Biocomposite Based on Polyhydroxybutyrate and Cellulose Acetate for the Adsorption of Methylene Blue
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Composite Biomaterials
2.2. Characterization of Composite Biomaterials
2.3. Adsorption Tests
3. Results
3.1. Preparation of Composite Biomaterials
3.2. Thermogravimetric Analysis
3.3. Characterization of the Biomaterial
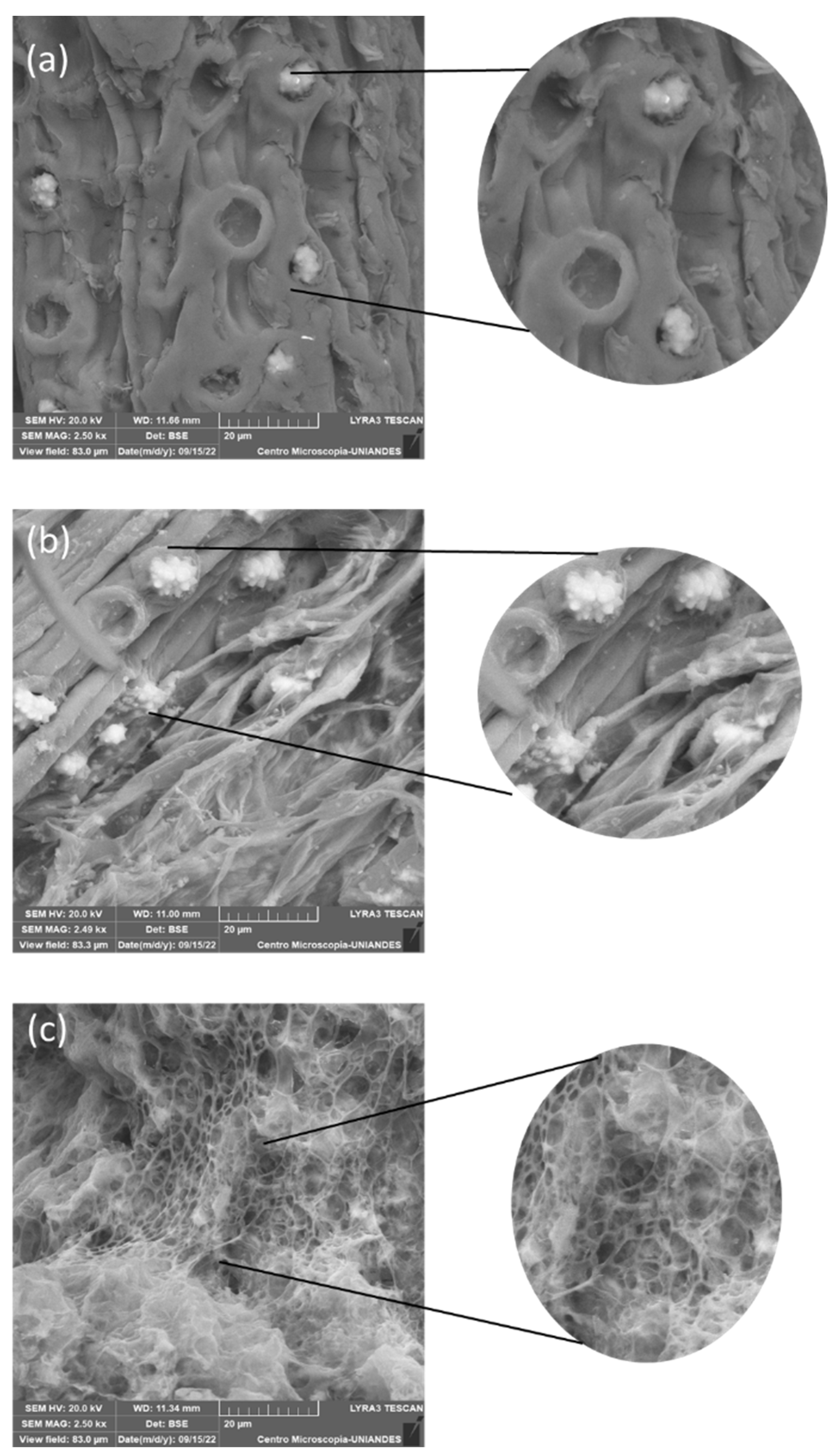
3.4. SEM Analysis
3.5. XRD Analysis for the CA/PHB
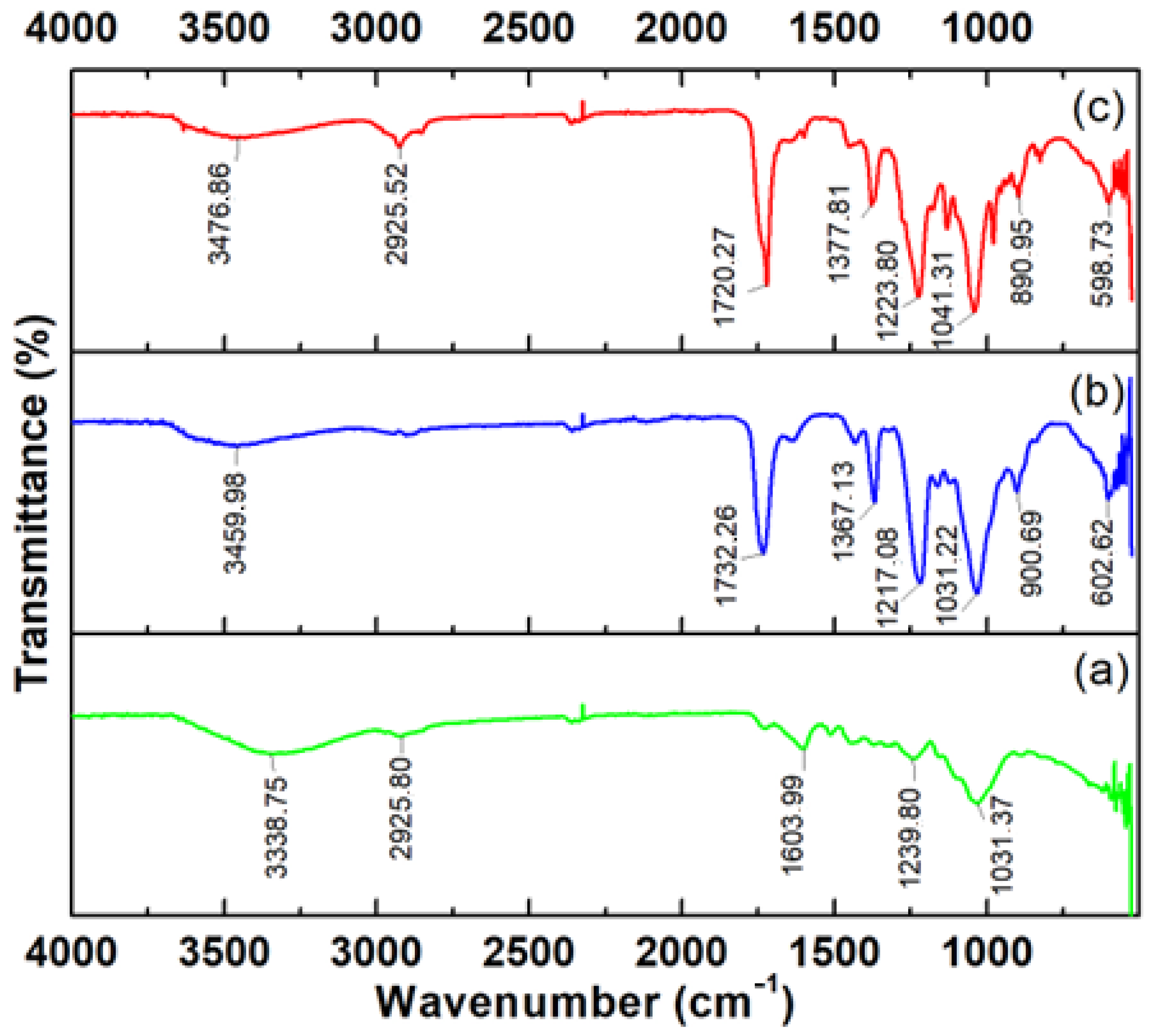
3.6. FTIR Analysis before Adsorption
3.7. Adsorption Test
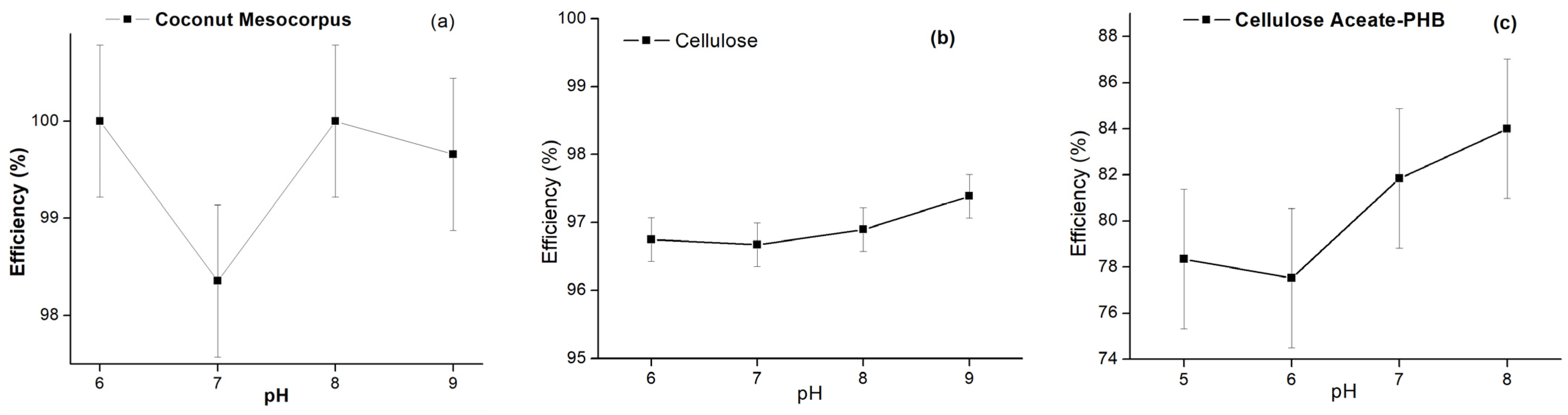
3.7.1. Influence of pH on the Adsorption Test
3.7.2. Effect of Adsorbent Dosage on Adsorption Efficiency
