Pseudocapacitive and Magnetic Properties of SrFe12O19–Polypyrrole Composites
Abstract
:1. Introduction
2. Materials and Methods
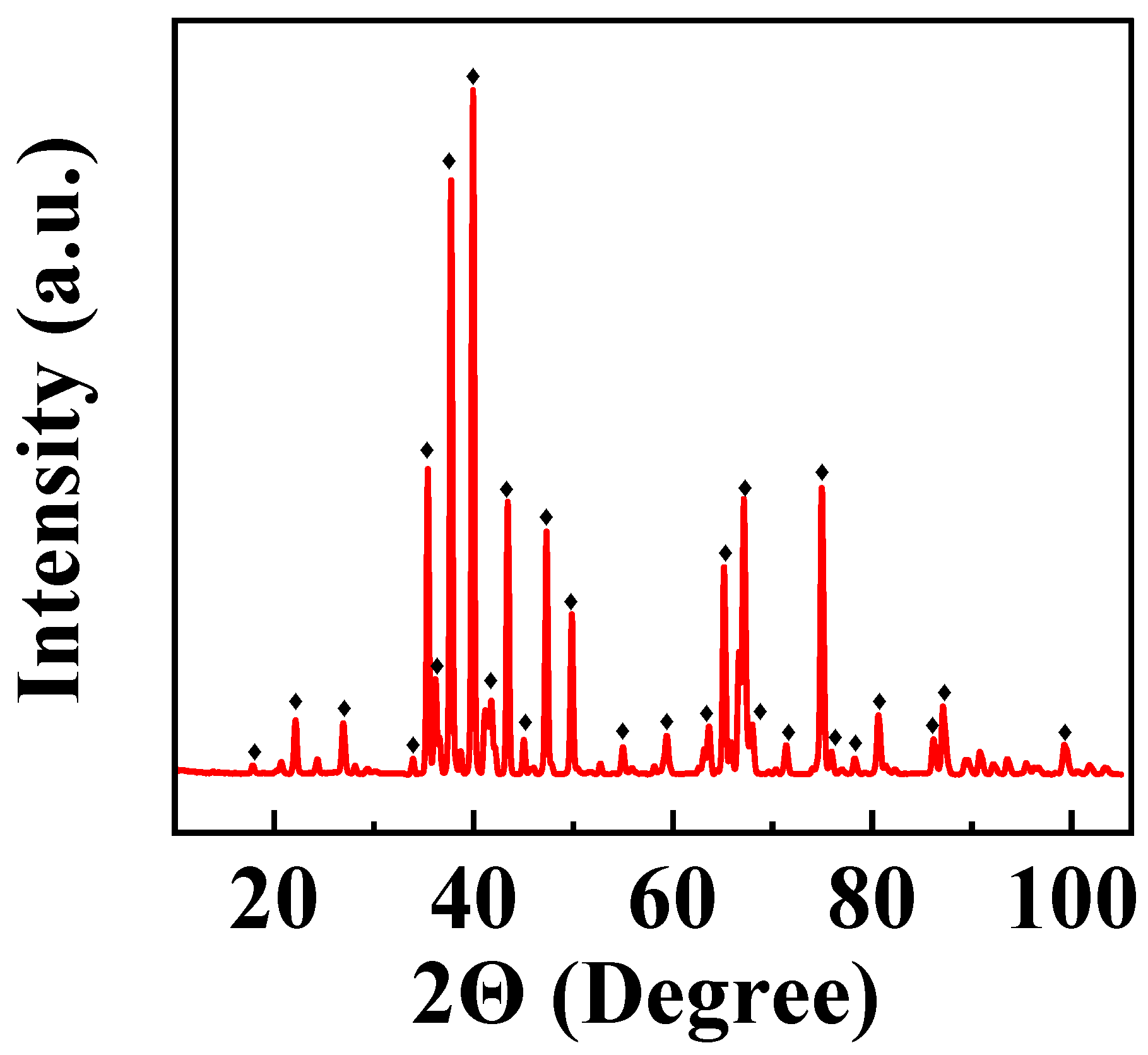
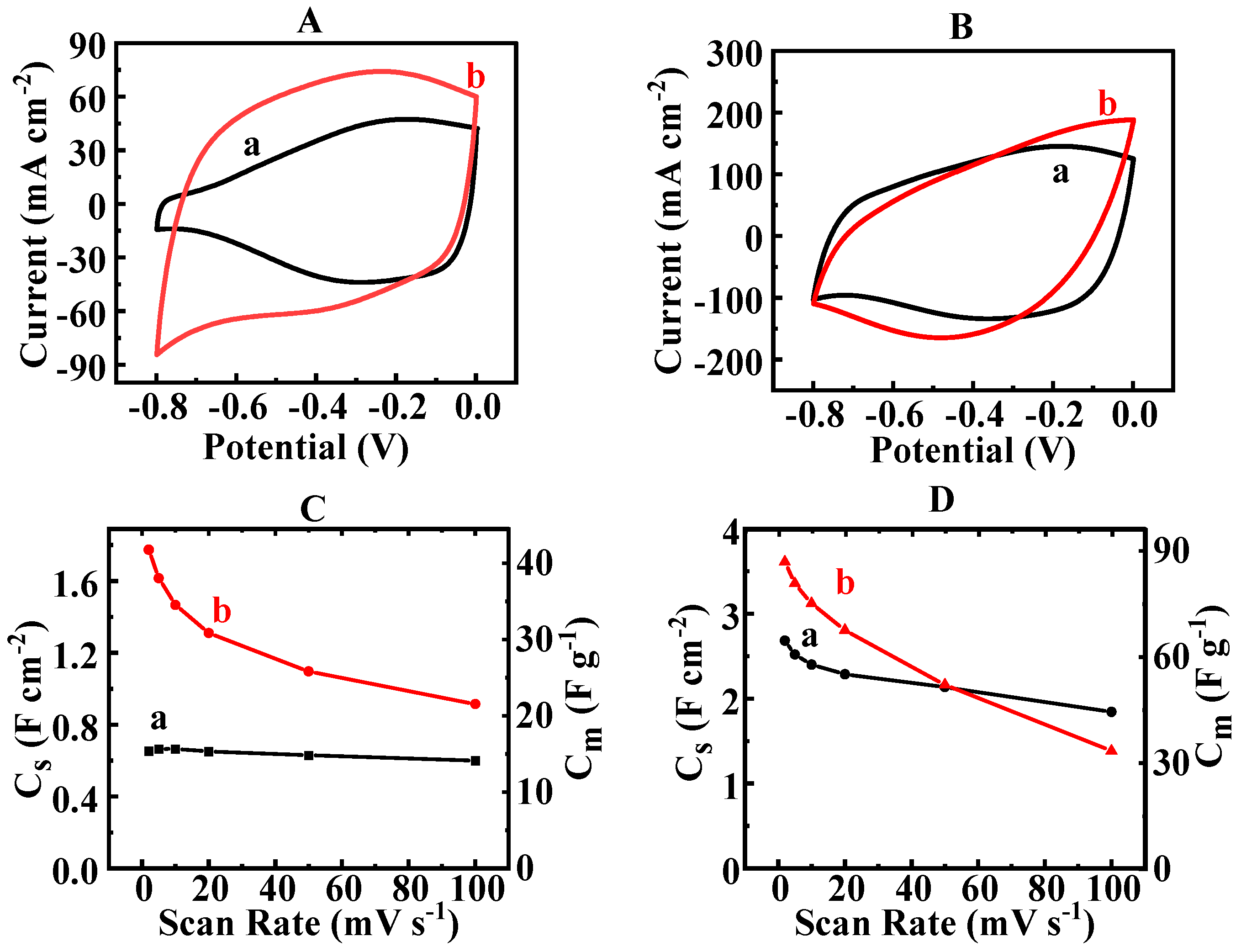
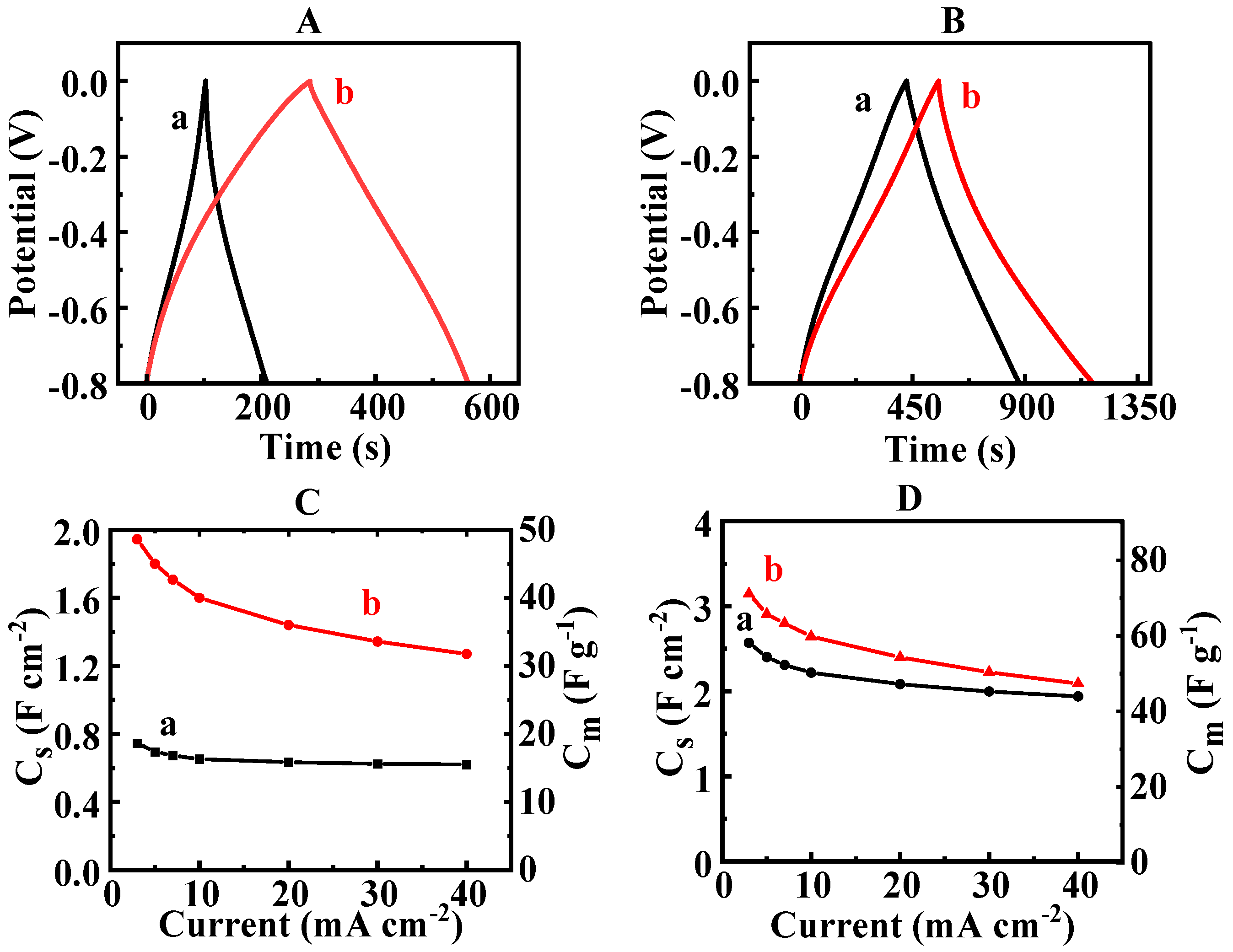
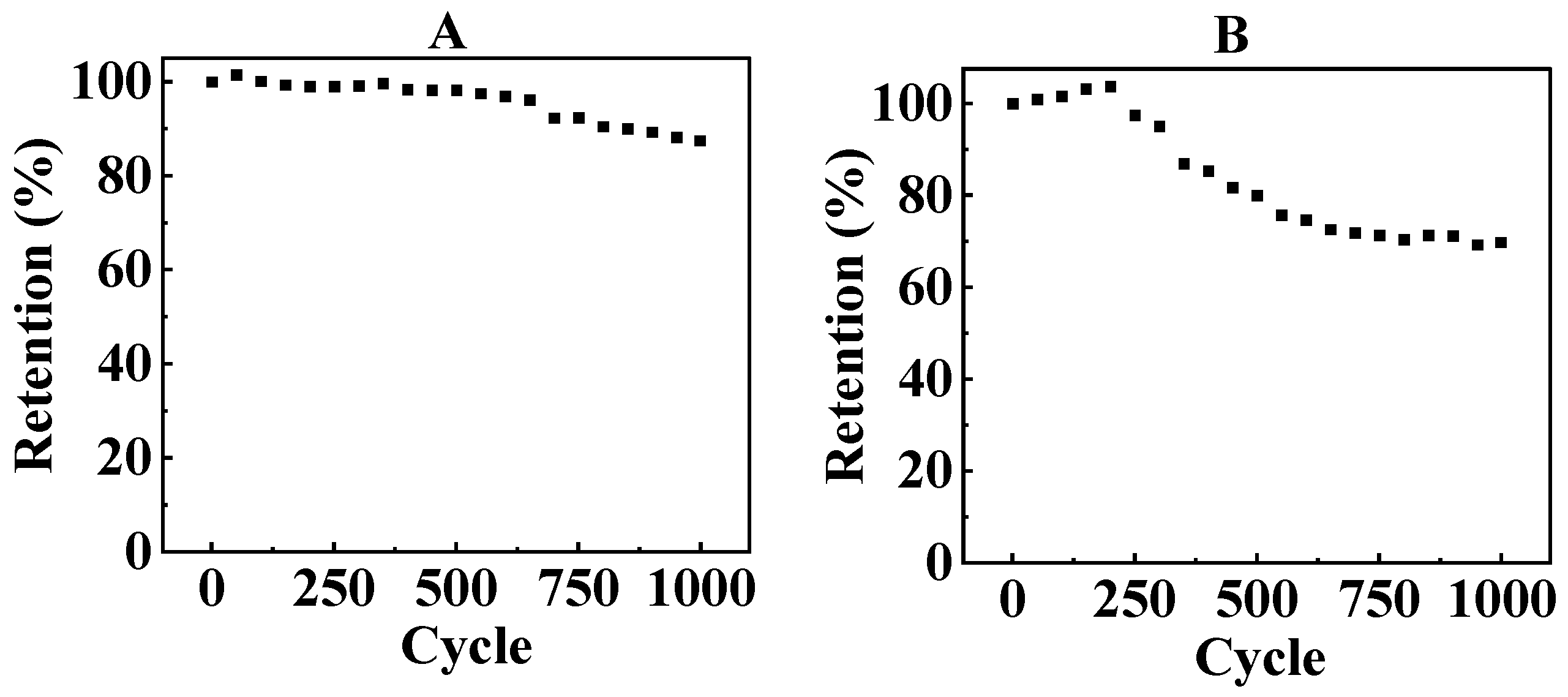
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srinivasan, G. Magnetoelectric composites. Annu. Rev. Mater. Res. 2010, 40, 153–178. [Google Scholar] [CrossRef]
- Eerenstein, W.; Mathur, N.; Scott, J.F. Multiferroic and magnetoelectric materials. Nature 2006, 442, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Nan, C.-W.; Bichurin, M.; Dong, S.; Viehland, D.; Srinivasan, G. Multiferroic magnetoelectric composites: Historical perspective, status, and future directions. J. Appl. Phys. 2008, 103, 031101. [Google Scholar] [CrossRef]
- Venevtsev, Y.N.; Gagulin, V.V.; Zhitomirsky, I.D. Material science aspects of seignette-magnetism problem. Ferroelectrics 1987, 73, 221–248. [Google Scholar] [CrossRef]
- Schmid, H. Multi-ferroic magnetoelectrics. Ferroelectrics 1994, 162, 317–338. [Google Scholar] [CrossRef]
- Fang, C.; Kools, F.; Metselaar, R.; De Groot, R. Magnetic and electronic properties of strontium hexaferrite SrFe12O19 from first-principles calculations. J. Phys. Condens. Matter 2003, 15, 6229. [Google Scholar] [CrossRef]
- Kostishyn, V.; Panina, L.; Timofeev, A.; Kozhitov, L.; Kovalev, A.; Zyuzin, A. Dual ferroic properties of hexagonal ferrite ceramics BaFe12O19 and SrFe12O19. J. Magn. Magn. Mater. 2016, 400, 327–332. [Google Scholar] [CrossRef]
- Lee, J.; Cho, S.Y.; Kim, I.; Rouleau, C.M.; Kang, K.; Ryu, S.; Heo, Y.; Keum, J.K.; Pajerowski, D.M.; Kim, Y. Multiferroism in strained strontium hexaferrite epitaxial thin films. Phys. Rev. Mater. 2024, 8, 024401. [Google Scholar] [CrossRef]
