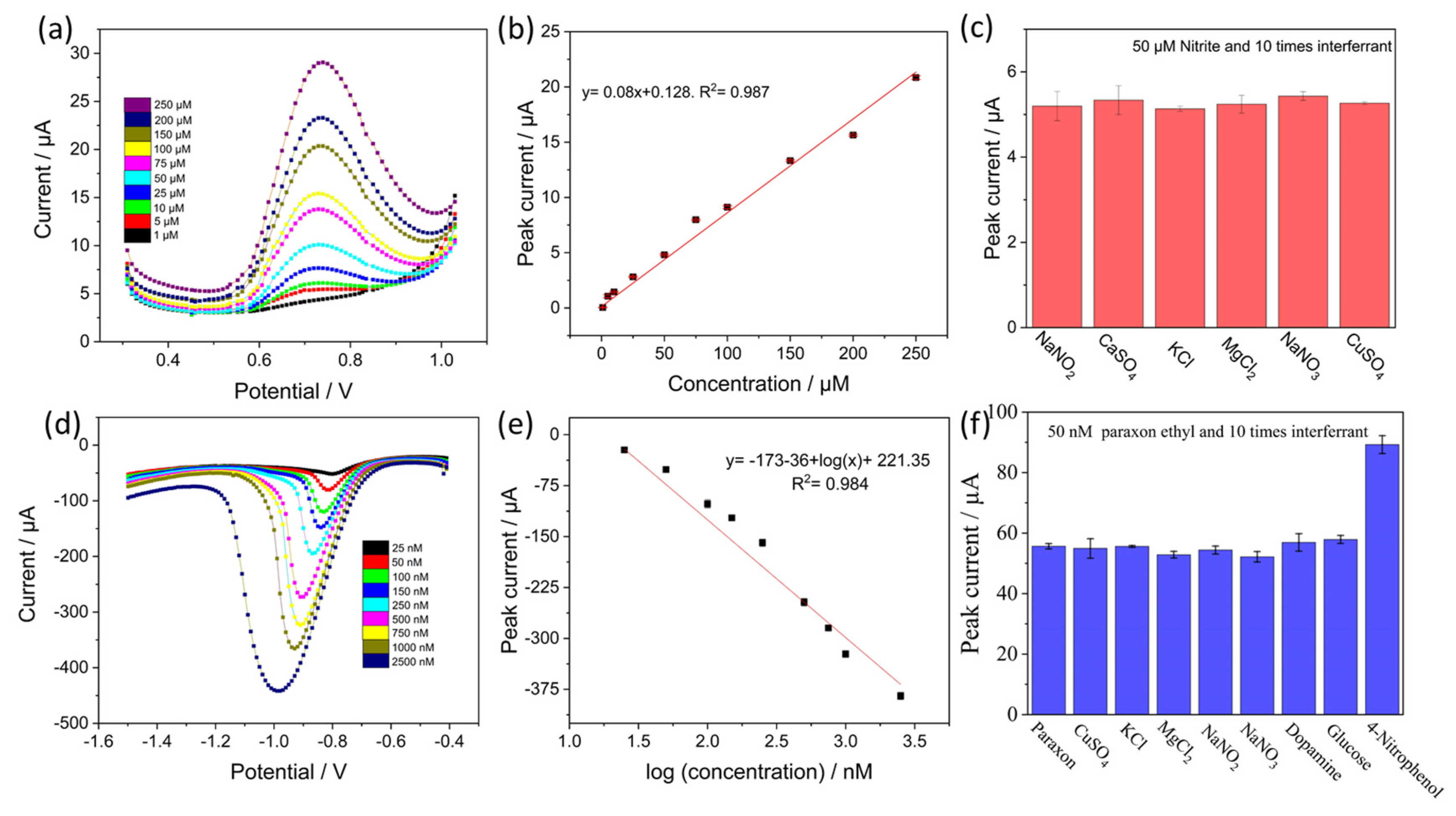
3.4. Electrochemical Detection and Selectivity Studies
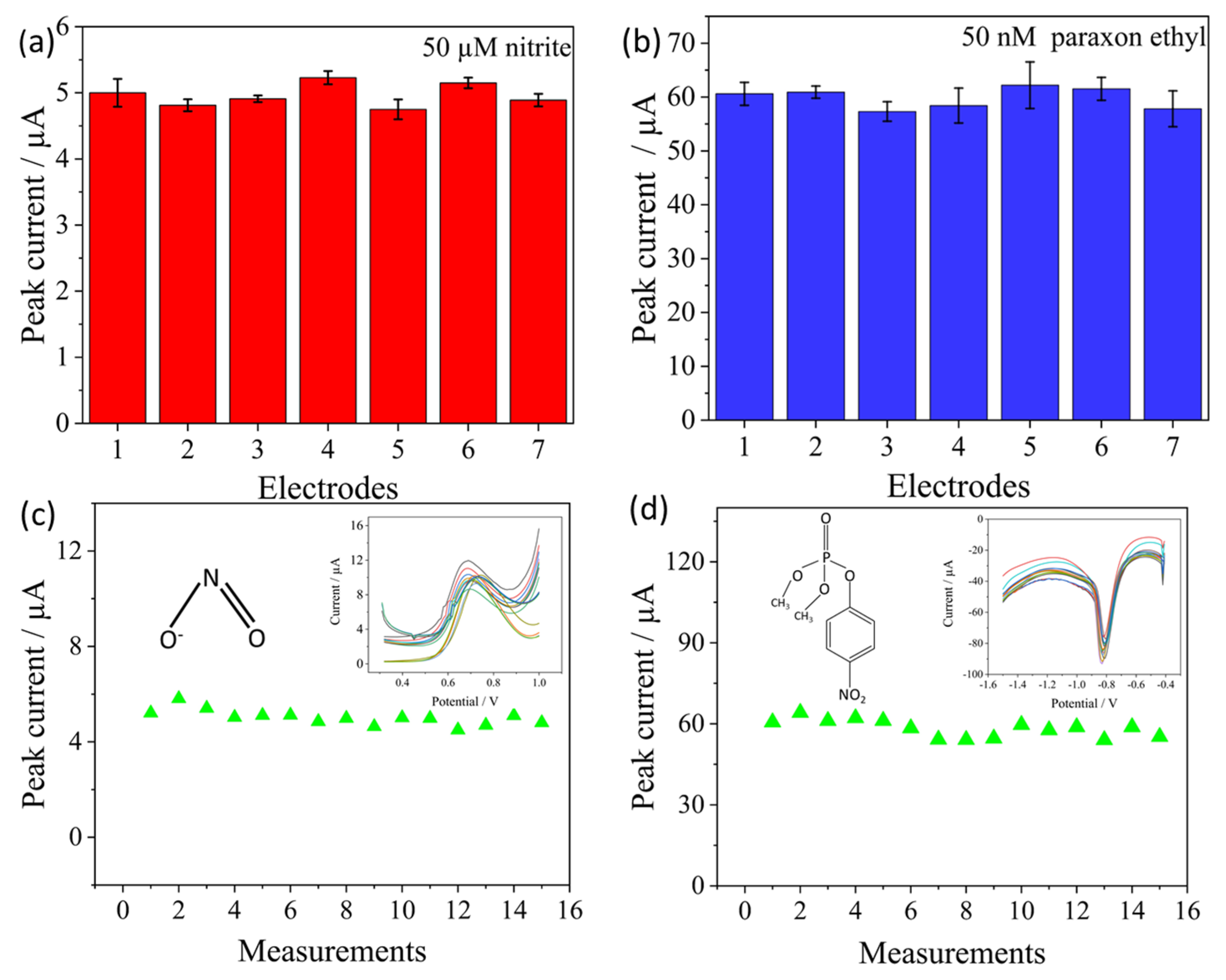
3.5. Reproducibility, Stability and Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, M.H.; Khan, R.; Andreescu, S. Advances in electrochemical detection methods for measuring contaminants of emerging concerns. Electrochem. Sci. Adv. 2022, 2, e2100184. [Google Scholar] [CrossRef]
- Kanoun, O.; Lazarević-Pašti, T.; Pašti, I.; Nasraoui, S.; Talbi, M.; Brahem, A.; Adiraju, A.; Sheremet, E.; Rodriguez, R.D.; Ben Ali, M.; et al. A Review of Nanocomposite-Modified Electrochemical Sensors for Water Quality Monitoring. Sensors 2021, 21, 4131. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Y.-T.; Li, D.-W.; Long, Y.-T. Recent developments and applications of screen-printed electrodes in environmental assays—A review. Anal. Chim. Acta 2012, 734, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Hui, T.S.; Zaini, M.A.A. Potassium hydroxide activation of activated carbon: A commentary. Carbon Lett. 2015, 16, 275–280. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Yorgun, S.; Vural, N.; Demiral, H. Preparation of high-surface area activated carbons from Paulownia wood by ZnCl2 activation. Microporous Mesoporous Mater. 2009, 122, 189–194. [Google Scholar] [CrossRef]
- Sakhiya, A.K.; Anand, A.; Kaushal, P. Production, activation, and applications of biochar in recent times. Biochar 2020, 2, 253–285. [Google Scholar] [CrossRef]
- Angın, D.; Altintig, E.; Köse, T.E. Influence of process parameters on the surface and chemical properties of activated carbon obtained from biochar by chemical activation. Bioresour. Technol. 2013, 148, 542–549. [Google Scholar] [CrossRef]
- Jin, H.; Capareda, S.; Chang, Z.; Gao, J.; Xu, Y.; Zhang, J. Biochar pyrolytically produced from municipal solid wastes for aqueous As(V) removal: Adsorption property and its improvement with KOH activation. Bioresour. Technol. 2014, 169, 622–629. [Google Scholar] [CrossRef]
- Kalinke, C.; Oliveira, P.R.; Oliveira, G.A.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Activated biochar: Preparation, characterization and electroanalytical application in an alternative strategy of nickel determination. Anal. Chim. Acta 2017, 983, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, L.; Wang, X.; Holm, N.; Rajagopalan, K.; Chen, F.; Ma, S. Highly ordered macroporous woody biochar with ultra-high carbon content as supercapacitor electrodes. Electrochim. Acta 2013, 113, 481–489. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; Sui, Z.; Zhang, Y.; Norris, P.; Sun, B.; Pan, W.-P. Plasma Induced Addition of Active Functional Groups to Biochar for Elemental Mercury Removal. Plasma Chem. Plasma Process. 2019, 39, 1449–1468. [Google Scholar] [CrossRef]