3.7.3. FITR Analysis after Adsorption Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chong, Z.T.; Soh, L.S.; Yong, W.F. Valorization of agriculture wastes as biosorbents for adsorption of emerging pollutants: Modification, remediation and industry application. Results Eng. 2023, 17, 100960. [Google Scholar] [CrossRef]
- Ahmadian, M.; Derakhshankhah, H.; Jaymand, M. Recent advances in adsorption of environmental pollutants using metal–organic frameworks-based hydrogels. Int. J. Biol. Macromol. 2023, 231, 123333. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Kamaruddin, M.A.; Abdul, A.K.; Yahya, E.B.; Muhammad, S.; Rizal, S.; Ahmad, M.I.; Surya, I.; Abdullah, C.K. Recent Advances in Nanocellulose Aerogels for Efficient Heavy Metal and Dye Removal. Gels 2023, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Sarria, N.V.; Rivera Velasco, D.M.; Larrahondo Chávez, D.A.; Mazuera Ríos, H.D.; Gandini Ayerbe, M.A.; Goyes López, C.E.; Mejía Villareal, I.M. Struvite and hydroxyapatite recovery from wastewater treatment plant at Autónoma de Occidente University, Colombia. Case Stud. Chem. Environ. Eng. 2022, 6, 100213. [Google Scholar] [CrossRef]
- UNESCO The United Nations World Water Development Report 2023: Partnerships and Cooperation for Water. UNESDOC. 2023. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000384655 (accessed on 21 March 2024).
