Thermal Stabilization Activities of Metal Oxide γ-Irradiated Styrene–Isoprene–Styrene Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. γ-Irradiation and Measurements
2.4. Chemiluminescence Measurements
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elkahlout, Z.I.; Al Ahzm, A.M.; Alejli, M.O.; Al Maadeed, F.Z.; Al-Maadeed, M.A. Polymer–metal oxide composite nanofibers. In Metal Oxide-Based Nanofibers and Their Applications; Esposito, V., Marani, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 89–109. [Google Scholar]
- Haider, S.; Haider, A. Renewable Polymers and Polymer-Metal Oxide Composites. Synthesis, Properties, and Applications; Elsevier: London, UK, 2022. [Google Scholar]
- Jacob, J.; Cacciotti, I.; Thomas, S. Nanostructured Materials for Food Packaging Applications; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Idumah, C.I.; Okonkwo, U.C.; Obele, C.M. Recently emerging advancements in montmorillonite polymeric nanoarchitectures and applications. Clean. Mater. 2022, 4, 100071. [Google Scholar] [CrossRef]
- Sawunyama, L.; Ajiboye, T.O.; Oyewo, O.; Onwudiwe, D.C. Ceramic-polymer composite membranes: Synthesis methods and environmental applications. Ceram. Int. 2024, 50, 5067–5079. [Google Scholar] [CrossRef]
- Jayalath, S.; Herath, M.; Epaarachchi, J.; Trifoni, E.; Gdoutos, E.E.; Fang, L. Durability and long-term behaviour of shape memory polymers and composites for the space industry—A review of current status and future perspectives. Polym. Degrad. Stab. 2023, 211, 10297. [Google Scholar] [CrossRef]
- Zaharescu, T. Stabilization effects of doped inorganic filler on EPDM for space and terrestrial applications. Mater. Chem. Phys. 2019, 234, 102–109. [Google Scholar] [CrossRef]
- Upadhyaya, P.; Singh, S.; Roy, S. A mechanism-based multi-scale model for predicting thermo-oxidative degradation in high temperature polymer matrix composites. Compos. Sci. Technol. 2011, 71, 1309–1315. [Google Scholar] [CrossRef]
