Carbon Nanotube–Carbon Nanocoil Hybrid Film Decorated by Amorphous Silicon as Anodes for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. The Synthesis of CNCs
2.2. The Synthesis of CNT Film and CNT–CNC Film
2.3. Electrode Fabrications
2.4. Electrode Characterization and Cell Measurement
3. Results
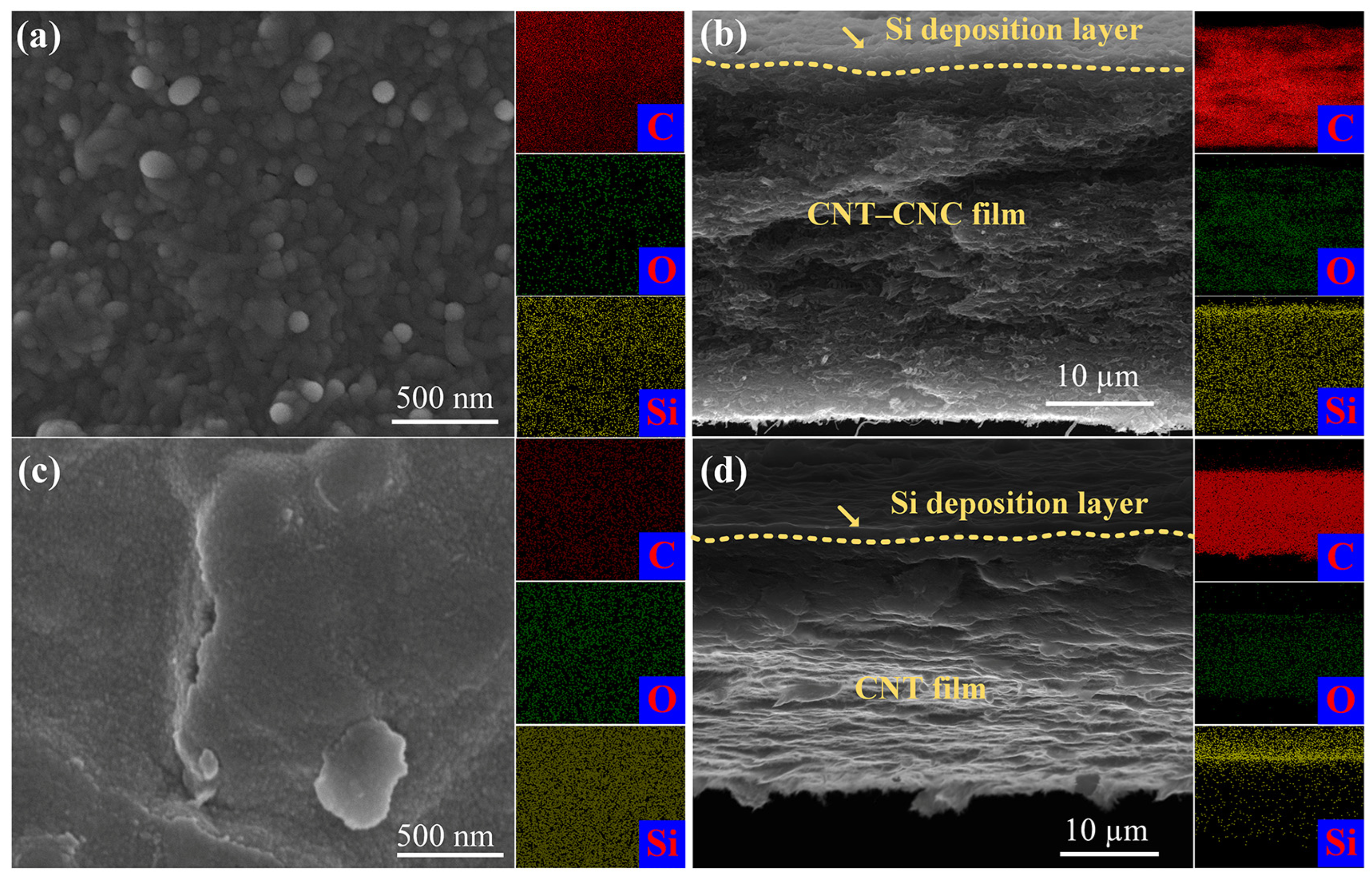
3.1. Characterization of the Film and Electrode
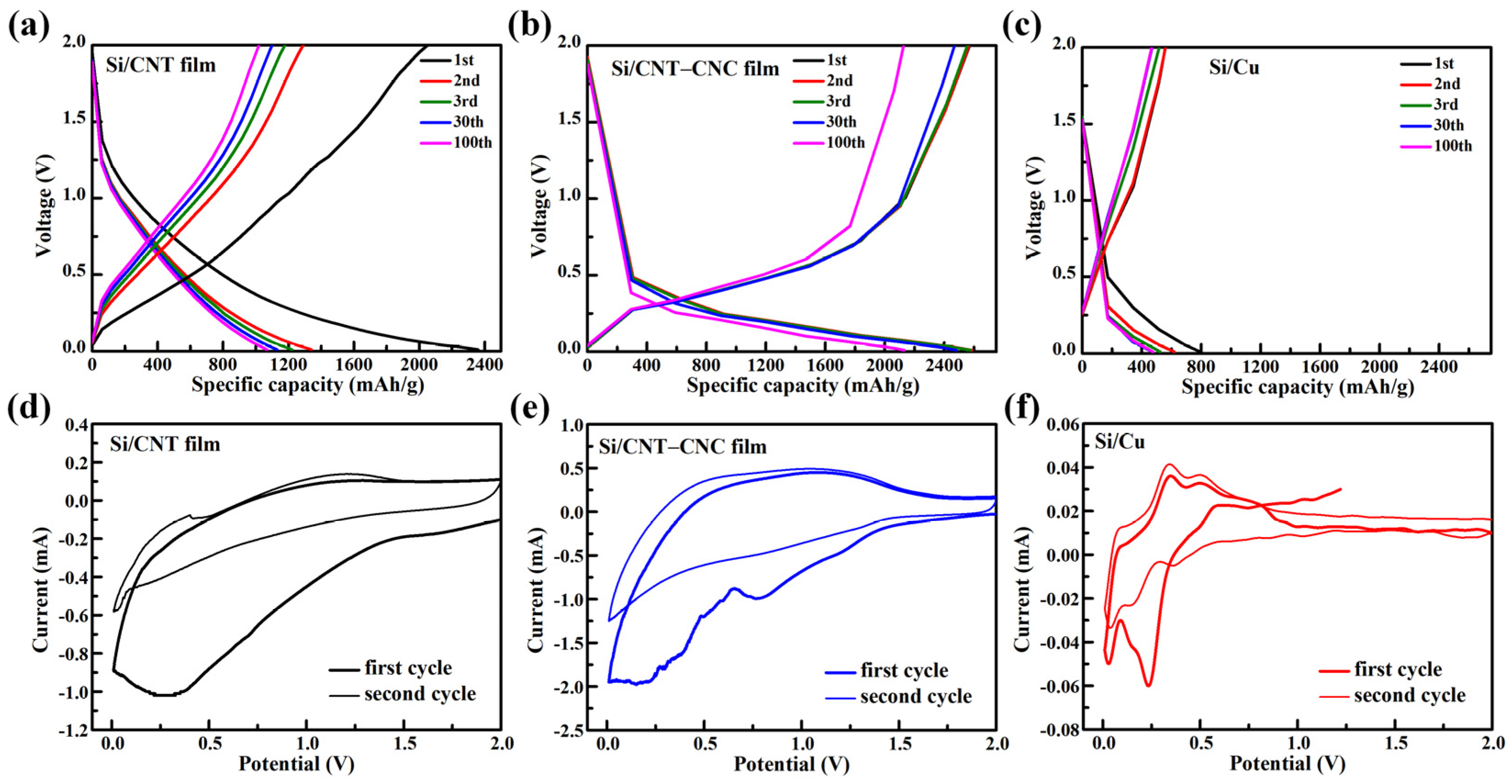
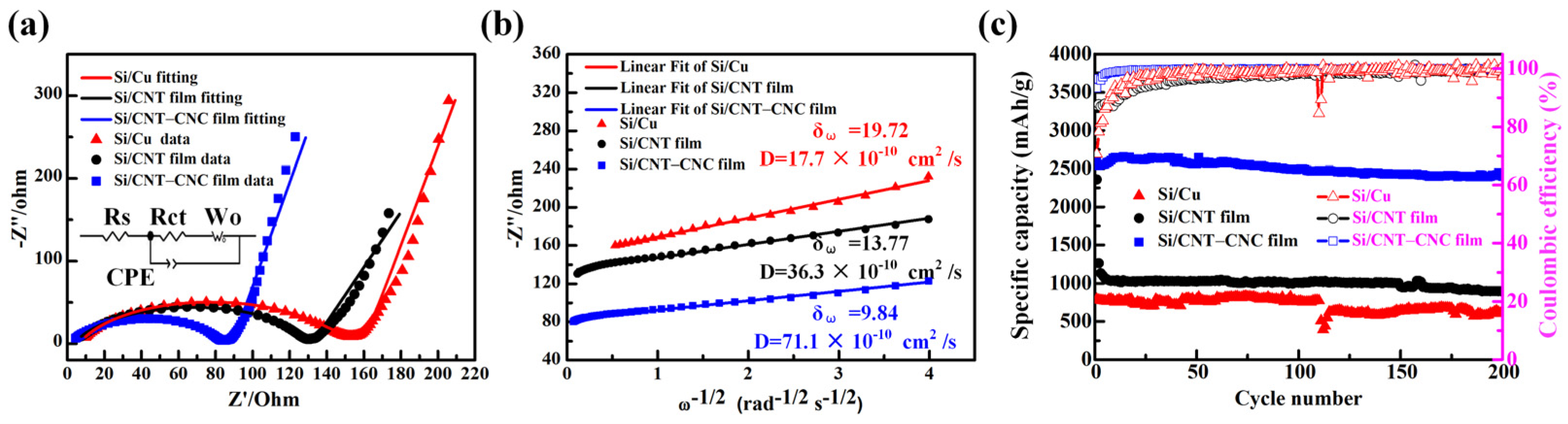
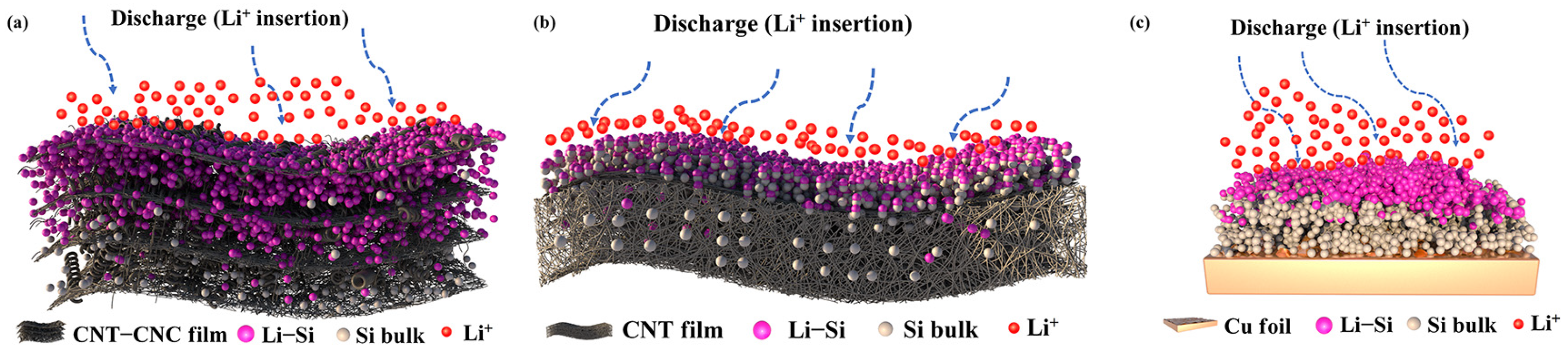
3.2. Electrochemical Performance of Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, T.; Song, W.T.; Son, D.Y.; Ono, L.K.; Qi, Y.B. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Wu, F.X.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.B.; Zhou, Q.F.; Li, X.D.; Xiong, X.H. Fast-charging anodes for lithium ion batteries: Progress and challenges. Chem. Commun. 2024, 60, 2472–2488. [Google Scholar] [CrossRef]
- Li, S.Q.; Wang, K.; Zhang, G.F.; Li, S.N.; Xu, Y.A.; Zhang, X.D.; Zhang, X.; Zheng, S.H.; Sun, X.Z.; Ma, Y.W. Fast Charging Anode Materials for Lithium-Ion Batteries: Current Status and Perspectives. Adv. Funct. Mater. 2022, 32, 2200796. [Google Scholar] [CrossRef]
- Zhang, S.C.; Du, Z.J.; Lin, R.X.; Jiang, T.; Liu, G.R.; Wu, X.M.; Weng, D.S. Nickel Nanocone-Array Supported Silicon Anode for High-Performance Lithium-Ion Batteries. Adv. Mater. 2010, 22, 5378–5382. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, Y.X.; Shao, R.; Wu, J.; Jiang, R.Y.; Jin, Z. Recent progress and future perspective on practical silicon anode-based lithium ion batteries. Energy Storage Mater. 2022, 46, 482–502. [Google Scholar] [CrossRef]
- Philippot, M.L.; Costa, D.; Cardellini, G.; De Sutter, L.; Smekens, J.; Van Mierlo, J.; Messagie, M. Life cycle assessment of a lithium-ion battery with a silicon anode for electric vehicles. J. Energy Storage 2023, 60, 106635. [Google Scholar] [CrossRef]
