An Overview of Nanotechnology in Dental Medicine
Abstract
1. Introduction
2. Nanomaterials in Preventive Dentistry
2.1. Nanoparticles in Oral Hygiene Products
2.2. Nanocoatings
3. Nanomaterials in Restorative Dentistry
3.1. Nanocomposites for Dental Fillings
3.2. Nanoadhesives
4. Nanomaterials in Endodontics
4.1. Nanoparticles in Root Canal Disinfection
4.2. Nano-Based Sealers
5. Nanomaterials in Periodontology
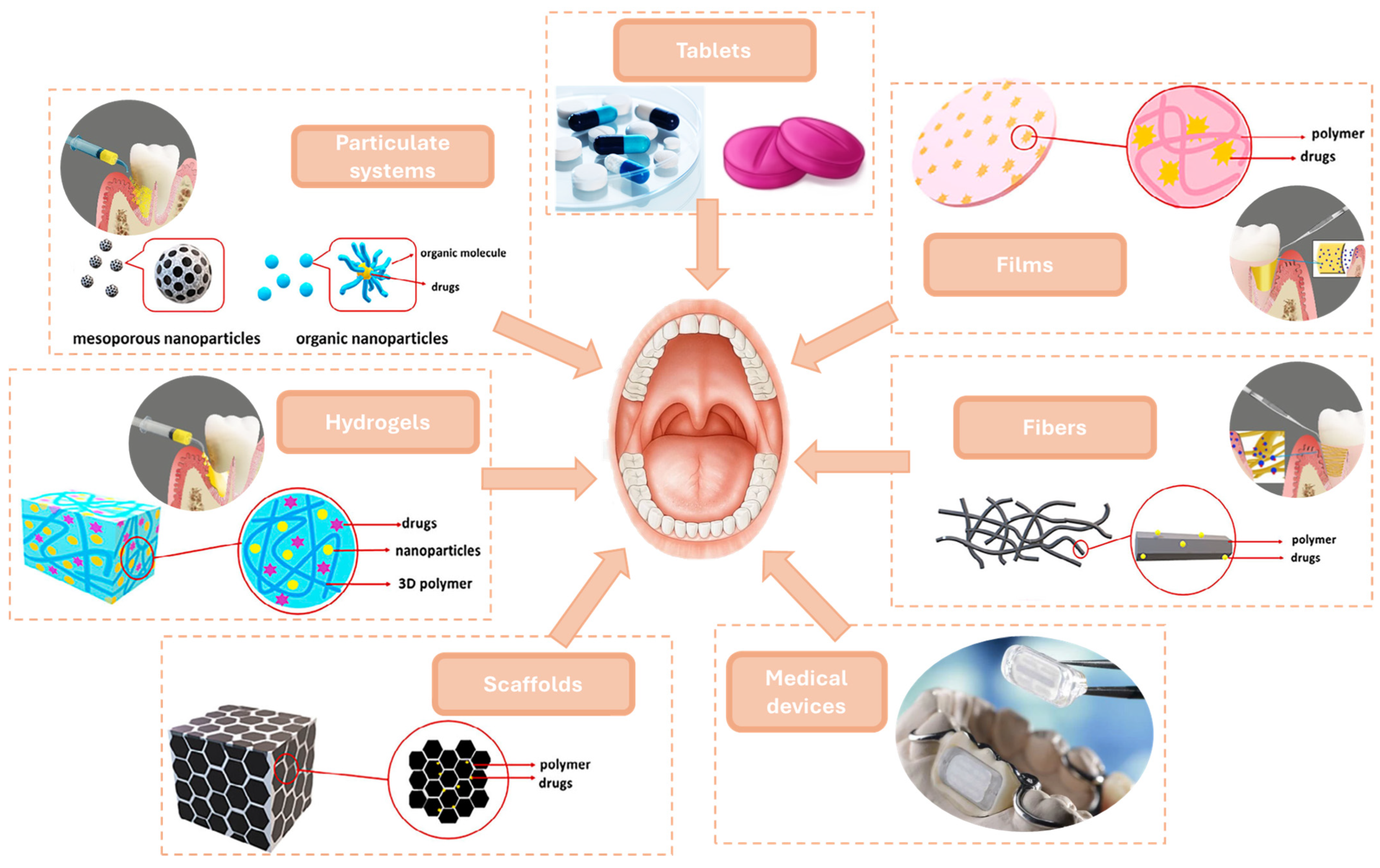
5.1. Nanoformulations for Targeted Drug Delivery in Periodontal Therapy
5.2. Nanoparticles in Regenerative Periodontal Treatment
6. Safety and Toxicity Considerations
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kandavalli, S.R.; Wang, Q.; Ebrahimi, M.; Gode, C.; Djavanroodi, F.; Attarilar, S.; Liu, S. A brief review on the evolution of metallic dental implants: History, design, and application. Front. Mater. 2021, 8, 646383. [Google Scholar] [CrossRef]
- Leek, F.F. The Practice of Dentistry in Ancient Egypt. J. Egypt. Archaeol. 1967, 53, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hyson, J.M., Jr. Amalgam: Its history and perils. J. Calif. Dent. Assoc. 2006, 34, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Marin, E. History of dental biomaterials: Biocompatibility, durability and still open challenges. Heritage Sci. 2023, 11, 207. [Google Scholar] [CrossRef]
- Rezaie, H.R.; Rizi, H.B.; Khamseh, M.M.R.; Öchsner, A. A Review on Dental Materials; Springer: New York, NY, USA, 2020. [Google Scholar]
- Burke, F.T.; Mackenzie, L.; Sands, P. Dental materials—What goes where? class I and II cavities. Dent. Updat. 2013, 40, 260–274. [Google Scholar] [CrossRef]
- Gajanan, K.; Tijare, S. Applications of nanomaterials. Mater. Today Proc. 2018, 5, 1093–1096. [Google Scholar] [CrossRef]
- Malik, S.; Waheed, Y. Emerging Applications of Nanotechnology in Dentistry. Dent. J. 2023, 11, 266. [Google Scholar] [CrossRef]
- Glowacka-Sobotta, A.; Ziental, D.; Czarczynska-Goslinska, B.; Michalak, M.; Wysocki, M.; Güzel, E.; Sobotta, L. Nanotechnology for Dentistry: Prospects and Applications. Nanomaterials 2023, 13, 2130. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Verma, S.; Chevvuri, R. Nanotechnology in dentistry: Unleashing the hidden gems. J. Indian Soc. Periodontol. 2018, 22, 196–200. [Google Scholar] [CrossRef]
- Sreenivasalu, P.K.P.; Dora, C.P.; Swami, R.; Jasthi, V.C.; Shiroorkar, P.N.; Nagaraja, S.; Asdaq, S.M.B.; Anwer, K. Nanomaterials in Dentistry: Current Applications and Future Scope. Nanomaterials 2022, 12, 1676. [Google Scholar] [CrossRef]
- Besinis, A.; De Peralta, T.; Tredwin, C.J.; Handy, R.D. Review of nanomaterials in dentistry: Interactions with the oral microenvironment, clinical applications, hazards, and benefits. ACS Nano 2015, 9, 2255–2289. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Alnazzawi, A.A.; Alrahabi, M.; Fareed, M.A.; Najeeb, S.; Khurshid, Z. Nanotechnology and nanomaterials in dentistry. In Advanced Dental Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 477–505. [Google Scholar]
- Mallineni, S.K.; Sakhamuri, S.; Kotha, S.L.; AlAsmari, A.R.G.M.; AlJefri, G.H.; Almotawah, F.N.; Mallineni, S.; Sajja, R. Silver Nanoparticles in Dental Applications: A Descriptive Review. Bioengineering 2023, 10, 327. [Google Scholar] [CrossRef]
- Bolenwar, A.; Reche, A.; Dhamdhere, N.; Rathi, S. Applications of Silver Nanoparticles in Dentistry. Cureus 2023, 15, e44090. [Google Scholar] [CrossRef] [PubMed]
- Agusnar, H.; Yunita, F.; Yuandani, Y.; Utami, N.M. Synthesis and characterization of silver nanoparticle chitosan as toothpaste with antimicrobial activity. In AIP Conference Proceedings; AIP Publishing: Medan, Indonesia, 2023; Volume 2626, p. 030018. [Google Scholar]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, applications, toxicity and toxicity mechanisms of silver nanoparticles: A review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef]
- Kaushik, A.; Gola, D.; Raghav, J.; Gupta, D.; Kumar, A.; Agarwal, M.; Chauhan, N.; Srivastava, S.K.; Tyagi, P.K. Synthesis of silver nanoparticles using egg white: Dye degradation and antimicrobial potential. Biointerface Res. Appl. Chem. 2022, 12, 2361–2372. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Mahmoud, Z.H.; Abdullaev, S.; Ali, F.K.; Naeem, Y.A.; Mizher, R.M.; Karim, M.M.; Abdulwahid, A.S.; Ahmadi, Z.; Habibzadeh, S.; et al. Nano titanium oxide (nano-TiO2): A review of synthesis methods, properties, and applications. Case Stud. Chem. Environ. Eng. 2024, 9, 100626. [Google Scholar] [CrossRef]
- Leynen, N.; Tytgat, J.; Bijnens, K.; Jaenen, V.; Verleysen, E.; Artois, T.; Van Belleghem, F.; Saenen, N.; Smeets, K. Assessing the in vivo toxicity of titanium dioxide nanoparticles in Schmidtea mediterranea: Uptake pathways and (neuro)developmental outcomes. Aquat. Toxicol. 2024, 270, 106895. [Google Scholar] [CrossRef]
- Abdulhameed, E.A.; Al-Rawi, N.H.; Omar, M.; Khalifa, N.; Samsudin, A.R. Titanium dioxide dental implants surfaces related oxidative stress in bone remodeling: A systematic review. PeerJ 2022, 10, e12951. [Google Scholar] [CrossRef]
- El-Khatib, E.M.; Ali, N.F.; Nassar, S.H.; El-Shemy, N.S. Functionalization of natural fibers properties by using TiO2 nanoparticles to improve its antimicrobial activity. Biointerface Res. Appl. Chem. 2022, 12, 4177–4191. [Google Scholar] [CrossRef]
- Pushpalatha, C.; Suresh, J.; Gayathri, V.; Sowmya, S.; Augustine, D.; Alamoudi, A.; Zidane, B.; Albar, N.H.M.; Patil, S. Zinc Oxide Nanoparticles: A Review on Its Applications in Dentistry. Front. Bioeng. Biotechnol. 2022, 10, 917990. [Google Scholar] [CrossRef]
- Barma, M.D.; Muthupandiyan, I.; Samuel, S.R.; Amaechi, B.T. Inhibition of Streptococcus mutans, antioxidant property and cytotoxicity of novel nano-zinc oxide varnish. Arch. Oral Biol. 2021, 126, 105132. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.K.; Jha, S.; Singh, A.K.; Mishra, S.K.; Pathak, A.K.; Ojha, R.P.; Yadav, R.S.; Dikshit, A. Innovative Investigation of Zinc Oxide Nanoparticles Used in Dentistry. Crystals 2022, 12, 1063. [Google Scholar] [CrossRef]
- El Shahawi, A.M. Incorporation of zinc oxide nanoparticles and it’s antibacterial effect on toothpaste. Bull. Natl. Res. Cent. 2023, 47, 2. [Google Scholar] [CrossRef]
- Florea, D.A.; Mocanu, A.; Pop, L.C.; Tomoaia, G.; Dobrota, C.-T.; Varhelyi, C., Jr.; Tomoaia-Cotisel, M. Remineralization of Tooth Enamel With Hydroxyapatite Nanoparticles: An In Vitro Study. Stud. Univ. Babes-Bolyai 2023, 68, 99–113. [Google Scholar] [CrossRef]
- Akhtar, K.; Pervez, C.; Zubair, N.; Khalid, H. Calcium hydroxyapatite nanoparticles as a reinforcement filler in dental resin nanocomposite. J. Mater. Sci. Mater. Med. 2021, 32, 129. [Google Scholar] [CrossRef] [PubMed]
- Balhuc, S.; Campian, R.; Labunet, A.; Negucioiu, M.; Buduru, S.; Kui, A. Dental applications of systems based on hydroxyapatite nanoparticles—An evidence-based update. Crystals 2021, 11, 674. [Google Scholar] [CrossRef]
- Abifarin, F.B.; Musa, Z.; Abifarin, J.K. mechanical processing of hydroxyapatite through sintering and multi-objective optimization technique for biomedical application. MRS Adv. 2023, 8, 532–537. [Google Scholar] [CrossRef]
