Does Applying Morpholine to Saliva-Contaminated Acrylic Resin Improve the Repair Bond Strength?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acrylic Resin Preparation
2.2. The Sandblast Method
2.3. Grouping of the Specimens
2.4. Artificial Saliva Contamination
2.5. Phosphoric Acid Etching Protocol
2.6. Morpholine Surface Treatment
2.7. MMA Monomer Treatment
2.8. The Universal Adhesive Protocol
2.9. Bonding with Light-Cured Resin Composites
2.10. Evaluation of SBS and Investigation of Failure Characteristics
2.11. Statistical Assessment of the Data
3. Results
3.1. SBS Data
3.2. Failure-Type Patterns
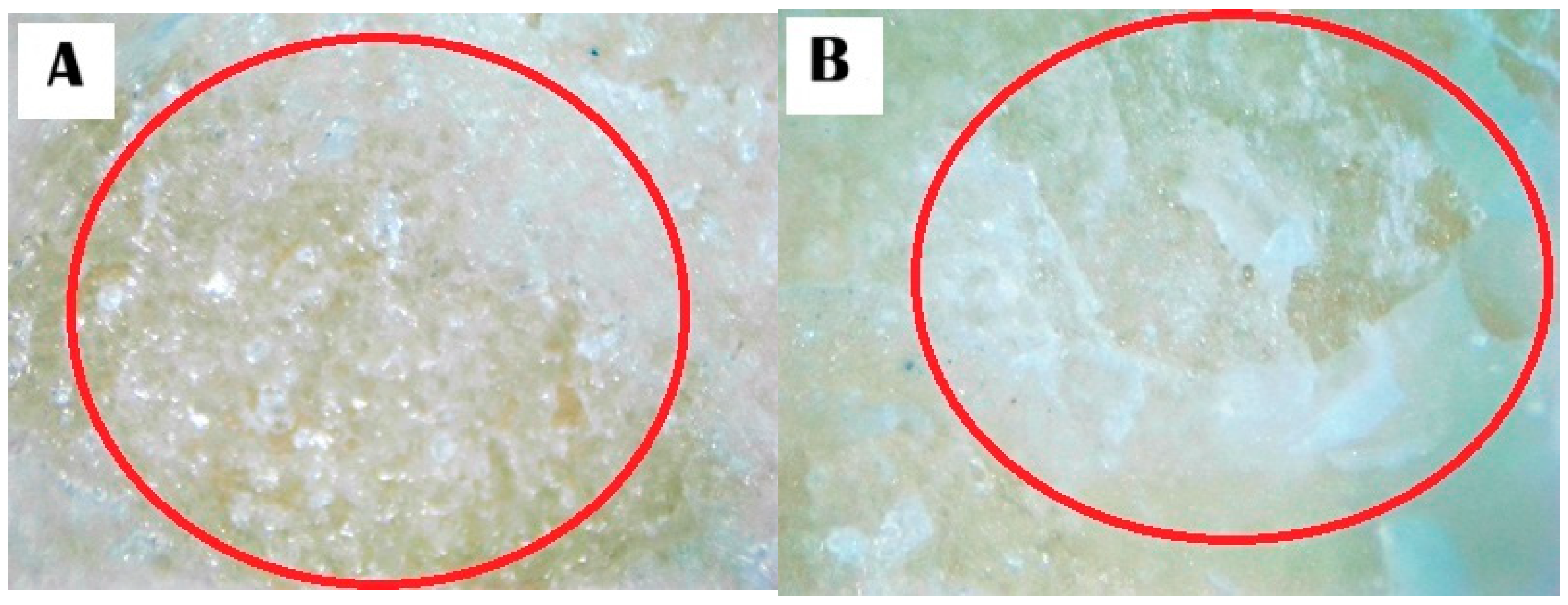
3.3. Stereomicroscope Images
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camacho, D.P.; Inez, T.; Svidzinski, E.; Furlaneto, M.C.; Lopes, M.B.; Corrêa, G.O. Acrylic Resins for Dental Use Based Polymethyl-methacrylate. Braz. J. Surg. Clin. Res. 2014, 6, 63–72. [Google Scholar]
- Silva, A.S.; Carvalho, A.; Barreiros, P.; de Sá, J.; Aroso, C.; Mendes, J.M. Comparison of fracture resistance in thermal and self-curing acrylic resins-an in vitro study. Polymers 2021, 13, 234. [Google Scholar] [CrossRef]
- Neppelenbroek, K.H.; Kuroishi, E.; Hotta, J.; Marques, V.R.; Moffa, E.B.; Soares, S.; Urban, V.M. Surface properties of multilayered, acrylic resin artificial teeth after immersion in staining beverages. J. Appl. Oral Sci. 2015, 23, 376–382. [Google Scholar] [CrossRef]
- Klaisiri, A.; Maneenacarith, A.; Krajangta, N.; Sukkee, A.; Hardy, N.S.; Wutikhun, T.; Supachartwong, C. Surface modification methods of self-cured acrylic resin repaired with resin composite using a universal adhesive. J. Compos. Sci. 2023, 7, 360. [Google Scholar] [CrossRef]
- Klaisiri, A.; Phumpatrakom, P.; Thamrongananskul, N. Chemical surface modification methods of resin composite repaired with resin-modified glass-ionomer cement. Eur. J. Dent. 2023, 17, 804–808. [Google Scholar] [CrossRef]
- Muhsin, S.A. Bond strength of repaired acrylic denture teeth using visible light cure composite resin. Open Dent. J. 2017, 11, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.B.; Naveen, B.H.; Patil, N.P. Bonding acrylic teeth to acrylic resin denture bases: A review. Gerodontology 2006, 23, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Infante, L.; Lee, H. An acrylic resin shell with guide extensions for accurate positioning of provisional restorations. J. Prosthet. Dent. 2011, 106, 340–342. [Google Scholar] [CrossRef]
- Chan, K.H.S.; Mai, Y.; Kim, H.; Tong, K.C.T.; Ng, D.; Hsiao, J.C.M. Review: Resin composite filling. Materials 2010, 3, 1228–1243. [Google Scholar] [CrossRef]
- Yadav, R.; Meena, A.; Lee, H.H.; Lee, S.Y.; Park, S.J. Tribological behavior of dental resin composites: A comprehensive review. Tribol. Int. 2023, 190, 109017. [Google Scholar] [CrossRef]
- Saini, S.; Yadav, R.; Sonwal, S.; Meena, A.; Huh, Y.S.; Brambilla, E.; Ionescu, A.C. Tribological, mechanical, and thermal properties of nano tricalcium phosphate and silver particulates reinforced Bis-GMA/TEGDMA dental resin composites. Tribol. Int. 2024, 199, 110010. [Google Scholar] [CrossRef]
