Effect of Copper Doping in Borate Bioactive Glass on Bacterial Colonization Prevention—An Insight Study on Protein/Carbohydrate Leakage for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Borate Bioactive Glass Synthesis
2.2. Characterization of BBG Powder
2.3. Hydroxyl Radical Generation
2.4. Minimum Inhibitory Concentration
2.5. Colony-Forming Unit
2.6. Protein Discharge Assay
2.7. Carbohydrate Discharge Assay
2.8. Live/Dead Cell Analysis
3. Results and Discussion
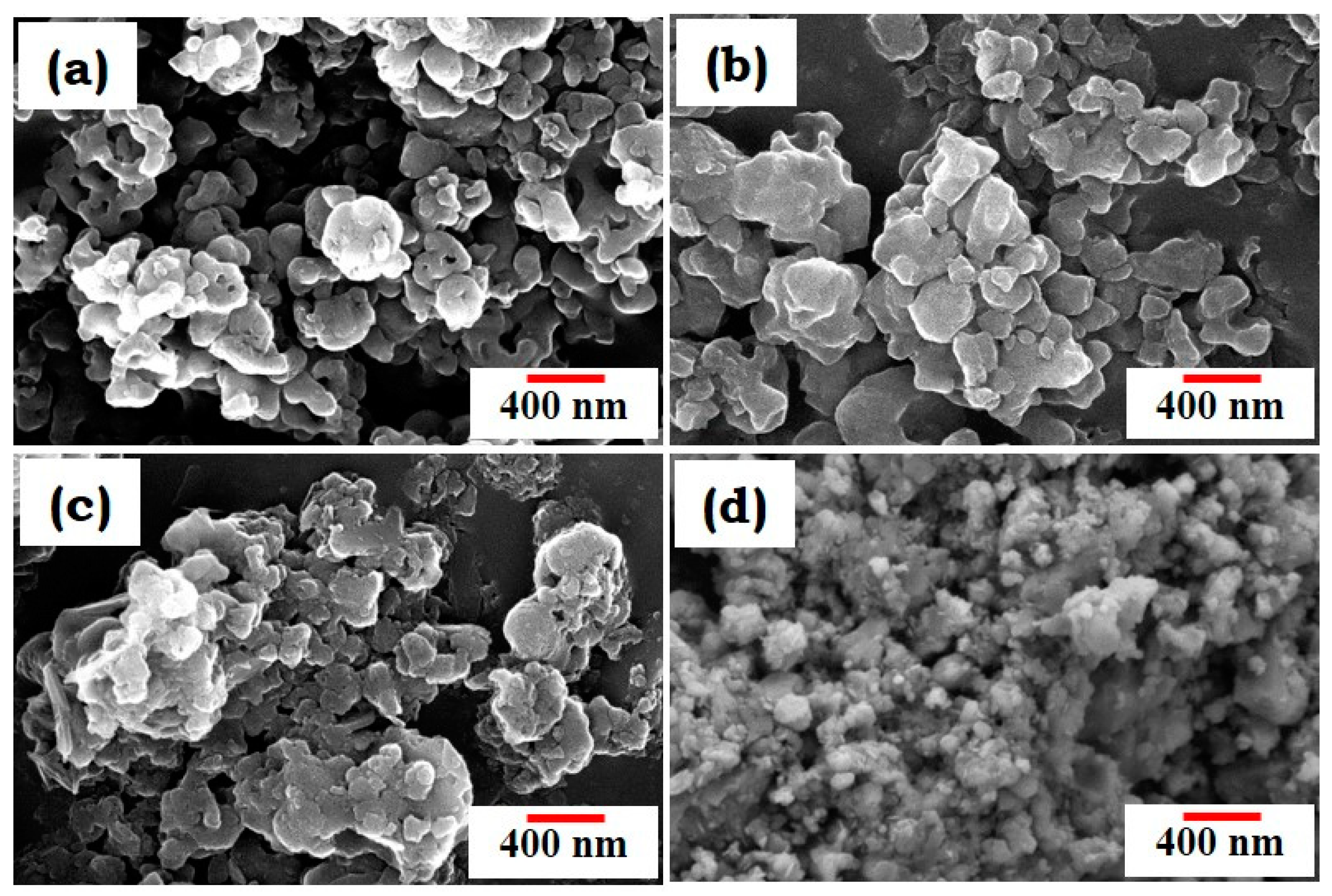
3.1. Characterization of BBG Powders Using XRD, Raman Spectroscopy, and FESEM
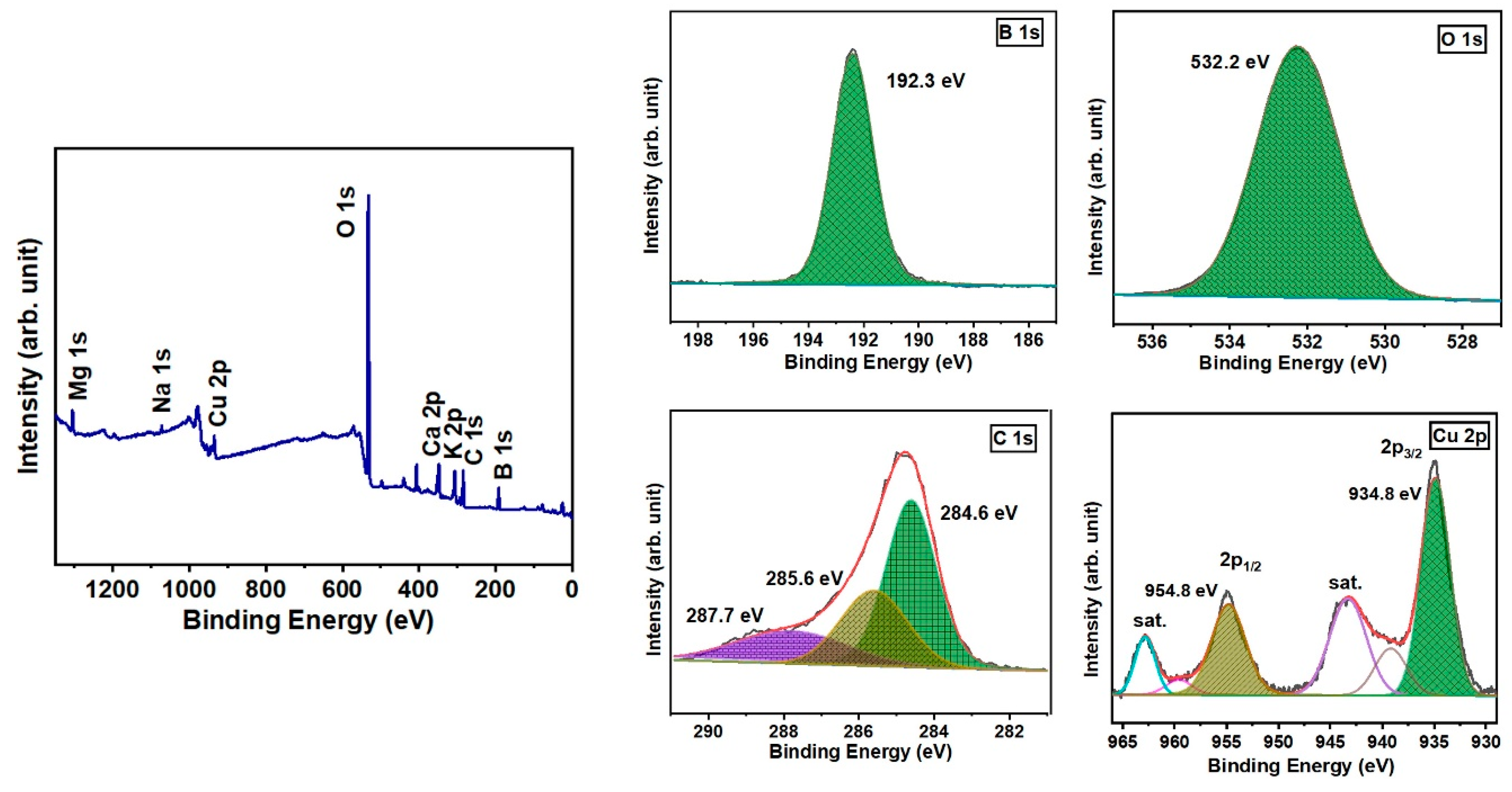
3.2. X-ray Photoelectron Spectroscopy Analysis
3.3. Epifluorescence Microscopy Analysis
3.4. Hydroxyl Radical Generation
3.5. Minimum Inhibitory Concentration (MIC)
3.6. Colony-Forming Unit (CFU)
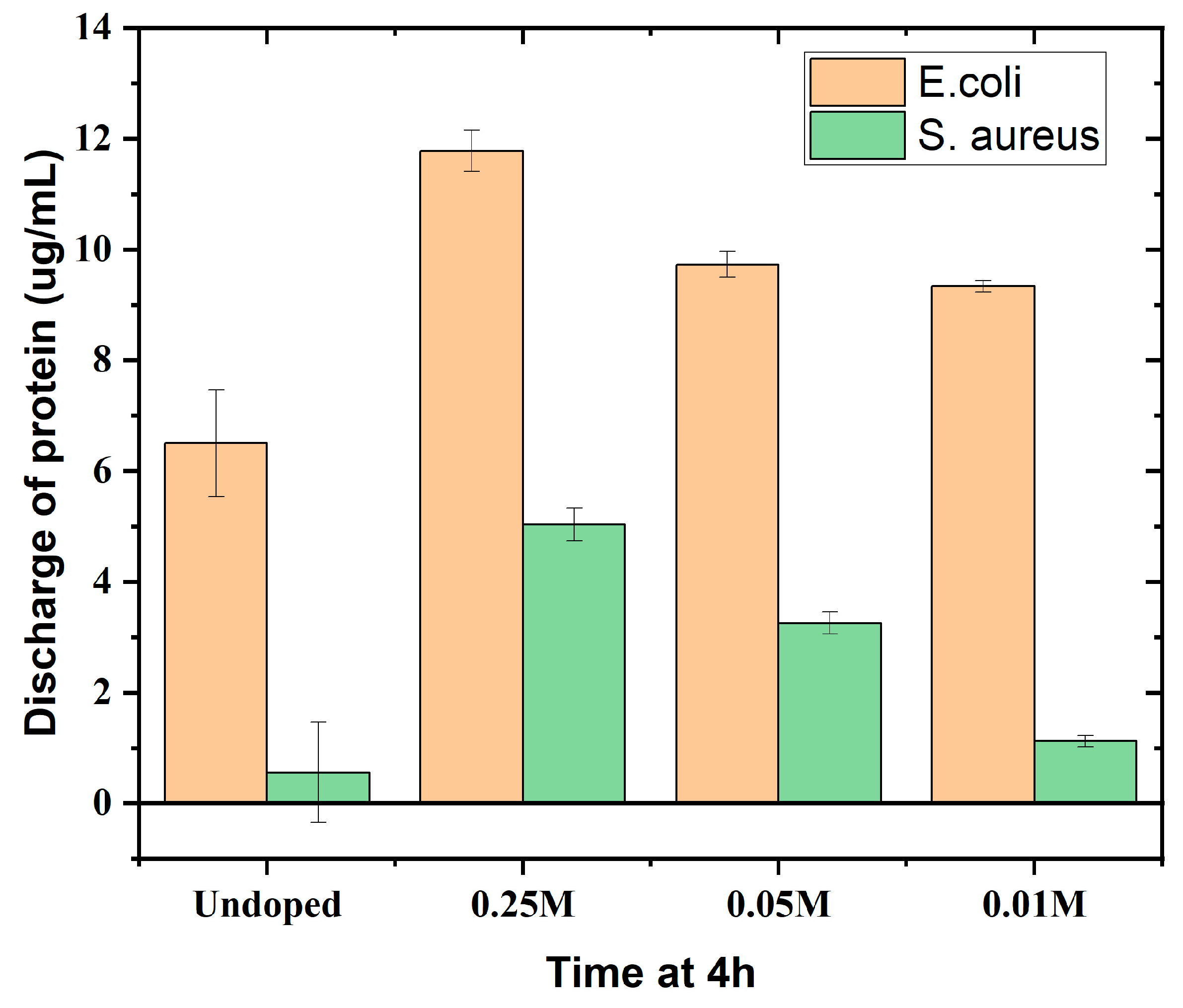
3.7. Protein and Carbohydrate Discharge Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, M.K.; Lavanya, M. Microbial Influenced Corrosion: Understanding Bioadhesion and Biofilm Formation. J. Bio- Tribo-Corros. 2022, 8, 76. [Google Scholar] [CrossRef]
- Malard, F.; Dore, J.; Gaugler, B.; Mohty, M. Introduction to Host Microbiome Symbiosis in Health and Disease. Mucosal Immunol. 2020, 14, 547–554. [Google Scholar] [CrossRef]
- Pugazhendhi, A.S.; Wei, F.; Hughes, M.; Coathup, M. Bacterial Adhesion, Virulence, and Biofilm Formation. In Musculoskeletal Infection; Springer: Cham, Switzerland, 2022; pp. 19–64. [Google Scholar] [CrossRef]
- Bamford, N.C.; Macphee, C.E.; Stanley-Wall, N.R. Microbial Primer: An Introduction to Biofilms—What They Are, Why They Form and Their Impact on Built and Natural Environments. Microbiology 2023, 169, 001338. [Google Scholar] [CrossRef]