- Lalander, C.; Dalahmeh, S.; Jönsson, H.; Vinnerås, B. Hygienic quality of artificial greywater subjected to aerobic treatment: A comparison of three filter media at increasing organic loading rates. Environ. Technol. 2013, 34, 2657–2662. [Google Scholar] [CrossRef] [PubMed]
- Creamer, A.E.; Gao, B.; Wang, S. Carbon dioxide capture using various metal oxyhydroxide–biochar composites. Chem. Eng. J. 2016, 283, 826–832. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Chen, Y.; Zhu, X. Biodiesel production from waste cooking oil using a heterogeneous catalyst from pyrolyzed rice husk. Bioresour. Technol. 2014, 154, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Gao, B.; Mosa, A.; Yu, H.; Yin, X.; Bashir, A.; Ghoveisi, H.; Wang, S. Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J. Clean. Prod. 2018, 180, 437–449. [Google Scholar] [CrossRef]
- Ramesh, T.; Rajalakshmi, N.; Dhathathreyan, K.S. Activated carbons derived from tamarind seeds for hydrogen storage. J. Energy Storage 2015, 4, 89–95. [Google Scholar] [CrossRef]
- Kalinke, C.; de Oliveira, P.R.; Bonacin, J.A.; Janegitz, B.C.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. State-of-the-art and perspectives in the use of biochar for electrochemical and electroanalytical applications. Green Chem. 2021, 23, 5272–5301. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Wang, H.; Xu, W.; Tian, L.; Huang, J.; Liang, C.; Zhang, Y. Recent Advances of Biochar-Based Electrochemical Sensors and Biosensors. Biosensors 2022, 12, 377. [Google Scholar] [CrossRef]
- Chingombe, P.; Saha, B.; Wakeman, R.J. Surface modification and characterisation of a coal-based activated carbon. Carbon 2005, 43, 3132–3143. [Google Scholar] [CrossRef]
- Wu, R.; Ye, Q.; Wu, K.; Wang, L.; Dai, H. Highly efficient CO2 adsorption of corn kernel-derived porous carbon with abundant oxygen functional groups. J. CO2 Util. 2021, 51, 101620. [Google Scholar] [CrossRef]
- Kim, J.-G.; Kim, H.-B.; Baek, K. Novel electrochemical method to activate biochar derived from spent coffee grounds for enhanced adsorption of lead (Pb). Sci. Total Environ. 2023, 886, 163891. [Google Scholar] [CrossRef]
- Soliman, M.; Eldyasti, A. Ammonia-Oxidizing Bacteria (AOB): Opportunities and applications—A review. Rev. Environ. Sci. Biotechnol. 2018, 17, 285–321. [Google Scholar] [CrossRef]