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Ajaj, Y.; Mahmoud, Z.H.; Kamil Ghadir, G.; Khalid Alani, Z.; Hussein, M.M.; Abed Hussein, S.; Morad Karim, M.; Al-khalidi, A.; Abbas, J.K.; et al. Adsorption of heavy metal ions use chitosan/graphene nanocomposites: A review study. Results Chem. 2024, 7, 101332. [Google Scholar] [CrossRef]
- Bahjat Kareem, A.; Al-Rawi, U.A.; Khalid, U.; Sher, F.; Zafar, F.; Naushad, M.; Nemțanu, M.R.; Lima, E.C. Functionalised graphene oxide dual nanocomposites for treatment of hazardous environmental contaminants. Sep. Purif. Technol. 2024, 342, 126959. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Villabona-Ortíz, Á.; Tejada-Tovar, C.; Herrera-Barros, A.; Cabrales-Sanjuan, D. Use of Sawdust (Aspidosperma polyneuron) in the Preparation of a Biocarbon-Type Adsorbent Material for Its Potential Use in the Elimination of Cationic Contaminants in Wastewater. Water 2023, 15, 3868. [Google Scholar] [CrossRef]
- Díaz, B.; Sommer-Márquez, A.; Ordoñez, P.E.; Bastardo-González, E.; Ricaurte, M.; Navas-Cárdenas, C. Synthesis Methods, Properties, and Modifications of Biochar-Based Materials for Wastewater Treatment: A Review. Resources 2024, 13, 8. [Google Scholar] [CrossRef]
- Younas, F.; Mustafa, A.; Farooqi, Z.U.R.; Wang, X.; Younas, S.; Mohy-Ud-din, W.; Hameed, M.A.; Abrar, M.M.; Maitlo, A.A.; Noreen, S.; et al. Current and Emerging Adsorbent Technologies for Wastewater Treatment: Trends, Limitations, and Environmental Implications. Water 2021, 13, 215. [Google Scholar] [CrossRef]
- Subash, A.; Naebe, M.; Wang, X.; Kandasubramanian, B. Tailoring electrospun nanocomposite fibers of polylactic acid for seamless methylene blue dye adsorption applications. Environ. Sci. Pollut. Res. 2024, 1, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Russo, N.; Sabir, A.; Ortega, D.H.; Quach, Q.; Abdel-Fattah, T.M. Application of Novel Magnetic Activated Carbon-Zeolite Y- Alginate Composites for Removing Organic Dyes. ECS Meet. Abstr. 2023, 65, 3025. [Google Scholar] [CrossRef]
- Ismail, U.M.; Vohra, M.S.; Onaizi, S.A. Adsorptive removal of heavy metals from aqueous solutions: Progress of adsorbents development and their effectiveness. Environ. Res. 2024, 251, 118562. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.T.; Lobo-Recio, M.Á.; Padilla, I.; Nagel-Hassemer, M.E.; Romero, M.; López-Delgado, A. Adsorption of Safranine-T dye using a waste-based zeolite: Optimization, kinetic and isothermal study. J. Ind. Eng. Chem. 2024, 136, 177–187. [Google Scholar] [CrossRef]
- Mubashar, M.; Naveed, M.; Mustafa, A.; Ashraf, S.; Baig, K.S.; Alamri, S.; Siddiqui, M.H.; Zabochnicka-światek, M.; Szota, M.; Kalaji, H.M. Experimental Investigation of Chlorella vulgaris and Enterobacter sp. MN17 for Decolorization and Removal of Heavy Metals from Textile Wastewater. Water 2020, 12, 3034. [Google Scholar] [CrossRef]
- Zarna, C.; Opedal, M.T.; Echtermeyer, A.T.; Chinga-Carrasco, G. Reinforcement ability of lignocellulosic components in biocomposites and their 3D printed applications—A review. Compos. Part C Open Access 2021, 6, 100171. [Google Scholar] [CrossRef]
- Wang, R.F.; Deng, L.G.; Li, K.; Fan, X.J.; Li, W.; Lu, H.Q. Fabrication and characterization of sugarcane bagasse–calcium carbonate composite for the efficient removal of crystal violet dye from wastewater. Ceram. Int. 2020, 46, 27484–27492. [Google Scholar] [CrossRef]
- Sukmono, Y.; Kristanti, R.A.; Foo, B.V.; Hadibarata, T. Adsorption of Fe and Pb from Aqueous Solution using Coconut Shell Activated Carbon. Biointerface Res. Appl. Chem. 2024, 14, 30. [Google Scholar]
- Chaudhary, S.; Goyal, S.; Umar, A. Fabrication of biogenic carbon-based materials from coconut husk for the eradication of dye. Chemosphere 2023, 340, 139823. [Google Scholar] [CrossRef]
- Benito-González, I.; López-Rubio, A.; Gómez-Mascaraque, L.G.; Martínez-Sanz, M. PLA coating improves the performance of renewable adsorbent pads based on cellulosic aerogels from aquatic waste biomass. Chem. Eng. J. 2020, 390, 124607. [Google Scholar] [CrossRef]
- Pang, Y.L.; Law, Z.X.; Lim, S.; Chan, Y.Y.; Shuit, S.H.; Chong, W.C.; Lai, C.W. Enhanced photocatalytic degradation of methyl orange by coconut shell–derived biochar composites under visible LED light irradiation. Environ. Sci. Pollut. Res. 2021, 28, 27457–27473. [Google Scholar] [CrossRef]
- Yuan, J.; Zhu, Y.; Wang, J.; Gan, L.; He, M.; Zhang, T.; Li, P.; Qiu, F. Preparation and application of Mg–Al composite oxide/coconut shell carbon fiber for effective removal of phosphorus from domestic sewage. Food Bioprod. Process. 2021, 126, 293–304. [Google Scholar] [CrossRef]
- Abu Aldam, S.; Dey, M.; Javaid, S.; Ji, Y.; Gupta, S. On the Synthesis and Characterization of Polylactic Acid, Polyhydroxyalkanoate, Cellulose Acetate, and Their Engineered Blends by Solvent Casting. J. Mater. Eng. Perform. 2020, 29, 5542–5556. [Google Scholar] [CrossRef]
- Krishnan, S.; Chinnadurai, G.S.; Ravishankar, K.; Raghavachari, D.; Perumal, P. Valorization of agro-wastes for the biosynthesis and characterization of polyhydroxybutyrate by Bacillus sp. isolated from rice bran dumping yard. 3 Biotech 2021, 11, 202. [Google Scholar] [CrossRef] [PubMed]
- ASTM D 570-98; Standard Test Method for Water Absorption of Plastics. ASTM: West Conshohocken, PA, USA, 2018.