- Zhao, H.S.; Li, J.B.; Zhao, Q.; Huang, X.B.; Jia, S.Y.; Ma, J.M.; Ren, Y.R. Si-Based Anodes: Advances and Challenges in Li-Ion Batteries for Enhanced Stability. Electrochem. Energy Rev. 2024, 7, 36. [Google Scholar] [CrossRef]
- Ezzedine, M.; Zamfir, M.R.; Jardali, F.; Leveau, L.; Caristan, E.; Ersen, O.; Cojocaru, C.S.; Florea, I. Insight into the Formation and Stability of Solid Electrolyte Interphase for Nanostructured Silicon-Based Anode Electrodes Used in Li-Ion Batteries. Acs Appl. Mater. Inter. 2021, 13, 24734–24746. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, G.; Li, S.; Liu, T.F.; Qiu, J.X.; Li, H.M. Recent progress of structural designs of silicon for performance -enhanced lithium -ion batteries. Chem. Eng. J. 2020, 397, 125380. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, M.G.; Joo, J.; Kim, K.; Kim, J.; Ahn, S.; Cui, Y.; Cho, J. Silicon Nanotube Battery Anodes. Nano Lett. 2009, 9, 3844–3847. [Google Scholar] [CrossRef]
- Ling, M.; Xu, Y.N.; Zhao, H.; Gu, X.X.; Qiu, J.X.; Li, S.; Wu, M.Y.; Song, X.Y.; Yan, C.; Liu, G.; et al. Dual-functional gum arabic binder for silicon anodes in lithium ion batteries. Nano Energy 2015, 12, 178–185. [Google Scholar] [CrossRef]
- Azam, M.A.; Safie, N.E.; Ahmad, A.S.; Yuza, N.A.; Zulkifli, N.S.A. Recent advances of silicon, carbon composites and tin oxide as new anode materials for lithium-ion battery: A comprehensive review. J. Energy Storage 2021, 33, 102096. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, R.R.; Yang, X.; Zhang, R.Y.; Guo, J.L.; Hao, J. Ionic liquids self-assembly preparation of binder-free composites anode with well-dispersed Si nanoparticles on CNTs networks for lithium-ion batteries. Appl. Surf. Sci. 2023, 618, 156655. [Google Scholar] [CrossRef]