- Brahem, A.; Al-Hamry, A.; Gross, M.A.; Paterno, L.G.; Ali, M.B.; Kanoun, O. Stability Enhancement of Laser-Scribed Reduced Graphene Oxide Electrodes Functionalized by Iron Oxide/Reduced Graphene Oxide Nanocomposites for Nitrite Sensors. J. Compos. Sci. 2022, 6, 221. [Google Scholar] [CrossRef]
- Xiao, B. Electrochemical sensor based on Bimetallic phosphosulfide Zn–Ni–P–S Nanocomposite-reduced graphene oxide for determination of Paraoxon Ethyl in agriculture wastewater. Int. J. Electrochem. Sci. 2022, 17, 220672. [Google Scholar] [CrossRef]
- Elleuch, A.; Boussetta, A.; Yu, J.; Halouani, K.; Li, Y. Experimental investigation of direct carbon fuel cell fueled by almond shell biochar: Part I. Physico-chemical characterization of the biochar fuel and cell performance examination. Int. J. Hydrogen Energy 2013, 38, 16590–16604. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Anurag, A.; Al-Hamry, A.; Attuluri, Y.; Palaniyappan, S.; Wagner, G.; Dentel, D.; Tegenkamp, C.; Kanoun, O. Optimized Reduction of a Graphene Oxide-MWCNT Composite with Electrochemically Deposited Copper Nanoparticles on Screen Printed Electrodes for a Wide Range of Detection of Nitrate. ChemElectroChem 2023, 10, e202200945. [Google Scholar] [CrossRef]
- Amin, F.R.; Huang, Y.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Biochar applications and modern techniques for characterization. Clean Technol. Environ. Policy 2016, 18, 1457–1473. [Google Scholar] [CrossRef]
- Tran, H.N.; Lee, C.-K.; Vu, M.T.; Chao, H.-P. Removal of Copper, Lead, Methylene Green 5, and Acid Red 1 by Saccharide-Derived Spherical Biochar Prepared at Low Calcination Temperatures: Adsorption Kinetics, Isotherms, and Thermodynamics. Water Air Soil Pollut. 2017, 228, 401. [Google Scholar] [CrossRef]
- Deng, J.; Xiong, T.; Wang, H.; Zheng, A.; Wang, Y. Effects of Cellulose, Hemicellulose, and Lignin on the Structure and Morphology of Porous Carbons. ACS Sustain. Chem. Eng. 2016, 4, 3750–3756. [Google Scholar] [CrossRef]
- Siipola, V.; Tamminen, T.; Källi, A.; Lahti, R.; Romar, H.; Rasa, K.; Keskinen, R.; Hyväluoma, J.; Hannula, M.; Wikberg, H. Effects of biomass type, carbonization process, and activation method on the properties of bio-based activated carbons. BioResources 2018, 13, 5976–6002. [Google Scholar] [CrossRef]
- Reza, M.S.; Afroze, S.; Bakar, M.S.; Saidur, R.; Aslfattahi, N.; Taweekun, J.; Azad, A.K. Biochar characterization of invasive Pennisetum purpureum grass: Effect of pyrolysis temperature. Biochar 2020, 2, 239–251. [Google Scholar] [CrossRef]
