Effect of Immunocastration on Culled Sows—A Preliminary Study on Reproductive Tract, Carcass Traits, and Meat Quality
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Animals
2.1.2. Immunization Treatments
- -
- Group 1: Inoculation in middle lactation (IM1; n = 5), two weeks after farrowing (lactational anestrus), when the endogenous GnRH patterns are low [36]; the females in this group were kept indoors in individual farrowing pens with the piglets until the end of farrowing (3 weeks after the first inoculation), when they were transferred outdoors.
- -
- Group 2: Inoculation at the beginning of estrus (IM2; n = 5), when the frequency of GnRH pulses increases; [34]; the first inoculation was performed the day cyclic sows showed signs of standing heat (standing reflex); the sows in this group were allocated to outdoor pens.
- -
- Group 3: Inoculation in diestrus, one week after the end of estrus (IM3; n = 5), when the pulsatile discharges of GnRH are decreased [37]; the sows in this group were also allocated to outdoor pens.
2.2. Sample Collection
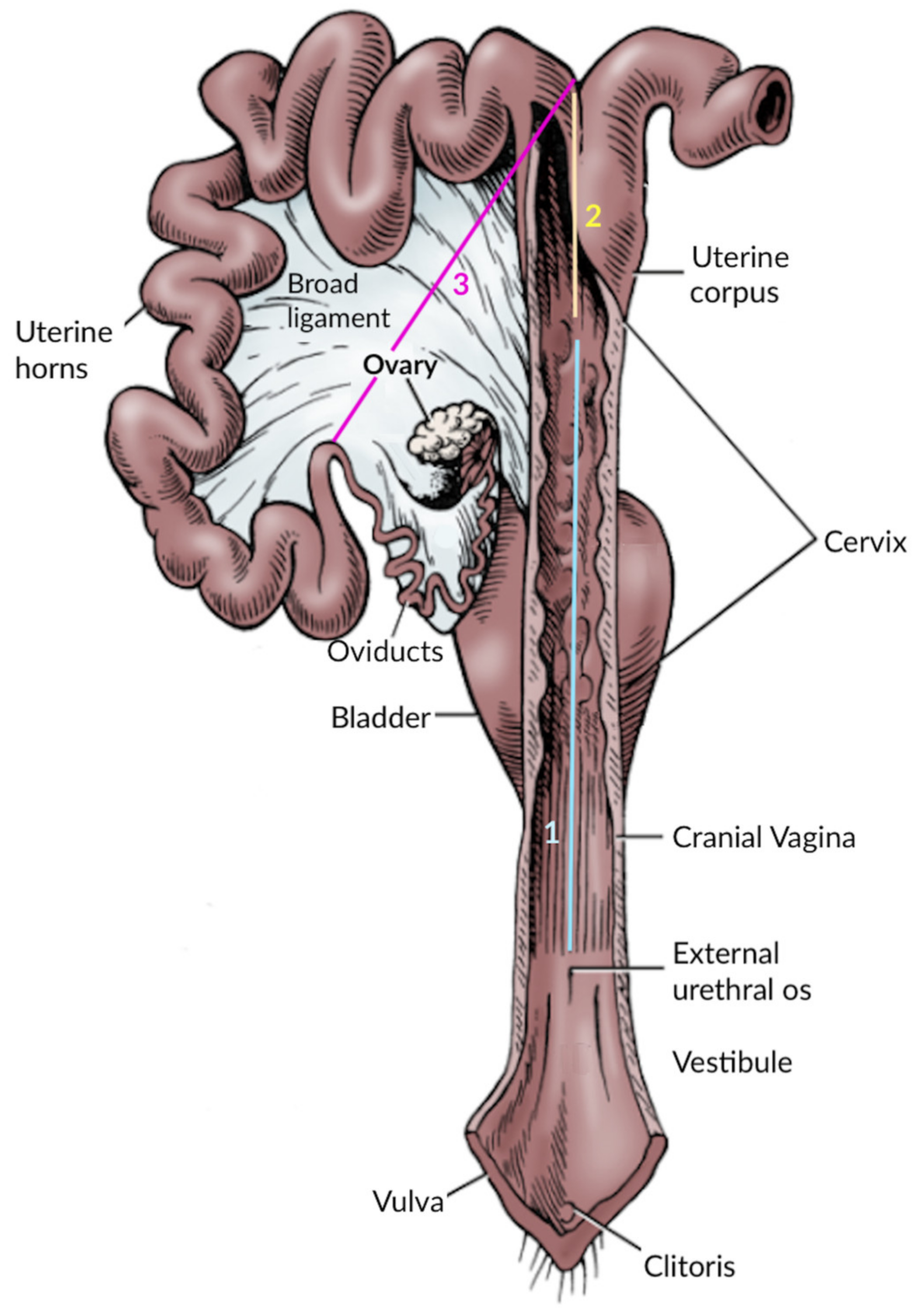
2.3. Reproductive Tract Morphometry
2.4. Physicochemical Analysis
2.5. Sensory Evaluation
2.6. Fatty Acid Profile
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, D.H.; Hwang, Y.J.; Ham, Y.K.; Ha, J.H.; Kim, Y.R.; Kim, H.W. Meat Quality Attributes and Oxidation Stability of Loin Chops from Finishing Gilts and Cull Sows. J. Food Sci. Technol. 2020, 57, 3142–3150. [Google Scholar] [CrossRef]
- Grahofer, A.; Björkman, S.; Peltoniemi, O. Diagnosis of Endometritis and Cystitis in Sows: Use of Biomarkers. J. Anim. Sci. 2020, 98, S107–S116. [Google Scholar] [CrossRef]
- Bogado Pascottini, O.; Aurich, C.; England, G.; Grahofer, A. General and Comparative Aspects of Endometritis in Domestic Species: A Review. Reprod. Domest. Anim. 2023, 58, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Fogsgaard, K.K.; Herskin, M.S.; Thodberg, K. Transportation of Cull Sows-a Descriptive Study of the Clinical Condition of Cull Sows before Transportation to Slaughter. Transl. Anim. Sci. 2018, 2, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Wientjes, J.G.M.; Soede, N.M.; Knol, E.F.; Van Den Brand, H.; Kemp, B. Piglet Birth Weight and Litter Uniformity: Effects of Weaning-to-Pregnancy Interval and Body Condition Changes in Sows of Different Parities and Crossbred Lines 1. J. Anim. Sci. 2013, 91, 2099–2107. [Google Scholar] [CrossRef]
- Herskin, M.S.; Holm, C.; Thodberg, K. Clinical and Behavioural Consequences of On-Farm Mixing of Cull Sows after Weaning. Appl. Anim. Behav. Sci. 2020, 228, 105028. [Google Scholar] [CrossRef]
- Thongkhuy, S.; Chuaychu, S.B.; Burarnrak, P.; Ruangjoy, P.; Juthamanee, P.; Nuntapaitoon, M.; Tummaruk, P. Effect of Backfat Thickness during Late Gestation on Farrowing Duration, Piglet Birth Weight, Colostrum Yield, Milk Yield and Reproductive Performance of Sows. Livest. Sci. 2020, 234, 103983. [Google Scholar] [CrossRef]
- Seoane, S.; De Palo, P.; Lorenzo, J.M.; Maggiolino, A.; González, P.; Pérez-Ciria, L.; Latorre, M.A. Effect of Increasing Dietary Aminoacid Concentration in Late Gestation on Body Condition and Reproductive Performance of Hyperprolific Sows. Animals 2020, 10, 99. [Google Scholar] [CrossRef]
- Grahofer, A.; Plush, K. Lactation in Swine: Review Article. Anim. Front. 2023, 13, 112–118. [Google Scholar] [CrossRef]