- Chen, X.X.; Chen, Z.H.; Ni, Y.; Wang, L.; Sun, Y.M. Double-shell interphase design enabling suppressed side reactions for stable Si battery anode. Appl. Phys. Lett. 2022, 121, 123905. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Chen, F.Q.; Han, J.W.; Kong, D.B.; Pan, S.Y.; Xiao, J.; Wu, S.C.; Yang, Q.H. Revival of Microparticular Silicon for Superior Lithium Storage. Adv. Energy Mater. 2023, 13, 2300367. [Google Scholar] [CrossRef]
- Elomari, G.; Hdidou, L.; Larhlimi, H.; Aqil, M.; Makha, M.; Alami, J.; Dahbi, M. Sputtered Silicon-Coated Graphite Electrodes as High Cycling Stability and Improved Kinetics Anodes for Lithium Ion Batteries. Acs Appl. Mater. Inter. 2024, 16, 2193–2203. [Google Scholar] [CrossRef]
- Jiménez, A.R.; Klöpsch, R.; Wagner, R.; Rodehorst, U.C.; Kolek, M.; Nölle, R.; Winter, M.; Placke, T. A Step toward High-Energy Silicon-Based Thin Film Lithium Ion Batteries. Acs Nano 2017, 11, 4731–4744. [Google Scholar] [CrossRef]
- Tocoglu, U.; Cetinkaya, T.; Cevher, O.; Guler, M.O.; Akbulut, H. Nanostructured Silicon Thin Film Electrodes for Li-Ion Batteries. Acta Phys. Pol. A 2013, 123, 380–382. [Google Scholar] [CrossRef]
- Demirkan, M.T.; Trahey, L.; Karabacak, T. Cycling performance of density modulated multilayer silicon thin film anodes in Li-ion batteries. J. Power Sources 2015, 273, 52–61. [Google Scholar] [CrossRef]
- Xie, J.; Tong, L.; Su, L.W.; Xu, Y.W.; Wang, L.B.; Wang, Y.H. Core-shell yolk-shell Si@C@Void@C nanohybrids as advanced lithium ion battery anodes with good electronic conductivity and corrosion resistance. J. Power Sources 2017, 342, 529–536. [Google Scholar] [CrossRef]
- Chockla, A.M.; Harris, J.T.; Akhavan, V.A.; Bogart, T.D.; Holmberg, V.C.; Steinhagen, C.; Mullins, C.B.; Stevenson, K.J.; Korgel, B.A. Silicon Nanowire Fabric as a Lithium Ion Battery Electrode Material. J. Am. Chem. Soc. 2011, 133, 20914–20921. [Google Scholar] [CrossRef]