- León, V.; Quintana, M.; Herrero, M.A.; Fierro, J.L.G.; de La Hoz, A.; Prato, M.; Vázquez, E. Few-layer graphenes from ball-milling of graphite with melamine. Chem. Commun. 2011, 47, 10936–10938. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferro, A.; Rovere, M.; Padovano, E.; Bartoli, M.; Giorcelli, M. Introducing the Novel Mixed Gaussian-Lorentzian Lineshape in the Analysis of the Raman Signal of Biochar. Nanomaterials 2020, 10, 1748. [Google Scholar] [CrossRef] [PubMed]
- González-Meza, O.A.; Larios-Durán, E.R.; Gutiérrez-Becerra, A.; Casillas, N.; Escalante, J.I.; Bárcena-Soto, M. Development of a Randles-Ševčík-like equation to predict the peak current of cyclic voltammetry for solid metal hexacyanoferrates. J. Solid State Electrochem. 2019, 23, 3123–3133. [Google Scholar] [CrossRef]
- Pham, X.-H.; Li, C.A.; Han, K.N.; Huynh-Nguyen, B.-C.; Le, T.-H.; Ko, E.; Kim, J.H.; Seong, G.H. Electrochemical detection of nitrite using urchin-like palladium nanostructures on carbon nanotube thin film electrodes. Sens. Actuators B Chem. 2014, 193, 815–822. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Durairaj, S.; Boateng, E.; Sidhureddy, B.; Chen, A. Electrochemical Detection of Nitrite Based on Co3O4-Au Nanocomposites for Food Quality Control. J. Electrochem. Soc. 2021, 168, 107505. [Google Scholar] [CrossRef]
- Brylev, O.; Sarrazin, M.; Roué, L.; Bélanger, D. Nitrate and nitrite electrocatalytic reduction on Rh-modified pyrolytic graphite electrodes. Electrochim. Acta 2007, 52, 6237–6247. [Google Scholar] [CrossRef]
- Mutharani, B.; Ranganathan, P.; Chen, S.-M.; Karuppiah, C. Enzyme-free electrochemical detection of nanomolar levels of the organophosphorus pesticide paraoxon-ethyl by using a poly(N-isopropyl acrylamide)-chitosan microgel decorated with palladium nanoparticles. Mikrochim. Acta 2019, 186, 167. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chatrathi, M.P.; Mulchandani, A.; Chen, W. Capillary electrophoresis microchips for separation and detection of organophosphate nerve agents. Anal. Chem. 2001, 73, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Aghaie, A.; Khanmohammadi, A.; Hajian, A.; Schmid, U.; Bagheri, H. Nonenzymatic Electrochemical Determination of Paraoxon Ethyl in Water and Fruits by Graphene-Based NiFe Bimetallic Phosphosulfide Nanocomposite as a Superior Sensing Layer. Food Anal. Methods 2019, 12, 1545–1555. [Google Scholar] [CrossRef]
- Zhang, C.; Govindaraju, S.; Giribabu, K.; Huh, Y.S.; Yun, K. AgNWs-PANI nanocomposite based electrochemical sensor for detection of 4-nitrophenol. Sens. Actuators B Chem. 2017, 252, 616–623. [Google Scholar] [CrossRef]
- Laviron, E. Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J. Electroanal. Chem. Interfacial Electrochem. 1974, 52, 355–393. [Google Scholar] [CrossRef]