- Peltoniemi, O.; Oliviero, C.; Yun, J.; Grahofer, A.; Björkman, S. Management Practices to Optimize the Parturition Process in the Hyperprolific Sow. J. Anim. Sci. 2020, 98, S96–S106. [Google Scholar] [CrossRef]
- Paixão, G.; Esteves, A.; Payan-Carreira, R. Characterization of a Non-Industrial Pig Production System: The Case of Bísaro Breed. Rev. Bras. Zootec. 2018, 47, e20170331. [Google Scholar] [CrossRef]
- Sindelar, J.J.; Prochaska, F.; Britt, J.; Smith, G.L.; Miller, R.K.; Templeman, R.; Osburn, W.N. Strategies to Eliminate Atypical Flavours and Aromas in Sow Loins. I. Optimization of Sodium Tripolyphosphate, Sodium Bicarbonate, and Injection Level. Meat Sci. 2003, 65, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Sindelar, J.J.; Prochaska, F.; Britt, J.; Smith, G.L.; Osburn, W.N. Strategies to Eliminate Atypical Aromas and Flavors in Sow Loins—Part II: Consumer Acceptance of Loins Marinated with Sodium Tripolyphosphate and Sodium Bicarbonate. Meat Sci. 2003, 65, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Hoa, V.B.; Cho, S.H.; Seong, P.N.; Kang, S.M.; Kim, Y.S.; Moon, S.S.; Choi, Y.M.; Kim, J.H.; Seol, K.H. Quality Characteristics, Fatty Acid Profiles, Flavor Compounds and Eating Quality of Cull Sow Meat in Comparison with Commercial Pork. Asian-Australas. J. Anim. Sci. 2020, 33, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Squires, E.J.; Bonneau, M. Boar Taint: Biological Causes and Practical Means to Alleviate It. In Encyclopedia of Meat Sciences; Elsevier: Amsterdam, The Netherlands, 2014; pp. 97–103. [Google Scholar] [CrossRef]
- Keenan, D.F. Pork Meat Quality, Production and Processing On. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 419–431. [Google Scholar] [CrossRef]
- Bonneau, M.; Weiler, U. Pros and Cons of Alternatives to Piglet Castration: Welfare, Boar Taint, and Other Meat Quality Traits. Animals 2019, 9, 884. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, M.; Čandek-Potokar, M.; Škrlep, M.; Font-I.-Furnols, M.; Aluwé, M.; Fontanesi, L. Potential Sensitivity of Pork Production Situations Aiming at High-Quality Products to the Use of Entire Male Pigs as an Alternative to Surgical Castrates. Animal 2018, 12, 1287–1295. [Google Scholar] [CrossRef]
- Prusa, K.; Nederveld, H.; Runnels, P.L.; Li, R.; King, V.L.; Crane, J.P. Prevalence and Relationships of Sensory Taint, 5α-Androstenone and Skatole in Fat and Lean Tissue from the Loin (Longissimus Dorsi) of Barrows, Gilts, Sows, and Boars from Selected Abattoirs in the United States. Meat Sci. 2011, 88, 96–101. [Google Scholar] [CrossRef]
- Aldal, I.; Kristin, A.; Haugen, J.; Gr, A.; Fjetland, O.; Leif, J.; Eikaas, H. Levels of Androstenone and Skatole and the Occurrence of Boar Taint in Fat from Young Boars. Livest. Prod. Sci. 2005, 95, 121–129. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, W.; Zhang, F.; Rao, S.; Peng, Z.; Yao, W. Effect of Immunocastration on Growth Performance, Gonadal Development and Carcass and Meat Quality of SuHuai Female Pigs. Anim. Prod. Sci. 2019, 59, 794–800. [Google Scholar] [CrossRef]
- Kress, K.; Millet, S.; Labussière, É.; Weiler, U.; Stefanski, V. Sustainability of Pork Production with Immunocastration in Europe. Sustainability 2019, 11, 3335. [Google Scholar] [CrossRef]
