Composite Coatings of Gellan Gum and Inulin with Lactobacillus casei: Enhancing the Post-Harvest Quality of Guava
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth of Lactobacillus casei
2.2. Preparation of Edible Coatings
2.3. Respiration Rate, Soluble Solids, and Titratable Acidity
2.4. Weight Loss
2.5. Total Phenol Content
2.6. Measurement of Color in Guava Fruits
2.7. Primary Modeling of Lactobacillus casei
2.8. Statistical Analysis
3. Results and Discussion
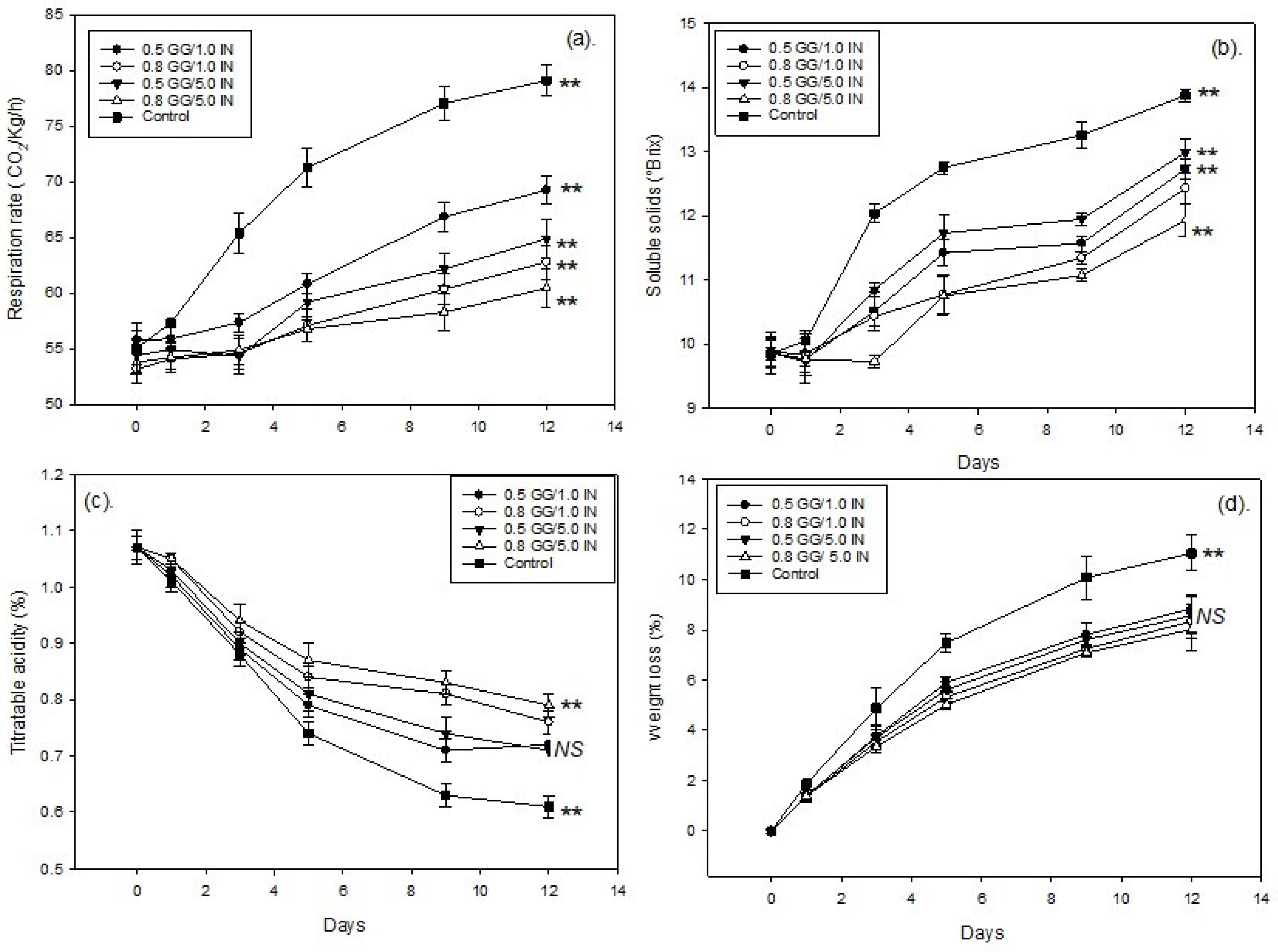
3.1. Respiration Rate, Soluble Solids, and Titratable Acidity
3.2. Guava Fruit Weight Loss
3.3. Total Phenol Content
3.4. Measurement of Color in Guava Fruits
3.5. Primary Modeling of Lactobacillus casei
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forato, L.A.; Britto, D.; De Rizzo, J.; Gastaldi, T.; Assis, O. Effect of cashew gum carboxymethylcellulose edible coatings in extending the shelf-life of fresh and cut guavas. Food Packag. Shelf Life 2015, 5, 68–74. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252. [Google Scholar] [CrossRef]
- Etemadipoor, R.; Ramezanian, A.; Dastjerdi, A.M.; Shamili, M. The potential of gum arabic enriched with cinnamon essential oil for improving the qualitative characteristics and storability of guava (Psidium guajava L.) fruit. Sci. Hortic. 2019, 251, 101–107. [Google Scholar] [CrossRef]
- Germano, T.A.; Aguiar, R.P.; Rocha, B.M.; Azevedo, M.R.; Ayala-Zavala, J.F.; Alcântara de Miranda, M.R. Galactomannan-carnauba wax coating improves the antioxidant status and reduces chilling injury of ‘Paluma’ guava. Postharvest Biol. Technol. 2019, 149, 9–17. [Google Scholar] [CrossRef]
- Santos, T.M.; de Souza, F.M.; Silva, D.O.; da Silveira, M.R.; de Mirand, M.R.; Lopes, M.A.; Azeredo, M.C. Enhancing storage stability of guava with tannic acid-crosslinked zein coatings. Food Chem. 2018, 257, 252–258. [Google Scholar] [CrossRef]
- Aquino, A.B.; Blank, A.F.; Santana, L.C. Impact of edible chitosan-cassava starch coatings enriched with Lippia gracilis Schauer genotype mixtures on the shelf life of guavas (Psidium guajava L.) during storage at room temperature. Food Chem. 2015, 15, 108–116. [Google Scholar] [CrossRef]