- Tan, G.; Chen, X. Synthesis, structures, and multiferroic properties of strontium hexaferrite ceramics. J. Electron. Mater. 2013, 42, 906–911. [Google Scholar] [CrossRef]
- Santos-López, F.; Díaz-Castañón, S. Magnetic Properties and Electric Hysteresis in SrFe12O19 Hexaferrites at Low Sintered Temperatures. J. Supercond. Nov. Magn. 2024, 37, 881–888. [Google Scholar] [CrossRef]
- Tan, G.; Huang, Y.; Sheng, H. Magnetoelectric response in multiferroic SrFe12O19 ceramics. PLoS ONE 2016, 11, e0167084. [Google Scholar] [CrossRef] [PubMed]
- Kostishyn, V.; Panina, L.; Kozhitov, L.; Timofeev, A.; Kovalev, A. Synthesis and multiferroic properties of M-type SrFe12O19 hexaferrite ceramics. J. Alloys Compd. 2015, 645, 297–300. [Google Scholar] [CrossRef]
- Qiang, G.; Jin, Y.; Lu, X.; Cui, X.; Deng, D.; Kang, B.; Yang, W.; Cao, S.; Zhang, J. Temperature effect on the magnetic property and ferroelectricity in hexaferrite SrFe12O19. Appl. Phys. A 2016, 122, 681. [Google Scholar] [CrossRef]
- Katlakunta, S.; Raju, P.; Meena, S.S.; Srinath, S.; Sandhya, R.; Kuruva, P.; Murthy, S.R. Multiferroic properties of microwave sintered BaTiO3–SrFe12O19 composites. Phys. B Condens. Matter 2014, 448, 323–326. [Google Scholar] [CrossRef]
- Stingaciu, M.; Reuvekamp, P.; Tai, C.-W.; Kremer, R.; Johnsson, M. The magnetodielectric effect in BaTiO3–SrFe12O19 nanocomposites. J. Mater. Chem. C 2014, 2, 325–330. [Google Scholar] [CrossRef]
- Singh, A.; Suri, S.; Kumar, P.; Kaur, B.; Thakur, A.K.; Singh, V. Effect of temperature and frequency on electrical properties of composite multiferroic of lead titanate and strontium hexaferrite (PbTiO3–SrFe12O19). J. Alloys Compd. 2018, 764, 599–615. [Google Scholar] [CrossRef]