- European Union. Council Directive 2008/120/EC of 18 December 2008 laying down minimum standards for the protection of pigs (Codified version). Off. J. Eur. Union 2009, L47, 5–13. [Google Scholar]
- Bohrer, B.M.; Flowers, W.L.; Kyle, J.M.; Johnson, S.S.; King, V.L.; Spruill, J.L.; Thompson, D.P.; Schroeder, A.L.; Boler, D.D. Effect of Gonadotropin Releasing Factor Suppression with an Immunological on Growth Performance, Estrus Activity, Carcass Characteristics, and Meat Quality of Market Gilts. J. Anim. Sci. 2014, 92, 4719–4724. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.A.; Almeida, F.R.C.L.; Peloso, J.V.; Ferreira, F.N.A.; Allison, J.; Fontes, D.O. The Effects of Immunization against Gonadotropin-Releasing Hormone on Growth Performance, Reproductive Activity and Carcass Traits of Heavy Weight Gilts. Animal 2019, 13, 1326–1331. [Google Scholar] [CrossRef]
- Pérez-Ciria, L.; Miana-Mena, F.J.; Falceto, M.V.; Mitjana, O.; Latorre, M.A. Effect of Immunocastration and Diet on Growth Performance, Serum Metabolites and Sex Hormones, Reproductive Organ Development and Carcass Quality of Heavy Gilts. Animals 2021, 11, 1900. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ciria, L.; Carcò, G.; Miana-Mena, F.J.; Mitjana, O.; Falceto, M.V.; Latorre, M.A. Immunocastration in Gilts: A Preliminary Study of the Effect of the Second Dose Administration Time on Growth, Reproductive Tract Development, and Carcass and Meat Quality. Animals 2021, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, F.I.; Duarte, J.L.; Pérez, M.A.; Raboso, C.; Del Rosario, A.I.; Izquierdo, M. Successful Long-Term Pre-Pubertal Immunocastration of Purebred Iberian Gilts Reared in Extensive Systems. In Proceedings of the 8th International Symposium on the Mediterranean Pig, Ljubljana, Slovenia, 10–12 October 2013; pp. 123–126. [Google Scholar]
- Mitjana, O.; Bonastre, C.; Tejedor, M.T.; Garza, L.; Latorre, M.Á.; Moreno, B.; Falceto, M.V. Immuno-Castration of Female and Male Pigs with Anti-Gonadotrophin Releasing Hormone Vaccine: Morphometric, Histopathological and Functional Studies of the Reproductive System. Anim. Reprod. Sci. 2020, 221, 106599. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.P.; Amorim, I.; Silva, J.S.; Pires, P.; Cerqueira, J. Outdoor Housing Systems for Bísaro Pig Breed with a Hoop Barn: Some Effects on Welfare. In Food Futures: Ethics, Science and Culture; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016; pp. 87–91. [Google Scholar] [CrossRef]
- Lebret, B.; Guillard, A.S. Outdoor Rearing of Cull Sows: Effects on Carcass, Tissue Composition and Meat Quality. Meat Sci. 2005, 70, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Pérez, M.A.; Del Rosario, A.I.; Rodriguez, P.; García-Gudiño, J.; Duarte, J.L.; Dalmau, J.L.; Hernández-García, F.I. The Effect of Immunocastration on Carcass and Meat Cut Yields in Extensively Reared Iberian Gilts. In Proceedings of the 8th International Symposium on the Mediterranean Pig, Ljubljana, Slovenia, 10–12 October 2013; pp. 151–154. [Google Scholar]
- Gómez-Fernández, J.; Horcajada, S.; Tomás, C.; Gómez-Izquierdo, E.; Mercado, E.D. The Effect of Immunocastration and Surgically Castration on Growth Performance and Carcass Quality in Fattening Period of Iberian Female Pigs. ITEA 2013, 109, 33–48. [Google Scholar]
- Decreto-Lei, n.o1/2019, de 10 de Janeiro|DR. Available online: https://diariodarepublica.pt/dr/detalhe/decreto-lei/1-2019-117663336 (accessed on 19 July 2023).