- Krishnan, S. Biofilm Formation on Medical Devices and Infection: Preventive Approaches. In Biofilm and Materials Science; Springer: Cham, Switzerland, 2015; pp. 93–108. [Google Scholar] [CrossRef]
- Troy, E.; Tilbury, M.A.; Power, A.M.; Wall, J.G. Nature-Based Biomaterials and Their Application in Biomedicine. Polymers 2021, 13, 3321. [Google Scholar] [CrossRef] [PubMed]
- Kreve, S.; Reis, A.C.D. Bacterial Adhesion to Biomaterials: What Regulates This Attachment? A Review. Jpn. Dent. Sci. Rev. 2021, 57, 85–96. [Google Scholar] [CrossRef]
- dos Santos, V.R.; Campos, T.M.B.; Anselmi, C.; Thim, G.P.; Bottino, M.C.; Borges, A.L.S.; Trichês, E. de S. Effect of Co, Cu, and Zn Ions on the Bioactivity and Antibacterial Properties of a Borate Bioactive Glass. J. Non Cryst. Solids 2023, 622, 122643. [Google Scholar] [CrossRef]
- Harrison, J.J.; Tremaroli, V.; Stan, M.A.; Chan, C.S.; Vacchi-Suzzi, C.; Heyne, B.J.; Parsek, M.R.; Ceri, H.; Turner, R.J. Chromosomal Antioxidant Genes Have Metal Ion-Specific Roles as Determinants of Bacterial Metal Tolerance. Environ. Microbiol. 2009, 11, 2491–2509. [Google Scholar] [CrossRef] [PubMed]
- Deliormanlı, A.M. Sol-Gel Synthesis of Borate-Based 13-93B3 Bioactive Glass Powders for Biomedical Applications. Mater. Technol. 2022, 37, 1808–1817. [Google Scholar] [CrossRef]
- Babbs, C.F.; Gale Steiner, M. [11] Detection and Quantitation of Hydroxyl Radical Using Dimethyl Sulfoxide as Molecular Probe. Methods Enzymol. 1990, 186, 137–147. [Google Scholar] [CrossRef]