- Martínez-Pérez, J.; Bolarín-Miró, A.; Pedro-García, F.; Cortés-Escobedo, C.; Barba-Pingarrón, A.; Sánchez-De Jesús, F. Magnetic and dielectric characterization of xBiFeO3:(1−x)SrFe12O19 multiferroic composites. J. Alloys Compd. 2019, 808, 151700. [Google Scholar] [CrossRef]
- Das, A.; Chatterjee, S.; Bandyopadhyay, S.; Das, D. Enhanced magnetoelectric properties of BiFeO3 on formation of BiFeO3/SrFe12O19 nanocomposites. J. Appl. Phys. 2016, 119, 234102. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, J.-P.; Zhang, X.-L.; Lei, R.-Y. Investigation on the electrical and magnetic properties of PVDF/SrFe12O19 composite membranes. J. Magn. Magn. Mater. 2023, 572, 170601. [Google Scholar] [CrossRef]
- Prathipkumar, S.; Hemalatha, J. Magnetoelectric behavior and magnetic field-tuned energy storage capacity of SrFe12O19 nanofiber reinforced P (VDF-HFP) composite films. J. Magn. Magn. Mater. 2022, 555, 169378. [Google Scholar] [CrossRef]
- Sikkema, R.; Zhitomirsky, I. Magnetic supercapacitors: Charge storage mechanisms, magnetocapacitance, and magnetoelectric phenomena. Appl. Phys. Rev. 2023, 10, 021307. [Google Scholar] [CrossRef]
- Elanthamilan, E.; Wang, S.-F. Surfactant assisted synthesis of strontium hexaferrite microspheres for the fabrication of high-performance asymmetric supercapacitors. New J. Chem. 2023, 47, 9174–9185. [Google Scholar] [CrossRef]
- Rezaie, E.; Rezanezhad, A.; Ghadimi, L.S.; Hajalilou, A.; Arsalani, N. Effect of calcination on structural and supercapacitance properties of hydrothermally synthesized plate-like SrFe12O19 hexaferrite nanoparticles. Ceram. Int. 2018, 44, 20285–20290. [Google Scholar] [CrossRef]