- Adiraju, A.; Jalasutram, A.; Wang, J.; Tegenkamp, C.; Kanoun, O. Electrodeposited Silver Dendrites on Laser-Induced Graphene for Electrochemical Detection of Nitrate with Tunable Sensor Properties. Adv. Mater. Interfaces 2024. [Google Scholar] [CrossRef]
- Cao, L.; Kang, Z.-W.; Ding, Q.; Zhang, X.; Lin, H.; Lin, M.; Yang, D.-P. Rapid pyrolysis of Cu2+-polluted eggshell membrane into a functional Cu2+-Cu+/biochar for ultrasensitive electrochemical detection of nitrite in water. Sci. Total Environ. 2020, 723, 138008. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, A.D.; Muchindu, M.; Jijana, A.; Mishra, S.; Nxumalo, E. Screen-printed electrode system based on carbon black/copper-organic framework hybrid nanocomposites for the electrochemical detection of nitrite. Mater. Today Commun. 2023, 35, 105567. [Google Scholar] [CrossRef]
- Allende, S.; Liu, Y.; Zafar, M.A.; Jacob, M.V. Nitrite sensor using activated biochar synthesised by microwave-assisted pyrolysis. Waste Dispos. Sustain. Energy 2023, 5, 1–11. [Google Scholar] [CrossRef]
- Jiang, J.; Nie, Y.; Fozia; Lin, J.; Dai, Z.; Xu, X.; Huang, X.; Wang, C.; Hu, Z.; Xu, H. Preparation of Spirogyra-derived biochar modified electrode and its application in nitrite detection. Biomass Conv. Bioref. 2023. [Google Scholar] [CrossRef]
- Renganathan, V.; Balaji, R.; Chen, S.-M.; Kokulnathan, T. Coherent design of palladium nanostructures adorned on the boron nitride heterojunctions for the unparalleled electrochemical determination of fatal organophosphorus pesticides. Sens. Actuators B Chem. 2020, 307, 127586. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Wang, T.-J.; Wang, Y.-Y.; Suvina, V.; Ahmed, F. Three-dimensional manganese cobaltate: A highly conductive electrocatalyst for paraoxon-ethyl detection. Mikrochim. Acta 2022, 189, 315. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Neagu, D.; Scognamiglio, V.; Patarino, S.; Moscone, D.; Palleschi, G. Automatable Flow System for Paraoxon Detection with an Embedded Screen-Printed Electrode Tailored with Butyrylcholinesterase and Prussian Blue Nanoparticles. Chemosensors 2015, 3, 129–145. [Google Scholar] [CrossRef]
- Deo, R.P.; Wang, J.; Block, I.; Mulchandani, A.; Joshi, K.A.; Trojanowicz, M.; Scholz, F.; Chen, W.; Lin, Y. Determination of organophosphate pesticides at a carbon nanotube/organophosphorus hydrolase electrochemical biosensor. Anal. Chim. Acta 2005, 530, 185–189. [Google Scholar] [CrossRef]



| Material Used | LOD (µM) | Sensitivity | Linear Range (µM) | Detection Method | Real Samples | Ref. |
|---|---|---|---|---|---|---|