- Yadav, R.; Saini, S.; Sonwal, S.; Meena, A.; Huh, Y.S.; Brambilla, E.; Ionescu, A.C. Optimization and ranking of dental restorative composites by ENTROPY-VIKOR and VIKOR-MATLAB. Polym. Adv. Technol. 2024, 35, e6526. [Google Scholar] [CrossRef]
- Alzraikat, H.; Burrow, M.F.; Maghaireh, G.A.; Taha, N.A. Nanofilled resin composite oroperties and clinical performance: A review. Oper. Dent. 2018, 43, E173–E190. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Resin composite--state of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Solow, R.A. Direct composite resin onlays: Rationale and clinical application. Gen. Dent. 2017, 65, 19–24. [Google Scholar]
- Alhejoury, H.A.; Mogharbel, L.F.; Al-Qadhi, M.A.; Shamlan, S.S.; Alturki, A.F.; Babatin, W.M.; Mohammed Alaishan, R.A.; Pullishery, F. Artificial saliva for therapeutic management of xerostomia: A narrative review. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. S2), S903–S907. [Google Scholar] [CrossRef]
- Eiriksson, S.O.; Pereira, P.N.; Swift, E.J., Jr.; Heymann, H.O.; Sigurdsson, A. Effects of saliva contamination on resin-resin bond strength. Dent. Mater. 2004, 20, 37–44. [Google Scholar] [CrossRef]
- Furuse, A.Y.; da Cunha, L.F.; Benetti, A.R.; Mondelli, J. Bond strength of resin-resin interfaces contaminated with saliva and submitted to different surface treatments. J. Appl. Oral Sci. 2007, 15, 501–505. [Google Scholar] [CrossRef]
- Bolme, J.; Gjerdet, N.R.; Laegreid, T. Effect of saliva contamination on the bond strength of single-step and three-step adhesive systems. Eur. J. Oral Sci. 2022, 130, e12838. [Google Scholar] [CrossRef]
- Sayinsu, K.; Isik, F.; Sezen, S.; Aydemir, B. Effect of blood and saliva contamination on bond strength of brackets bonded with a protective liquid polish and a light-cured adhesive. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 391–394. [Google Scholar] [CrossRef]
- Arshad, F.; Khan, M.F.; Akhtar, W.; Alam, M.M.; Nainwal, L.M.; Kaushik, S.K.; Akhter, M.; Parvez, S.; Hasan, S.M.; Shaquiquzzaman, M. Revealing quinquennial anticancer journey of morpholine: A SAR based review. Eur. J. Med. Chem. 2019, 167, 324–356. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, R.K. Morpholine as ubiquitous pharmacophore in medicinal chemistry: Deep insight into the structure-activity relationship (SAR). Bioorg. Chem. 2020, 96, 103578. [Google Scholar] [CrossRef] [PubMed]
- Klaisiri, A.; Suebnukarn, S.; Krajangta, N.; Rakmanee, T.; Sriamporn, T.; Thamrongananskul, N. The effect of morpholine on composite-to-composite repair strength contaminated with saliva. Polymers 2022, 14, 4718. [Google Scholar] [CrossRef]
- Prawatvatchara, W.; Angkanawiriyarak, S.; Klaisiri, A.; Sriamporn, T.; Thamrongananskul, N. Effect of aprotic solvents on the microtensile bond strength of composite core and fiber-reinforced composite posts. Polymers 2023, 15, 3984. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Pal, G. Photopolymerization of methyl methacrylate using morpholine–bromine charge transfer complex as the photoinitiator. Eur. Polym. J. 1998, 35, 677–682. [Google Scholar] [CrossRef]