- Yazdanian, M.; Rostamzadeh, P.; Rahbar, M.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Yazdanian, A. The potential application of green-synthesized metal nanoparticles in dentistry: A comprehensive review. Bioinorg. Chem. Appl. 2022, 2022, 2311910. [Google Scholar] [CrossRef]
- Jadhav, K.; Hr, R.; Deshpande, S.; Jagwani, S.; Dhamecha, D.; Jalalpure, S.; Subburayan, K.; Baheti, D. Phytosynthesis of gold nanoparticles: Characterization, biocompatibility, and evaluation of its osteoinductive potential for application in implant dentistry. Mater. Sci. Eng. C 2018, 93, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, P.; Zhao, R.; Zhao, L.; Liu, J.; Peng, S.; Fu, X.; Wang, X.; Luo, R.; Wang, R.; et al. Silica nanoparticles: Biomedical applications and toxicity. Biomed. Pharmacother. 2022, 151, 113053. [Google Scholar] [CrossRef]
- Pavanello, L.; Cortês, I.T.; de Carvalho, R.D.P.; Picolo, M.Z.D.; Cavalli, V.; Silva, L.T.S.; Boaro, L.C.C.; Prokopovich, P.; Cogo-Müller, K. Physicochemical and biological properties of dental materials and formulations with silica nanoparticles: A narrative review. Dent. Mater. 2024. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, A.K.; Koulaouzidou, E.A.; Gogos, C.; Achilias, D.S. Synthesis of Novel Dental Nanocomposite Resins by Incorporating Polymerizable, Quaternary Ammonium Silane-Modified Silica Nanoparticles. Polymers 2021, 13, 1682. [Google Scholar] [CrossRef] [PubMed]
- Rojas, B.; Soto, N.; Villalba, M.; Bello-Toledo, H.; Meléndrez-Castro, M.; Sánchez-Sanhueza, G. Antibacterial Activity of Copper Nanoparticles (CuNPs) against a Resistant Calcium Hydroxide Multispecies Endodontic Biofilm. Nanomaterials 2021, 11, 2254. [Google Scholar] [CrossRef]
- Maawadh, A.; AlDeeb, L.; Almohareb, T.; Alahdal, K.; Alshamrani, A.S.; Alrahlah, A. Copper Oxide Nanoparticles Loaded in Adhesive to Carious Dentin. An In Vitro Study Assessing μTBS, Degree of Conversion, EDX, and SEM. Sci. Adv. Mater. 2024, 16, 1109–1115. [Google Scholar] [CrossRef]
- Xu, V.W.; Nizami, M.Z.I.; Yin, I.X.; Yu, O.Y.; Lung, C.Y.K.; Chu, C.H. Application of Copper Nanoparticles in Dentistry. Nanomaterials 2022, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, J.; Karlsson, H.L.; Hedberg, Y.; Blomberg, E.; Wallinder, I.O. The importance of extracellular speciation and corrosion of copper nanoparticles on lung cell membrane integrity. Colloids Surfaces B Biointerfaces 2016, 141, 291–300. [Google Scholar] [CrossRef]
- Higino, T.; França, R. Drug-delivery nanoparticles for bone-tissue and dental applications. Biomed. Phys. Eng. Express 2022, 8, 042001. [Google Scholar] [CrossRef]
- Yudaev, P.; Chuev, V.; Klyukin, B.; Kuskov, A.; Mezhuev, Y.; Chistyakov, E. Polymeric Dental Nanomaterials: Antimicrobial Action. Polymers 2022, 14, 864. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Osorio, M.; Osorio, R.; Aguilera, F.S.; Medina-Castillo, A.L.; Toledano, M.; Osorio, E.; Acosta, S.; Chen, R.; Aparicio, C. Polymeric nanoparticles protect the resin-dentin bonded interface from cariogenic biofilm degradation. Acta Biomater. 2020, 111, 316–326. [Google Scholar] [CrossRef]
- Prabha, A.S.; Dorothy, R.; Jancirani, S.; Rajendran, S.; Singh, G.; Kumaran, S.S. Recent advances in the study of toxicity of polymer-based nanomaterials. Nanotoxicity 2020, 143–165. [Google Scholar]
- Montoya, C.; Roldan, L.; Yu, M.; Valliani, S.; Ta, C.; Yang, M.; Orrego, S. Smart dental materials for antimicrobial applications. Bioact. Mater. 2023, 24, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, X. Current Application of Magnetic Materials in the Dental Field. Magnetochemistry 2024, 10, 46. [Google Scholar] [CrossRef]
- Vakili-Ghartavol, R.; Momtazi-Borojeni, A.A.; Vakili-Ghartavol, Z.; Aiyelabegan, H.T.; Jaafari, M.R.; Rezayat, S.M.; Bidgoli, S.A. Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artif. Cells Nanomed. Biotechnol. 2020, 48, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Lee, J.-S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.-R.; Hsiao, C.-D. Potential toxicity of iron oxide magnetic nanoparticles: A review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.; Mitsidis, D.; Xie, Z.; McNeilly, O.; Ng, Y.H.; Amal, R.; Gunawan, C. Antibacterial activity of reduced graphene oxide. J. Nanomater. 2021, 2021, 9941577. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, J.; Gao, Y.; Li, T.; Wang, H.; Yan, H.; Niu, B.; Guo, R. Antibacterial graphene oxide coatings on polymer substrate. Appl. Surf. Sci. 2018, 436, 624–630. [Google Scholar] [CrossRef]
- Bregnocchi, A.; Zanni, E.; Uccelletti, D.; Marra, F.; Cavallini, D.; De Angelis, F.; De Bellis, G.; Bossù, M.; Ierardo, G.; Polimeni, A.; et al. Graphene-based dental adhesive with anti-biofilm activity. J. Nanobiotechnology 2017, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Rhazouani, A.; Gamrani, H.; El Achaby, M.; Aziz, K.; Gebrati, L.; Uddin, M.S.; Aziz, F. Synthesis and toxicity of graphene oxide nanoparticles: A literature review of in vitro and in vivo studies. BioMed Res. Int. 2021, 2021, 5518999. [Google Scholar] [CrossRef]
- Abedi, M.; Ghasemi, Y.; Nemati, M.M. Nanotechnology in toothpaste: Fundamentals, trends, and safety. Heliyon 2024, 10, e24949. [Google Scholar] [CrossRef]
- Loesche, W.J. Microbiology of Dental Decay and Periodontal Disease. Medical Microbiology, 4th ed.; Wiley: Hoboken, NJ, USA, 1996. [Google Scholar]
- Rezaei, T.; Mehramouz, B.; Gholizadeh, P.; Yousefi, L.; Ganbarov, K.; Ghotaslou, R.; Taghizadeh, S.; Kafil, H.S. Factors associated with Streptococcus mutans pathogenicity in the oral cavity. Biointerface Res. Appl. Chem. 2023, 13, 368. [Google Scholar]
- Carrouel, F.; Viennot, S.; Ottolenghi, L.; Gaillard, C.; Bourgeois, D. Nanoparticles as Anti-Microbial, Anti-Inflammatory, and Remineralizing Agents in Oral Care Cosmetics: A Review of the Current Situation. Nanomaterials 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Abadi, M.F.; Mehrabian, S.; Asghari, B.; Namvar, A.E.; Ezzatifar, F.; Lari, A.R. Silver nanoparticles as active ingredient used for alcohol-free mouthwash. GMS Hyg. Infect. Control 2013, 8, Doc05. [Google Scholar] [CrossRef]
- Ahmed, O.A.K.; Sibuyi, N.R.S.; Fadaka, A.O.; Maboza, E.; Olivier, A.; Madiehe, A.M.; Meyer, M.; Geerts, G. Prospects of Using Gum Arabic Silver Nanoparticles in Toothpaste to Prevent Dental Caries. Pharmaceutics 2023, 15, 871. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Saliminasab, M.; Jabbari, H.; Farahmand, H.; Asadi, M.; Soleimani, M.; Fathi, A. Study of antibacterial performance of synthesized silver nanoparticles on Streptococcus mutans bacteria. Nanomed. Res. J. 2022, 7, 391–396. [Google Scholar] [CrossRef]
- Sathiyaraj, S.; Suriyakala, G.; Gandhi, A.D.; Babujanarthanam, R.; Almaary, K.S.; Chen, T.-W.; Kaviyarasu, K. Biosynthesis, characterization, and antibacterial activity of gold nanoparticles. J. Infect. Public Health 2021, 14, 1842–1847. [Google Scholar] [CrossRef]
- Al-Fahham, B.M.; Mohamed, R.A.; Al-Talqani, J.M.T.; Fahad, A.H.; Haider, J. Evaluating Antimicrobial Effectiveness of Gold Nanoparticles against Streptococcus oralis. Int. J. Dent. 2023, 2023, 9935556. [Google Scholar] [CrossRef]