- Wang, L.; Gao, B.; Peng, C.J.; Peng, X.; Fu, J.J.; Chu, P.K.; Huo, K.F. Bamboo leaf derived ultrafine Si nanoparticles and Si/C nanocomposites for high-performance Li-ion battery anodes. Nanoscale 2015, 7, 13840–13847. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.H.; Lu, G.H.; Mao, S.; Kim, H.; Cui, S.M.; Yu, K.H.; Huang, X.K.; Hurley, P.T.; Mao, O.; Chen, J.H. Silicon nanotube anode for lithium-ion batteries. Electrochem. Commun. 2013, 29, 67–70. [Google Scholar] [CrossRef]
- Polat, B.D.; Keles, O. Improving Si Anode Performance by Forming Copper Capped Copper-Silicon Thin Film Anodes for Rechargeable Lithium Ion Batteries. Electrochim. Acta 2015, 170, 63–71. [Google Scholar] [CrossRef]
- Xie, J.; Imanishi, N.; Zhang, T.; Hirano, A.; Takeda, Y.; Yamamoto, O. Li-ion diffusion in amorphous Si films prepared by RF magnetron sputtering: A comparison of using liquid and polymer electrolytes. Mater. Chem. Phys. 2010, 120, 421–425. [Google Scholar] [CrossRef]
- Chen, X.; Bi, Q.S.; Sajjad, M.; Wang, X.; Ren, Y.; Zhou, X.W.; Xu, W.; Liu, Z. One-Dimensional Porous Silicon Nanowires with Large Surface Area for Fast Charge-Discharge Lithium-Ion Batteries. Nanomaterials 2018, 8, 285. [Google Scholar] [CrossRef]
- Schmuelling, G.; Winter, M.; Placke, T. Investigating the Mg-Si Binary System via Combinatorial Sputter Deposition As High Energy Density Anodes for Lithium-Ion Batteries. Acs Appl. Mater. Inter. 2015, 7, 20124–20133. [Google Scholar] [CrossRef]
- Tong, L.; Wang, P.; Chen, A.R.; Qiu, F.; Fang, W.Z.; Yang, J.; Wang, C.; Yang, Y. Improved electrochemical performance of binder-free multi-layered silicon/carbon thin film electrode for lithium-ion batteries. Carbon 2019, 153, 592–601. [Google Scholar] [CrossRef]
- Chai, L.; Wang, X.Y.; Su, B.; Li, X.G.; Xue, W.D. Insight into the decay mechanism of non-ultra-thin silicon film anode for lithium-ion batteries. Electrochim. Acta 2023, 448, 142112. [Google Scholar] [CrossRef]