- Fu, M.; Chen, W.; Zhu, X.; Yang, B.; Liu, Q. Crab shell derived multi-hierarchical carbon materials as a typical recycling of waste for high performance supercapacitors. Carbon 2019, 141, 748–757. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Pana, O.; Soran, M.; Leostean, C.; Macavei, S.; Gautron, E.; Teodorescu, C.; Gheorghe, N.; Chauvet, O. Interface charge transfer in polypyrrole coated perovskite manganite magnetic nanoparticles. J. Appl. Phys. 2012, 111, 044309. [Google Scholar] [CrossRef]
- Xu, J.; Wang, D.; Yuan, Y.; Wei, W.; Gu, S.; Liu, R.; Wang, X.; Liu, L.; Xu, W. Polypyrrole-coated cotton fabrics for flexible supercapacitor electrodes prepared using CuO nanoparticles as template. Cellulose 2015, 22, 1355–1363. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhitomirsky, I. Surface modification of MnO2 and carbon nanotubes using organic dyes for nanotechnology of electrochemical supercapacitors. J. Mater. Chem. A 2013, 1, 12519–12526. [Google Scholar] [CrossRef]
- Chen, R.; Yu, M.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. The development of pseudocapacitor electrodes and devices with high active mass loading. Adv. Energy Mater. 2020, 10, 1903848. [Google Scholar] [CrossRef]
- Reddy, R.N.; Reddy, R.G. Sol–gel MnO2 as an electrode material for electrochemical capacitors. J. Power Sources 2003, 124, 330–337. [Google Scholar] [CrossRef]
- Jeong, Y.; Manthiram, A. Nanocrystalline manganese oxides for electrochemical capacitors with neutral electrolytes. J. Electrochem. Soc. 2002, 149, A1419. [Google Scholar] [CrossRef]