- Punniyakotti, P.; Panneerselvam, P.; Perumal, D.; Aruliah, R.; Angaiah, S. Anti-bacterial and anti-biofilm properties of green synthesized copper nanoparticles from Cardiospermum halicacabum leaf extract. Bioprocess Biosyst. Eng. 2020, 43, 1649–1657. [Google Scholar] [CrossRef]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116. [Google Scholar] [CrossRef]
- Agarwal, H.; Shanmugam, V. A review on anti-inflammatory activity of green synthesized zinc oxide nanoparticle: Mechanism-based approach. Bioorganic Chem. 2020, 94, 103423. [Google Scholar] [CrossRef]
- Matsumoto-Nakano, M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci. Rev. 2018, 54, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Emad, M.; Salama, K. A comparison of the effects of lemon peel-silver nanoparticles versus brand toothpastes and mouthwashes on staphylococcus spp. isolated from teeth caries. Iraqi J. Sci. 2020, 61, 1894–1901. [Google Scholar] [CrossRef]
- Junevičius, J.; Žilinskas, J.; Česaitis, K.; Česaitienė, G.; Gleiznys, D.; Maželienė, Ž. Antimicrobial activity of silver and gold in toothpastes: A comparative analysis. Stomatologija 2015, 17, 9–12. [Google Scholar]
- O’hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontology 2021, 110, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Kaisarly, D.; Kunzelmann, K.-H. Tooth whitening with an experimental toothpaste containing hydroxyapatite nanoparticles. BMC Oral Health 2022, 22, 331. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Kunzelmann, K.-H. Biomimetic tooth-whitening effect of hydroxyapatite-containing mouthrinses after long-term simulated oral rinsing. Am. J. Dent. 2021, 34, 307–312. [Google Scholar] [PubMed]
- Florea, A.-D.; Dobrota, C.T.; Carpa, R.; Racz, C.-P.; Tomoaia, G.; Mocanu, A.; Avram, A.; Soritau, O.; Pop, L.C.; Tomoaia-Cotisel, M. Optimization of Functional Toothpaste Formulation Containing Nano-Hydroxyapatite and Birch Extract for Daily Oral Care. Materials 2023, 16, 7143. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.C.; Cazzaniga, G.; Ottobelli, M.; Garcia-Godoy, F.; Brambilla, E. Substituted Nano-Hydroxyapatite Toothpastes Reduce Biofilm Formation on Enamel and Resin-Based Composite Surfaces. J. Funct. Biomater. 2020, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Amaechi, B.T.; Lemke, K.C.; Saha, S.; Luong, M.N.; Gelfond, J. Clinical efficacy of nanohydroxyapatite-containing toothpaste at relieving dentin hypersensitivity: An 8 weeks randomized control trial. BDJ Open 2021, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Barma, M.D.; Kannan, S.D.; Indiran, M.A.; Rajeshkumar, S.; Kumar, R.P. Antibacterial Activity of Mouthwash Incorporated with Silica Nanoparticles against S. aureus, S. mutans, E. faecalis: An in-vitro Study. J. Pharm. Res. Int. 2020, 32, 25–33. [Google Scholar] [CrossRef]
- Wang, S.; Fang, L.; Zhou, H.; Wang, M.; Zheng, H.; Wang, Y.; Weir, M.D.; Masri, R.; Oates, T.W.; Cheng, L.; et al. Silica nanoparticles containing nano-silver and chlorhexidine respond to pH to suppress biofilm acids and modulate biofilms toward a non-cariogenic composition. Dent. Mater. 2024, 40, 179–189. [Google Scholar] [CrossRef]
- Aspinall, S.R.; Khutoryanskiy, V.V. Surface Modification of Silica Particles with Adhesive Functional Groups or Their Coating with Chitosan to Improve the Retention of Toothpastes in the Mouth. Langmuir 2023, 39, 1677–1685. [Google Scholar] [CrossRef]
- Parnia, F.; Yazdani, J.; Javaherzadeh, V.; Dizaj, S.M. Overview of nanoparticle coating of dental implants for enhanced osseointegration and antimicrobial purposes. J. Pharm. Pharm. Sci. 2017, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, R.; Barhoum, A.; Uludag, H. A review of nanostructured surfaces and materials for dental implants: Surface coating, patterning and functionalization for improved performance. Biomater. Sci. 2018, 6, 1312–1338. [Google Scholar] [CrossRef]
- Memarzadeh, K.; Sharili, A.S.; Huang, J.; Rawlinson, S.C.; Allaker, R.P. Nanoparticulate zinc oxide as a coating material for orthopedic and dental implants. J. Biomed. Mater. Res. A 2015, 103, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, Z.; Liu, G.; Wang, Z.; Guo, X.; Guo, C.; Han, J. TiO2 Nanocoatings with Controllable Crystal Type and Nanoscale Topography on Zirconia Implants to Accelerate Bone Formation. Bioinorg. Chem. Appl. 2022, 2022, 8650659. [Google Scholar] [CrossRef]
- Hammad, S.M.; El-Wassefy, N.A.; Shamaa, M.S.; Fathy, A. Evaluation of zinc-oxide nanocoating on the characteristics and antibacterial behavior of nickel-titanium alloy. Dent. Press J. Orthod. 2020, 25, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gulati, K.; Li, Z.; Di, P.; Liu, Y. Dental implant nano-engineering: Advances, limitations and future directions. Nanomaterials 2021, 11, 2489. [Google Scholar] [CrossRef]
- Besinis, A.; Hadi, S.D.; Le, H.R.; Tredwin, C.; Handy, R.D. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Seo, Y.-K.; Lee, J.-H. Effects of the combination of bone morphogenetic protein-2 and nano-hydroxyapatite on the osseointegration of dental implants. J. Korean Assoc. Oral Maxillofac. Surg. 2021, 47, 454–464. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.G.F.P.; de Melo Soares, M.S.; Silveira e Souza, A.M.M.; Taba Jr, M.; Palioto, D.B.; Messora, M.R.; Ghiraldini, B.; Nunes, F.A.d.S.; de Souza, S.L.S. Influence of nano-hydroxyapatite coating implants on gene expression of osteogenic markers and micro-CT parameters. An in vivo study in diabetic rats. J. Biomed. Mater. Res. Part A 2021, 109, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Lamkhao, S.; Phaya, M.; Jansakun, C.; Chandet, N.; Thongkorn, K.; Rujijanagul, G.; Bangrak, P.; Randorn, C. Synthesis of Hydroxyapatite with Antibacterial Properties Using a Microwave-Assisted Combustion Method. Sci. Rep. 2019, 9, 4015. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, A.; Bruno, G.; Preo, G.; Gracco, A. Application of Nanotechnology in Orthodontic Materials: A State-of-the-Art Review. Dent. J. 2020, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Ilić, B.; Petrović, B.; Marinković, J.; Miletić Vukajlović, J.; Stevanović, M.; Potočnik, J.; Jokanović, V. Investigation of Ion Release and Antibacterial Properties of TiN-Cu-Nanocoated Nitinol Archwires. Coatings 2023, 13, 1587. [Google Scholar] [CrossRef]
- Selvaraj, A.; George, A.M.; Rajeshkumar, S. Efficacy of zirconium oxide nanoparticles coated on stainless steel and nickel titanium wires in orthodontic treatment. Bioinformation 2021, 17, 760–766. [Google Scholar] [CrossRef]
- Golshah, A.; Feyli, S.A. Effect of zirconium oxide nano-coating on frictional resistance of orthodontic wires. J. Orthod. Sci. 2022, 11, 35. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.; Zheng, Z.; Xia, Y. Bio-inspired nanocomposite coatings on orthodontic archwires with corrosion resistant and antibacterial properties. Front. Bioeng. Biotechnol. 2023, 11, 1272527. [Google Scholar] [CrossRef]
- Gracco, A.; Dandrea, M.; Deflorian, F.; Zanella, C.; De Stefani, A.; Bruno, G.; Stellini, E. Application of a Molybdenum and Tungsten Disulfide Coating to Improve Tribological Properties of Orthodontic Archwires. Nanomaterials 2019, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Aminoroaya, A.; Neisiany, R.E.; Khorasani, S.N.; Panahi, P.; Das, O.; Madry, H.; Cucchiarini, M.; Ramakrishna, S. A review of dental composites: Challenges, chemistry aspects, filler influences, and future insights. Compos. Part B Eng. 2021, 216, 108852. [Google Scholar] [CrossRef]