- Blanco, I.; Cicala, G.; Tosto, C.; Bottino, F.A. Kinetic study of the thermal and thermo-oxidative degradations of polystyrene reinforced with multiple-cages POSS. Polymers 2020, 12, 2742. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kumar, N.; Sachdeva, A. Factors affecting the ageing of polymer composite: A state of art. Polym. Degrad. Stab. 2024, 21, 110670. [Google Scholar] [CrossRef]
- Bernstein, R.; Thornberg, S.M.; Irwin, A.N.; Hochrein, J.M.; Derzon, D.K.; Klamo, S.B.; Clough, R.L. Radiation-oxidation mechanisms: Volatile organic degradation products from polypropylene having selective C-13 labeling studied by GC/MS. Polym. Degrad. Stab. 2008, 93, 854–870. [Google Scholar] [CrossRef]
- Kumar, A.P.; Depan, D.; Tomer, N.S.; Singh, R.P. Nanoscale particles for polymer degradation and stabilization mechanism—Trends and future perspectives. Prog. Polym. Sci. 2009, 34, 479–515. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O. Nanostructuring of polymers by controlling of ionizing radiation-induced free radical polymerization, copolymerization, grafting and crosslinking by RAFT mechanism. Radiat. Phys. Chem. 2020, 169, 107816. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedulli, G.F.; Matyakin, M.V.; Schlick, S. Electron spin resonance imaging of polymer degradation and stabilization. Prog. Polym. Sci. 2003, 28, 331–340. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Latteri, A.; Recca, A. A kinetic study of the thermal and thermal oxidative degradations of new bridged POSS/PS nanocomposites. Polym. Degrad. Stab. 2013, 98, 2564–2570. [Google Scholar] [CrossRef]
- Davachi, S.M.; Heidari, B.S.; Hejazi, I.; Seyfi, J.; Oliaei, E.; Farzaneh, A.; Rashedi, H. Interface modified polylactic acid/starch/poly ε-caprolactone antibacterial nanocomposite blends for medical applications. Carbohyd. Polym. 2017, 155, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Hore, M.J.A.; Clarke, N.; Winey, K.I.; Composto, R.J. Nanoparticle brush architecture controls polymer diffusion in nanocomposites. Macromolecules 2014, 47, 2404–2410. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Alessi, S.; Arrig, R.; Przybytniak, G.; Spadaro, G. Influence of the e-beam irradiation and photo-oxidation aging on the structure and properties of LDPE-OMMT nanocomposite films. Radiat. Phys. Chem. 2013, 81, 432–436. [Google Scholar] [CrossRef]