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, 276, 33–79.
- Quesnel, H.; Prunier, A. Endocrine Bases of Lactational Anoestrus in the Sow. Reprod. Nutr. Dev. 1995, 395–414. [Google Scholar] [CrossRef]
- Clarke, I.J. Two Decades of Measuring GnRH Secretion. Reprod. Suppl. 2002, 59, 1–13. [Google Scholar]
- European Commission. Council Regulation (EC) 1/2005 of 22 December 2004 on the protection of animals during transport and related operations and amending Directives 64/432/EEC and 93/119/EC and Regulation (EC) No 1255/97. Off. J. Eur. Union 2005, L3, 1–44. [Google Scholar]
- European Council. Council Directive 93/119/EC on the Protection of Animals at the Time of Slaughter or Killing. Off. J. Eur. Union 1993, L340, 21–34. [Google Scholar]
- European Commission. Regulation of the European Parliament and of the Council (29 April 2004). Laying down specific hygiene rules for the hygiene of foodstuffs, 853/2004/EC. Off. J. Eur. Union 2004, L139, 55–206. [Google Scholar]
- Pires, M.A.; Payan-Carreira, R. Procedimentos Para Análise Histopatológica Do Aparelho Genital Feminino. In Descrição Anotomopatológica em Medicina Veterinária; Pires, M.A., Payan-Carreira, R., Eds.; CECAV, UTAD: Vila Real, Portugal, 2016; pp. 223–233. ISBN 978-989-704-220-1. [Google Scholar]
- Leonhardt, H.; Gull, B.; Stener-Victorin, E.; Hellström, M. Ovarian Volume and Antral Follicle Count Assessed by MRI and Transvaginal Ultrasonography: A Methodological Study. Acta Radiol. 2014, 55, 248–256. [Google Scholar] [CrossRef]
- Cardoso, L.; Rodrigues, L.; Fontes, D.; Allison, J.; Chiarini-Garcia, H.; Almeida, F. Ovarian Morphometrical Evaluation to Assess Reproductive Activity Suppression in Heavy Weight Finishing Gilts Immunized against Gonadotropin-Releasing Hormone. Res. Vet. Sci. 2021, 136, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Commission Internationale de L’Eclairage, (CIE). Colorimetry, 2nd ed.; Commission Internationale de lÉclairage: Vienna, Austria, 1986. [Google Scholar]
- Hornsey, H.C. The Colour of Cooked Cured Pork. II.—Estimation of the Nitric Oxide-Haem Pigments. J. Sci. Food Agric. 1956, 7, 534–540. [Google Scholar] [CrossRef]
- DeVore, D.P.; Solberg, M. Oxygen Uptake in Postrigor Bovine Muscle. J. Food Sci. 1974, 39, 22–28. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference Methods for the Assessment of Physical Characteristics of Meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Honikel, K.O. How to Measure the Water-Holding Capacity of Meat? Recommendation of Standardized Methods. In Evaluation and Control of Meat Quality in Pigs; Springer: Berlin/Heidelberg, Germany, 1987; pp. 129–142. [Google Scholar] [CrossRef]
- NP 1614/2002; Determination of Moisture Content. Reference Method (ISO 1442:1197). In Portuguese Norm–Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy Innovation: Caparica, Portugal, 2002.
- NP 1612/2002; Determination of Total Nitrogen Content. Reference Method (ISO 937:1978). In Portuguese Norm–Meat and Meat Products. Portuguese Institute of Quality, Ministry of Economy and Innovation: Caparica, Portugal, 2002.
- NP-ISO-1615/2002; Determination of Total Ashes. Reference Method. In Portuguese Norm–Meat and Meat Products. International Organization for Standardization: Geneva, Switzerland, 2002.
- Hansen-Møller, J. Rapid High-Performance Liquid Chromatographic Method for Simultaneous Determination of Androstenone, Skatole and Indole in Back Fat from Pigs. J. Chromatogr. B Biomed. Sci. Appl. 1994, 661, 219–230. [Google Scholar] [CrossRef]
- Pinto, R.P.; Vaz-velho, M.; Mata, F.; Pires, P. A Modified High-Performance Liquid Chromatographic Method for Simultaneous Quantification of Skatole and Androstenone in Pig’s Backfat. In Proceedings of the XVI Food Chemistry Meeting (Portuguese Chemistry Society), Castelo Branco, Portugal, 23–26 October 2022; pp. 111–112. [Google Scholar]
- ISO 8586:2012; Sensory Analysis. General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2012.