- Steiner, M.G.; Babbs, C.F. Quantitation of the Hydroxyl Radical by Reaction with Dimethyl Sulfoxide. Arch. Biochem. Biophys. 1990, 278, 478–481. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Dey, A.; Neogi, S. An Insight into the Mechanism of Antibacterial Activity by Magnesium Oxide Nanoparticles. J. Mater. Chem. B 2021, 9, 5329–5339. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.H.M.L.M.; Insa, S.; Arxé, M.; Buttiglieri, G.; Rodríguez-Mozaz, S.; Barceló, D. Analysis of Microplastics in the Environment: Identification and Quantification of Trace Levels of Common Types of Plastic Polymers Using Pyrolysis-GC/MS. MethodsX 2023, 10, 102143. [Google Scholar] [CrossRef]
- Lepry, W.C.; Smith, S.; Nazhat, S.N. Effect of Sodium on Bioactive Sol-Gel-Derived Borate Glasses. J. Non Cryst. Solids 2018, 500, 141–148. [Google Scholar] [CrossRef]
- Bento, R.; Gaddam, A.; Ferreira, J.M.F. Sol–Gel Synthesis and Characterization of a Quaternary Bioglass for Bone Regeneration and Tissue Engineering. Materials 2021, 14, 4515. [Google Scholar] [CrossRef]
- Sreedhar, M.; Reddy, I.N.; Bera, P.; Ramachandran, D.; Gobi Saravanan, K.; Rabel, A.M.; Anandan, C.; Kuppusami, P.; Brijitta, J. Cu/TiO2 Thin Films Prepared by Reactive RF Magnetron Sputtering. Appl. Phys. A Mater. Sci. Process 2015, 120, 765–773. [Google Scholar] [CrossRef]
- Konijnendijk, W.L.; Stevels, J.M. Structure of Borate and Borosilicate Glasses by Raman Spectroscopy. In Borate Glasses; Springer: Boston, MA, USA, 1978; pp. 259–279. [Google Scholar] [CrossRef]
- Padmaja, G.; Kistaiah, P. Infrared and Raman Spectroscopic Studies on Alkali Borate Glasses: Evidence of Mixed Alkali Effect. J. Phys. Chem. A 2009, 113, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.R.; Kukulka, E.C.; Araújo, J.C.R.; Campos, T.M.B.; do Prado, R.F.; de Vasconcellos, L.M.R.; Thin, G.P.; Borges, A.L.S. Electrospun Polylactic Acid Scaffolds with Strontium- and Cobalt-Doped Bioglass for Potential Use in Bone Tissue Engineering Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Shi, Z.; Reddy, R.G. Mechanism Study of Cu-Zn Alloys Electrodeposition in Deep Eutectic Solvents. Ionics 2020, 26, 3161–3172. [Google Scholar] [CrossRef]