- Chai, L.; Wang, X.Y.; Bi, C.J.; Su, B.; Zhang, C.; Li, X.G.; Xue, W.D. Lifetime Optimization of Amorphous Silicon Thin-Film Anodes for Lithium-Ion Batteries. Acs Appl. Energy Mater. 2023, 6, 8388–8396. [Google Scholar] [CrossRef]
- Tong, Y.F.; Xu, Z.; Liu, C.; Zhang, G.A.; Wang, J.; Wu, Z.G. Magnetic sputtered amorphous Si/C multilayer thin films as anode materials for lithium ion batteries. J. Power Sources 2014, 247, 78–83. [Google Scholar] [CrossRef]
- Ma, Y.T.; Li, L.; Qian, J.; Qu, W.J.; Luo, R.; Wu, F.; Chen, R.J. Materials and structure engineering by magnetron sputtering for advanced lithium batteries. Energy Storage Mater. 2021, 39, 203–224. [Google Scholar] [CrossRef]
- Tocoglu, U.; Cevher, O.; Guler, M.O.; Akbulut, H. Coaxial silicon/multi-walled carbon nanotube nanocomposite anodes for long cycle life lithium-ion batteries. Appl. Surf. Sci. 2014, 305, 402–411. [Google Scholar] [CrossRef]
- Tong, L.; Wang, P.; Fang, W.Z.; Guo, X.J.; Bao, W.Z.; Yang, Y.; Shen, S.L.; Qiu, F. Interface Engineering of Silicon/Carbon Thin-Film Anodes for High-Rate Lithium-Ion Batteries. Acs Appl. Mater. Inter. 2020, 12, 29242–29252. [Google Scholar] [CrossRef]
- Farmakis, F.; Elmasides, C.; Fanz, P.; Hagen, M.; Georgoulas, N. High energy density amorphous silicon anodes for lithium ion batteries deposited by DC sputtering. J. Power Sources 2015, 293, 301–305. [Google Scholar] [CrossRef]
- Schulze, M.C.; Urias, F.; Dutta, N.S.; Huey, Z.; Coyle, J.; Teeter, G.; Doeren, R.; de Villers, B.T.J.; Han, S.D.; Neale, N.R.; et al. Control of nanoparticle dispersion, SEI composition, and electrode morphology enables long cycle life in high silicon content nanoparticle-based composite anodes for lithium-ion batteries. J. Mater. Chem. A 2023, 11, 5257–5266. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Yang, Z.; Li, Z.L.; Wang, J.; He, X.M.; Zhao, H.L. Rational Electrolyte Design for Interfacial Chemistry Modulation to Enable Long-Term Cycling Si Anode. Adv. Energy Mater. 2023, 13, 2302068. [Google Scholar] [CrossRef]