- Oliveira, V.R.; Santos, F.K.; Leite, R.H.; Aroucha, E.M.; Silva, K.N. Use of biopolymeric coating hydrophobized with beeswax in postharvest conservation of guavas. Food Chem. 2018, 259, 55–64. [Google Scholar] [CrossRef] [PubMed]
- González-Cuello, R.E.; Moron-Alcazar, L.B.; Pérez-Mendoza, J.A. Recubrimientos a base de goma gelana de bajo acilo conteniendo α-pineno y extracto de arándano para la conservación de la calidad post-cosecha de fresas. Inf. Tecnol. 2022, 33, 93–102. [Google Scholar] [CrossRef]
- González-Cuello, R.E.; Ramos-Ramírez, E.G.; Cruz-Orea, A.; Salazar-Montoya, J.A. Rheological characterization and activation energy values of binary mixtures of gellan. Eur. Food Res. Technol. 2012, 234, 305–313. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Krol, M.; Varzakas, T. Lactic acid bacteria as antibacterial agents to extend the shelf life of fresh and minimally processed fruits and vegetables: Quality and safety aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef]
- Marín, A.; Plotto, A.; Atarés, L.; Chiralt, A. Lactic acid bacteria incorporated into edible coatings to control fungal growth and maintain postharvest quality of grapes. HortScience 2019, 54, 337–343. [Google Scholar] [CrossRef]
- Silva, W.B.; Cosme, G.M.; Bortolini, S.D.; Rodrigues, S.A.; Barbosa, M.D.; Belghith, I.; Martins, N.; Menezes, M.H.; Polete, G.M. Chitosan delays ripening and ROS production in guava (Casei guajava L.) fruit. Food Chem. 2018, 242, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.; Villarroel, M.; Rubilar, M.; Shene, C. Lactobacillus acidophilus La-05 encapsulated by spray drying: Effect of mucilage and protein from flaxseed (Linum usitatissimum L.). LWT-Food Sci. Technol. 2015, 62, 1162–1168. [Google Scholar] [CrossRef]
- Gull, S.; Ejaz, S.; Ali, S.; Ali, M.; Hussain, S.; Sardar, H.; Azam, M.; Nawaz, A.; Naz, S.; Maqbool, M. A novel edible coating based on Albizia [Albizia lebbeck (L.) Benth.] gum delays softening and maintains quality of harvested guava fruits during storage. Int. J. Biol. Macromol. 2024, 277, 134096. [Google Scholar] [CrossRef]
- Beaudry, R.M. Responses of horticultural commodities to low oxygen: Modified atmosphere packaging. HortTechnology 2000, 10, 491–500. [Google Scholar] [CrossRef]