- Siddiqui, H.; Parra, M.R.; Qureshi, M.S.; Malik, M.M.; Haque, F.Z. Studies of Structural, Optical, and Electrical Properties Associated with Defects in Sodium-Doped Copper Oxide (CuO/Na) Nanostructures. J. Mater. Sci. 2018, 53, 8826–8843. [Google Scholar] [CrossRef]
- ElBaz, N.; El-Damrawi, G.; Abdelghany, A.M.; ElBaz, N.; El-Damrawi, G.; Abdelghany, A.M. Structural Role of CeO2 in the Modified Borate Glass-Ceramics. New J. Glass Ceram. 2021, 11, 34–43. [Google Scholar] [CrossRef]
- Iqbal, M.; Ali, A.; Ahmad, K.S.; Rana, F.M.; Khan, J.; Khan, K.; Thebo, K.H. Synthesis and Characterization of Transition Metals Doped CuO Nanostructure and Their Application in Hybrid Bulk Heterojunction Solar Cells. SN Appl. Sci. 2019, 1, 647. [Google Scholar] [CrossRef]
- Lallukka, M.; Miola, M.; Najmi, Z.; Cochis, A.; Spriano, S.; Rimondini, L.; Verné, E. Cu-Doped Bioactive Glass with Enhanced In Vitro Bioactivity and Antibacterial Properties. Ceram. Int. 2024, 50, 5091–5103. [Google Scholar] [CrossRef]
- Senna, M.; Noda, H.; Xin, Y.; Hasegawa, H.; Takai, C.; Shirai, T.; Fuji, M. Solid-State Reduction of Silica Nanoparticles via Oxygen Abstraction from SiO4 Units by Polyolefins under Mechanical Stressing. RSC Adv. 2018, 8, 36338. [Google Scholar] [CrossRef] [PubMed]
- Nandanwar, S.; Borkar, S.; Cho, J.H.; Kim, H.J. Microwave-assisted Synthesis and Characterization of Solar-Light-active Copper–Vanadium Oxide: Evaluation of Antialgal and Dye Degradation Activity. Catalysts 2021, 11, 36. [Google Scholar] [CrossRef]