- Dong, W.; Sakamoto, J.S.; Dunn, B. Electrochemical properties of vanadium oxide aerogels. Sci. Technol. Adv. Mater. 2003, 4, 3–11. [Google Scholar] [CrossRef]
- Kim, E.; Liu, Y.; Shi, X.W.; Yang, X.; Bentley, W.E.; Payne, G.F. Biomimetic approach to confer redox activity to thin chitosan films. Adv. Funct. Mater. 2010, 20, 2683–2694. [Google Scholar] [CrossRef]
- Kim, E.; Liu, Y.; Bentley, W.E.; Payne, G.F. Redox capacitor to establish bio-device redox-connectivity. Adv. Funct. Mater. 2012, 22, 1409–1416. [Google Scholar] [CrossRef]
- Wang, G.-L.; Xu, J.-J.; Chen, H.-Y. Dopamine sensitized nanoporous TiO2 film on electrodes: Photoelectrochemical sensing of NADH under visible irradiation. Biosens. Bioelectron. 2009, 24, 2494–2498. [Google Scholar] [CrossRef]
- Radich, E.J.; Peeples, N.R.; Santra, P.K.; Kamat, P.V. Charge transfer mediation through CuxS. The hole story of CdSe in polysulfide. J. Phys. Chem. C 2014, 118, 16463–16471. [Google Scholar] [CrossRef]
- Tallman, D.; Vang, C.; Wallace, G.; Bierwagen, G. Direct electrodeposition of polypyrrole on aluminum and aluminum alloy by electron transfer mediation. J. Electrochem. Soc. 2002, 149, C173. [Google Scholar] [CrossRef]
- Ariyanayagamkumarappa, D.; Zhitomirsky, I. Electropolymerization of polypyrrole films on stainless steel substrates for electrodes of electrochemical supercapacitors. Synth. Met. 2012, 162, 868–872. [Google Scholar] [CrossRef]
- Seung-Hoon, S.; Young-Je, Y. Characteristics of mediated enzymatic nitrate reduction by gallocyanine-bound nanoporous electrode. J. Microbiol. Biotechnol. 2006, 16, 505–510. [Google Scholar]
- Berkowitz, A.; Schuele, W.; Flanders, P. Permanent magnets and fine particles. J. Appl. Phys 1968, 39, 1261. [Google Scholar] [CrossRef]