- Matinlinna, J.P.; Lassila, L.V. Enhanced resin-composite bonding to zirconia framework after pretreatment with selected silane monomers. Dent. Mater. 2011, 27, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Klaisiri, A.; Krajangta, N.; Peampring, C.; Sriamporn, T.; Thamrongananskul, N.; Neff, A.; Pitak-Arnnop, P. Shear bond strength of different functional monomer in universal adhesives at the resin composite/base metal alloys interface. J. Int. Dent. Med. Res. 2021, 14, 187–191. [Google Scholar]
- Klaisiri, A.; Krajangta, N.; Thamrongananskul, N. The durability of zirconia/resin composite shear bond strength using different functional monomer of universal adhesives. Eur. J. Dent. 2022, 16, 756–760. [Google Scholar] [CrossRef]
- Chatterjee, N.; Gupta, T.K.; Banerjee, A. A study on effect of surface treatments on the shear bond strength between composite resin and acrylic resin denture teeth. J. Indian Prosthodont. Soc. 2011, 11, 20–25. [Google Scholar] [CrossRef]
- Qaw, M.S.; Abushowmi, T.H.; Almaskin, D.F.; AlZaher, Z.A.; Gad, M.M.; Al-Harbi, F.A.; Abualsaud, R.; Ammar, M.M. A novel approach to improve repair bond strength of repaired acrylic resin: An in vitro study on the shear bond strength. J. Prosthodont. 2020, 29, 323–333. [Google Scholar] [CrossRef]
- Dal Piva, A.M.d.O.; Tribst, J.P.M.; de Carvalho, P.C.K.; Uemura, E.S.; de Arruda Paes Junior, T.J.; Borges, A.L.S. Effect of surface treatments on the bond repair strength of resin composite to different artificial teeth. Appl. Adhes. Sci. 2018, 6, 7. [Google Scholar] [CrossRef]
- Gad, M.M.; Albazroun, Z.; Aldajani, F.; Elakel, A.M.; Zayat, M.E.; Akhtar, S.; Khan, S.Q.; Ali, S.; Rahoma, A.M. Repair bond strength of conventionally and digitally fabricated denture base resins to auto-polymerized acrylic resin: Surface treatment effects in vitro. Materials 2022, 15, 9062. [Google Scholar] [CrossRef] [PubMed]
- Alkurt, M.; Yeşil Duymuş, Z.; Gundogdu, M. Effect of repair resin type and surface treatment on the repair strength of heat-polymerized denture base resin. J. Prosthet. Dent. 2014, 111, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Neelagiri, K.; Kundabala, M.; Shashi, R.A.; Thomas, M.S.; Parolia, A. Effects of saliva contamination and decontamination procedures on shear bond strength of self-etch dentine bonding systems: An invitro study. J. Conserv. Dent. 2010, 13, 71–75. [Google Scholar] [PubMed]
- Ulker, E.; Bilgin, S.; Kahvecioglu, F.; Erkan, A.I. Effect of saliva decontamination procedures on shear bond strength of a one-step adhesive system. Niger. J. Clin. Pract. 2017, 20, 1201–1205. [Google Scholar]
- Pinzon, L.M.; Powers, J.M.; O’Keefe, K.L.; Dusevish, V.; Spencer, P.; Marshall, G.W. Effect of mucoprotein on the bond strength of resin composite to human dentin. Odontology 2011, 99, 119–128. [Google Scholar] [CrossRef]