| Cu2+-Cu/Biochar | 0.63 | 30.0 μA·mM−1·cm−2 | 1–300 | DPV | Mineral water, tap water, and sausage | [47] |
| ZnCl2-biochar/GCE | 0.97 | - | 100–1400 | CA | - | [49] |
| SBPDH/CS/GCE | 8.29 | - | 20–6000 | DPV | - | [50] |
| A.AS-BioC/SPCE | 0.375 | 0.08 µA µM−1 | 1–250 | SWV | Ground water | This work |
| Material Used | LOD (nM) | Sensitivity | Linear Range (nM) | Detection Method | Real Samples | Ref. |
|---|---|---|---|---|---|---|
| NiFE SP@Gr | 3.7 | - | 12–1000 | SWV | Tap water, tomato juice, and cucumber juice | [43] |
| Pd NP/BN | 3 | 2.23 μA μM−1 cm−2 | 10–21,000 | LSV | River sample | [51] |
| MnCO2O4 | 2 | 2.3 µA µM−1 cm2 | 15–43,500 | CA | - | [52] |
| BuChE/PBNPs/SPE | 4 | - | 7–10 | CA | Albano Lake, tap water and river river | [53] |
| SWCNTS/OPH/GCE | 150 | - | 250–400 | CA | - | [54] |
| Zn-Ni-P-S/GCE | 35 | 0.06369 μA μM−1 | 1000–200,000 | CA | Wastewater | [26] |
| A.AS-BioC/SPCE | 1.63 | 17.3 µA nM−1 | 25–2500 | SWV | Ground water | This work |
| Target | Spiked | Peak Current Obtained | Found | Recovery |
|---|---|---|---|---|
| Nitrite | 50 µM | 4.91 µA | 54.03 µM | 108.6% |
| Paraxon ethyl | 50 nM | 54.32 µA | 48.21 µM | 96.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adiraju, A.; Brahem, A.; Lu, T.; Al-Hamry, A.; Zhou, Y.; Wei, L.; Jalasutram, A.; Tegenkamp, C.; Halouani, K.; Kanoun, O. Electrochemical Enrichment of Biocharcoal Modified on Carbon Electrodes for the Detection of Nitrite and Paraxon Ethyl Pesticide. J. Compos. Sci. 2024, 8, 217. https://doi.org/10.3390/jcs8060217
Adiraju A, Brahem A, Lu T, Al-Hamry A, Zhou Y, Wei L, Jalasutram A, Tegenkamp C, Halouani K, Kanoun O. Electrochemical Enrichment of Biocharcoal Modified on Carbon Electrodes for the Detection of Nitrite and Paraxon Ethyl Pesticide. Journal of Composites Science. 2024; 8(6):217. https://doi.org/10.3390/jcs8060217
Chicago/Turabian StyleAdiraju, Anurag, Amina Brahem, Tianqi Lu, Ammar Al-Hamry, Yu Zhou, Leixin Wei, Aditya Jalasutram, Christoph Tegenkamp, Kamel Halouani, and Olfa Kanoun. 2024. "Electrochemical Enrichment of Biocharcoal Modified on Carbon Electrodes for the Detection of Nitrite and Paraxon Ethyl Pesticide" Journal of Composites Science 8, no. 6: 217. https://doi.org/10.3390/jcs8060217
APA StyleAdiraju, A., Brahem, A., Lu, T., Al-Hamry, A., Zhou, Y., Wei, L., Jalasutram, A., Tegenkamp, C., Halouani, K., & Kanoun, O. (2024). Electrochemical Enrichment of Biocharcoal Modified on Carbon Electrodes for the Detection of Nitrite and Paraxon Ethyl Pesticide. Journal of Composites Science, 8(6), 217. https://doi.org/10.3390/jcs8060217