- Garrido, M.D.; Egea, M.; Linares, M.B.; Martínez, B.; Viera, C.; Rubio, B.; Borrisser-Pairó, F. A Procedure for Sensory Detection of Androstenone in Meat and Meat Products from Entire Male Pigs: Development of a Panel Training. Meat Sci. 2016, 122, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Sinkinson, C. Triangle Test. In Discrimination Testing in Sensory Science: A Practical Handbook; Woodhead Publishing: Sawston, UK, 2017; pp. 153–170. [Google Scholar] [CrossRef]
- Sødring, M.; Thauland Håseth, T.; Rasten Brunsdon, E.; Bjørnstad, P.H.; Sandnes, R.; Røtterud, O.J.; Mason, A.; de Medeiros Esper, I.; Hallenstvedt, E.; Agerup, P.; et al. Effects of Meat Factory Cell on Pork Qualities, Sensory Characteristics and Carcass Hygiene: An Exploratory Study. Acta Agric. Scand. A Anim. Sci. 2022, 71, 98–113. [Google Scholar] [CrossRef]
- Silva, J.A.; Cardoso, R.; Vieira, R.; Almeida, J.C.; Gomes, M.J.; Venâncio, C.; Patarata, L. The Effect of Weaning and Slaughter Age on the Physicochemical and Sensory Characteristics of Arouquesa Beef—A PDO Portuguese Meat. Foods 2022, 11, 2505. [Google Scholar] [CrossRef]
- Lunde, K.; Egelandsdal, B.; Choinski, J.; Mielnik, M.; Flåtten, A.; Kubberød, E. Marinating as a Technology to Shift Sensory Thresholds in Ready-to-Eat Entire Male Pork Meat. Meat Sci. 2008, 80, 1264–1272. [Google Scholar] [CrossRef]
- Domínguez, R.; Purriños, L.; Pateiro, M.; Campagnol, P.C.B.; Reyes, J.F.; Munekata, P.E.S.; Lorenzo, J.M. Fatty Acids. In Methods to Assess the Quality of Meat Products; Springer: Berlin/Heidelberg, Germany, 2022; pp. 41–52. [Google Scholar] [CrossRef]
- Cittadini, A.; Munekata, P.E.S.; Pateiro, M.; Sarriés, M.V.; Domínguez, R.; Lorenzo, J.M. Physicochemical Composition and Nutritional Properties of Foal Burgers Enhanced with Healthy Oil Emulsion Hydrogels. Int. J. Food Sci. Technol. 2021, 56, 6182–6191. [Google Scholar] [CrossRef]
- Di Martino, G.; Scollo, A.; Garbo, A.; Lega, F.; Stefani, A.L.; Vascellari, M.; Natale, A.; Zuliani, F.; Zanardello, C.; Tonon, F.; et al. Impact of Sexual Maturity on the Welfare of Immunocastrated v. Entire Heavy Female Pigs. Animal 2018, 12, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, A.; Velarde, A.; Rodríguez, P.; Pedernera, C.; Llonch, P.; Fàbrega, E.; Casal, N.; Mainau, E.; Gispert, M.; King, V.; et al. Use of an Anti-GnRF Vaccine to Suppress Estrus in Crossbred Iberian Female Pigs. Theriogenology 2015, 84, 342–347. [Google Scholar] [CrossRef]
- Daza, A.; Latorre, M.A.; Olivares, A.; López Bote, C.J. The Effects of Male and Female Immunocastration on Growth Performances and Carcass and Meat Quality of Pigs Intended for Dry-Cured Ham Production: A Preliminary Study. Livest. Sci. 2016, 190, 20–26. [Google Scholar] [CrossRef]
- Daza, A.; Latorre, M.A.; Olivares, A.; López-Bote, C.J. The Effect of Immunocastration and a Diet Based on Granulated Barley on Growth Performance and Carcass, Meat and Fat Quality in Heavy Gilts. Animal 2014, 8, 484–493. [Google Scholar] [CrossRef]
- Gamero-Negrón, R.; Sánchez del Pulgar, J.; Ventanas, J.; García, C. Immune-Spaying as an Alternative to Surgical Spaying in Iberian×Duroc Females: Effect on Carcass Traits and Meat Quality Characteristics. Meat Sci. 2015, 99, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Van Den Broeke, A.; Leen, F.; Aluwé, M.; Ampe, B.; Van Meensel, J.; Millet, S. The Effect of GnRH Vaccination on Performance, Carcass, and Meat Quality and Hormonal Regulation in Boars, Barrows, and Gilts. J. Anim. Sci. 2016, 2811–2820. [Google Scholar] [CrossRef]