- Formiga, A.S.; Pinsetta, J.S.; Pereira, E.; Cordeiro, I.; Mattiuz, B. Use of edible coatings based on hydroxypropyl methylcellulose and beeswax in the conservation of red guava ‘Pedro Sato’. Food Chem. 2019, 290, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, J.G.; Montenegro, S.T.; Castelo, N.F.; dos Santos, N.I.; Barbosa, M.A.; Estevez, M.M.; Stamford-Arnaud, T.M.; Pereira, S.N.; Montenegro, T.L. Chitosan–citric acid edible coating to control Colletotrichum gloeosporioides and maintain quality parameters of fresh-cut guava. Int. J. Biol. Macromol. 2020, 63, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.; Akram, H.; Zaidi, M.; Ali, S. Effect of gum arabic and Aloe vera gel based edible coatings in combination with plant extracts on postharvest quality and storability of ‘Gola’ guava fruits. Sci. Hortic. 2020, 271, 109506. [Google Scholar] [CrossRef]
- Parven, A.; Sarker, M.R.; Megharaj, M.; Meftaul, I.M. Prolonging the shelf life of Papaya (Carica papaya L.) using Aloe vera gel at ambient temperature. Sci. Hortic. 2020, 265, 109228. [Google Scholar] [CrossRef]
- Arroyo, B.J.; Campos, B.A.; Lins, O.L.; Jarma, A.S.; Almeida de Melo, E.; Pinheiro, A.M. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.). Food Chem. 2020, 309, 125566. [Google Scholar] [CrossRef]
- Gol, N.B.; Patel, P.R.; Rao, T.V. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Khaliq, G.; Abbas, H.T.; Ali, I.; Waseem, M. Aloe vera gel enriched with garlic essential oil effectively controls anthracnose disease and maintains postharvest quality of banana fruit during storage. Hortic. Environ. Biotechnol. 2019, 60, 659–669. [Google Scholar] [CrossRef]
- Hagenmaier, R.D.; Baker, R.A. Reduction in gas exchange of citrus fruit by wax coatings. J. Agric. Food Chem. 1993, 41, 283–287. [Google Scholar] [CrossRef]
- Navarro-Tarazag, M.L.; Massa, A.; Pérez-Gago, M.B. Effect of beeswax content on hydroxypropyl methylcellulose-based edible film properties and postharvest quality of coated plums (Cv. Angeleno). LWT-Food Sci. Technol. 2011, 44, 2328–2334. [Google Scholar] [CrossRef]
- Chen, H.; Sun, Z.; Yang, H. Effect of carnauba wax-based coating containing glycerol monolaurate on the quality maintenance and shelf-life of Indian jujube (Zizyphus mauritiana Lamk.) fruit during storage. Sci. Hortic. 2019, 244, 157–164. [Google Scholar] [CrossRef]
- Pérez-Guzmán, A.E.; Saucedo-Veloz, C.; Arana-Errasquin, R. Effect of individual seal packaging in plastic films on the quality of Dancy mandarins stored under refrigeration. Food Sci. Technol. Int. 1999, 5, 215–222. [Google Scholar] [CrossRef]
- Abreu, J.R.; Santos, C.D.; Abreu, M.C.; Castro, E.M. Histochemistry and morphoanatomy study on guava fruit during ripening. Ciênc Tecnol. Aliment. 2012, 32, 179–186. [Google Scholar] [CrossRef]
- Tran, D.T.; Verlinden, B.E.; Hertog, M.; Nicolaï, B. Monitoring of extremely low oxygen control atmosphere storage of “Greenstar” apples using chlorophyll fluorescence. Sci. Hortic. 2015, 184, 18–22. [Google Scholar] [CrossRef]
- Santos, D.X.; Casazza, A.A.; Aliakbarian, B.; Bedani, R.; Isay, S.M.; Perego, P. Improved probiotic survival to in vitro gastrointestinal stress in a mousse containing Lactobacillus acidophilus La-5 microencapsulated with inulin by spray drying. LWT-Food Sci. Technol. 2019, 99, 404–410. [Google Scholar] [CrossRef]

| Color Parameters | Days | Control | 0.5 GG/1.0 IN | 0.8 GG/1.0 IN | 0.5 GG/5.0 IN | 0.8 GG/5.0 IN |
|---|---|---|---|---|---|---|
| L* | 0 | 44.97 ± 0.02 a | 44.72 ± 0.04 a | 45.80 ± 0.02 a | 46.30 ± 0.02 b | 44.37 ± 0.04 a |
| 1 | 48.80 ± 0.00 a | 47.15 ± 0.04 a | 44.82 ± 0.04 b | 46.77 ± 0.02 a | 44.62 ± 0.02 b | |
| 3 | 54.10 ± 0.05 a | 51.75 ± 0.00 b | 49.20 ± 0.02 cd | 50.17 ± 0.02 c | 48.17 ± 0.00 d | |
| 5 | 56.72 ± 0.00 a | 53.30 ± 0.02 b | 51.95 ± 0.02 b | 51.45 ± 0.04 b | 49.33 ± 0.00 c | |
| 9 | 60.95 ± 0.00 a | 54.32 ± 0.03 b | 51.32 ± 0.04 c | 52.65 ± 0.04 c | 51.02 ± 0.02 c | |
| 12 | 63.57 ± 0.04 a | 54.47 ± 0.02 b | 53.10 ± 0.02 b | 53.05 ± 0.02 b | 52.3 ± 0.05 b | |
| a* | 0 | −5.90 ± 0.04 a | −5.90 ± 0.02 b | −5.87 ± 0.00 b | −6.00 ± 0.02 b | −5.90 ± 0.00 b |
| 1 | −6.00 ± 0.02 a | −4.77 ± 0.00 b | −5.55 ± 0.02 b | −5.20 ± 0.00 b | −5.65 ± 0.02 b | |
| 3 | −2.65 ± 0.02 a | 0.87 ± 0.00 b | −3.25 ± 0.00 a | −3.14 ± 0.00 a | −3.85 ± 0.00 c | |
| 5 | 0.20 ± 0.00 a | 0.70 ± 0.02 b | −1.45 ± 0.00 c | −0.90 ± 0.00 d | −1.55 ± 0.02 e | |
| 9 | 7.40 ± 0.02 a | 2.90 ± 0.02 b | 0.85 ± 0.00 c | 1.22 ± 0.00 d | −0.47 ± 0.00 e | |
| 12 | 10.35 ± 0.00 a | 3.73 ± 0.01 b | 2.85 ± 0.02 c | 3.52 ± 0.02 d | 1.05 ± 0.01 e | |
| b* | 0 | 31.05 ± 0.02 a | 36.72 ± 0.12 b | 36.47 ± 0.05 b | 36.85 ± 0.10 b | 36.87 ± 0.05 b |
| 1 | 41.85 ± 0.08 a | 40.82 ± 0.10 a | 39.82 ± 0.10 a | 39.77 ± 0.10 a | 37.3 ± 0.05 b | |
| 3 | 45.97 ± 0.04 a | 42.72 ± 0.04 b | 40.5 ± 0.12 c | 41.3 ± 0.04 c | 39.77 ± 0.02 d | |
| 5 | 49.85 ± 0.04 a | 44.15 ± 0.08 b | 42.22 ± 0.12 c | 44.25 ± 0.04 b | 41.08 ± 0.02 c | |
| 9 | 47.25 ± 0.12 a | 44.52 ± 0.08 b | 43.00 ± 0.02 b | 44.27 ± 0.04 b | 41.95 ± 0.00 c | |
| 12 | 38.27 ± 0.10 a | 47.7 ± 0.04 b | 43.75 ± 0.08 c | 45.07 ± 0.05 d | 41.47 ± 0.05 e | |
| C* | 0 | 31.60 | 37.19 | 36.93 | 37.33 | 37.33 |
| 1 | 42.27 | 41.09 | 40.20 | 40.10 | 37.72 | |
| 3 | 46.04 | 42.72 | 40.63 | 41.41 | 39.95 | |
| 5 | 49.80 | 44.15 | 42.24 | 44.25 | 41.02 | |
| 9 | 47.82 | 44.61 | 43.00 | 44.28 | 41.95 | |
| 12 | 39.64 | 45.60 | 43.84 | 45.20 | 41.48 | |
| ΔE | 1 | 11.45 | 4.89 | 3.50 | 3.06 | 0.55 |
| 3 | 17.79 | 11.45 | 5.88 | 6.55 | 5.20 | |
| 5 | 22.95 | 13.12 | 9.50 | 10.35 | 7.78 | |
| 9 | 26.35 | 15.18 | 10.87 | 12.14 | 9.97 | |
| 12 | 25.73 | 17.56 | 13.50 | 14.26 | 11.50 |
| Parameter | 0.5 GG/ 1.0 IN | 0.8 GG/ 1.0 IN | 0.5 GG/ 5.0 IN | 0.8 GG/ 5.0 IN |
|---|---|---|---|---|
| Y0 | 4.29 | 4.35 | 4.23 | 4.32 |
| Ymax | 9.99 | 10.01 | 9.88 | 9.45 |
| µmax | 0.03 | 0.03 | 0.05 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Cuello, R.E.; Mendoza-Nova, L.; Rodriguez-Rodriguez, V.C.; Hernández-Fernández, J.; Ortega-Toro, R. Composite Coatings of Gellan Gum and Inulin with Lactobacillus casei: Enhancing the Post-Harvest Quality of Guava. J. Compos. Sci. 2024, 8, 353. https://doi.org/10.3390/jcs8090353
González-Cuello RE, Mendoza-Nova L, Rodriguez-Rodriguez VC, Hernández-Fernández J, Ortega-Toro R. Composite Coatings of Gellan Gum and Inulin with Lactobacillus casei: Enhancing the Post-Harvest Quality of Guava. Journal of Composites Science. 2024; 8(9):353. https://doi.org/10.3390/jcs8090353
Chicago/Turabian StyleGonzález-Cuello, Rafael Emilio, Leidy Mendoza-Nova, Virginia Consuelo Rodriguez-Rodriguez, Joaquín Hernández-Fernández, and Rodrigo Ortega-Toro. 2024. "Composite Coatings of Gellan Gum and Inulin with Lactobacillus casei: Enhancing the Post-Harvest Quality of Guava" Journal of Composites Science 8, no. 9: 353. https://doi.org/10.3390/jcs8090353