- Gholampour, S.-S.; Zoorazma, G.; Shakouri, E. Evaluating the effect of dental filling material and filling depth on the strength and deformation of filled teeth. Dent. Mater. Tech. 2016, 5, 172–180. [Google Scholar] [CrossRef]
- Ab Rahman, I.; Padavettan, V. Synthesis of Silica Nanoparticles by Sol-Gel: Size-Dependent Properties, Surface Modification, and Applications in Silica-Polymer Nanocomposites—A Review. J. Nanomater. 2012, 2012, 132424. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Rong, M.Z.; Yu, S.L.; Wetzel, B.; Friedrich, K. Effect of particle surface treatment on the tribological performance of epoxy based nanocomposites. Wear 2002, 253, 1086–1093. [Google Scholar] [CrossRef]
- Pinto, D.; Bernardo, L.; Amaro, A.; Lopes, S.M.R. Mechanical properties of epoxy nanocomposites using titanium dioxide as reinforcement—A review. Constr. Build. Mater. 2015, 95, 506–524. [Google Scholar] [CrossRef]
- Carballeira, P.; Haupert, F. Toughening effects of titanium dioxide nanoparticles on TiO2/epoxy resin nanocomposites. Polym. Compos. 2010, 31, 1241–1246. [Google Scholar] [CrossRef]
- Moreau, J.L.; Weir, M.D.; Giuseppetti, A.A.; Chow, L.C.; Antonucci, J.M.; Xu, H.H.K. Long-term mechanical durability of dental nanocomposites containing amorphous calcium phosphate nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1264–1273. [Google Scholar] [CrossRef]
- Fathy, A.; Elkady, O.; Abu-Oqail, A. Production and properties of Cu-ZrO2 nanocomposites. J. Compos. Mater. 2018, 52, 1519–1529. [Google Scholar] [CrossRef]
- Velo, M.M.d.A.C.; Filho, F.G.N.; Nascimento, T.R.d.L.; Obeid, A.T.; Castellano, L.C.; Costa, R.M.; Brondino, N.C.M.; Fonseca, M.G.; Silikas, N.; Mondelli, R.F.L. Enhancing the mechanical properties and providing bioactive potential for graphene oxide/montmorillonite hybrid dental resin composites. Sci. Rep. 2022, 12, 10259. [Google Scholar] [CrossRef] [PubMed]
- Aati, S.; Chauhan, A.; Shrestha, B.; Rajan, S.M.; Aati, H.; Fawzy, A. Development of 3D printed dental resin nanocomposite with graphene nanoplatelets enhanced mechanical properties and induced drug-free antimicrobial activity. Dent. Mater. 2022, 38, 1921–1933. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Q.; Peng, J.; Yang, X.; Yu, D.; Zhao, W. Antibacterial and mechanical properties of reduced graphene-silver nanoparticle nanocomposite modified glass ionomer cements. J. Dent. 2020, 96, 103332. [Google Scholar] [CrossRef] [PubMed]
- Barot, T.; Rawtani, D.; Kulkarni, P. Physicochemical and biological assessment of silver nanoparticles immobilized Halloysite nanotubes-based resin composite for dental applications. Heliyon 2020, 6, e03601. [Google Scholar] [CrossRef] [PubMed]
- Comeau, P.; Burgess, J.; Malekafzali, N.; Leite, M.L.; Lee, A.; Manso, A. Exploring the Physicochemical, Mechanical, and Photocatalytic Antibacterial Properties of a Methacrylate-Based Dental Material Loaded with ZnO Nanoparticles. Materials 2022, 15, 5075. [Google Scholar] [CrossRef]
- Javed, R.; Rais, F.; Fatima, H.; Haq, I.U.; Kaleem, M.; Naz, S.S.; Ao, Q. Chitosan encapsulated ZnO nanocomposites: Fabrication, characterization, and functionalization of bio-dental approaches. Mater. Sci. Eng. C 2020, 116, 111184. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.A.A.; Silva, R.M.F.d.C.e.; Ferreira, L.d.A.Q.; Branco, N.T.T.; Ávila, D.S.; Peres, A.M.; Fernandes-Braga, W.; Sette-Dias, A.C.; Andrade, L.; Palma-Dibb, R.G.; et al. Enhanced mechanical properties, anti-biofilm activity, and cytocompatibility of a methacrylate-based polymer loaded with native multiwalled carbon nanotubes. J. Mech. Behav. Biomed. Mater. 2022, 136, 105511. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, R.; Kumar, S. Synergistic effect of carbon nanotubes and nano-hydroxyapatite on mechanical properties of polyetheretherketone based hybrid nanocomposites. Polym. Polym. Compos. 2021, 29, 1365–1376. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mukherjee, S.; Mishra, M. Nanoparticles used in dentistry: A review. J. Oral Biol. Craniofacial Res. 2018, 8, 58–67. [Google Scholar] [CrossRef]
- Mandhalkar, R.; Paul, P.; Reche, A. Application of Nanomaterials in Restorative Dentistry. Cureus 2023, 15, e33779. [Google Scholar] [CrossRef]
- Azmy, E.; Al-Kholy, M.R.Z.; Fattouh, M.; Kenawi, L.M.M.; Helal, M.A. Impact of Nanoparticles Additions on the Strength of Dental Composite Resin. Int. J. Biomater. 2022, 2022, 1165431. [Google Scholar] [CrossRef]
- Saridou, M.; Nikolaidis, A.K.; Koulaouzidou, E.A.; Achilias, D.S. Synthesis and Characterization of Dental Nanocomposite Resins Reinforced with Dual Organomodified Silica/Clay Nanofiller Systems. J. Funct. Biomater. 2023, 14, 405. [Google Scholar] [CrossRef]
- Toledano, M.; Vallecillo-Rivas, M.; Aguilera, F.S.; Osorio, M.T.; Osorio, E.; Osorio, R. Polymeric zinc-doped nanoparticles for high performance in restorative dentistry. J. Dent. 2021, 107, 103616. [Google Scholar] [CrossRef]
- Alshamrani, A.; Alhotan, A.; Kelly, E.; Ellakwa, A. Mechanical and Biocompatibility Properties of 3D-Printed Dental Resin Reinforced with Glass Silica and Zirconia Nanoparticles: In Vitro Study. Polymers 2023, 15, 2523. [Google Scholar] [CrossRef]
- Rudolf, R.; Popović, D.; Tomić, S.; Bobovnik, R.; Lazić, V.; Majerič, P.; Anžel, I.; Čolić, M. Microstructure Characterisation and Identification of the Mechanical and Functional Properties of a New PMMA-ZnO Composite. Materials 2020, 13, 2717. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, M.I.; Ahmed, M.A.; Felemban, N.H. Effect of Nanoparticles Reinforced Adhesive Layers on Microleakage of Tooth Restorations. World J. Nano Sci. Eng. 2016, 06, 64–69. [Google Scholar] [CrossRef][Green Version]
- Balhaddad, A.A.; Garcia, I.M.; Mokeem, L.; Alsahafi, R.; Collares, F.M.; de Melo, M.A.S. Metal Oxide Nanoparticles and Nanotubes: Ultrasmall Nanostructures to Engineer Antibacterial and Improved Dental Adhesives and Composites. Bioengineering 2021, 8, 146. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhao, Q.; Niu, Y.; Zhao, D. Adhesion Advances: From Nanomaterials to Biomimetic Adhesion and Applications. Soft Matter 2022, 18, 3447–3464. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Alhamdan, Y.; Alibrahim, H.; Almulhim, K.S.; Nawaz, M.; Ahmed, S.Z.; Aljuaid, K.; Ateeq, I.S.; Akhtar, S.; Ansari, M.A.; et al. Analyses of Experimental Dental Adhesives Based on Zirconia/Silver Phosphate Nanoparticles. Polymers 2023, 15, 2614. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Cheng, L.; Zhang, K.; Weir, M.D.; Rodrigues, L.K.; Xu, H.H. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent. Mater. 2013, 29, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.C.; Kondas, V.V.; Nandini, V.; Kirana, R.; Yadalam, P.K.; Eswaramoorthy, R. Evaluating the effect of poly (amidoamine) treated bioactive glass nanoparticle incorporated in universal adhesive on bonding to artificially induced caries affected dentin. BMC Oral Health 2023, 23, 810. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, M.; Kreutz, C.; Kanzow, P.; Tauböck, T.T.; Burrer, P.; Noll, C.; Bader, O.; Rohland, B.; Wiegand, A.; Rizk, M. Effect of Bioactive and Antimicrobial Nanoparticles on Properties and Applicability of Dental Adhesives. Nanomaterials 2022, 12, 3862. [Google Scholar] [CrossRef] [PubMed]