- Behera, S.; Gautam, R.K.; Mohan, S. Polylactic acid and polyhydroxybutyrate coating on hemp fiber: Its effect on hemp fiber reinforced epoxy composites performance. J. Compos. Mater. 2022, 56, 929–939. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Villabona-Ortíz, Á.; Ortega-Toro, R. Removal of Metals and Dyes in Water Using Low-Cost Agro-Industrial Waste Materials. Appl. Sci. 2023, 13, 8481. [Google Scholar] [CrossRef]
- Fernández-Pérez, A.; Marbán, G. Visible Light Spectroscopic Analysis of Methylene Blue in Water. J. Appl. Spectrosc. 2022, 88, 1284–1290. [Google Scholar] [CrossRef]
- González-Delgado, A.; Villabona-Ortíz, A.; Tejada-Tovar, C. Evaluation of Three Biomaterials from Coconut Mesocarp for Use in Water Treatments Polluted with an Anionic Dye. Water 2022, 14, 408. [Google Scholar] [CrossRef]
- Pérez-Calderón, J.; Scian, A.; Ducos, M.; Santos, V.; Zaritzky, N. Performance of oxalic acid-chitosan/alumina ceramic biocomposite for the adsorption of a reactive anionic azo dye. Environ. Sci. Pollut. Res. 2021, 28, 67032–67052. [Google Scholar] [CrossRef]
- Kathirselvam, M.; Kumaravel, A.; Arthanarieswaran, V.P.; Saravanakumar, S.S. Characterization of cellulose fibers in Thespesia populnea barks: Influence of alkali treatment. Carbohydr. Polym. 2019, 217, 178–189. [Google Scholar] [CrossRef]
- Shanmugasundaram, N.; Rajendran, I.; Ramkumar, T. Characterization of untreated and alkali treated new cellulosic fiber from an Areca palm leaf stalk as potential reinforcement in polymer composites. Carbohydr. Polym. 2018, 195, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Satha, H.; Kouadri, I.; Benachour, D. Thermal, Structural and Morphological Studies of Cellulose and Cellulose Nanofibers Extracted from Bitter Watermelon of the Cucurbitaceae Family. J. Polym. Environ. 2020, 28, 1914–1920. [Google Scholar] [CrossRef]
- Zhao, X.; Anwar, I.; Zhang, X.; Pellicciotti, A.; Storts, S.; Nagib, D.A.; Vodovotz, Y. Thermal and Barrier Characterizations of Cellulose Esters with Variable Side-Chain Lengths and Their Effect on PHBV and PLA Bioplastic Film Properties. ACS Omega 2021, 6, 24700–24708. [Google Scholar] [CrossRef]
- Vijay, R.; Lenin Singaravelu, D.; Vinod, A.; Sanjay, M.R.; Siengchin, S.; Jawaid, M.; Khan, A.; Parameswaranpillai, J. Characterization of raw and alkali treated new natural cellulosic fibers from Tridax procumbens. Int. J. Biol. Macromol. 2019, 125, 99–108. [Google Scholar] [CrossRef]
- Guerrero, Y. Extracción de la Celulosa a Partir de los Residuos de pasto Común (Festuca arundinacea) para la Elaboración de Acetato de Celulosa. Bachelor’s Thesis, University Politécnica Salesiana, Sede Cuenca, Cuenca, 2021. [Google Scholar]
- Wang, J.; Cahyadi, A.; Wu, B.; Pee, W.; Fane, A.G.; Chew, J.W. The roles of particles in enhancing membrane filtration: A review. J. Memb. Sci. 2020, 595, 117570. [Google Scholar] [CrossRef]
- Aiman Suhaimi, U.; Mohamed, R.M. The properties of coconut coir fiber reinforced epoxy composites. Int. J. Synerg. Eng. Technol. 2021, 2, 16–35. [Google Scholar]
- Battisti, R.; Hafemann, E.; Claumann, C.A.; Machado, R.A.F.; Marangoni, C. Synthesis and characterization of cellulose acetate from royal palm tree agroindustrial waste. Polym. Eng. Sci. 2019, 59, 891–898. [Google Scholar] [CrossRef]