- Pauly, C.; Luginbühl, W.; Ampuero, S.; Bee, G. Expected Effects on Carcass and Pork Quality When Surgical Castration Is Omitted—Results of a Meta-Analysis Study. Meat Sci. 2012, 92, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Trefan, L.; Doeschl-Wilson, A.; Rooke, J.A.; Terlouw, C.; Bünger, L. Meta-Analysis of Effects of Gender in Combination with Carcass Weight and Breed on Pork Quality. J. Anim. Sci. 2013, 91, 1480–1492. [Google Scholar] [CrossRef]
- Martinez-Macipe, M.; Rodríguez, P.; Izquierdo, M.; Gispert, M.; Manteca, X.; Mainau, E.; Hernández, F.I.; Claret, A.; Guerrero, L.; Dalmau, A. Comparison of Meat Quality Parameters in Surgical Castrated versus Vaccinated against Gonadotrophin-Releasing Factor Male and Female Iberian Pigs Reared in Free-Ranging Conditions. Meat Sci. 2016, 111, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Warriss, P.D. Meat Science: An Introductory Text; CAB Publishing: Wallingford, UK, 2000. [Google Scholar] [CrossRef]
- Kyriazakis, I.; Whittemore, C.T. Whittemore’s Science and Practice of Pig Production; Blackwell Publishing: Hoboken, NJ, USA, 2006; ISBN 9781405124485. [Google Scholar]
- Peinado, J.; Medel, P.; Fuentetaja, A.; Mateos, G.G. Influence of Sex and Castration of Females on Growth Performance and Carcass and Meat Quality of Heavy Pigs Destined for the Dry-Cured Industry. J. Anim. Sci. 2008, 86, 1410–1417. [Google Scholar] [CrossRef]
- Peinado, J.; Serrano, M.P.; Medel, P.; Fuentetaja, A.; Mateos, G.G. Productive Performance, Carcass and Meat Quality of Intact and Castrated Gilts Slaughtered at 106 or 122 Kg BW. Animal 2011, 5, 1131–1140. [Google Scholar] [CrossRef]
- Serrano, M.P.; Valencia, D.G.; Fuentetaja, A.; Lázaro, R.; Mateos, G.G. Effect of Gender and Castration of Females and Slaughter Weight on Performance and Carcass and Meat Quality of Iberian Pigs Reared under Intensive Management Systems. Meat Sci. 2008, 80, 1122–1128. [Google Scholar] [CrossRef]
- Santos e Silva, J.; Bernardo, A.; Pires da Costa, J. Genetic Characterization and Inventory of the Bísaro Pig through Visible Effect Genes. Their Utilization in the Genotypic Comparison between Populations and in the Establishing of a Nucleus for in Vivo Genetic Conservation. Tradit. Innov. Mediterr. Pig Prod. 2000, 51, 39–51. [Google Scholar]
- Bonneau, M.; Chevillon, P. Acceptability of Entire Male Pork with Various Levels of Androstenone and Skatole by Consumers According to Their Sensitivity to Androstenone. Meat Sci. 2012, 90, 330–337. [Google Scholar] [CrossRef]
- Thomsen, R.; Edwards, S.A.; Jensen, B.B.; Rousing, T.; Sorensen, J.T. Effect of Faecal Soiling on Skatole and Androstenone Occurrence in Organic Entire Male Pigs. Animal 2015, 9, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Narendran, R.; Etches, R.J.; Hacker, R.R.; Bowman, G.H. 5α-Androstenone Concentrations in Sow Plasma during the Estrous Cycle. Theriogenology 1980, 13, 263–269. [Google Scholar] [CrossRef]
- Van Oeckel, M.J.; Casteels, M.; Warnants, N.; De Boever, J.L.; Van Renterghem, R.; Boucqué, C.V. Production of Entire Males with Belgian Landrace and Hybrid Pigs: The Incidence of Sensory Aberrations*. J. Anim. Physiol. Anim. Nutr. 1996, 76, 111–121. [Google Scholar] [CrossRef]
- Robic, A.; Faraut, T.; Prunier, A. Pathways and Genes Involved in Steroid Hormone Metabolism in Male Pigs: A Review and Update. J. Steroid Biochem. Mol. Biol. 2014, 140, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Jeremiah, L.E.; Squires, E.J.; Canada, G.O.; Sather, A.P.; Jeremiah, L.E.; Sather, A.P. Gender and Diet Influences on Pork Palatability and Consumer Acceptance II. Sex Taint Compounds and Their Relationshiop to Sensory Properties. J. Muscle Foods 1999, 10, 317–331. [Google Scholar] [CrossRef]
- Serrano, M.P.; Valencia, D.G.; Fuentetaja, A.; Lázaro, R.; Mateos, G.G. Effect of Castration on Productive Performance, Carcass Characteristics and Meat Quality of Iberian Pig Females Reared under Intensive Management Systems. Livest. Sci. 2009, 123, 147–153. [Google Scholar] [CrossRef]
- Škrlep, M.; Poklukar, K.; Kress, K.; Vrecl, M.; Fazarinc, G.; Lukač, N.B.; Weiler, U.; Stefanski, V.; Čandek-Potokar, M. Effect of Immunocastration and Housing Conditions on Pig Carcass and Meat Quality Traits. Transl. Anim. Sci. 2020, 4, 1224–1237. [Google Scholar] [CrossRef]
- Pérez-Ciria, L.; Miana-Mena, F.J.; López-Mendoza, M.C.; Álvarez-Rodríguez, J.; Latorre, M.A. Influence of Immunocastration and Diet on Meat and Fat Quality of Heavy Female and Male Pigs. Animals 2021, 11, 3355. [Google Scholar] [CrossRef] [PubMed]
- Pauly, C.; Spring, P.; Odoherty, J.V.; Ampuero Kragten, S.; Bee, G. Growth Performance, Carcass Characteristics and Meat Quality of Group-Penned Surgically Castrated, Immunocastrated (Improvac) and Entire Male Pigs and Individually Penned Entire Male Pigs. Animal 2009, 3, 1057–1066. [Google Scholar] [CrossRef]
- Seiquer, I.; Palma-Granados, P.; Haro, A.; Lara, L.; Lachica, M.; Fernández-Fígares, I.; Nieto, R. Meat Quality Traits in Longissimus Lumborum and Gluteus Medius Muscles from Immunocastrated and Surgically Castrated Iberian Pigs. Meat Sci. 2019, 150, 77–84. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 365–379. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat Deposition, Fatty Acid Composition and Meat Quality: A Review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]

| Groups | Age | Parity |
|---|---|---|
| Median (Q1–Q3) | Median (Q1–Q3) | |
| C (n = 5) | 39 (24–53) | 4 (2–6) |
| IM1 (n = 5) | 27 (23–41) | 3 (3–5) |
| IM2 (n = 5) | 24 (24–47) | 2 (2–5) |
| IM3 (n = 5) | 37 (32–44) | 4 (3–5) |
| p value | 0.538 | 0.582 |
| Parameter | C (n = 5) | IM1 (n = 5) | IM2 (n = 5) | IM3 (n = 5) | SEM | p Value |
|---|---|---|---|---|---|---|
| Total genital tract weight (kg) | 1.403 a | 0.508 b | 0.590 ab | 0.599 ab | 0.10 | 0.024 |
| Segment cervix–vagina (cm) | 40.60 a | 29.20 bc | 37.60 ab | 24.80 c | 1.82 | 0.001 |
| Uterine corpus (cm) | 11.20 | 14.40 | 11.00 | 13.00 | 0.90 | 0.523 |
| Uterine horns | ||||||
| Length (cm) | 120.65 | 75.90 | 80.80 | 107.30 | 7.25 | 0.091 |
| Diameter (cm) | 4.95 a | 2.85 ab | 2.55 b | 2.50 b | 0.26 | 0.005 |
| Ovaries | ||||||
| Length (cm) | 4.22 a | 2.99 b | 2.75 b | 3.25 ab | 0.18 | 0.040 |
| Width (cm) | 2.82 a | 2.27 b | 1.85 b | 2.45 ab | 0.13 | 0.039 |
| Depth (cm) | 2.00 a | 1.30 b | 1.10 b | 1.37 ab | 0.12 | 0.020 |
| Volume (ml) | 13.25 a | 4.72 b | 2.91 b | 6.58 ab | 1.29 | 0.015 |
| Weight (g) | 12.00 a | 3.50 b | 4.20 ab | 5.10 ab | 0.94 | 0.027 |
| GSI | 5.33 a | 1.57 b | 2.09 ab | 2.31 ab | 0.40 | 0.014 |
| Parameter | C (n = 5) | IM1 (n = 5) | IM2 (n = 5) | IM3 (n = 5) | SEM | p Value |
|---|---|---|---|---|---|---|
| Live weight (kg) | 219.36 | 223.18 | 209.96 | 222.40 | 7.47 | 0.933 |
| Carcass weight (kg) | 160.40 | 167.00 | 158.50 | 158.60 | 6.56 | 0.970 |
| Carcass yield (%) | 73.20 | 74.20 | 75.90 | 70.92 | 1.25 | 0.512 |
| pH45min | 6.45 | 6.14 | 6.27 | 6.30 | 0.10 | 0.846 |
| pH24h | 5.50 | 5.67 | 5.58 | 5.62 | 0.03 | 0.183 |
| L* | 48.2 | 47.2 | 45.1 | 48.1 | 0.80 | 0.542 |
| a* | 21.7 | 21.1 | 22.5 | 22.3 | 0.29 | 0.335 |
| b* | 6.8 | 5.8 | 6.5 | 6.6 | 0.34 | 0.791 |
| C* | 22.7 | 21.9 | 23.4 | 23.2 | 0.35 | 0.487 |
| h° | 17.3 | 15.3 | 15.9 | 16.4 | 0.65 | 0.768 |
| Heme (mg/g) | 1.92 | 1.78 | 2.30 | 1.89 | 0.08 | 0.105 |
| Drip loss (%) | 3.25 | 2.33 | 3.22 | 3.40 | 0.23 | 0.206 |
| Cooking loss (%) | 23.31 | 23.73 | 21.06 | 24.09 | 0.61 | 0.299 |
| Shear force (N/cm2) | 56.89 | 64.46 | 81.63 | 69.81 | 4.68 | 0.311 |
| Moisture (%) | 71.09 b | 72.78 a | 71.19b | 73.16 a | 0.27 | 0.001 |
| Protein (%) | 23.18 | 23.08 | 24.29 | 23.21 | 0.24 | 0.206 |
| Intramuscular fat (%) | 2.89 | 2.40 | 2.26 | 2.02 | 0.24 | 0.652 |
| Ashes (%) | 1.13 | 1.11 | 1.08 | 1.10 | 0.01 | 0.655 |
| Androstenone (ng/g) | 8.83 | 4.36 | 12.02 | 4.29 | 1.77 | 0.441 |
| Skatole (ng/g) | 4.07 | 7.25 | 3.91 | 3.51 | 0.56 | 0.109 |
| Fatty Acid (g/100 g Fat) | C (n = 5) | IM1 (n = 5) | IM2 (n = 5) | IM3 (n = 5) | SEM | p Value |
|---|---|---|---|---|---|---|
| Total saturated fatty acid (SFA) | 30.24 | 32.92 | 29.86 | 32.46 | 0.58 | 0.206 |
| Myristic acid (C14:0) | 1.04 | 1.12 | 1.00 | 1.10 | 0.03 | 0.648 |
| Pentadecanoic acid (C15:0) | 0.02 | 0.02 | 0.02 | 0.02 | 0.00 | 0.299 |
| Palmitic acid (C16:0) | 20.28 | 21.65 | 19.87 | 21.14 | 0.36 | 0.440 |
| Margaric acid (C17:0) | 0.11 | 0.11 | 0.12 | 0.13 | 0.00 | 0.309 |
| Stearic acid (C18:0) | 8.44 | 9.60 | 8.47 | 9.67 | 0.21 | 0.059 |
| Arachidic acid (C20:0) | 0.20 | 0.24 | 0.23 | 0.24 | 0.01 | 0.309 |
| Total monounsaturated fatty acid (MUFA) | 49.65 | 51.52 | 49.03 | 48.18 | 0.80 | 0.521 |
| Palmitoleic acid (C16:1 n-7) | 3.62 | 3.64 | 3.42 | 3.46 | 0.12 | 0.851 |
| Oleic acid (C18:1 n-9) | 38.61 | 40.48 | 38.09 | 37.51 | 0.72 | 0.444 |
| Gondoic acid (C20:1 n-9) | 0.84 | 0.92 | 0.87 | 0.88 | 0.03 | 0.550 |
| Total polyunsaturated fatty acid (PUFA) | 10.85 | 10.99 | 11.76 | 11.73 | 0.55 | 0.690 |
| Linoleic acid (C18:2 n-6) | 7.18 | 7.71 | 7.88 | 8.38 | 0.31 | 0.727 |
| Alpha-linolenic acid (C18:3 n-3) | 0.18 | 0.22 | 0.20 | 0.22 | 0.01 | 0.205 |
| Dihomoγ-linolenic acid (C20:3 n-6) | 0.19 | 0.21 | 0.23 | 0.21 | 0.01 | 0.374 |
| Arachidonic acid (C20:4 n-6) | 2.52 | 2.15 | 2.63 | 2.25 | 0.21 | 0.771 |
| Eicosatrienoic acid (C20:3 n-3) | 0.05 | 0.05 | 0.05 | 0.05 | 0.00 | 0.769 |
| Eicosapentaenoic acid (C20:5 n-3) | 0.05 | 0.05 | 0.06 | 0.04 | 0.00 | 0.452 |
| Docosapentaenoic acid (C22:5 n-3) | 0.31 | 0.23 | 0.30 | 0.22 | 0.03 | 0.690 |
| Docosaheptaenoic acid (C22:6 n-3) | 0.07 ab | 0.04 ab | 0.09 a | 0.02 b | 0.01 | 0.015 |
| PUFA/SFA | 0.37 | 0.34 | 0.39 | 0.37 | 0.02 | 0.612 |
| Total n-3 | 0.65 | 0.59 | 0.70 | 0.55 | 0.04 | 0.666 |
| Total n-6 | 10.20 | 10.40 | 11.06 | 11.18 | 0.51 | 0.684 |
| n-6/n-3 | 15.74 b | 17.78 ab | 16.04 b | 20.42 a | 0.51 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botelho-Fontela, S.; Paixão, G.; Pereira-Pinto, R.; Vaz-Velho, M.; Pires, M.d.A.; Payan-Carreira, R.; Patarata, L.; Lorenzo, J.; Silva, A.; Esteves, A. Effect of Immunocastration on Culled Sows—A Preliminary Study on Reproductive Tract, Carcass Traits, and Meat Quality. Vet. Sci. 2023, 10, 600. https://doi.org/10.3390/vetsci10100600
Botelho-Fontela S, Paixão G, Pereira-Pinto R, Vaz-Velho M, Pires MdA, Payan-Carreira R, Patarata L, Lorenzo J, Silva A, Esteves A. Effect of Immunocastration on Culled Sows—A Preliminary Study on Reproductive Tract, Carcass Traits, and Meat Quality. Veterinary Sciences. 2023; 10(10):600. https://doi.org/10.3390/vetsci10100600
Chicago/Turabian StyleBotelho-Fontela, Sofia, Gustavo Paixão, Ricardo Pereira-Pinto, Manuela Vaz-Velho, Maria dos Anjos Pires, Rita Payan-Carreira, Luís Patarata, José Lorenzo, António Silva, and Alexandra Esteves. 2023. "Effect of Immunocastration on Culled Sows—A Preliminary Study on Reproductive Tract, Carcass Traits, and Meat Quality" Veterinary Sciences 10, no. 10: 600. https://doi.org/10.3390/vetsci10100600
APA StyleBotelho-Fontela, S., Paixão, G., Pereira-Pinto, R., Vaz-Velho, M., Pires, M. d. A., Payan-Carreira, R., Patarata, L., Lorenzo, J., Silva, A., & Esteves, A. (2023). Effect of Immunocastration on Culled Sows—A Preliminary Study on Reproductive Tract, Carcass Traits, and Meat Quality. Veterinary Sciences, 10(10), 600. https://doi.org/10.3390/vetsci10100600