- Svintsitskiy, D.A.; Yu Kardash, T.; Boronin, A.I. Surface Dynamics of Mixed Silver-Copper Oxide AgCuO2 during X-ray Photoelectron Spectroscopy Study. Appl. Surf. Sci. 2019, 463, 300–309. [Google Scholar] [CrossRef]
- Kumar, D.D.; Kaliaraj, G.S. Multifunctional Zirconium Nitride/Copper Multilayer Coatings on Medical Grade 316L SS and Titanium Substrates for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2018, 77, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of Antibacterial Activity of Copper Nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Lu, H.; Wu, Q.; Li, L.; Zhao, R.; Long, C.; Cui, C. Hydroxyl Radicals Dominate Reoxidation of Oxide-Derived Cu in Electrochemical CO2 Reduction. Nat. Commun. 2022, 13, 3694. [Google Scholar] [CrossRef] [PubMed]
- Rundén-Pran, E.; Mariussen, E.; El Yamani, N.; Elje, E.; Longhin, E.M.; Dusinska, M. The Colony Forming Efficiency Assay for Toxicity Testing of Nanomaterials—Modifications for Higher-Throughput. Front. Toxicol. 2022, 4, 983316. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhang, Y.; He, G.X.; Katagori, N.; Chen, H. A Comparison of Conventional Methods for the Quantification of Bacterial Cells after Exposure to Metal Oxide Nanoparticles. BMC Microbiol. 2014, 14, 222. [Google Scholar] [CrossRef]
- Toplitsch, D.; Lackner, J.M.; Schwan, A.M.; Hinterer, A.; Stögmüller, P.; Horn, K.; Fritzlar, N.; Pfuch, A.; Kittinger, C. Antimicrobial Activity of a Novel Cu(NO3)2-Containing Sol–Gel Surface under Different Testing Conditions. Materials 2021, 14, 6488. [Google Scholar] [CrossRef] [PubMed]







| Chemicals | CuNO3 (Molar Concentration) | ||
|---|---|---|---|
| 0.25 | 0.05 | 0.01 | |
| TEP | 0.54 mL | 0.54 mL | 0.54 mL |
| Sodium nitrate (NaNO3) | 0.72 g | 0.72 g | 0.72 g |
| Calcium nitrate (Ca(NO3)2·4H2O) | 2.95 g | 5.31 g | 5.31 g |
| Magnesium nitrate (Mg(NO3)2·6H2O) | 3.02 g | 0.64 g | 0.64 g |
| Potassium nitrate (KNO3) | 1.31 g | 1.31 g | 1.31 g |
| Copper nitrate (CuNO3) | 3.20 g | 0.60 g | 0.12 g |
| BBG Powder | E. coli (Zone of Inhibition) | S. aureus (Zone of Inhibition) |
|---|---|---|
| BBG + 0.25 M Cu | 16 mm | 14 mm |
| BBG + 0.05 M Cu | 10 mm | 11 mm |
| BBG + 0.01 M Cu | 8 mm | 9 mm |
| Undoped BBG | 4 mm | 2 mm |
| BBG Concentration (M) | E. coli (CFU/µL) | S. aureus (CFU/µL) |
|---|---|---|
| Undoped | 2.79 | 2.67 |
| 0.25 | 1.11 | 1.07 |
| 0.05 | 2.01 | 1.33 |
| 0.01 | 269.0 | 2.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sankaralingam, B.; Kaliaraj, G.S.; Rameshbabu, I.; Rajendran, P.; Amirtharaj Mosas, K.K. Effect of Copper Doping in Borate Bioactive Glass on Bacterial Colonization Prevention—An Insight Study on Protein/Carbohydrate Leakage for Biomedical Applications. J. Compos. Sci. 2024, 8, 245. https://doi.org/10.3390/jcs8070245
Sankaralingam B, Kaliaraj GS, Rameshbabu I, Rajendran P, Amirtharaj Mosas KK. Effect of Copper Doping in Borate Bioactive Glass on Bacterial Colonization Prevention—An Insight Study on Protein/Carbohydrate Leakage for Biomedical Applications. Journal of Composites Science. 2024; 8(7):245. https://doi.org/10.3390/jcs8070245
Chicago/Turabian StyleSankaralingam, Bharath, Gobi Saravanan Kaliaraj, Isha Rameshbabu, Padmapriya Rajendran, and Kamalan Kirubaharan Amirtharaj Mosas. 2024. "Effect of Copper Doping in Borate Bioactive Glass on Bacterial Colonization Prevention—An Insight Study on Protein/Carbohydrate Leakage for Biomedical Applications" Journal of Composites Science 8, no. 7: 245. https://doi.org/10.3390/jcs8070245