- Cindradewi, A.W.; Bandi, R.; Park, C.W.; Park, J.S.; Lee, E.A.; Kim, J.K.; Kwon, G.J.; Han, S.Y.; Lee, S.H. Preparation and characterization of cellulose acetate film reinforced with cellulose nanofibril. Polymers 2021, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zheng, Y.M.; Zhang, B.G.; Dai, Y.R. A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J. Hazard. Mater. 2021, 401, 123608. [Google Scholar] [CrossRef]
- Unugul, T.; Nigiz, F.U. Preparation and Characterization an Active Carbon Adsorbent from Waste Mandarin Peel and Determination of Adsorption Behavior on Removal of Synthetic Dye Solutions. Water. Air. Soil Pollut. 2020, 231, 538. [Google Scholar] [CrossRef]
- Nandiyanto, A.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Gheju, M.; Boran, S. Syringa vulgaris leaves powder a novel low-cost adsorbent for methylene blue removal: Isotherms, kinetics, thermodynamic and optimization by Taguchi method. Sci. Rep. 2020, 10, 17676. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Luo, Z.; Shen, J.; Ni, Q.; Yao, J. Facile preparation of a cellulose-based bioadsorbent modified by hPEI in heterogeneous system for high-efficiency removal of multiple types of dyes. React. Funct. Polym. 2018, 125, 77–83. [Google Scholar] [CrossRef]
- Luis-Zarate, V.H.; Rodriguez-Hernandez, M.C.; Alatriste-Mondragon, F.; Chazaro-Ruiz, L.F.; Rangel-Mendez, J.R. Coconut endocarp and mesocarp as both biosorbents of dissolved hydrocarbons in fuel spills and as a power source when exhausted. J. Environ. Manag. 2018, 211, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Bakatula, E.N.; Richard, D.; Neculita, C.M.; Zagury, G.J. Determination of point of zero charge of natural organic materials. Environ. Sci. Pollut. Res. 2018, 25, 7823–7833. [Google Scholar] [CrossRef]
- Shoukat, S.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Mango stone biocomposite preparation and application for crystal violet adsorption: A mechanistic study. Microporous Mesoporous Mater. 2017, 239, 180–189. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Ghassabian, S. Linearity of Calibration Curves for Analytical Methods: A Review of Criteria for Assessment of Method Reliability. In Calibration and Validation of Analytical Methods—A Sampling of Current Approaches; IntechOpen: London, UK, 2018. [Google Scholar]
- Consiglio Kasemodel, M.; Romão, E.L.; Bueno Ruiz Papa, T. Adsorption of methylene blue on babassu coconut (Orbignya speciosa) mesocarp commercial biochar. Int. J. Environ. Sci. Technol. 2023, 21, 1671–1682. [Google Scholar] [CrossRef]
- Pastre, M.M.G.; Cunha, D.L.; Kuznetsov, A.; Archanjo, B.S.; Marques, M. Optimization of Methylene Blue Removal from Aqueous Media by Photocatalysis and Adsorption Processes Using Coconut Biomass-Based Composite Photocatalysts. Water. Air. Soil Pollut. 2024, 235, 207. [Google Scholar] [CrossRef]
- Mishra, L.; Basu, G. Coconut fibre: Its structure, properties and applications. In Handbook of Natural Fibres, 2nd ed.; Kozłowski, R.M., Mackiewicz-Talarczyk, M., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 231–255. [Google Scholar]
- Etim, U.J.; Umoren, S.A.; Eduok, U.M. Coconut coir dust as a low cost adsorbent for the removal of cationic dye from aqueous solution. J. Saudi Chem. Soc. 2016, 20, S67–S76. [Google Scholar] [CrossRef]
- de Oliveira, F.M.; Coelho, L.M.; de Melo, E.I. Avaliação de processo adsortivo utilizando mesocarpo de coco verde para remoção do corante azul de metileno. Matéria 2018, 23, e12223. [Google Scholar] [CrossRef]