- Delozier, D.M.; Watson, K.A.; Smith, J.G.; Connell, J.W. Preparation and characterization of space durable polymer nanocomposite films. Compos. Sci. Technol. 2003, 65, 749–755. [Google Scholar] [CrossRef]
- Zegebreal, L.T.; Tegegne, N.A.; Hon, F.G. Recent progress in hybrid conducting polymers and metal oxide nanocomposite for room-temperature gas sensor applications: A review. Sens. Actuators A Phys. 2023, 359, 114472. [Google Scholar] [CrossRef]
- Chmielewska, D. Radiation methods and uses in nanotechnology. In Applications of Ionizing Radiation in Materials Processing; Sun, Y., Chmielewski, A.G., Eds.; Institute of Nuclear Chemistry and Technology: Warsaw, Poland, 2017; pp. 395–414. [Google Scholar]
- Zaharescu, T.; Blanco, I.; Bottino, F.A. Antioxidant activity assisted by modified particle surface in POSS/EPDM hybrids. Appl. Surf. Sci. 2020, 509, 144702. [Google Scholar] [CrossRef]
- Singh, N.; Gaur, S.; Chawla, S.; Singh, S.; Husen, A. Use of smart nanomaterials in food packaging. In Advances in Smart Nanomaterials and Their Applications; Husen, A., Siddiqi, K.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 233–245. [Google Scholar]
- Sanu, S.M.; Gejo, G.; Sajna, M.S.; Prakashan, V.P.; Twinkle, A.J.; Prathibha, V.; Saritha, A.C.; Biju, P.R.; Joseph, C. Unnikrishnan NV, Recent advancements in multifunctional applications of sol-gel derived polymer incorporated TiO2-ZrO2 composite coatings: A comprehensive review. Appl. Surface Sci. Adv. 2021, 6, 100173. [Google Scholar] [CrossRef]
- Zezina, E.A.; Emel’yanov, A.I.; Pozdnyakov, A.S.; Prozorova, G.F.; Abramchuk, S.S.; Feldman, V.I.; Zezin, A.A. Radiation-induced synthesis of copper nanostructures in the films of interpolymer complexes. Radiat. Phys. Chem. 2019, 158, 115–121. [Google Scholar] [CrossRef]
- Zaharescu, T.; Bumbac, M.; Nicolescu, C.M.; Blanco, I. The contribution of silica nanoparticles to the stability of styrene-isoprene-styrene triblock copolymer (SIS). II. Energetic peculiarities. Radiat. Phys. Chem. 2024, 215, 111318. [Google Scholar] [CrossRef]
- Rychlý, J.; Rychlá, L.; Novák, I.; Vanko, V.; Preƭo, J.; Janigová, I.; Chodák, I. Thermooxidative stability of hot melt adhesives based on metallocene polyolefins grafted with polar acrylic acid moieties. Polym. Test. 2020, 85, 106422. [Google Scholar] [CrossRef]
- Okamba-Diogo, O.; Fernagut, F.; Guilment, J.; Pery, F.; Fayolle, B.; Richaud, E. Thermal stabilization of polyamide 11 by phenolic antioxidants. Polym. Degrad. Stab. 2020, 79, 109206. [Google Scholar] [CrossRef]
- Tayouri, M.I.; Estaji, S.; Mousavi, S.R.; Khasraghi, S.S.; Jahanmardi, R.; Nouranian, S.; Arjmand, M.; Khonakdar, H.A. Degradation of polymer nanocomposites filled with graphene oxide and reduced graphene oxide nanoparticles: A review of current status. Polym. Degrad. Stab. 2022, 206, 110179. [Google Scholar] [CrossRef]
- Hearon, K.; Smith, S.E.; Maher, C.A.; Wilson, T.S.; Maitland, D.J. The effect of free radical inhibitor on the sensitized radiation crosslinking and thermal processing stabilization of polyurethane shape memory polymers. Radiat. Phys. Chem. 2013, 83, 111–121. [Google Scholar] [CrossRef]
- Morsch, S.; Wand, C.R.; Gibbon, S.; Irwin, M.; Siperstein, F.; Lyon, S. The effect of cross-linker structure on interfacial interactions, polymer dynamics and network composition in an epoxy-amine resin. Appl. Surf. Sci. 2023, 609, 155380. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhu, H.; Shuai, S.; Zhao, C.; Zhou, K.; Ge, W.; Hao, J. Degradation of methoxy-poly (ethylene glycol)-block-poly(a-carboxyl-ε-caprolactone)/magnetite nanocomposites in vitro polymer degradation and stability. Polym. Degrad. Stab. 2020, 177, 109191. [Google Scholar] [CrossRef]
- Vassiliou, A.; Bikiaris, D.; Chrissafis, K.; Paraskevopoulos, K.M.; Stavrev, S.Y.; Docoslis, A. Nanocomposites of isotactic polypropylene with carbon nanoparticles exhibiting enhanced stiffness, thermal stability and gas barrier properties. Compos. Sci. Technol. 2008, 68, 933–943. [Google Scholar] [CrossRef]
- Cheikh, D.; Majdoub, H.; Darder, M. An overview of clay-polymer nanocomposites containing bioactive compounds for food packaging applications. Appl. Clay Sci. 2022, 216, 106335. [Google Scholar] [CrossRef]
- Ferry, M.; Ngono, Y. Energy transfer in polymers submitted to ionizing radiation: A review. Radiat. Phys. Chem. 2021, 180, 109320. [Google Scholar] [CrossRef]
- Tanaka, T.; Kozako, M.; Fuse, N.; Okhi, Y. Proposal of a multi-core model for polymer nanocomposite dielectrics. IEEE Trans. Dielect. Electr. Insul. 2005, 12, 669–681. [Google Scholar] [CrossRef]
- Fatimah, I.; Fadillah, G.; Purwiandono, G.; Sahroni, I.; Purwaningsih, D.; Riantana, H.; Avif, A.N.; Sagadevan, S. Magnetic-silica nanocomposites and the functionalized forms for environment and medical applications: A review. Inorg. Chem. Commun. 2022, 137, 109213. [Google Scholar] [CrossRef]
- Lupu (Luchian), A.-M.; Zaharescu, T.; Râpă, M.; Mariș, M.; Iovu, H. Availability of PLA/SIS blends for packaging and medical applications. Part II: Contribution of stabilizer agents. Radiat. Phys. Chem. 2022, 201, 110446. [Google Scholar] [CrossRef]
- Champa-Bujaico, E.; Díez-Pascual, A.M.; Redondo, A.L.; Garcia-Diaz, P. Optimization of mechanical properties of multiscale hybrid polymer nanocomposites: A combination of experimental and machine learning techniques. Compos. Part B Eng. 2024, 269, 111099. [Google Scholar] [CrossRef]
- Singh, M.; Pandey, J.C. Effect of particle surface treatment on dielectric properties of epoxy-alumina nano-compositesIn Proceedings of the 2016 IEEE 7th Power India International Conference (PIICON), Bikaner, India, 25–27 November 2016. [CrossRef]
- Kornacka, E.M. Radiation induced oxidation in polymers. In Applications of Ionizing Radiation in Materials Processing; Sun, Y., Chmielewski, A.G., Eds.; Institute of Nuclear Chemistry and Technology: Warsaw, Poland, 2017; pp. 183–193. [Google Scholar]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Zaharescu, T.; Lugaõ, A.B. Stability improvement of irradiated polymer composites by inorganic compounds—A pertinent solution with respect to phenolic antioxidants. J. Compos. Sci. 2025, 9, 47. [Google Scholar] [CrossRef]
- Huang, G.; Gao, G.; Leng, J.; Wu, Y.; Chen, L.; Jing, R.; Xie, P.; Deng, H.; Shi, Q. Controlling terahertz dielectric responses in polymer composites by engineering α-Al2O3 whisker filler distribution. J. Compos. Sci. 2025, 9, 136. [Google Scholar] [CrossRef]
- Ma, Q.; Dong, K.; Li, F.; Yu, M.; Xiong, Y. Inverse design of material, structure, and process for dielectric properties of additively manufactured PLA/BaTiO3 polymer composites. Compos. Commun. 2025, 55, 102314. [Google Scholar] [CrossRef]
- Myrthong, B.; Ansari, S.; Choudhary, R.B. Bimetallic oxide-conducting polymer composites for robust supercapacitor performance and applications: A review. J. Energy Storage 2025, 117, 116182. [Google Scholar] [CrossRef]
- Alonzo, S.M.M.; Ashie, M.D.; Pathiraja, G.; Bastakoti, B.P. A novel organic-inorganic hybrid polymerization strategy for titanium oxide/polymer nanocomposites with supercapacitive properties. Chm. Eng. J. 2025, 505, 159208. [Google Scholar] [CrossRef]
- Milazzo, M.; Serena Danti, S.; Livens, P.; Dirckx, J.; Scaffaro, R.; Gammino, M. Electrospun graphene oxide/polymeric nanocomposites for eardrum replacements. Compos. Commum. 2024, 51, 102048. [Google Scholar] [CrossRef]
- Romero, L.M.; Palacio, D.A.; Esquivel, S.; Sánchez-Sanhueza, G.A.; Montaño, M.; Rojas, D.; Jaramillo, A.F.; Medina, C.; Montalba, C.; Meléndrez, M.F. Contact antibacterial and biocompatible polymeric, composite with copper zeolite filler and copper oxide, nanoparticles: A step towards new raw materials for the biomedical industry. Polymer 2024, 315, 127795. [Google Scholar] [CrossRef]
- Gadtaya, A.S.; Sahu, B.B.; Sagadevan, S.; Mahaling, R.N.; Moharana, S. Piezoelectric polymer composites for energy harvesting. In Polymer Composites. Fundamentals and Applications; Moharana, S., Sahu, B.B., Nayak, A.K., Tiwari, S.K., Eds.; Springer Nature: Singapore, 2024; pp. 459–488. [Google Scholar]
- Saini, A.; Saini, S.K. Polymer composites for environmental pollution and remediation. In Polymer Compositions: From Computational to Experimental Aspects; Sethi, S.K., Gupta, H.S., Verma, A., Eds.; Springer Nature: Singapore, 2024; pp. 151–180. [Google Scholar]
- Jia, W.; Lin, F.; Li, Z.; Hao Zhang, H.; Liu, F.; Yang, Y. Novel experimental approach to evaluate silica–elastomer interactions of vulcanizates. Polym. Eng. Sci. 2024, 64, 5551–5565. [Google Scholar] [CrossRef]
- Drakopoulos, S.X.; Wu, J.; Maguire, S.M.; Srinivasan, S.; Randazzo, K.; Davidson, E.C.; Priestley, R.D. Polymer nanocomposites: Interfacial properties and capacitive energy storage. Prog. Polym. Sci. 2024, 156, 101870. [Google Scholar] [CrossRef]
- Bian, J.; Liao, L.; Lv, G. Preparation strategy of photo-thermal composite phase change materials: A review. J. Energy Storage 2024, 102, 114155. [Google Scholar] [CrossRef]
- Arif, M.; Rauf, A.; Akhter, T. A comprehensive review on crosslinked network systems of zinc oxide-organic polymer composites. Int. J. Biol. Macromol. 2024, 274, 133250. [Google Scholar] [CrossRef]
- Osakoo, N.; Khemthong, P.; Roessner, F.; Kidkhunthod, P.; Chanlek, N.; Prayoonpokarach, S.; Wittayakun, J. Development and characterization of silica supported cobalt oxides for ethanol oxidation using different preparation methods. Radiat. Phys. Chem. 2020, 171, 108718. [Google Scholar] [CrossRef]
- Bansal, N.; Arora, S. Exploring the impact of gamma rays and electron beam irradiation on physico-mechanical properties of polymers & polymer composites: A comprehensive review. Nucl. Instrum. Meth. Phys. Res. 2024, 549, 165297. [Google Scholar] [CrossRef]
- Żenkiewicz, M.; Rauchfleisz, M.; Czupryńska, J.; Polański, J.; Karasiewicz, T.; Engelgard, W. Effects of electron-beam irradiation on surface oxidation of polymer composites. Appl. Surf. Sci. 2007, 253, 8992–8999. [Google Scholar] [CrossRef]
- Shi, Y.; Shao, J.; Wang, J.; Guo, R.; Li, Z.; Li, N.; Zhang, Q.; Chu, L. Solvent-induced polyelectrolyte conjugation for fluorescence-dependence solvent detection. Adv. Funct. Mater, 2024; Early View. [Google Scholar] [CrossRef]
- Zaharescu, T.; Ilieş, C.-D.; Roşu, T. Thermal and spectroscopic analysis of stabilization effect of copper complexes in EPDM. J. Therm. Anal. Calorim. 2016, 123, 231–239. [Google Scholar] [CrossRef]
- Gupta, R.; Singh, M.K.; Rangappa, S.M.; Siengchin, S.; Dhakal, H.N.; Zafar, S. Recent progress in additive inorganic flame retardants polymer composites: Degradation mechanisms, modeling and applications. Heliyon 2024, 10, e39662. [Google Scholar] [CrossRef] [PubMed]
- Zaharescu, T.; Tardei, C.; Marinescu, V.; Râpă, M.; Iordoc, M. Interphase surface effects on the thermal stability of hydroxyapatite/poly(lactic acid) hybrids. Ceram. Int. 2020, 46, 7288–7297. [Google Scholar] [CrossRef]
- Kolluru, S.; Thakur, A.; Tamakuwala, D.; Kumar, V.V.; Ramakrishna, S.; Chandran, S. Sustainable recycling of polymers: A comprehensive review. Polym. Bull. 2024, 81, 9569–9610. [Google Scholar] [CrossRef]



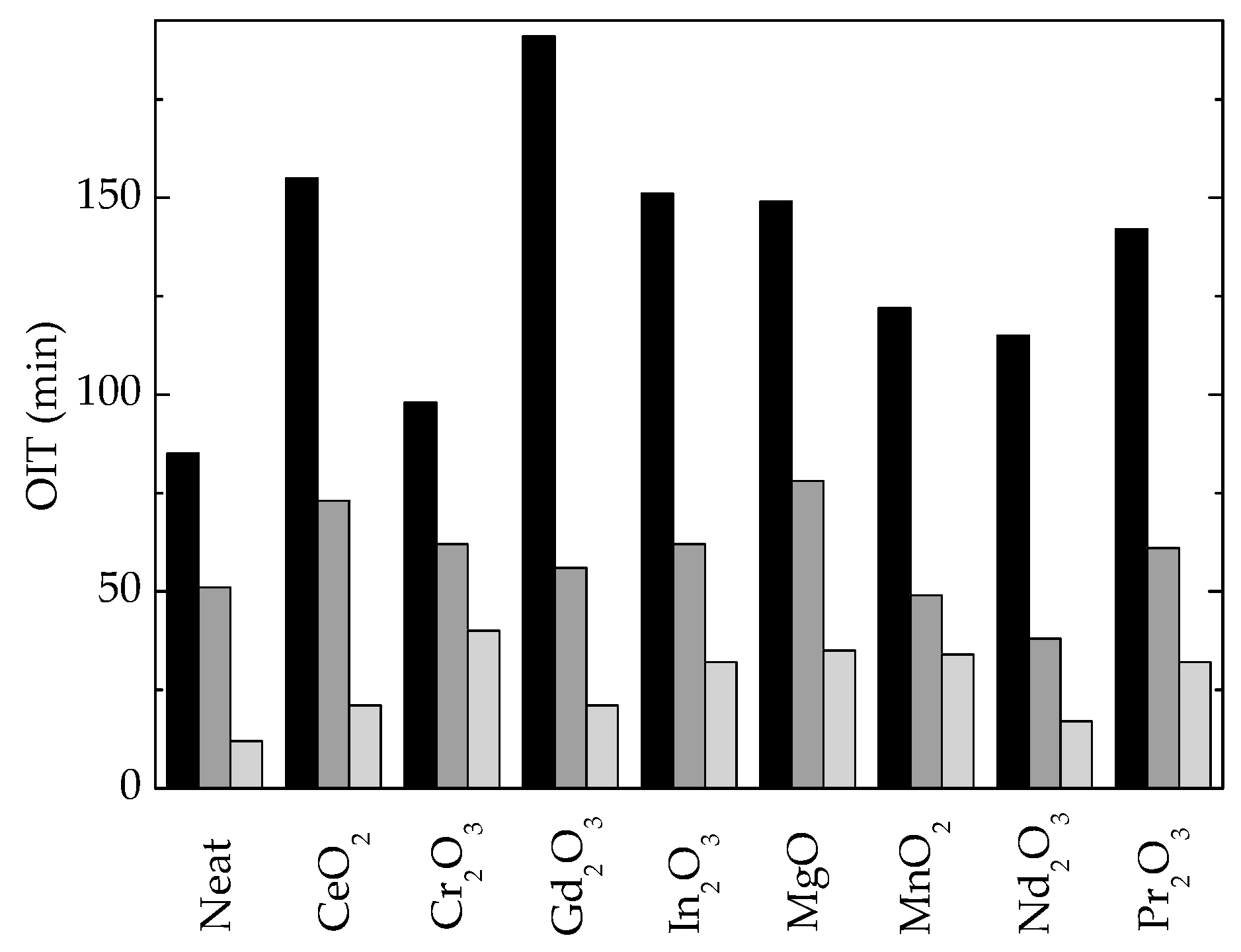
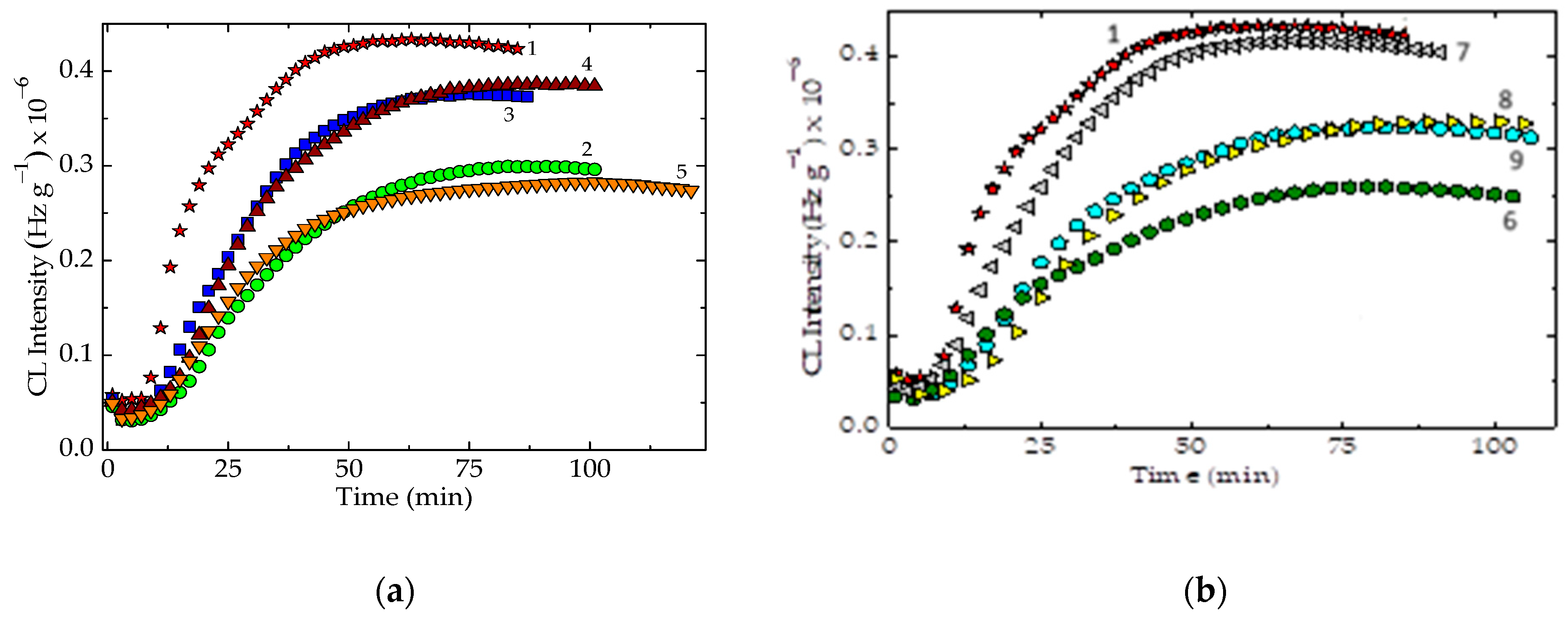

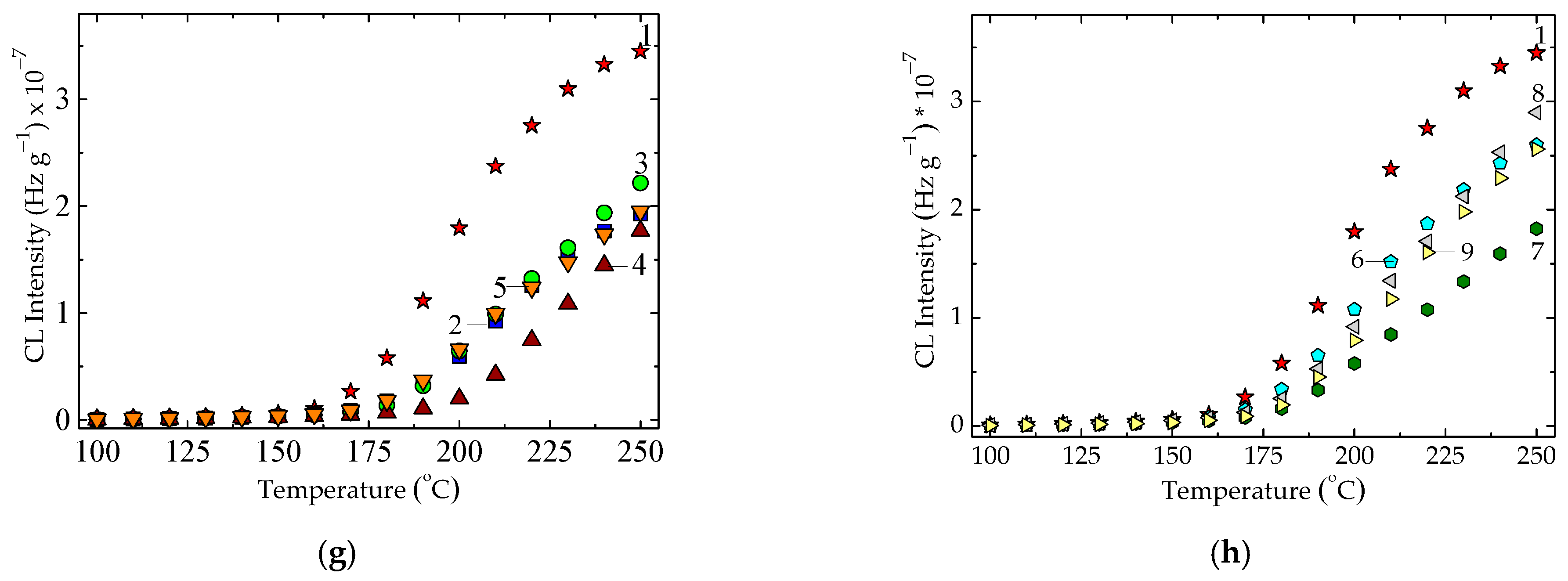
| Composition | Oxidation Induction Time (OIT) (min) | Correlation Factor | Activation Energy, Ea (kJ mol−1) | ||
|---|---|---|---|---|---|
| 140 °C | 150 °C | 160 °C | |||
| SIS | 75 | 42 | 15 | 0.97584 | 135 |
| SIS/CeO2 | 155 | 73 | 21 | 0.98800 | 148 |
| SIS/Cr2O3 | 112 | 44 | 15 | 0.99769 | 150 |
| SIS/Gd2O3 | 180 | 56 | 25 | 0.99581 | 147 |
| SIS/In2O3 | 151 | 62 | 32 | 0.99804 | 155 |
| SIS/MgO | 149 | 78 | 35 | 0.99071 | 139 |
| SIS/MnO2 | 182 | 49 | 34 | 0.99938 | 148 |
| SIS/Nd2O3 | 115 | 38 | 17 | 0.99749 | 140 |
| SIS/Pr2O3 | 142 | 61 | 28 | 0.99956 | 143 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaharescu, T.; Lugāo, A.B.; Mangalagiu, V.; Mirea, R. Thermal Stabilization Activities of Metal Oxide γ-Irradiated Styrene–Isoprene–Styrene Nanocomposites. J. Compos. Sci. 2025, 9, 192. https://doi.org/10.3390/jcs9040192
Zaharescu T, Lugāo AB, Mangalagiu V, Mirea R. Thermal Stabilization Activities of Metal Oxide γ-Irradiated Styrene–Isoprene–Styrene Nanocomposites. Journal of Composites Science. 2025; 9(4):192. https://doi.org/10.3390/jcs9040192
Chicago/Turabian StyleZaharescu, Traian, Ademar B. Lugāo, Violeta Mangalagiu, and Radu Mirea. 2025. "Thermal Stabilization Activities of Metal Oxide γ-Irradiated Styrene–Isoprene–Styrene Nanocomposites" Journal of Composites Science 9, no. 4: 192. https://doi.org/10.3390/jcs9040192
APA StyleZaharescu, T., Lugāo, A. B., Mangalagiu, V., & Mirea, R. (2025). Thermal Stabilization Activities of Metal Oxide γ-Irradiated Styrene–Isoprene–Styrene Nanocomposites. Journal of Composites Science, 9(4), 192. https://doi.org/10.3390/jcs9040192