- Mirhashemi, A.; Akhondi, M.S.A.; Sodagar, A.; Jalali, Y.F.; Jazi, L. Effect of nano-zinc oxide and nano-chitosan particles on the shear bond strength of dental composites used as orthodontic adhesive. J. World Fed. Orthod. 2021, 10, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Binhasan, M.; Al-Habeeb, K.M.; Almuqbil, A.S.; Alhaidary, T.A.; Alfawaz, Y.F.; Farooq, I.; Vohra, F.; Abduljabbar, T. Assessment of the Physical Properties of an Experimental Adhesive Dentin Bonding Agent with Carbon Nanoparticles. Crystals 2022, 12, 1441. [Google Scholar] [CrossRef]
- Allende, J.B.; Nascimiento, F.D.; Damasceno, M.; Chiari, S.; Aliaga-Galvez, R.; Ñaupari-Villasante, R.; Miranda, C.B.; Pardo-Díaz, C.; FelipeGutiérrez, M.; Covarrubias, C.; et al. Evaluation of adhesive properties and enzymatic activity at the hybrid layer of a simplified adhesive loaded with 0.2% Cu and 5% ZnO nanoparticles: A Randomized Clinical Trial and ex vivo analysis. J. Dent. 2024, 149, 105283. [Google Scholar] [CrossRef]
- Roig-Soriano, X.; Souto, E.B.; Elmsmari, F.; Garcia, M.L.; Espina, M.; Duran-Sindreu, F.; Sánchez-López, E.; Sánchez, J.A.G. Nanoparticles in Endodontics Disinfection: State of the Art. Pharmaceutics 2022, 14, 1519. [Google Scholar] [CrossRef] [PubMed]
- Capuano, N.; Amato, A.; Dell’annunziata, F.; Giordano, F.; Folliero, V.; Di Spirito, F.; More, P.R.; De Filippis, A.; Martina, S.; Amato, M.; et al. Nanoparticles and Their Antibacterial Application in Endodontics. Antibiotics 2023, 12, 1690. [Google Scholar] [CrossRef]
- Raura, N.; Garg, A.; Arora, A.; Roma, M. Nanoparticle technology and its implications in endodontics: A review. Biomater. Res. 2020, 24, 21. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, R.; Lau, M.; Sheah, M.; Montagner, F.; Quiram, G.; Palmer, K.; Stefan, M.C.; Rodrigues, D.C. Synthesis and Characterization of New Chlorhexidine-Containing Nanoparticles for Root Canal Disinfection. Materials 2016, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Parolia, A.; Kumar, H.; Ramamurthy, S.; Madheswaran, T.; Davamani, F.; Pichika, M.R.; Mak, K.-K.; Fawzy, A.S.; Daood, U.; Pau, A. Effect of Propolis Nanoparticles against Enterococcus faecalis Biofilm in the Root Canal. Molecules 2021, 26, 715. [Google Scholar] [CrossRef]
- Elmsmari, F.; Delgado, L.M.; Duran-Sindreu, F.; Pérez, R.A.; García, M.L.; Trull, M.T.; Afrashtehfar, K.I.; González, J.A.; Sánchez-López, E. Novel strategies enhancing endodontic disinfection: Antibacterial biodegradable calcium hydroxide nanoparticles in an ex vivo model. Int. J. Pharm. 2023, 648, 123627. [Google Scholar] [CrossRef]
- Marín-Correa, B.M.; Guzmán-Martínez, N.; Gómez-Ramírez, M.; Pless, R.C.; Mundo, J.R.; García-Ramos, J.C.; Rojas-Avelizapa, N.G.; Pestryakov, A.; Bogdanchikova, N.; Fierros-Romero, G. Nanosilver gel as an endodontic alternative against Enterococcus faecalis in an in vitro root canal system in Mexican dental specimens. New Microbiol. 2020, 43, 166–170. [Google Scholar]
- Gholami, A.; Ghezelbash, K.; Asheghi, B.; Abbaszadegan, A.; Amini, A. An In Vitro Study on the Antibacterial Effects of Chlorhexidine-Loaded Positively Charged Silver Nanoparticles on Enterococcus faecalis. J. Nanomater. 2022, 2022, 6405772. [Google Scholar] [CrossRef]
- Razumova, S.; Brago, A.; Serebrov, D.; Barakat, H.; Kozlova, Y.; Howijieh, A.; Guryeva, Z.; Enina, Y.; Troitskiy, V. The Application of Nano Silver Argitos as a Final Root Canal Irrigation for the Treatment of Pulpitis and Apical Periodontitis. In Vitro Study. Nanomaterials 2022, 12, 248. [Google Scholar] [CrossRef]
- Tonini, R.; Giovarruscio, M.; Gorni, F.; Ionescu, A.; Brambilla, E.; Mikhailovna, I.M.; Luzi, A.; Maciel Pires, P.; Sauro, S. In Vitro Evaluation of Antibacterial Properties and Smear Layer Removal/Sealer Penetration of a Novel Silver-Citrate Root Canal Irrigant. Materials 2020, 13, 194. [Google Scholar] [CrossRef]
- Ravi, V.; Kini, S.; Shenoy, N.; Somayaji, K.; Shenoy, P. Comparative Evaluation of the Antimicrobial Efficacy of Sodium Hypochlorite, Silver Nanoparticles, and Zinc Nanoparticles against Candidal Biofilm: An In Vitro Study. Eng. Proc. 2023, 59, 170. [Google Scholar]
- Husein, A.; Said, H.M.; Bakar, W.Z.W.; Farea, M. The effect of different sealer placement techniques on sealing Ability: An in vitro study. J. Conserv. Dent. 2012, 15, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Worlikar, N.; Shah, K.; Sharma, Y. Recent advancements in root canal sealers-An overview. J. Adv. Med. Dent. Sci. Res. 2023, 11, 82–91. [Google Scholar]
- Marica, A.; Sipos, L.; Iurcov, R.; Stefanescu, T.; Gabriela, C.; Ioanalucan, A. Current use of nanoparticles in endodontics: A sytematic review. Rom. J. Oral. Rehabil. 2022, 14, 115–126. [Google Scholar]
- Behnaz, M.; Kasraei, S.; Yadegari, Z.; Zare, F.; Nahvi, G. Effects of orthodontic bonding containing TiO2 and ZnO nanoparticles on prevention of white spot lesions: An in vitro study. Biointerface Res. Appl. Chem 2022, 12, 431–440. [Google Scholar]
- Droepenu, E.K.; Wee, B.S.; Chin, S.F.; Kok, K.Y.; Maligan, M.F. Zinc Oxide nanoparticles synthesis methods and its effect on morphology: A review. Biointerface Res. Appl. Chem. 2022, 12, 4261–4292. [Google Scholar] [CrossRef]
- Choi, J.-W.; Yang, S.-Y. Effect of Zinc Oxide Incorporation on the Antibacterial, Physicochemical, and Mechanical Properties of Pit and Fissure Sealants. Polymers 2023, 15, 529. [Google Scholar] [CrossRef]
- Collares, F.M.; Garcia, I.M.; Klein, M.; Parolo, C.F.; Sánchez, F.A.L.; Takimi, A.; Bergmann, C.P.; Samuel, S.M.W.; Melo, M.A.; Leitune, V.C. Exploring Needle-Like Zinc Oxide Nanostructures for Improving Dental Resin Sealers: Design and Evaluation of Antibacterial, Physical and Chemical Properties. Polymers 2020, 12, 789. [Google Scholar] [CrossRef]
- Zubizarreta-Macho, Á.; Rico-Romano, C.; Fernández-Aceñero, M.J.; Mena-Álvarez, J.; Cabal, B.; Díaz, L.A.; Torrecillas, R.; Moya, J.S.; López-Píriz, R. Adding Two Antimicrobial Glasses to an Endodontic Sealer to Prevent Bacterial Root Canal Reinfection: An In Vivo Pilot Study in Dogs. Antibiotics 2021, 10, 1183. [Google Scholar] [CrossRef]
- Bertacci, A.; Moro, D.; Ulian, G.; Valdrè, G. Development of A Nano-Apatite Based Composite Sealer for Endodontic Root Canal Filling. J. Compos. Sci. 2021, 5, 30. [Google Scholar] [CrossRef]
- Al-Sabawi, N.A.; Al-Jubori, S.H. Preparation and characterization of novel nano-tricalcium silicate-58s bioactive glass-based root canal sealer. Saudi Endod. J. 2024, 14, 90–99. [Google Scholar] [CrossRef]
- Raheem, I.A.A.; Razek, A.A.; Elgendy, A.A.; Labah, D.A.; Saleh, N.M. Egyptian Propolis-loaded nanoparticles as a root canal nanosealer: Sealing ability and in vivo biocompatibility. Int. J. Nanomed. 2020, 15, 5265–5277. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, B.H.; Al-Huwaizi, H. Evaluation of Antimicrobial Activity and Cytotoxicity of an Epoxy Resin-Based Endodontic Sealer Containing Nanoparticles Amorphous Calcium Phosphate. Int. J. Dent. 2023, 2023, 8717655. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Rajeshwari, H.R.; Dhamecha, D.; Jagwani, S.; Rao, M.; Jadhav, K.; Shaikh, S.; Puzhankara, L.; Jalalpure, S. Local drug delivery systems in the management of periodontitis: A scientific review. J. Control. Release 2019, 307, 393–409. [Google Scholar] [CrossRef]
- Wei, Y.; Deng, Y.; Ma, S.; Ran, M.; Jia, Y.; Meng, J.; Han, F.; Gou, J.; Yin, T.; He, H.; et al. Local drug delivery systems as therapeutic strategies against periodontitis: A systematic review. J. Control. Release 2021, 333, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Şenel, S.; Özdoğan, A.I.; Akca, G. Current status and future of delivery systems for prevention and treatment of infections in the oral cavity. Drug Deliv. Transl. Res. 2021, 11, 1703–1734. [Google Scholar] [CrossRef]
- Alghanem, S.; Dziurkowska, E.; Ordyniec-Kwaśnica, I.; Sznitowska, M. Intraoral medical devices for sustained drug delivery. Clin. Oral Investig. 2023, 27, 7157–7169. [Google Scholar] [CrossRef]
- Paul, M.; Das Pramanik, S.; Sahoo, R.N.; Dey, Y.N.; Nayak, A.K. Dental delivery systems of antimicrobial drugs using chitosan, alginate, dextran, cellulose and other polysaccharides: A review. Int. J. Biol. Macromol. 2023, 247, 125808. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, R.; Lei, L.; Yang, Y.; Hu, T. Drug delivery systems for oral disease applications. J. Appl. Oral Sci. 2022, 30, e20210349. [Google Scholar] [CrossRef]
- Joshi, D.; Garg, T.; Goyal, A.K.; Rath, G. Advanced drug delivery approaches against periodontitis. Drug Deliv. 2016, 23, 363–377. [Google Scholar] [CrossRef]
- Chandramouli, M.; Shivalingappa, R.P.; Basavanna, V.; Doddamani, S.; Shanthakumar, D.C.; Nagarajaiah, S.R.; Ningaiah, S. Oral Thin-films from Design to Delivery: A Pharmaceutical Viewpoint. Biointerface Res. Appl. Chem. 2023, 13, 2023. [Google Scholar]
- Steckiewicz, K.P.; Cieciórski, P.; Barcińska, E.; Jaśkiewicz, M.; Narajczyk, M.; Bauer, M.; Kamysz, W.; Megiel, E.; Inkielewicz-Stepniak, I. Silver Nanoparticles as Chlorhexidine and Metronidazole Drug Delivery Platforms: Their Potential Use in Treating Periodontitis. Int. J. Nanomed. 2022, 17, 495–517. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.; Lupei, M.; Bucatariu, S.-M.; Pelin, I.M.; Doroftei, F.; Ichim, D.L.; Daraba, O.M.; Fundueanu, G. PVA/Chitosan Thin Films Containing Silver Nanoparticles and Ibuprofen for the Treatment of Periodontal Disease. Polymers 2023, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Wang, P.; Chen, Z.; Liu, Y.; Wang, L.; Guo, J.; Li, Z.; Cai, H.; Wei, J. Combined Ferromagnetic Nanoparticles for Effective Periodontal Biofilm Eradication in Rat Model. Int. J. Nanomed. 2023, 18, 2371–2388. [Google Scholar] [CrossRef]
- Sahu, S.A.; Panda, S.; Das, A.C.; Mishra, L.; Rath, S.; Sokolowski, K.; Kumar, M.; Mohanty, R.; Nayak, R.; Satpathy, A.; et al. Efficacy of Sub-Gingivally Delivered Propolis Nanoparticle in Non-Surgical Management of Periodontal Pocket: A Randomized Clinical Trial. Biomolecules 2023, 13, 1576. [Google Scholar] [CrossRef]
- Kadam, P.; Mahale, S.; Sonar, P.; Chaudhari, D.; Shimpi, S.; Kathurwar, A. Efficacy of silver nanoparticles in chronic periodontitis patients: A clinico-microbiological study. Iberoam. J. Med. 2020, 2, 142–147. [Google Scholar] [CrossRef]
- Yıldırım, Y.; İnce, I.; Gümüştaş, B.; Vardar, Ö.; Yakar, N.; Munjaković, H.; Özdemir, G.; Emingil, G. Development of doxycycline and atorvastatin-loaded chitosan nanoparticles for local delivery in periodontal disease. J. Drug Deliv. Sci. Technol. 2023, 82, 104322. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Gkiliopoulos, D.; Pouroutzidou, G.K.; Lymperaki, E.; Papoulia, C.; Reybier, K.; Perio, P.; Paraskevopoulos, K.M.; Kontonasaki, E.; Theocharidou, A. Effect of Artemisinin-Loaded Mesoporous Cerium-Doped Calcium Silicate Nanopowder on Cell Proliferation of Human Periodontal Ligament Fibroblasts. Nanomaterials 2021, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Gu, C.; Lu, X.; Ge, X.; Yang, J.; Wang, C.; Gu, Y.; Deng, A.; Guo, Y.; Feng, X.; et al. Polydopamine functionalized mesoporous silica as ROS-sensitive drug delivery vehicles for periodontitis treatment by modulating macrophage polarization. Nano Res. 2021, 14, 4577–4583. [Google Scholar] [CrossRef]
- Bako, J.; Toth, F.; Gall, J.; Kovacs, R.; Csík, A.; Varga, I.; Sculean, A.; Zelko, R.; Hegedus, C. Combined Release of Antiseptic and Antibiotic Drugs from Visible Light Polymerized Biodegradable Nanocomposite Hydrogels for Periodontitis Treatment. Pharmaceutics 2022, 14, 957. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Li, G.; Wang, Z.; Zhang, H.; Shan, Y.; Yuan, X.; Shi, Q.; Dou, X.; Zhou, Q.; Xu, Q. Protease-Loaded CuS Nanoparticles with Synergistic Photothermal/Dynamic Therapy against F. nucleatum-Induced Periodontitis. ACS Appl. Mater. Interfaces 2023, 15, 32215–32225. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.d.S.B.F.; Lima, M.L.d.S.; da Silva-Junior, A.A.; Silva, E.d.S.; Júnior, R.F.d.A.; Martins, A.A.; Alves, J.S.F.; Oliveira, A.d.S.; Ferreira, L.D.S.; Costa, E.C.T.d.A.; et al. In vitro-in vivo availability of metformin hydrochloride-PLGA nanoparticles in diabetic rats in a periodontal disease experimental model. Pharm. Biol. 2021, 59, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Parmar, R.; Salman, M.M.; Chauhan, P. Fabrication of Cefixime Nanoparticles Loaded Films and their Ex Vivo Antimicrobial Effect on Periodontitis Patient’s Saliva. Pharm. Nanotechnol. 2021, 9, 361–371. [Google Scholar] [CrossRef]
- Cao, B.; Da, X.; Wu, W.; Xie, J.; Li, X.; Wang, X.; Xu, H.; Gao, J.; Yang, H.; Su, J. Multifunctional human serum albumin-crosslinked and self-assembling nanoparticles for therapy of periodontitis by anti-oxidation, anti-inflammation and osteogenesis. Mater. Today Bio 2024, 28, 101163. [Google Scholar] [CrossRef]
- Zong, C.; Bronckaers, A.; Willems, G.; He, H.; de Llano-Pérula, M.C. Nanomaterials for Periodontal Tissue Regeneration: Progress, Challenges and Future Perspectives. J. Funct. Biomater. 2023, 14, 290. [Google Scholar] [CrossRef]
- Hollý, D.; Klein, M.; Mazreku, M.; Zamborský, R.; Polák, Š.; Danišovič, Ľ.; Csöbönyeiová, M. Stem Cells and Their Derivatives—Implications for Alveolar Bone Regeneration: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 11746. [Google Scholar] [CrossRef]
- Huck, O.; Stutz, C.; Gegout, P.-Y.; Özçelik, H.; Benkirane-Jessel, N.; Petit, C.; Batool, F. Nanomedicine and Periodontal Regenerative Treatment. Dent. Clin. N. Am. 2022, 66, 131–155. [Google Scholar] [CrossRef]
- Takallu, S.; Kakian, F.; Bazargani, A.; Khorshidi, H.; Mirzaei, E. Development of antibacterial collagen membranes with optimal silver nanoparticle content for periodontal regeneration. Sci. Rep. 2024, 14, 7262. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Zhou, Y.; Zheng, K.; Xu, X.; Yang, J.; Wang, X.; Miao, L.; Wei, H.; Xu, Y. Cerium oxide nanoparticles loaded nanofibrous membranes promote bone regeneration for periodontal tissue engineering. Bioact. Mater. 2022, 7, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.S.; Zafar, M.S.; Alnazzawi, A.; Javed, F. Nanocrystalline hydroxyapatite in regeneration of periodontal intrabony defects: A systematic review and meta-analysis. Ann. Anat.-Anat. Anz. 2022, 240, 151877. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.C.; Luo, H.T.; Lin, Z.J.; Tai, W.C.; Chang, C.H.; Chang, Y.C.; Cochran, D.L.; Chen, M.H. Regeneration of critical-sized mandibular defect using a 3D-printed hydroxyapatite-based scaffold: An exploratory study. J. Periodontol. 2021, 92, 428–435. [Google Scholar] [CrossRef]
- Tamburaci, S.; Tihminlioglu, F. Development of Si doped nano hydroxyapatite reinforced bilayer chitosan nanocomposite barrier membranes for guided bone regeneration. Mater. Sci. Eng. C 2021, 128, 112298. [Google Scholar] [CrossRef] [PubMed]
- Vani, T.M.S.; Paramashivaiah, R.; Prabhuji, M.L.V.; Peeran, S.W.; Fageeh, H.; Tasleem, R.; Bahamdan, G.K.; Aldosari, L.I.N.; Bhavikatti, S.K.; Scardina, G.A. Regeneration of Intrabony Defects with Nano Hydroxyapatite Graft, Derived from Eggshell along with Periosteum as Barrier Membrane under Magnification—An Interventional Study. Appl. Sci. 2023, 13, 1693. [Google Scholar] [CrossRef]
- Huang, B.; Chen, M.; Tian, J.; Zhang, Y.; Dai, Z.; Li, J.; Zhang, W. Oxygen-Carrying and Antibacterial Fluorinated Nano-Hydroxyapatite Incorporated Hydrogels for Enhanced Bone Regeneration. Adv. Health Mater. 2022, 11, 2102540. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Feng, X.; Chen, A.; Zhang, Y.; Wang, J.; Shao, L. Application of dental nanomaterials: Potential toxicity to the central nervous system. Int. J. Nanomed. 2015, 10, 3547–3565. [Google Scholar] [CrossRef]
- Karunakaran, H.; Krithikadatta, J.; Doble, M. Local and systemic adverse effects of nanoparticles incorporated in dental materials- a critical review. Saudi Dent. J. 2024, 36, 158–167. [Google Scholar] [CrossRef]
- Tang, X.; Li, L.; Meng, X.; Liu, T.; Hu, Q.; Miao, L. Cytotoxicity of silver nanoparticles on human periodontal ligament fibroblasts. Nanosci. Nanotechnol. Lett. 2017, 9, 1015–1022. [Google Scholar] [CrossRef]
- Cunningham, B.; Engstrom, A.M.; Harper, B.J.; Harper, S.L.; Mackiewicz, M.R. Silver Nanoparticles Stable to Oxidation and Silver Ion Release Show Size-Dependent Toxicity In Vivo. Nanomaterials 2021, 11, 1516. [Google Scholar] [CrossRef] [PubMed]
- Filip, G.A.; Florea, A.; Olteanu, D.; Clichici, S.; David, L.; Moldovan, B.; Cenariu, M.; Scrobota, I.; Potara, M.; Baldea, I. Biosynthesis of silver nanoparticles using Sambucus nigra L. fruit extract for targeting cell death in oral dysplastic cells. Mater. Sci. Eng. C 2021, 123, 111974. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed]
- Vandebriel, R.J.; De Jong, W.H. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol. Sci. Appl. 2012, 5, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-B.; Kim, Y.W.; Lim, S.K.; Roh, T.H.; Bang, D.Y.; Choi, S.M.; Lim, D.S.; Kim, Y.J.; Baek, S.-H.; Kim, M.-K.; et al. Risk assessment of zinc oxide, a cosmetic ingredient used as a UV filter of sunscreens. J. Toxicol. Environ. Health Part B 2017, 20, 155–182. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Li, Z.; Xie, Z.; Sokolova, I.M.; Song, L.; Peijnenburg, W.J.; Hu, M.; Wang, Y. Rethinking nano-TiO2 safety: Overview of toxic effects in humans and aquatic animals. Small 2020, 16, 2002019. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhang, T.; Xue, Y.; Wang, S.; Huang, M.; Yang, Y.; Lu, M.; Lei, H.; Kong, L.; Wang, Y.; et al. Metabonomic studies of biochemical changes in the serum of rats by intratracheally instilled TiO2 nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 3065–3074. [Google Scholar] [CrossRef]
- Lammel, T.; Mackevica, A.; Johansson, B.R.; Sturve, J. Endocytosis, intracellular fate, accumulation, and agglomeration of titanium dioxide (TiO2) nanoparticles in the rainbow trout liver cell line RTL-W1. Environ. Sci. Pollut. Res. 2019, 26, 15354–15372. [Google Scholar] [CrossRef]
- Vranic, S.; Shimada, Y.; Ichihara, S.; Kimata, M.; Wu, W.; Tanaka, T.; Boland, S.; Tran, L.; Ichihara, G. Toxicological Evaluation of SiO2 Nanoparticles by Zebrafish Embryo Toxicity Test. Int. J. Mol. Sci. 2019, 20, 882. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, L.; Ma, Y.; Liu, J.; Huang, Y.; Fu, X.; Peng, S.; Wang, X.; Yang, Y.; Zhang, X.; et al. Mechanistic study of silica nanoparticles on the size-dependent retinal toxicity in vitro and in vivo. J. Nanobiotechnol. 2022, 20, 146. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, I.Y.; Heo, M.B.; Moon, J.H.; Son, J.G.; Lee, T.G. Global proteomics to study silica nanoparticle-induced cytotoxicity and its mechanisms in HepG2 cells. Biomolecules 2021, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Putthanuparp, T.; Rirattanapong, P.; Ruangsawasdi, N. The cytotoxicity of silver nanoparticles on human gingival fibroblast cells: An in vitro study. Mahidol Dent. J. 2023, 43, 107–114. [Google Scholar]
- Wang, Z.; Zhang, C.; Liu, X.; Huang, F.; Wang, Z.; Yan, B. Oral intake of ZrO2 nanoparticles by pregnant mice results in nanoparticles’ deposition in fetal brains. Ecotoxicol. Environ. Saf. 2020, 202, 110884. [Google Scholar] [CrossRef] [PubMed]
- Barbasz, A.M.; Dyba, B. Direct Interaction of Zirconia Nanoparticles with Human Immune Cells. Biophysica 2024, 4, 83–91. [Google Scholar] [CrossRef]
- Chen, F.-C.; Huang, C.-M.; Yu, X.-W.; Chen, Y.-Y. Effect of nano zinc oxide on proliferation and toxicity of human gingival cells. Hum. Exp. Toxicol. 2022, 41, 09603271221080236. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.M.; El-Mansy, M.N.; El-Borady, O.M.; Hegazy, E.M. Impact of biosynthesized silver nanoparticles cytotoxicity on dental pulp of albino rats (histological and immunohistochemical study). J. Oral Biol. Craniofacial Res. 2021, 11, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Al-Saeed, F.A.; Abduallah, A.M.; Ahmed, A.E.; Shahzad, A.; Amjad, N.; Ali, A.; Mostafa, M.S.; Hussain, R. Calcium Nanoparticles Induce Oxidative Stress in Erythrocytes, Neurotoxicity and Testicular Toxicity in Albino Rats (Rattus norvegicus). Pak. Vet. J. 2023, 43, 241–247. [Google Scholar] [CrossRef]
- Solanki, L.A.; Sundari, K.S.; Rajeshkumar, S. In-vitro cytotoxicity evaluation of green synthesized gold nanoparticles and its indigenous mouthwash. J. Pure Appl. Microbiol. 2021, 15, 735–742. [Google Scholar] [CrossRef]
- Khanna, N.; Chokkattu, J.J.; Neeharika, S.; Ramakrishnan, M.; Shanmugam, R.; Thangavelu, L. Anti-inflammatory Activity and Cytotoxic Effect of Ginger and Rosemary-mediated Titanium Oxide Nanoparticles-based Dental Varnish. World J. Dent. 2023, 14, 761–765. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Wu, F.; Sajjad, W.; Zhang, Q.; Khan, I.; Ullah, K.; Wang, W. Long-term exposure to high-concentration silver nanoparticles induced toxicity, fatality, bioaccumulation, and histological alteration in fish (Cyprinus carpio). Environ. Sci. Eur. 2021, 33, 14. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Cheney, D.L.; Grunberger, J.W.; Yazdimamaghani, M.; Jedrzkiewicz, J.; Isaacson, K.J.; Dobrovolskaia, M.A.; Ghandehari, H. One-year chronic toxicity evaluation of single dose intravenously administered silica nanoparticles in mice and their Ex vivo human hemocompatibility. J. Control. Release 2020, 324, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Bengalli, R.; Colantuoni, A.; Perelshtein, I.; Gedanken, A.; Collini, M.; Mantecca, P.; Fiandra, L. In vitro skin toxicity of CuO and ZnO nanoparticles: Application in the safety assessment of antimicrobial coated textiles. NanoImpact 2021, 21, 100282. [Google Scholar] [CrossRef] [PubMed]

