Thermally Activated Al(OH)3: Part I—Morphology and Porosity Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization Methods
- mi—the initial mass of the sample, before drying (g);
- md—the mass of the dried sample (g); and
- mt—the mass of the sample when calcined at temperature t (g).
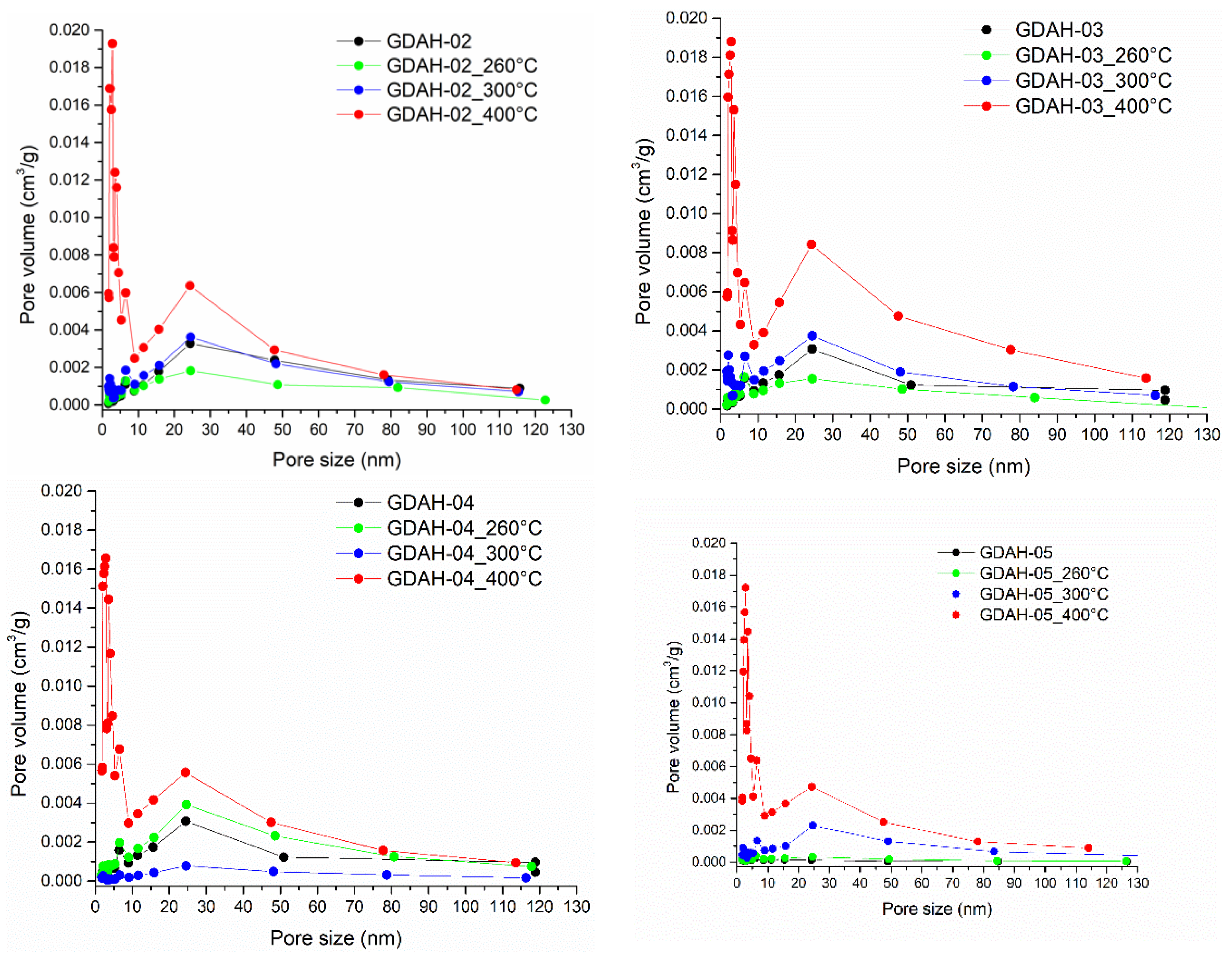
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Padilla, I.; López-Andrés, S.; López-Delgado, A. Effects of Different Raw Materials in the Synthesis of Boehmite and γ- and α-Alumina. J. Chem. 2016, 2016, 5353490. [Google Scholar] [CrossRef] [Green Version]
- Žumbar, T.; Ristic, A.; Dražić, G.; Lazarova, H.; Volvašek, J.; Pintar, A.; Logar, N.Z.; Tusar, N.N. Influence of alumina precursor properties on cu-fe alumina supported catalysts for total toluene oxidation as a model volatile organic air pollutant. Catalysts 2021, 11, 252. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; Rooney, D.W.; Halawy, S.A.; Mohamed, M.A.; Abdelkader, A. Effect of precursor on the performance of alumina for the dehydration of methanol to dimethyl ether. Appl. Catal. B Environ. 2012, 127, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Shirai, T.; Watanabe, H.; Fuji, M.; Takahashi, M. Structural Properties and Surface Characteristics on Aluminum Oxide Powders. Annu. Rep. Ceram. Res. Lab. Nagoya Inst. Technol. 2010, 9, 23–31. [Google Scholar]
- Pyzalski, M.; Wojcik, M. The dehydroxylation of aluminium hydroxides and the kinetics of α-Al2O3 formation. J. Therm. Anal. 1990, 36, 2147–2151. [Google Scholar] [CrossRef]
- Said, S.; Mikhail, S.; Riad, M. Recent processes for the production of alumina nano-particles. Mater. Sci. Energy Technol. 2020, 3, 344–363. [Google Scholar] [CrossRef]
- Abdelkader, A.; Hussien, B.M.; Fawzy, E.M.; Ibrahim, A.A. Boehmite nanopowder recovered from aluminum cans waste as a potential adsorbent for the treatment of oilfield produced water. Appl. Petrochem. Res. 2021, 1, 3. [Google Scholar]
- Mohammadi, M.; Khodamorady, M.; Tahmasbi, B.; Bahrami, K.; Ghorbani-Choghamarani, A. Boehmite nanoparticles as versatile support for organic–inorganic hybrid materials: Synthesis, functionalization, and applications in eco-friendly catalysis. J. Ind. Eng. Chem. 2021, 97, 1–78. [Google Scholar] [CrossRef]
- Ptacek, P. Processes during Thermal Treatment. In Strontium Aluminate-Cement Fundamentals, Manufacturing, Hydration, Setting Behaviour and Applications, 1st ed.; IntechOpen Limited: London, UK, 2014; pp. 97–128. [Google Scholar]
- van Gog, H. First-principles study of dehydration interfaces between diaspore and corundum, gibbsite and boehmite, and boehmite and γ-Al2O3: Energetic stability, interface charge effects, and dehydration defects. Appl. Surf. Sci. 2021, 541, 148501. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; McLaren, M.; Laffir, F.; Rooney, D.W. Characterisation of Robust Combustion Catalyst from Aluminium Foil Waste. ChemistrySelect 2018, 3, 1545–1550. [Google Scholar] [CrossRef] [Green Version]