- Morales, M.; Andres-Verges, M.; Veintemillas-Verdaguer, S.; Montero, M.; Serna, C. Structural effects on the magnetic properties of γ-Fe2O3 nanoparticles. J. Magn. Magn. Mater. 1999, 203, 146–148. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Zhang, L. Effects of crystalline phase and particle size on the properties of plate-like Fe2O3 nanoparticles during γ-to α-phase transformation. J. Phys. Chem. C 2011, 115, 3602–3611. [Google Scholar] [CrossRef]
- Jeong, J.R.; Lee, S.J.; Kim, J.D.; Shin, S.C. Magnetic properties of γ-Fe2O3 nanoparticles made by coprecipitation method. Phys. Status Solidi 2004, 241, 1593–1596. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, P.; Zhang, H.; Zhang, D.; Sun, X.; Ma, Y. Rapid hydrothermal synthesis of hierarchical nanostructures assembled from ultrathin birnessite-type MnO2 nanosheets for supercapacitor applications. Electrochim. Acta 2013, 89, 523–529. [Google Scholar] [CrossRef]
- Jayachandran, M.; Rose, A.; Maiyalagan, T.; Poongodi, N.; Vijayakumar, T. Effect of various aqueous electrolytes on the electrochemical performance of α-MnO2 nanorods as electrode materials for supercapacitor application. Electrochim. Acta 2021, 366, 137412. [Google Scholar] [CrossRef]
- Srither, S.; Karthik, A.; Arunmetha, S.; Murugesan, D.; Rajendran, V. Electrochemical supercapacitor studies of porous MnO2 nanoparticles in neutral electrolytes. Mater. Chem. Phys. 2016, 183, 375–382. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Zhou, Y.; Ouyang, J.-H.; Jia, D.; Guo, L. High voltage asymmetric supercapacitor based on MnO2 and graphene electrodes. J. Electroanal. Chem. 2013, 689, 201–206. [Google Scholar] [CrossRef]