- Landi, B.J.; Ganter, M.J.; Cress, C.D.; DiLeo, R.A.; Raffaelle, R.P. Carbon nanotubes for lithium ion batteries. Energ. Environ. Sci. 2009, 2, 638–654. [Google Scholar] [CrossRef]
- Coppey, N.; Noé, L.; Dupin, J.C.; Puech, P.; Vergnes, H.; Monthioux, M.; Caussat, B. Silicon Nanoparticle/Carbon Nanotube Composites for LI-ION Battery Anodes. Carbon 2012, 17–22. [Google Scholar]
- Shi, Q.T.; Zhou, J.H.; Ullah, S.; Yang, X.Q.; Tokarska, K.; Trzebicka, B.; Ta, H.Q.; Rümmeli, M.H. A review of recent developments in Si/C composite materials for Li-ion batteries. Energy Storage Mater. 2021, 34, 735–754. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Y.P.; Zhao, H.T.; Huang, H.; Wen, N.X.; Wang, C.; Fan, Z.; Hao, L.; Pan, L.J. Hybrid films constructed by carbon nanotubes and carbon nanocoils as current collectors for lithium-ion batteries. J. Electroanal. Chem. 2023, 935, 117288. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Fan, Z.; Hao, L.; Pan, L.J. Facile fabrication of binder-free carbon nanotube-carbon nanocoil hybrid films for anodes of lithium-ion batteries. J. Solid. State Electr. 2024, 28, 3325–3335. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Wang, J.Z.; Huang, H.; Cong, T.Z.; Yang, S.T.; Chen, H.; Qin, J.Q.; Usman, M.; Fan, Z.; Pan, L.J. Growth of Carbon Nanocoils by Porous α-Fe2O3/SnO2 Catalyst and Its Buckypaper for High Efficient Adsorption. Nano-Micro Lett. 2020, 12, 23. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Zhang, H.; Yang, X.; Huang, H.; Zhao, G.L.; Cong, T.Z.; Zuo, X.Q.; Fan, Z.; Yang, S.T.; Pan, L.J. In situ construction of hierarchical core-shell Fe3O4@C nanoparticles-helical carbon nanocoil hybrid composites for highly efficient electromagnetic wave absorption. Carbon 2021, 171, 395–408. [Google Scholar] [CrossRef]