- Yin, H.; Kwon, S.; Chung, S.H.; Kim, R.J.Y. Performance of universal adhesives in composite resin repair. BioMed Res. Int. 2022, 2022, 7663490. [Google Scholar] [CrossRef]
- Ishii, R.; Tsujimoto, A.; Takamizawa, T.; Tsubota, K.; Suzuki, T.; Shimamura, Y.; Miyazaki, M. Influence of surface treatment of contaminated zirconia on surface free energy and resin cement bonding. Dent. Mater. J. 2015, 34, 91–97. [Google Scholar] [CrossRef]





| Material | Manufacturer | Chemical Composition |
|---|---|---|
| Self-curing acrylic resin | Shofu Inc., Kyoto, Japan | Powder: MMA-EMA copolymer, pigments, and others Liquid: MMA and others |
| Morpholine | Loba Chemie PVT Ltd., Mumbai, India | 98% extra pure O(CH2CH2)2NH |
| Singlebond Universal | 3M, Neuss, Germany | 10-MDP, Bis-GMA, HEMA, DMA, methacrylate functional copolymer, silane, filler, initiators, ethanol, water |
| Resin composite, Harmonize A2E | Kerr Corporation, Orange, CA, USA | Bis-GMA, TEGDMA, EBPADMA, zirconia/silica cluster filler (2–3 m) comprising 20 nm spherical fumed silica and 5 nm zirconia particles, and prepolymerized filler (filler loading; 81.5% by weight) |
| Groups | Surface Treatment |
|---|---|
| 1 | Saliva-contaminated acrylic resin (saliva) |
| 2 | Saliva-contaminated acrylic resin treated with phosphoric acid prior to the application of MMA monomer liquid and universal adhesive (saliva + PH + MMA + UA) |
| 3 | Saliva-contaminated acrylic resin treated with MMA monomer liquid and universal adhesive (saliva + MMA + UA) |
| 4 | Saliva-contaminated acrylic resin treated with morpholine prior to the application of MMA monomer liquid and universal adhesive (saliva + MR + MMA + UA) |
| Group | Mean SBS (SD) | Failure Pattern | ||
|---|---|---|---|---|
| Adhesive | Mixed | Cohesive | ||
| 1. Saliva | 1.38 ± 0.87 a | 100 | 0 | 0 |
| 2. Saliva + PH + MMA + UA | 30.46 ± 2.26 b | 0 | 20 | 80 |
| 3. Saliva + MMA + UA | 17.36 ± 2.81 c | 0 | 33.33 | 66.67 |
| 4. Saliva + MR + MMA + UA | 32.10 ± 2.72 b | 0 | 6.67 | 93.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klaisiri, A.; Krajangta, N.; Assawarattanaphan, K.; Sriperm, J.; Prawatvatchara, W.; Thamrongananskul, N.; Sriamporn, T. Does Applying Morpholine to Saliva-Contaminated Acrylic Resin Improve the Repair Bond Strength? J. Compos. Sci. 2024, 8, 349. https://doi.org/10.3390/jcs8090349
Klaisiri A, Krajangta N, Assawarattanaphan K, Sriperm J, Prawatvatchara W, Thamrongananskul N, Sriamporn T. Does Applying Morpholine to Saliva-Contaminated Acrylic Resin Improve the Repair Bond Strength? Journal of Composites Science. 2024; 8(9):349. https://doi.org/10.3390/jcs8090349
Chicago/Turabian StyleKlaisiri, Awiruth, Nantawan Krajangta, Kasidit Assawarattanaphan, Jaratchom Sriperm, Wisarut Prawatvatchara, Niyom Thamrongananskul, and Tool Sriamporn. 2024. "Does Applying Morpholine to Saliva-Contaminated Acrylic Resin Improve the Repair Bond Strength?" Journal of Composites Science 8, no. 9: 349. https://doi.org/10.3390/jcs8090349