- Ou, T.-M.; Hsu, C.-T.; Hu, C.-C. Synthesis and characterization of sodium-doped MnO2 for the aqueous asymmetric supercapacitor application. J. Electrochem. Soc. 2015, 162, A5124. [Google Scholar] [CrossRef]
- Zhang, G.; Ren, L.; Hu, D.; Gu, H.; Zhang, S. Sulfuric acid etching for fabrication of porous MnO2 for high-performance supercapacitor. J. Colloid Interface Sci. 2018, 518, 84–91. [Google Scholar] [CrossRef]
- Demarconnay, L.; Raymundo-Piñero, E.; Béguin, F. Adjustment of electrodes potential window in an asymmetric carbon/MnO2 supercapacitor. J. Power Sources 2011, 196, 580–586. [Google Scholar] [CrossRef]
- Athouël, L.; Moser, F.; Dugas, R.; Crosnier, O.; Bélanger, D.; Brousse, T. Variation of the MnO2 Birnessite Structure upon Charge/Discharge in an Electrochemical Supercapacitor Electrode in Aqueous Na2SO4 Electrolyte. J. Phys. Chem. C 2008, 112, 7270–7277. [Google Scholar] [CrossRef]
- Ming, B.; Li, J.; Kang, F.; Pang, G.; Zhang, Y.; Chen, L.; Xu, J.; Wang, X. Microwave–hydrothermal synthesis of birnessite-type MnO2 nanospheres as supercapacitor electrode materials. J. Power Sources 2012, 198, 428–431. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacDonald, M.; Zhitomirsky, I. Pseudocapacitive and Magnetic Properties of SrFe12O19–Polypyrrole Composites. J. Compos. Sci. 2024, 8, 351. https://doi.org/10.3390/jcs8090351
MacDonald M, Zhitomirsky I. Pseudocapacitive and Magnetic Properties of SrFe12O19–Polypyrrole Composites. Journal of Composites Science. 2024; 8(9):351. https://doi.org/10.3390/jcs8090351
Chicago/Turabian StyleMacDonald, Michael, and Igor Zhitomirsky. 2024. "Pseudocapacitive and Magnetic Properties of SrFe12O19–Polypyrrole Composites" Journal of Composites Science 8, no. 9: 351. https://doi.org/10.3390/jcs8090351