- Zhao, H.T.; Chen, H.; Wang, C.; Fan, Z.; Hao, L.; Pan, L.J. Three-dimensional porous framework constructed by hybrid of carbon nanotubes and carbon nanocoils for stable lithium metal anode. J. Mater. Res. 2022, 37, 2073–2081. [Google Scholar] [CrossRef]
- Leong, I.W.; Tsutsui, M.; Yokota, K.; Murayama, S.; Taniguchi, M. Regulating Nonlinear Ion Transport through a Solid-State Pore by Partial Surface Coatings. Acs Appl. Mater. Inter. 2023, 15, 6123–6132. [Google Scholar] [CrossRef]
- Divya, M.L.; Lee, Y.S.; Aravindan, V. Solvent Co-intercalation: An Emerging Mechanism in Li-, Na-, and K-Ion Capacitors. Acs Energy Lett. 2021, 6, 4228–4244. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, C.; Fan, Z.; Cheng, C.; Hao, L.; Pan, L. Carbon Nanotube–Carbon Nanocoil Hybrid Film Decorated by Amorphous Silicon as Anodes for Lithium-Ion Batteries. J. Compos. Sci. 2024, 8, 350. https://doi.org/10.3390/jcs8090350
Chen H, Wang C, Fan Z, Cheng C, Hao L, Pan L. Carbon Nanotube–Carbon Nanocoil Hybrid Film Decorated by Amorphous Silicon as Anodes for Lithium-Ion Batteries. Journal of Composites Science. 2024; 8(9):350. https://doi.org/10.3390/jcs8090350
Chicago/Turabian StyleChen, Huan, Chen Wang, Zeng Fan, Chuanhui Cheng, Liang Hao, and Lujun Pan. 2024. "Carbon Nanotube–Carbon Nanocoil Hybrid Film Decorated by Amorphous Silicon as Anodes for Lithium-Ion Batteries" Journal of Composites Science 8, no. 9: 350. https://doi.org/10.3390/jcs8090350
APA StyleChen, H., Wang, C., Fan, Z., Cheng, C., Hao, L., & Pan, L. (2024). Carbon Nanotube–Carbon Nanocoil Hybrid Film Decorated by Amorphous Silicon as Anodes for Lithium-Ion Batteries. Journal of Composites Science, 8(9), 350. https://doi.org/10.3390/jcs8090350