- Benhalima, T.; Ferfera-Harrar, H. Eco-friendly porous carboxymethyl cellulose/dextran sulfate composite beads as reusable and efficient adsorbents of cationic dye methylene blue. Int. J. Biol. Macromol. 2019, 132, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, C.; Zhao, L.; Meng, G.; Wu, J.; Liu, Z. Cellulose-based porous adsorbents with high capacity for methylene blue adsorption from aqueous solutions. Fibers Polym. 2017, 18, 891–899. [Google Scholar] [CrossRef]
- Putri, K.N.A.; Keereerak, A.; Chinpa, W. Novel cellulose-based biosorbent from lemongrass leaf combined with cellulose acetate for adsorption of crystal violet. Int. J. Biol. Macromol. 2020, 156, 762–772. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef] [PubMed]
- Padmavathy, K.S.; Madhu, G.; Haseena, P.V. A study on Effects of pH, Adsorbent Dosage, Time, Initial Concentration and Adsorption Isotherm Study for the Removal of Hexavalent Chromium (Cr (VI)) from Wastewater by Magnetite Nanoparticles. Procedia Technol. 2016, 24, 585–594. [Google Scholar] [CrossRef]
- Sarker, N.; Fakhruddin, A.N.M. Removal of phenol from aqueous solution using rice straw as adsorbent. Appl. Water Sci. 2017, 7, 1459–1465. [Google Scholar] [CrossRef]
- Somsesta, N.; Piyamawadee, C.; Sricharoenchaikul, V.; Aht-Ong, D. Adsorption isotherms and kinetics for the removal of cationic dye by Cellulose-based adsorbent biocomposite films. Korean J. Chem. Eng. 2020, 37, 1999–2010. [Google Scholar] [CrossRef]
- Hariani, P.L.; Riyanti, F.; Kurniaty, A. Modification of cellulose with acetic acid to removal of methylene blue dye. J. Phys. Conf. Ser. 2019, 1282, 012079. [Google Scholar] [CrossRef]
- Salah omer, A.; AEl Naeem, G.; Abd-Elhamid, A.I.; OMFarahat, O.; AEl-Bardan, A.; MASoliman, H.; Nayl, A.A. Adsorption of Crystal violet and Methylene blue Dyes using a Cellulose-based adsorbent from Sugercane bagasse: Characterization, kinetic and Isotherm studies. J. Mater. Res. Technol. 2022, 19, 3241–3254. [Google Scholar] [CrossRef]
- Alibak, A.H.; Khodarahmi, M.; Fayyazsanavi, P.; Alizadeh, S.M.; Hadi, A.J.; Aminzadehsarikhanbeglou, E. Simulation the adsorption capacity of polyvinyl alcohol/carboxymethyl cellulose based hydrogels towards methylene blue in aqueous solutions using cascade correlation neural network (CCNN) technique. J. Clean. Prod. 2022, 337, 130509. [Google Scholar] [CrossRef]
- Araújo, R.F.; Bezerra, L.C.A.; de Novais, L.M.R.; D’Oca, C.D.R.M.; Avelino, F. Unveiling the mechanistic aspects of methylene blue adsorption onto a novel phosphate-decorated coconut fiber lignin. Int. J. Biol. Macromol. 2023, 253, 127011. [Google Scholar] [CrossRef] [PubMed]
- Goscianska, J.; Ciesielczyk, F. Lanthanum enriched aminosilane-grafted mesoporous carbon material for efficient adsorption of tartrazine azo dye. Microporous Mesoporous Mater. 2019, 280, 7–19. [Google Scholar] [CrossRef]
- Bhowmik, S.; Chakraborty, V.; Das, P. Batch adsorption of indigo carmine on activated carbon prepared from sawdust: A comparative study and optimization of operating conditions using Response Surface Methodology. Results Surf. Interfaces 2021, 3, 100011. [Google Scholar] [CrossRef]
- Castro, D.; Rosas-Laverde, N.M.; Belén Aldás, M.; Almeida-Naranjo, C.E.; Guerrero, V.H.; Iuliana Pruna, A.; Aldás, N.M.; Almeida-Naranjo, M.B.; Guerrero, C.E.; Pruna, V.H.; et al. Chemical Modification of Agro-Industrial Waste-Based Bioadsorbents for Enhanced Removal of Zn(II) Ions from Aqueous Solutions. Materials 2021, 14, 2134. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.H.; Abdulhameed, A.S.; Mastuli, M.S. Acid-factionalized biomass material for methylene blue dye removal: A comprehensive adsorption and mechanism study. J. Taibah Univ. Sci. 2020, 14, 305–313. [Google Scholar] [CrossRef]
- Jasper, E.E.; Ajibola, V.O.; Onwuka, J.C. Nonlinear regression analysis of the sorption of crystal violet and methylene blue from aqueous solutions onto an agro-waste derived activated carbon. Appl. Water Sci. 2020, 10, 132. [Google Scholar] [CrossRef]








| Precursor | Synthesis Variables | Contaminate Removed | Removal Capacity (mg/g) | pH | Adsorbent Dose (g) | Pollutant Concentration (mg/L) | Reference | |
|---|---|---|---|---|---|---|---|---|
| Mesoporous carbon grafted with aminosilane | T (°C) | 60 | Tartrazine | 143.09 in 2 h | 6 | 0.02 | 12.5–250 | [67] |
| t (h) | 6 | |||||||
| Modification | Impregnation with LaCl3 | |||||||
| Raw sawdust | T (°C) | 700 | Indigo Carmine | 9.39 in 3 h | 2.5 | 5 | 10–50 | [68] |
| t (h) | 2 | |||||||
| Modification | Activation with NaOH | |||||||
| Banana peels | T (°C) | 60 | Zn(II) | 25.59 in 2 h | 5 | 1 | 50 | [69] |
| t (h) | 24 | |||||||
| Modification | Modified with NaOH and Ca(CH3OO)2 | |||||||
| Passion fruit peels | T (°C) | 60 | 27.48 in 2 h | 5 | 1 | 50 | ||
| t (h) | 24 | |||||||
| Modification | Modified with NaOH and Ca(CH3OO)2 | |||||||
| Orange peels | T (°C) | 60 | 16.61 in 2 h | 5 | 1 | 50 | ||
| t (h) | 24 | |||||||
| Modification | Modified with NaOH and Ca(CH3OO)2 | |||||||
| Coconut shell | T (°C) | 100 | Methylene blue | 50.6 in 1 h | 8 | 0.02–0.2 | 25–200 | [70] |
| t (h) | 24 | |||||||
| Modification | Activation with H2SO4 | |||||||
| Millettia Thonningii seed pod | T (°C) | 400 | Methylene blue | 2.55 in 3 h | 7 | 0.5 | 10–50 | [71] |
| t (h) | 0.5 | |||||||
| Modification | Activation with H3PO4 (2:1) | |||||||
| Aspidosperma polyneuron sawdust | T (°C) | 250 | Methylene blue | 12.45 in 24 h | 7 | 3.5 | 60 | [9] |
| t (h) | 0.5 | |||||||
| Modification | H3PO4 urea 6 M | |||||||
| Coconut shell | T (°C) | 60 | Methylene blue | 35.98 in 24 h | 8 | 0.01 | 40 | Present study |
| t (h) | 24 | |||||||
| Modification | Modified with polyhydroxybutyrate (PHB) | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villabona-Ortíz, Á.; Ortega-Toro, R.; Pedroza-Hernández, J. Biocomposite Based on Polyhydroxybutyrate and Cellulose Acetate for the Adsorption of Methylene Blue. J. Compos. Sci. 2024, 8, 234. https://doi.org/10.3390/jcs8070234
Villabona-Ortíz Á, Ortega-Toro R, Pedroza-Hernández J. Biocomposite Based on Polyhydroxybutyrate and Cellulose Acetate for the Adsorption of Methylene Blue. Journal of Composites Science. 2024; 8(7):234. https://doi.org/10.3390/jcs8070234
Chicago/Turabian StyleVillabona-Ortíz, Ángel, Rodrigo Ortega-Toro, and Jenyfer Pedroza-Hernández. 2024. "Biocomposite Based on Polyhydroxybutyrate and Cellulose Acetate for the Adsorption of Methylene Blue" Journal of Composites Science 8, no. 7: 234. https://doi.org/10.3390/jcs8070234
APA StyleVillabona-Ortíz, Á., Ortega-Toro, R., & Pedroza-Hernández, J. (2024). Biocomposite Based on Polyhydroxybutyrate and Cellulose Acetate for the Adsorption of Methylene Blue. Journal of Composites Science, 8(7), 234. https://doi.org/10.3390/jcs8070234