- Mercury, J.M.R.; Sucupira, J.R.M.; Rodríguez, M.A.; Cabral, A.A.; de Aza, A.H.; Pena, P. Influence of the milling conditions on the thermal decomposition of Bayer gibbsite. Powder Technol. 2020, 362, 188–196. [Google Scholar] [CrossRef]
- Jiao, W.Q.; Yue, M.B.; Wang, Y.M.; He, M.Y. Synthesis of morphology-controlled mesoporous transition aluminas derived from the decomposition of alumina hydrates. Microporous Mesoporous Mater. 2012, 147, 167–177. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, W.; Hu, J.Z.; Wang, H.-W.; Prange, M.P.; Wan, C.; Jaegers, N.R.; Zong, M.; Zhang, H.; Pearce, C.I.; et al. Transformation of Gibbsite to Boehmite in Caustic Aqueous Solution at Hydrothermal Conditions. Cryst. Growth Des. 2019, 19, 5557–5567. [Google Scholar] [CrossRef]
- Brown, J.F.; Clark, D.; Elliott, W.W. The thermal decomposition of the alumina trihydrate, gibbsite. J. Chem. Soc. 1953, 84–88. [Google Scholar] [CrossRef]
- Golafshan, N.; Vorndran, E.; Zaharievski, S.; Brommer, H.; Kadumudi, F.B.; Dolatshahi-Pirouz, A.; Gbureck, U.; van Weeren, R.; Castilho, M.; Malda, J. Tough magnesium phosphate-based 3D-printed implants induce bone regeneration in an equine defect model. Biomaterials 2020, 261, 120302. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.C.V.; Santos, H.d.; Kiyohara, P.K.; Marcos, K.N.P.; Santos, P.d. Surface area, crystal morphology and characterization of transition alumina powders from a new gibbsite precursor. Mater. Res. 2007, 10, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Sweegers, C.; de Coninck, H.C.; Meekes, H.; van Enckevort, W.J.P.; Hiralal, I.D.K.; Rijkeboer, A. Morphology, evolution and other characteristics of gibbsite crystals grown from pure and impure aqueous sodium aluminate solutions. J. Cryst. Growth 2001, 233, 567–582. [Google Scholar] [CrossRef]
- Filho, R.W.N.D.; Rocha, G.D.; Montes, C.R.; Vieira-Coelho, A.C. Synthesis and characterization of boehmites obtained from gibbsite in presence of different environments. Mater. Res. 2016, 19, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.; Park, J.H.; Rhee, C.K.; Lee, J.; Kim, H. Effect of thermal treatment on the aluminum hydroxide nanofibers synthesized by electrolysis of Al plates. Microelectron. Eng. 2012, 89, 89–91. [Google Scholar] [CrossRef]
- Dobra, G.; Garcie-Granda, S.; IIiev, S.; Cotet, L.; Hulka, I.; Negrea, P.; Duteanu, N.; Boiangiu, A.; Laurentiu, F. Aluminum hydroxide impurities occlusions and contamination sources. Rev. Chim. 2020, 70, 65–76. [Google Scholar] [CrossRef]
- Baranyai, V.Z.; Kristály, F.; Szűcs, I. Influence of grain and crystallite size on the gibbsite to boehmite thermal transformation. Stud. Univ. Babes Bolyai Chem. 2015, 60, 27–44. [Google Scholar]
- Alex, T.C.; Kailath, A.J.; Kumar, R. Al-Monohydrate (Boehmite) to Al-Trihydrate (Bayerite/Gibbsite) Transformation During High-Energy Milling. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2020, 51, 443–451. [Google Scholar] [CrossRef]
- Keselj, D.; Lazic, D.; Penavin-skundric, J.; Sladojevic, S.; Vasiljevic, L. Determination of Alumina Oxide in Bauxites by X-Ray Fluorescence Analysis. Glob. J. Sci. Front. Res. Chem. 2012, 12, 1–6. [Google Scholar]
- Dobra, G.; