Abstract
Background: Approximately 15% to 20% of patients will experience disease recurrence following surgical removal of renal cell carcinoma. A range of pharmacological agents is prescribed for metastatic renal cell carcinoma, but there are trials testing whether these have an earlier role in the adjuvant setting. We aim to assess the efficacy of adjuvant systemic treatment following surgery in patients with renal cell carcinoma and to determine the most effective treatment. Methods: The protocol for this review was published in PROSPERO (CRD42021281588). We searched multiple databases up to August 2021. We included only randomized trials of patients with renal cell carcinoma that had been completely resected. We included patients with locoregional nodal disease if it was surgically removed, and excluded all cases of metastatic disease. We included all adjuvant systemic therapies that were commenced within 90 days of renal surgery. A network meta-analysis was performed using a frequentist approach. Results: A total of 13 studies with 8103 patients were included for analysis. Only pembrolizumab (HR 0.74; 95%CI 0.57 to 0.96) and pazopanib (HR 0.80; 95%CI 0.68 to 0.95) improved disease-free survival compared with observation. These 2 treatments were the 2 highest ranked comparisons with a P-score of 0.87 and 0.80. No agent improved overall survival. All agents increased the risk of severe adverse events compared with observation. Conclusions: Pembrolizumab and pazopanib were the only 2 adjuvant agents that improved time to disease recurrence compared with observation, with the former likely being the more efficacious. None of the treatments improved overall survival and almost all increased severe adverse events.
Introduction
There has been an increased incidence of renal cell carcinoma, especially in developed countries[1]. Most of these cancers are localized to the kidney at the time of presentation and are curable by surgery. However, approximately 20% of patients will experience disease recurrence following surgery[2]. Overall prognosis for advanced disease is poor with a median survival time of 21 months after recurrence[3].
A range of pharmacological agents has been used to treat metastatic renal cell carcinoma (mRCC) with varying efficacy, including chemotherapy, immunotherapy, tyrosine kinase inhibitors, monoclonal antibody against circulating vascular endothelial growth factor, and mTOR inhibitors. A network meta-analysis found that combination immunotherapy likely represents the current best available treatment[4]. The European Association of Urology guidelines support this by suggesting that immunotherapy (including combinations) should be used as first-line treatment in this setting[5]. As immunotherapy has come to the forefront of mRCC management, there has been increasing interest in employing these treatments at earlier stages of disease. Many of the aforementioned treatments have been trialled in the adjuvant setting with varying results, and there are recent reports of use of adjuvant immunotherapy. However, these trials have primarily been conducted using observation or placebo as a comparator arm, which has not permitted direct comparisons of active agents.
We therefore aimed to perform a systematic review and network-meta-analysis of systemic agents used in the adjuvant setting after surgery for kidney cancer.
Methods
We registered the protocol of this systematic review in PROSPERO (CRD42021281588). We searched multiple databases (MEDLINE, EMBASE, ScienceDirect, Cochrane Libraries, HTA database, and Web of Science) up to 20 August 2021, with a range of keywords associated with “renal carcinoma” and “adjuvant therapy.” We also searched the abstracts from leading urological and oncological meetings, including those of the European Association of Urology, American Urological Association, American Society of Clinical Oncology, and European Society of Medical Oncology in the last 5 years. We also searched trial registries such as ClinicalTrials.gov. We did not place any restriction on language or date of publication. We included only randomized studies.
Our population of interest was patients with RCC that had been completely resected. Surgical treatment included both radical and partial nephrectomy. We included patients with locoregional nodal disease if they underwent surgical removal at the time of kidney extirpation, ie, N+ cases were eligible. We included all histological subtypes of renal carcinoma. We excluded all patients with distant metastatic disease even if they had undergone metastectomy, ie, M1 cases were not eligible for inclusion.
We included all adjuvant systematic therapies that were commenced within 90 days of renal surgery. We excluded autologous vaccine-based treatments because they are not widely available in clinical practice. We did not include adjuvant radiotherapy. Control arms eligible for analysis were observation, placebo, and active treatments, although we did not find any studies with the last.
Following our search, titles and abstracts were screened by 2 independent authors according to the inclusion/exclusion criteria. Full texts of relevant abstracts were then reviewed by 2 independent authors to confirm eligibility. Any disagreements were resolved by a third senior author. Data were then extracted independently.
The efficacy outcomes of interest were disease-free survival (DFS), defined as time from randomization to disease recurrence (local or distant) and/or death; and overall survival (OS), defined as time from randomization to death from any cause.
The safety outcome of interest was severe adverse events defined as incidence of grade III to V events per patient.
We also intended to perform subgroup analysis on the efficacy outcome according to histological subtype (clear-cell versus other subtypes) and nodal disease (no nodal disease [N0] versus nodal disease [N1]).
Statistical Analysis
We first performed traditional pairwise meta-analysis of the included studies (data not shown). To do this, we applied the model proposed by Woods et al. by extracting hazard rates for DFS and OS and number of severe adverse events from each of the included studies[6].
We then performed a network meta-analysis of all included trials which enables indirect comparisons of treatments based on a common comparator arm. We adopted a frequentist approach and performed a fixed-effect consistency network meta-analysis. As a sensitivity analysis, we used the same approach with a random-effects model. We used P-scores that estimate the extent that one treatment is superior to another, averaged over all competing treatments, to determine which agent is the most efficacious.
All analyses were performed using RJAGS and R (R Foundation for Statistical Computing, Vienna, Austria) version 3.4. Risk of bias was performed according to the Cochrane framework[7].
Results
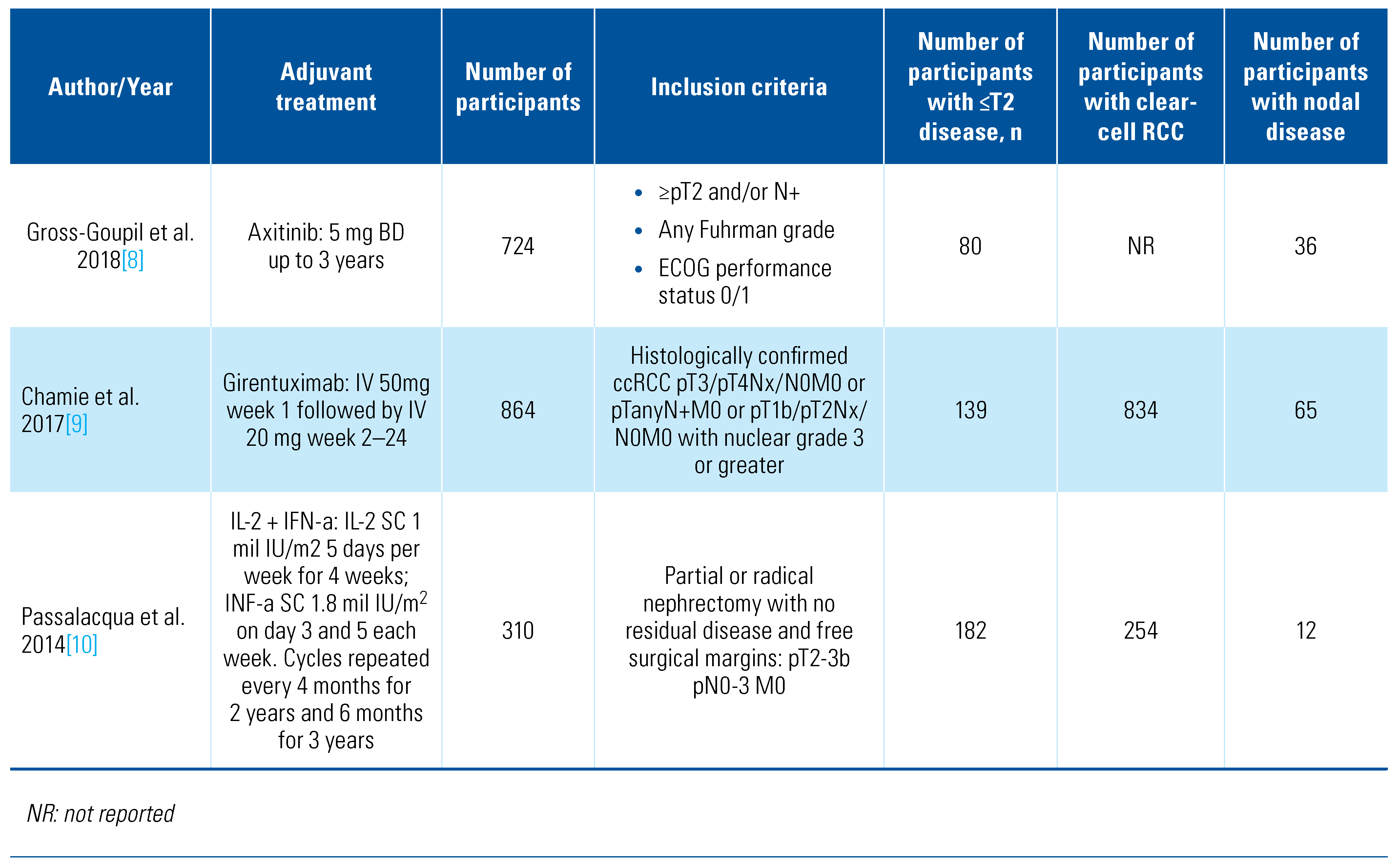
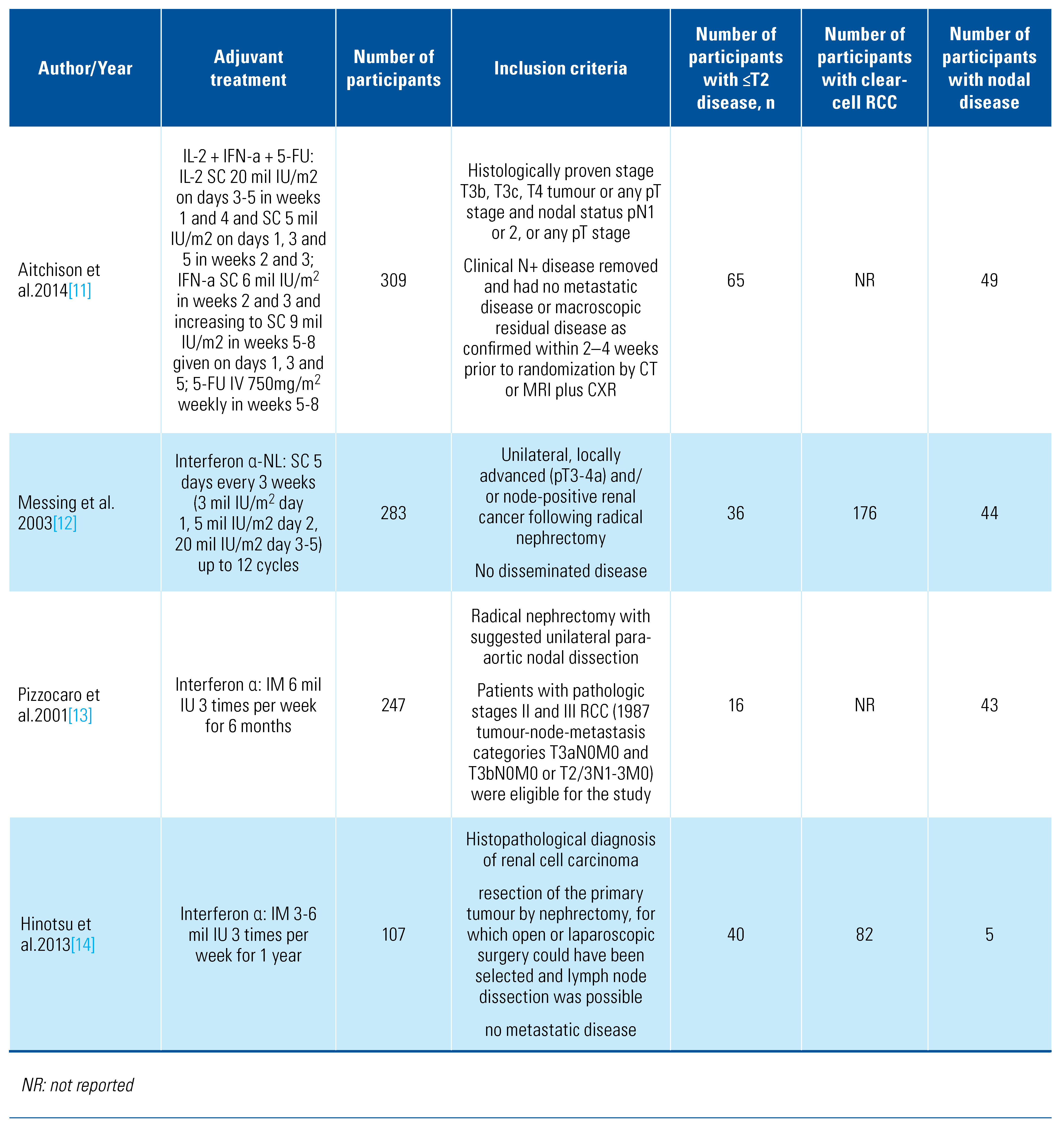
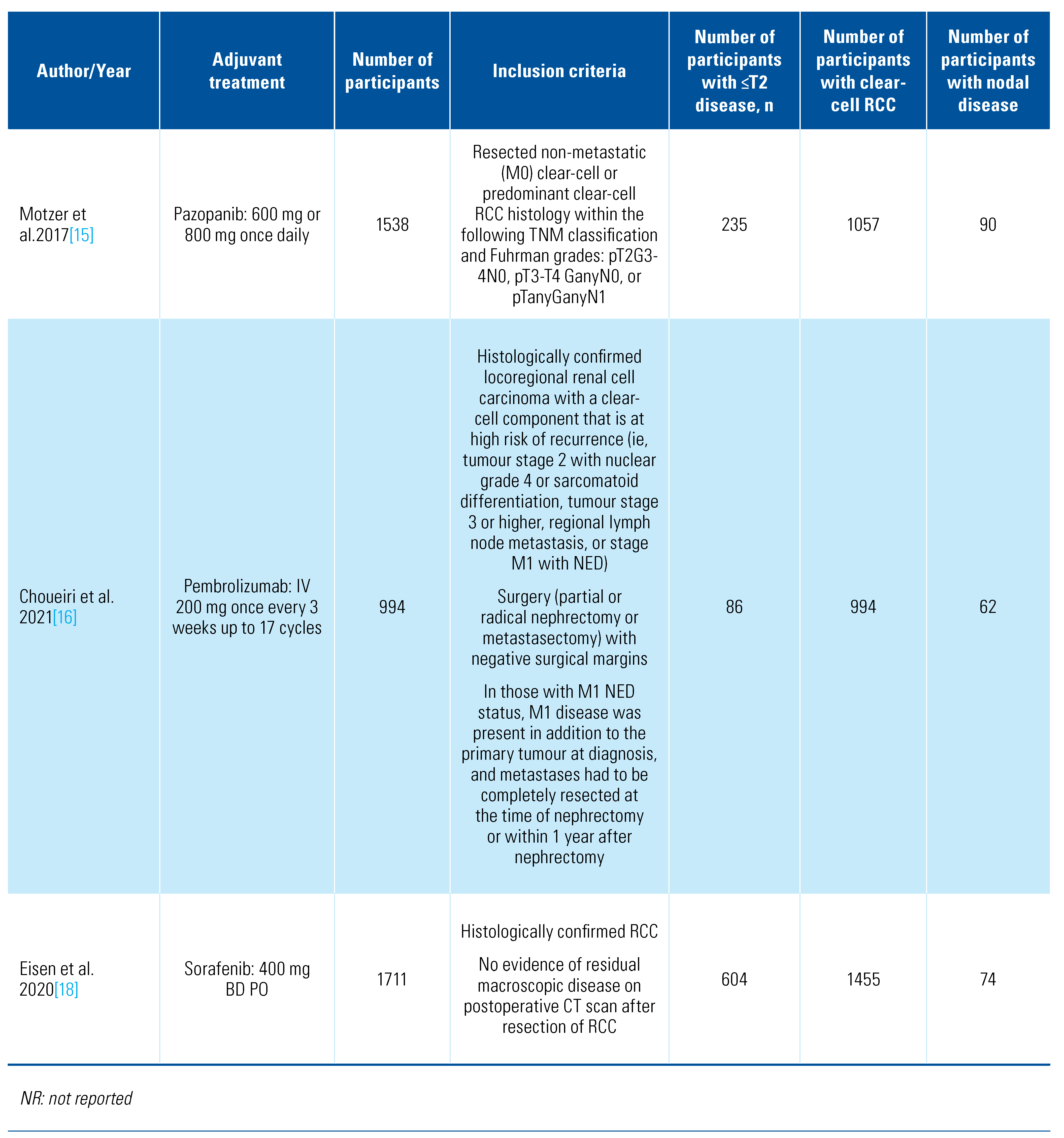
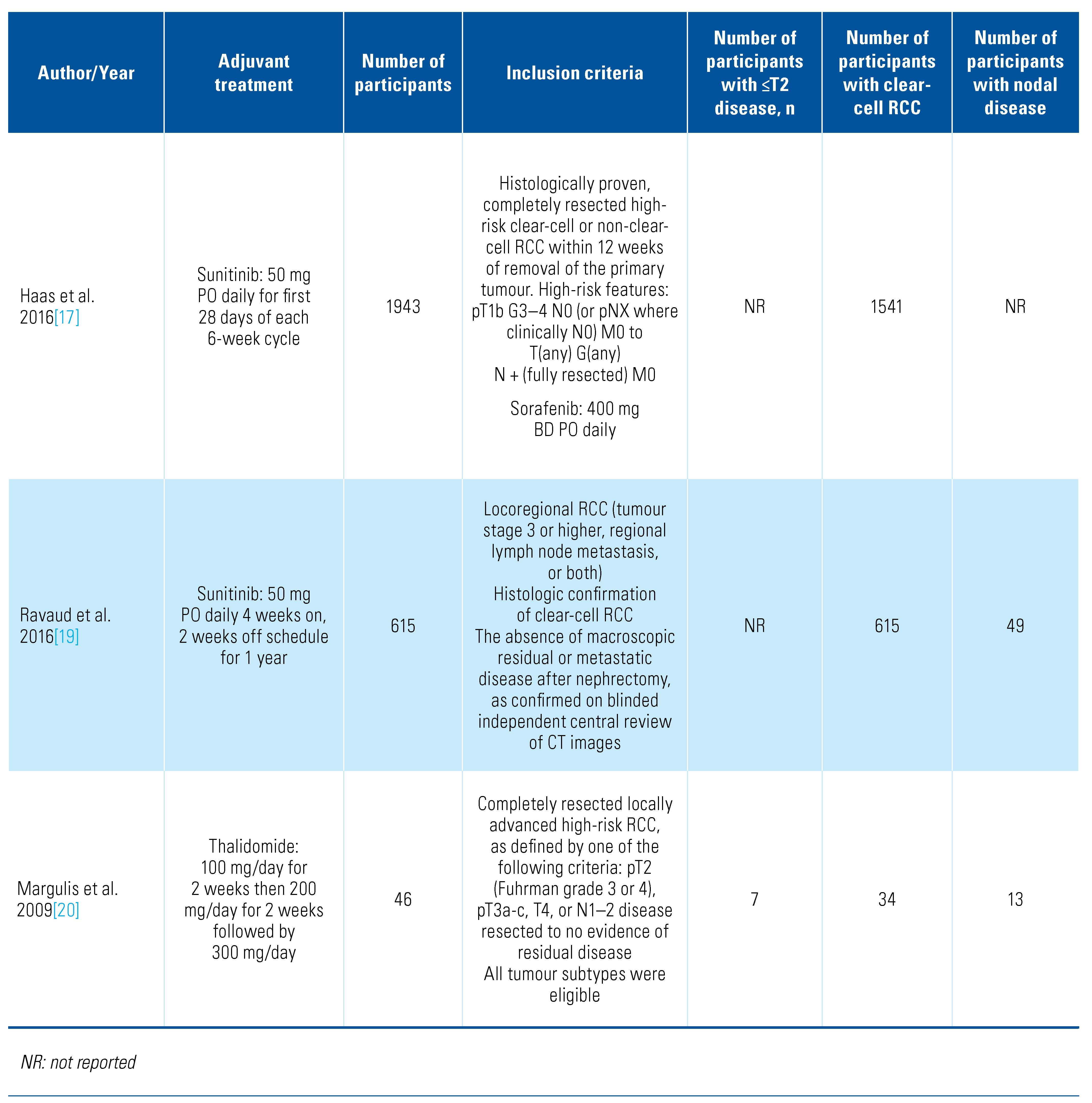
Our search retrieved 4088 abstracts of which 41 proceeded to full text review. After inclusion/exclusion criteria were applied, 13 studies were eligible and included for analysis (Online Supplementary Figure S1). The details of included studies are shown in Table 1.

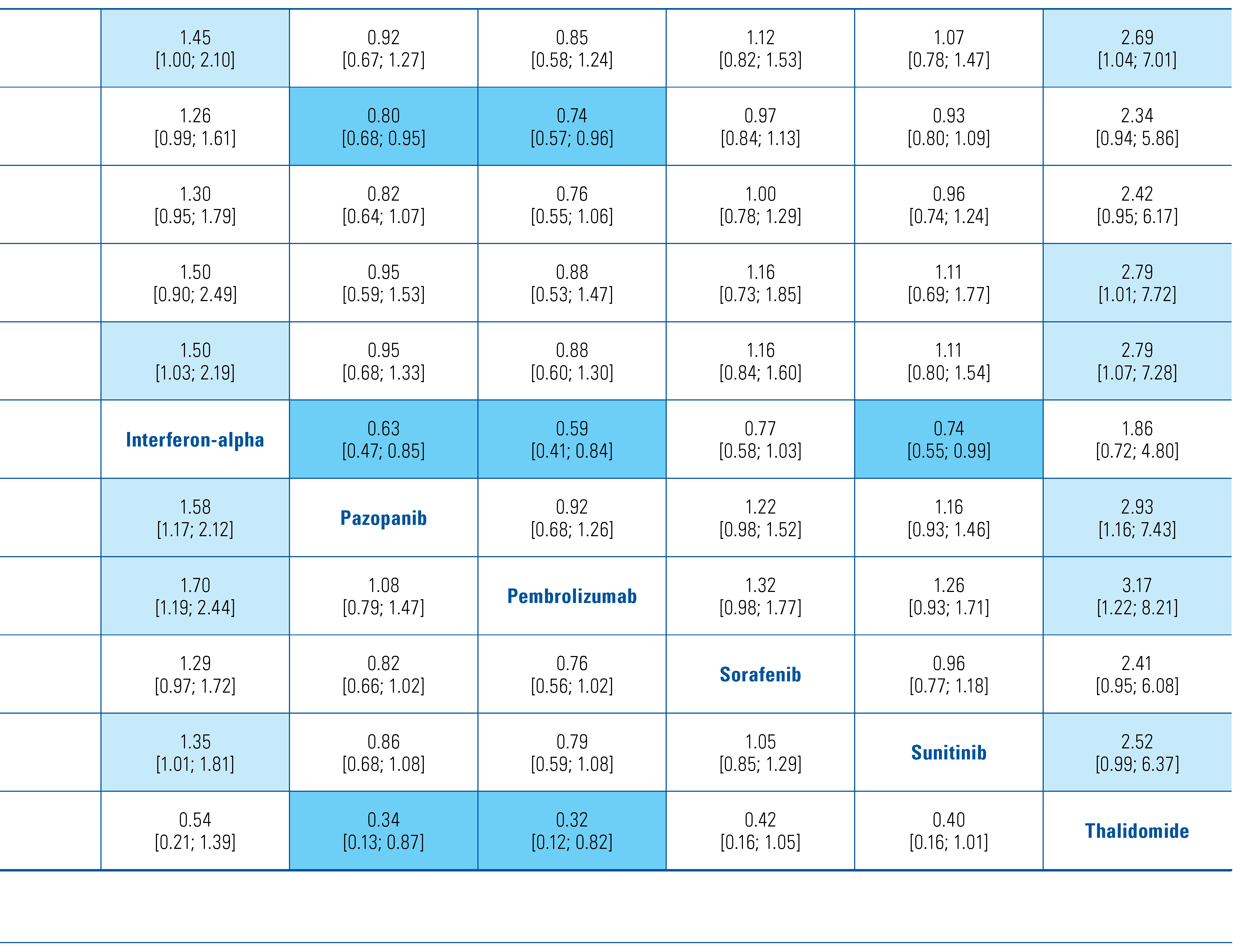
Table 1.
Characteristics of included studies.
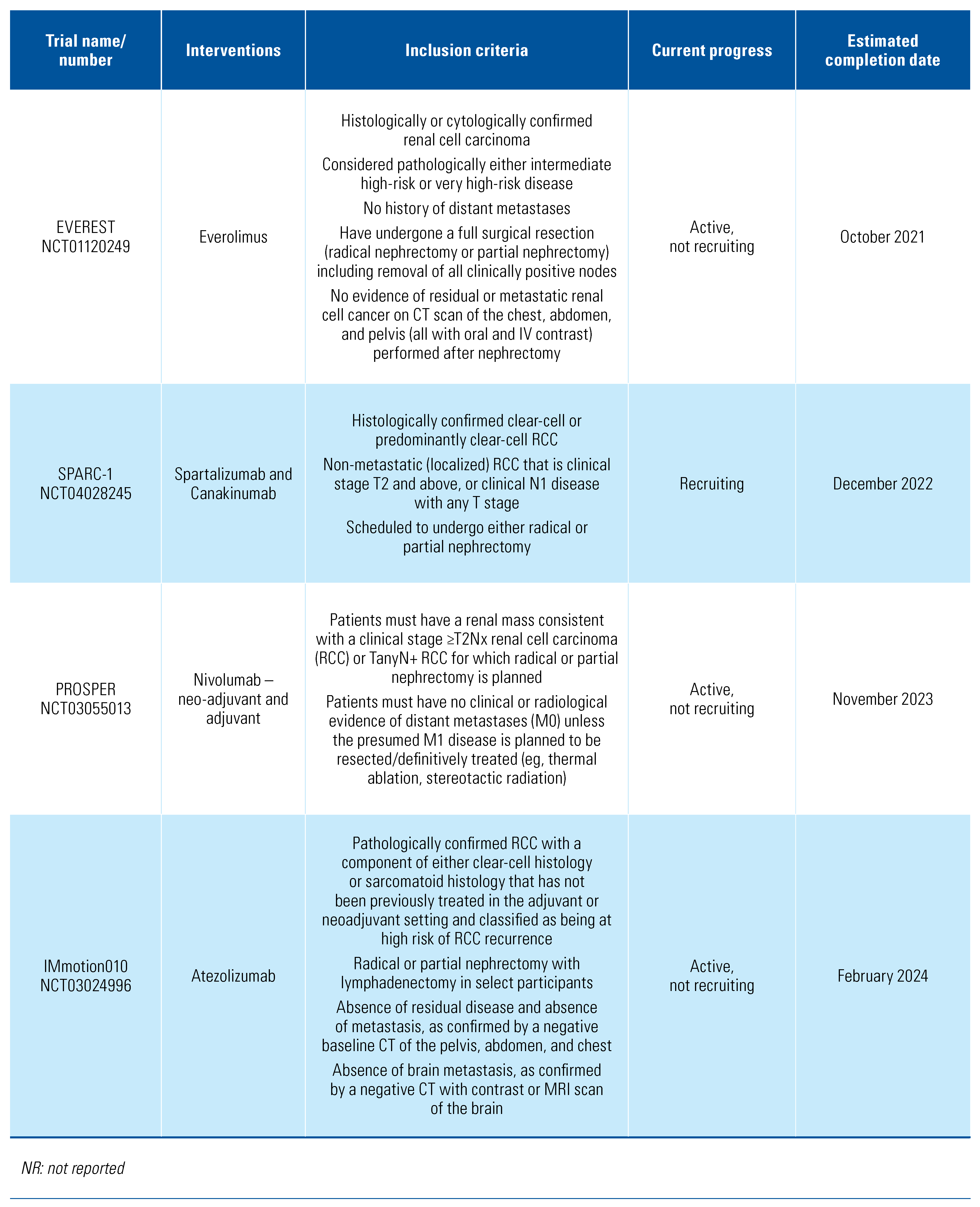
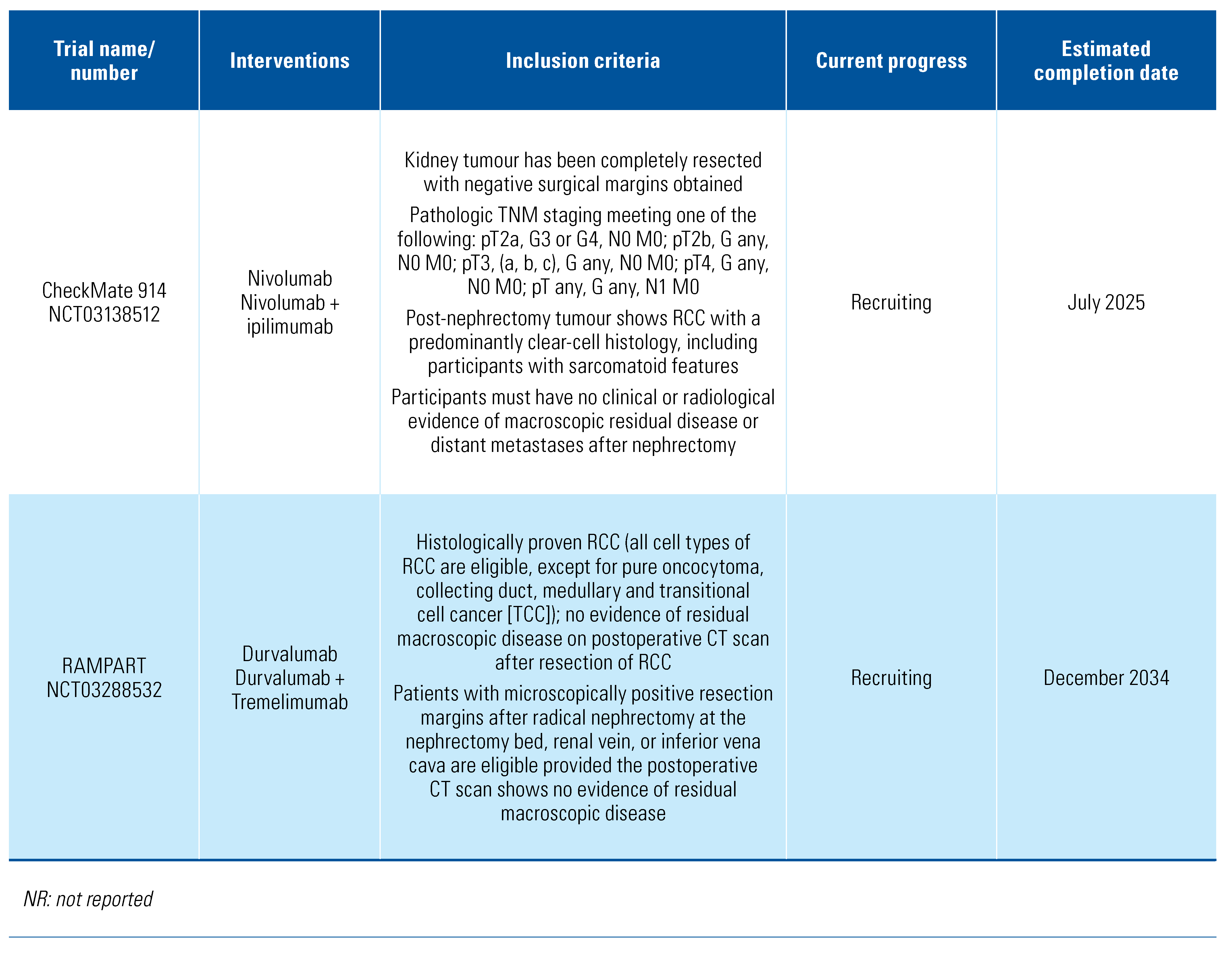
The included trials tested a range of adjuvant treatments: axitinib[8], girentuximab[9], interferon-alpha[10,11,12,13,14], interleukin-2 [10,11], pazopanib[15], pembrolizumab[16], sorafenib[17,18], sunitinib[17,19], and thalidomide[20]. Two of the trials tested combination adjuvant therapies of interleukin-2+interferon-alpha[10] and interleukin-2+interferon-alpha+5-flurouracil[11]. The PROTECT trial that compared pazopanib with placebo included patients who received either 600 mg or 800 mg, and we included both in this analysis[15]. The trials were overall of moderate quality, and the detailed risk of bias classification can be found in Online Supplementary Table S1. We also found a further 6 trials in progress (Table 2).

Table 2.
Trials in progress.
Disease-Free Survival
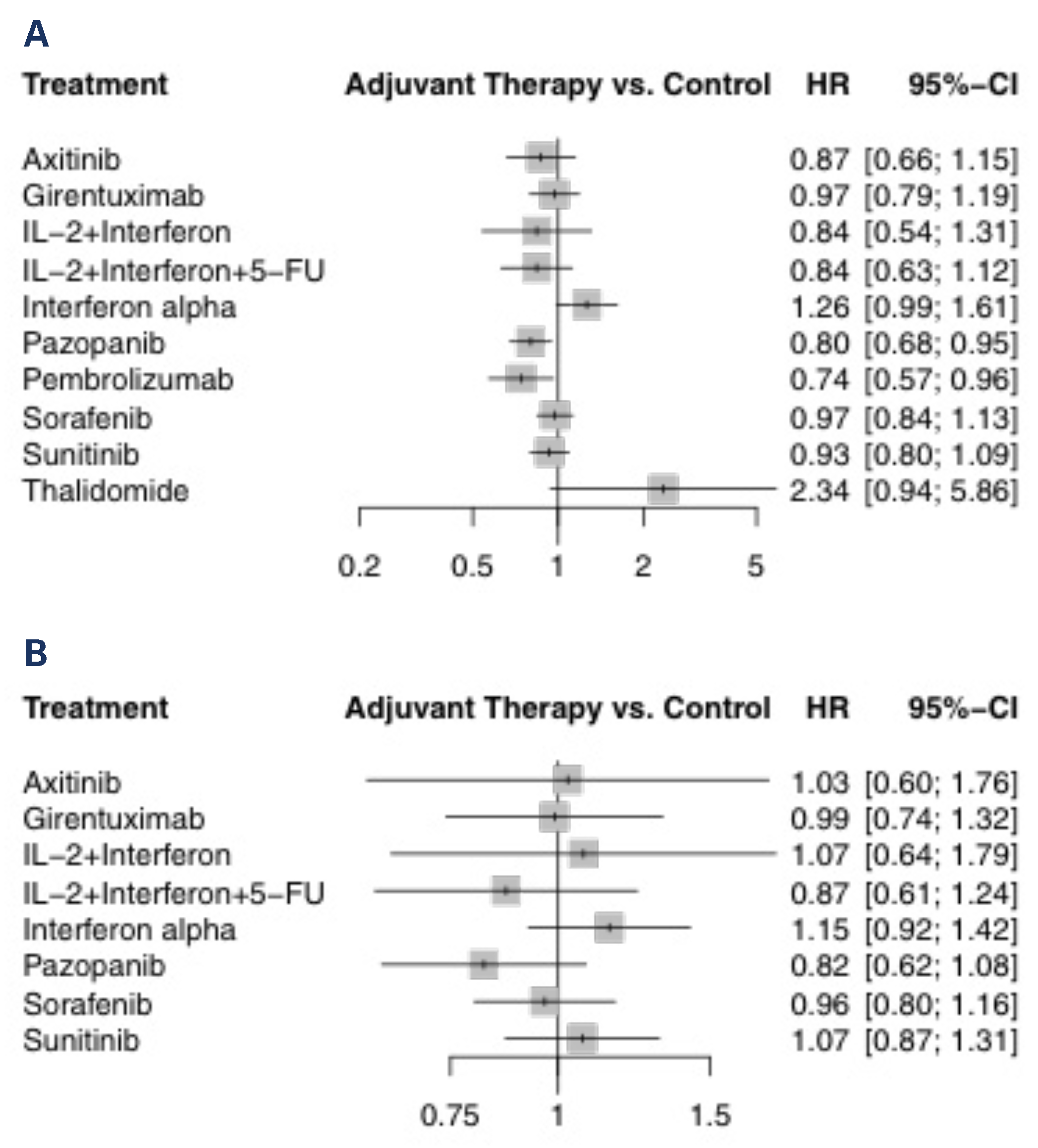
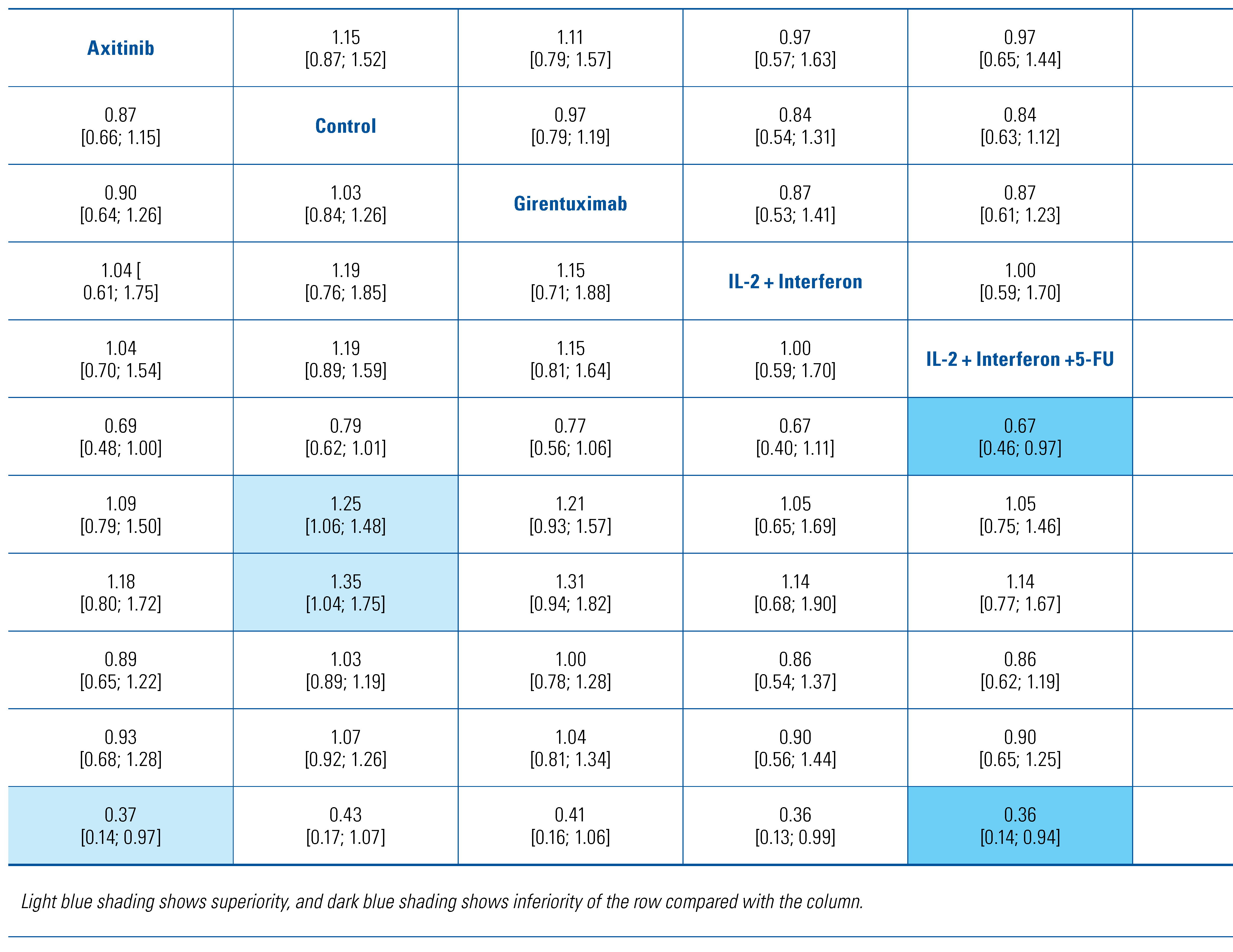
All eligible studies reported on DFS and were included in this analysis of 8103 patients. Only the 2016 study by Haas et al. reported on direct comparison between active agents[17]. The forest plot of HRs compared with control arm for each agent is shown in Figure 1A. Only pembrolizumab (HR 0.74; 95% CI 0.57 to 0.96) and pazopanib (HR 0.80; 95% CI 0.68 to 0.95) prolonged DFS compared with observation. These 2 treatments were the 2 highest ranked comparisons with a P-score of 0.87 and 0.80, respectively. Thalidomide was the lowest ranked treatment with a P-score of 0.03. Interferon-alpha was inferior to axitinib, pazopanib, pembrolizumab and sunitinib. Comparisons of all treatments are shown in Table 3. These findings were the same in the sensitivity analysis when using a random-effects model (data not shown).
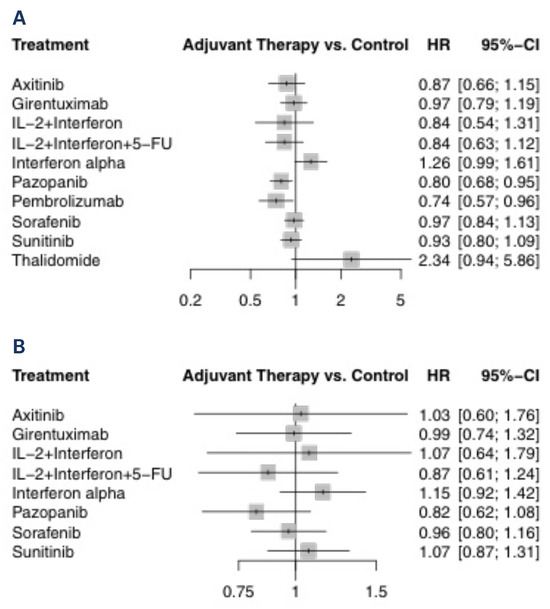
Figure 1.
Treatment versus control for (A) DFS and (B) OS.

Table 3.
Matrix comparing hazard ratios [confidence intervals] for DFS between all therapies.
Overall Survival
The OS analysis included 7063 patients from all the studies from above except Margulis et al. (thalidomide, 2009)[20] and Choueiri et al. (pembrolizumab, 2021) [16]. The forest plot of HRs compared with the control arm for each agent is shown in Figure 1B. None of the agents demonstrated a survival benefit compared with observation. Pazopanib was the highest ranked treatment with a P-score of 0.83. Comparisons of all treatments are shown in Online Supplementary Table S2. There was no difference between any of the treatment comparisons. These findings were the same in the sensitivity analysis when using a random-effects model (data not shown).
Severe Adverse Events
Data from 8 trials using the following interventions were included in the safety analysis: axitinib, girentuximab, interferon-alpha, pazopanib, pembrolizumab, sorafenib, sunitinib, and thalidomide[8,9,12,15,16,17,19,20]. The forest plots of ORs compared with control are shown in Online Supplementary Figure S2. All of the active treatments except girentuximab significantly increased the likelihood of severe adverse events compared with observation. These findings were the same in the sensitivity analysis when using a random-effects model.
Subgroup Analyses
There were insufficient data to perform a network meta- analysis on the planned subgroups.
Discussion
Our network meta-analysis report found that pembrolizumab is likely the most efficacious adjuvant agent in prolonging time to disease recurrence compared with other tyrosine kinase inhibitors, monoclonal antibody against circulating vascular endothelial growth factor, and/or chemotherapies. The only other therapy that was shown to improve DFS compared with observation was pazopanib. However, the absolute difference in recurrence-free survival at 3 years was only 3% between pazopanib and placebo[15]. We used data from patients who received both 600 mg and 800 mg where there were discrepancies in the results related to dose. Although patients receiving the lower dose did not experience improved DFS, those receiving the higher dose were noted to have a prolonged disease- free survival. Therefore, it is likely that the benefit of pazopanib 800 mg is greater than the overall estimates in this review. It should also be noted that the sunitinib did show an improvement in DFS in the S-TRAC trial, although the estimates from this meta-analysis were not significant when including the ECOG trial. The results from this network meta-analysis are consistent with those of previously published meta-analyses on the topic[21,22]. Despite these positive findings in delaying disease recurrence, none of the treatments improved overall survival. We acknowledge that the overall survival data have not matured for most of the recent studies and that there may still be a benefit with adjuvant therapy. Importantly, there was an increased risk of severe adverse events with adjuvant treatment compared with observation.
It should be considered that there are several factors that impact the use of adjuvant treatment and choice of agent. This review represented a population of patients with locoregional renal cancer who had undergone surgery, but there are sub-populations within this group in whom treatment effect may differ. For example, we believed there may have been differences based on histological subtype and nodal status but were unable to perform the preplanned subgroup analyses due to a lack of data. The POLAR-01 trial reported that patients with N0 disease had better outcomes with combination IL-2 and IFN-alpha treatment than did those with N+ disease[10]. In contrast, the ATLAS trial demonstrated that patients with highest risk (pT3 with grade ≥ 3 or pT4 and/or N+, any T, any grade) benefitted with axitinib treatment compared with those with low risk (pT2 or pT3 with grade ≤ 2) who had no difference in outcomes with axitinib[8]. The wider literature, especially in the metastatic setting, highlights the increasing use of molecular biomarkers to tailor treatment choices[23]. This will increase in importance as immunotherapies are used more in this setting. Therefore, patient selection is key in determining the benefit of adjuvant therapy and the choice of agent.
The findings of this meta-analysis should be contextualised within its limitations. As mentioned above, there is heterogeneity within the populations of the included studies, and we were unable to perform the pre-planned subgroup analyses. There were also individual study limitations, especially with respect to blinding, that may have introduced bias into the estimates. Additionally, we did not assess patient-reported outcomes, which is critical in determining whether adjuvant treatment improves quality in life[24]. Future studies will need to assess the cost-effectiveness of these treatments because immunotherapies are expensive and thus may not be cost-effective[25]. Health economics studies of advanced RCC have reported that a significant decrease in the cost of immunotherapy is required for it to be cost-effective at generally accepted thresholds[26]. It is likely that these would be generalizable to the use of immunotherapy in the adjuvant setting as the absolute benefits of treatment are small, albeit statistically significant, and come at significant cost. Furthermore, this study will need to be updated following the publication of trials in progress.
Conclusions
Pembrolizumab and pazopanib were the only 2 adjuvant agents that improved time to disease recurrence compared with observation, with the former likely being the more efficacious. None of the treatments improved overall survival, and almost all increased severe adverse events. While it is promising to see these agents show efficacy in this setting, the duration and cost of treatment also need to be considered when determining utility.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/2563-6499/3/5/341/s1.
Competing Interests
None declared.
References
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; et al. Epidemiology of renal cell carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef]
- Cindolo, L.; Patard, J.J.; Chiodini, P.; Schips, L.; Ficarra, V.; Tostain, J.; et al. Comparison of predictive accuracy of four prognostic models for nonmetastatic renal cell carcinoma after nephrectomy: A multicenter European study. Cancer 2005, 104, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Eggener, S.E.; Yossepowitch, O.; Pettus, J.A.; Snyder, M.E.; Motzer, R.J.; Russo, P. Renal cell carcinoma recurrence after nephrectomy for localized disease: Predicting survival from time of recurrence. J. Clin. Oncol. 2006, 24, 3101–3106. [Google Scholar] [CrossRef]
- Riaz, I.B.; He, H.; Ryu, A.J.; Siddiqi, R.; Naqvi, S.A.A.; Yao, Y.; et al. A living, interactive systematic review and network meta-analysis of first-line treatment of metastatic renal cell carcinoma. Eur. Urol. 2021. [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bensalah, K.; Dabestani, S.; Fernández-Pello, S.; et al. European Association of Urology Guidelines on renal cell carcinoma: The 2019 update. Eur. Urol. 2019, 75, 799–810. [Google Scholar] [CrossRef]
- Woods, B.S.; Hawkins, N.; Scott, D.A. Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: A tutorial. BMC Med. Res. Methodol. 2010, 10, 54. [Google Scholar] [CrossRef]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing risk of bias in a randomized trial. Cochrane Handb. Syst. Rev. Interv. 2019, 205–228. [Google Scholar]
- Gross-Goupil, M.; Kwon, T.G.; Eto, M.; Ye, D.; Miyake, H.; Seo, S.I.; et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: Results from the phase III, randomized ATLAS trial. Ann. Oncol. 2018, 29, 2371–2378. [Google Scholar] [CrossRef]
- Chamie, K.; Donin, N.M.; Klöpfer, P.; Bevan, P.; Fall, B.; Wilhelm, O.; et al. Adjuvant weekly girentuximab following nephrectomy for high-risk renal cell carcinoma: The ARISER Randomized Clinical Trial. JAMA Oncol. 2017, 3, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Passalacqua, R.; Caminiti, C.; Buti, S.; Porta, C.; Camisa, R.; Braglia, L.; et al. Adjuvant low-dose interleukin-2 (IL-2) plus interferon-α (IFN-α) in operable renal cell carcinoma (RCC): A phase III, randomized, multicentre trial of the Italian Oncology Group for Clinical Research (GOIRC). J. Immunother. 2014, 37, 440–447. [Google Scholar] [CrossRef]
- Aitchison, M.; Bray, C.A.; Van Poppel, H.; Sylvester, R.; Graham, J.; Innes, C.; et al. Adjuvant 5-flurouracil, alpha-interferon and interleukin-2 versus observation in patients at high risk of recurrence after nephrectomy for renal cell carcinoma: Results of a phase III randomised European Organisation for Research and Treatment of Cancer (Genito-Urinary Cancers Group)/National Cancer Research Institute trial. Eur. J. Cancer 2014, 50, 70–77. [Google Scholar] [PubMed]
- Messing, E.M.; Manola, J.; Wilding, G.; Propert, K.; Fleischmann, J.; Crawford, E.D.; et al. Phase III study of interferon alfa-NL as adjuvant treatment for resectable renal cell carcinoma: An Eastern Cooperative Oncology Group/Intergroup trial. J. Clin. Oncol. 2003, 21, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Pizzocaro, G.; Piva, L.; Colavita, M.; Ferri, S.; Artusi, R.; Boracchi, P.; et al. Interferon adjuvant to radical nephrectomy in Robson stages II and III renal cell carcinoma: A multicentric randomized study. J. Clin. Oncol. 2001, 19, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Hinotsu, S.; Kawai, K.; Ozono, S.; Tsushima, T.; Tokuda, N.; Nomata, K.; et al. Randomized controlled study of natural interferon α as adjuvant treatment for stage II or III renal cell carcinoma. Int. J. Clin. Oncol. 2013, 18, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Haas, N.B.; Donskov, F.; Gross-Goupil, M.; Varlamov, S.; Kopyltsov, E.; et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J. Clin. Oncol. 2017, 35, 3916–3923. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Chang, Y.-H.; et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N. Engl. J. Med. 2021, 385, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Haas, N.B.; Manola, J.; Uzzo, R.G.; Flaherty, K.T.; Wood, C.G.; Kane, C.; et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016, 387, 2008–2016. [Google Scholar] [CrossRef]
- Eisen, T.; Frangou, E.; Oza, B.; Ritchie, A.W.S.; Smith, B.; Kaplan, R.; et al. Adjuvant sorafenib for renal cell carcinoma at intermediate or high risk of relapse: Results from the SORCE randomized phase III intergroup trial. J. Clin. Oncol. 2020, 38, 4064–4075. [Google Scholar] [CrossRef]
- Ravaud, A.; Motzer, R.J.; Pandha, H.S.; George, D.J.; Pantuck, A.J.; Patel, A.; et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N. Engl. J. Med. 2016, 375, 2246–2254. [Google Scholar] [CrossRef]
- Margulis, V.; Matin, S.F.; Tannir, N.; Tamboli, P.; Shen, Y.; Lozano, M.; et al. Randomized trial of adjuvant thalidomide versus observation in patients with completely resected high-risk renal cell carcinoma. Urology 2009, 73, 337–341. [Google Scholar] [CrossRef][Green Version]
- Bai, Y.; Li, S.; Jia, Z.; Ding, Y.; Gu, C.; Yang, J. Adjuvant therapy for locally advanced renal cell carcinoma: A meta-analysis and systematic review. Urol. Oncol. 2018, 36, 79.e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Di Nunno, V.; Mollica, V.; Graham, J.; Gatto, L.; Heng, D. Adjuvant tyrosine kinase inhibitors in treatment of renal cell carcinoma: A meta-analysis of available clinical trials. Clin. Genitourin. Cancer 2019, 17, e339–e344. [Google Scholar] [CrossRef] [PubMed]
- Pourmir, I.; Noel, J.; Simonaggio, A.; Oudard, S.; Vano, Y.A. Update on the most promising biomarkers of response to immune checkpoint inhibitors in clear cell renal cell carcinoma. World J. Urol. 2021, 39, 1377–1385. [Google Scholar] [CrossRef]
- Efficace, F.; Fayers, P.; Pusic, A.; Cemal, Y.; Yanagawa, J.; Jacobs, M.; et al. Quality of patient-reported outcome reporting across cancer randomized controlled trials according to the CONSORT patient- reported outcome extension: A pooled analysis of 557 trials. Cancer 2015, 121, 3335–3342. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.-R.; Geynisman, D.M.; Kim, B.; Xu, Y.; Shih, Y.-C.T. Economic burden of renal cell carcinoma-part I: An updated review. Pharmacoeconomics 2019, 37, 301–331. [Google Scholar] [CrossRef]
- Watson, T.R.; Gao, X.; Reynolds, K.L.; Kong, C.Y. Cost-effectiveness of pembrolizumab plus axitinib vs nivolumab plus ipilimumab as first-line treatment of advanced renal cell carcinoma in the US. JAMA Netw. Open. 2020, 3, e2016144. [Google Scholar] [CrossRef]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2022 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.