| NPs | Applications | Mechanism of Action | Limitations | Refs. |
|---|---|---|---|---|
| Silver NPs (AgNPs) | Composites Antibacterial coatings Toothpaste additives Dental implants | Release silver ions that disrupt bacterial cell walls Bacterial growth inhibition Reduce biofilm formation | Potential toxicity to human cells | [14,15,16,17,18] |
| Titanium Dioxide (TiO2) NPs | Implants Antibacterial coatings Composites | Photocatalytic activity under UV light breaks down organic stains Improves mechanical properties | Potential for UV-induced damage Can cause oxidative stress | [19,20,21,22] |
| Zinc Oxide NPs (ZnO NPs) | Toothpaste formulations Dental varnishes Antibacterial agents | Disrupts microbial cell membranes Provides a protective barrier | Potential cytotoxic effects | [23,24,25,26] |
| Hydroxyapatite NPs (HAp NPs) | Tooth enamel remineralization Dental fillers Bone regeneration | Similar to natural tooth mineral structure—it repairs and strengthens enamel Promotes bone growth in implants | Poor mechanical properties | [27,28,29,30] |
| Gold NPs (AuNPs) | Mouthwashes formulations Toothpaste additives Implants | Antibacterial properties Osteoinductive action | High cost Potential toxicity | [31,32,33] |
| Silica NPs | Dental fillers Coatings | Improves mechanical properties and durability of fillers | Potential toxicity | [34,35,36] |
| Copper NPs (CuNPs) | Toothpaste formulations Coatings | Prevents biofilm formation Release copper ions that disrupt microbial cell membranes and inhibit bacterial growth | Potential toxicity Risk of corrosion | [37,38,39,40] |
| Polymeric NPs | Drug delivery Adhesives | Controlled release of drugs Improves adhesiveness | Potential toxicity | [41,42,43,44] |
| Magnetic NPs | TDD Antibacterial agents | Uses magnetic fields to guide particles to targeted locations Antibacterial properties | Biocompatibility issues | [45,46,47,48] |
| Graphene oxide | Dental implants Coatings | Enhances mechanical strength and bioactivity in implants Antibacterial activity through oxidative stress and physical disruption | Potential toxicity | [49,50,51,52] |
| NPs | Properties | Application in Oral Hygiene | Refs. |
|---|---|---|---|
| AgNPs | Strong antimicrobial activity Bacterial growth inhibition | Toothpaste—to prevent dental caries Alcohol-free mouthwashes | [57,58,59,60] |
| AuNPs | Antimicrobial activity Effective against S. oralis | AuNP-based mouthwashes Toothpaste formulations | [61,62,63] |
| CuNPs | Anti-biofilm formation Antimicrobial activity | Toothpaste formulations | [63,64] |
| ZnO NPs | Anti-inflammatory and antibacterial activity | Toothpaste formulations | [26,65] |
| Nanocomposite | Properties | Refs. |
|---|---|---|
| Silica NPs | Improved mechanical properties Reduced polymerization shrinkage Enhanced wear resistance | [36,96,97] |
| TiO2 NPs | Increased tensile strength Improved stiffness and toughness | [98,99] |
| Calcium phosphate (CaP) NPs | Increased wear resistance Stress-bearing capacities | [100] |
| Zirconium Oxide (ZrO2) NPs | Enhanced wear resistance and hardness | [101] |
| Graphene Oxide NPs | Improved mechanical strength Crack propagation resistance | [102,103] |
| AgNPs | Improved flexural strength Increased surface microhardness | [104,105] |
| ZnO NPs | Improved microhardness High flexural strength and modulus | [106,107] |
| Carbon nanotubes | Increased elastic modulus Increased compressive strength Improved flexural strength | [108,109] |
| Nanomaterial | Effect | Refs. |
|---|---|---|
| Chitosan NPs loaded with atorvastatin (AS) and doxycycline (DOX) | AS showed a sustained release over 9 days, while DS had a quicker release, stabilizing around 5 days AS/DS chitosan NPs were more effective against Staphylococcus aureus compared to Escherichia coli The system was non-cytotoxic | [164] |
| Ce-doped mesoporous calcium silicate nanopowders loaded with artemisinin (ART) | Hemocompatible and promoted cell proliferation of human periodontal ligament fibroblasts (hPDLFs) Protected cells from oxidative damage by neutralizing ROS | [165] |
| Polydopamine (PDA)-functionalized MS NPs loaded with minocycline hydrochloride (MH) | PDA and MH shifted macrophages from a pro-inflammatory (M1) to an anti-inflammatory (M2) state Reduced bone loss Prevented inflammation | [166] |
| Nanocomposite hydrogel (NCHG) loaded with CHX and metronidazole | The NCHG released metronidazole within 12 h and CHX over more than 7 days, with the release strongly dependent on pH Biocompatible Targeted bacteria in acidic, inflamed environments | [167] |
| Protease-loaded CuS NPs | Eliminated bacterial biofilms, particularly Fusobacterium nucleatum Biocompatible Reduced bone resorption and inflammation | [168] |
| Metformin hydrochloride-loaded PLGA NPs | Controlled drug release, sustained metformin’s plasma concentration for over 72 h, and required a lower dosage Slower elimination rate, resulting in a more efficient and long-lasting treatment option | [169] |
| Cefixime-loaded NPs within chitosan films | Sustained drug release Better antimicrobial activity against periodontal bacteria than conventional mouthwash Maintained their drug release profile and structural integrity over six months | [170] |
| Human serum albumin (HSA)-crosslinked manganese-doped Prussian blue NPs (HSA-MDSPB NPs) | Antioxidant, anti-inflammatory, and osteogenic properties Reduced inflammation, oxidative stress, and bone loss in periodontal tissues Promoted macrophage polarization toward an anti-inflammatory state | [171] |
| Nanomaterial | Potential Toxic Effect | Refs. |
|---|---|---|
| AgNPs | DNA damage, oxidative stress, inflammatory response | [186,187] |
| ZnO | DNA damage, triggers inflammatory response, oxidative stress, ROS generation | [188,189] |
| ZrO2 | Tissue accumulation, gene alterations, oxidative stress | [190] |
| TiO2 | Tissue accumulation, ROS generation, oxidative stress, environmental damage | [190,191,192] |
| SiO2 | Genotoxicity, tissue accumulation, ROS generation, aggregation, oxidative stress | [193,194,195] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolae, C.-L.; Pîrvulescu, D.-C.; Niculescu, A.-G.; Rădulescu, M.; Grumezescu, A.M.; Croitoru, G.-A. An Overview of Nanotechnology in Dental Medicine. J. Compos. Sci. 2024, 8, 352. https://doi.org/10.3390/jcs8090352
Nicolae C-L, Pîrvulescu D-C, Niculescu A-G, Rădulescu M, Grumezescu AM, Croitoru G-A. An Overview of Nanotechnology in Dental Medicine. Journal of Composites Science. 2024; 8(9):352. https://doi.org/10.3390/jcs8090352
Chicago/Turabian StyleNicolae, Carmen-Larisa, Diana-Cristina Pîrvulescu, Adelina-Gabriela Niculescu, Marius Rădulescu, Alexandru Mihai Grumezescu, and George-Alexandru Croitoru. 2024. "An Overview of Nanotechnology in Dental Medicine" Journal of Composites Science 8, no. 9: 352. https://doi.org/10.3390/jcs8090352
APA StyleNicolae, C.-L., Pîrvulescu, D.-C., Niculescu, A.-G., Rădulescu, M., Grumezescu, A. M., & Croitoru, G.-A. (2024). An Overview of Nanotechnology in Dental Medicine. Journal of Composites Science, 8(9), 352. https://doi.org/10.3390/jcs8090352











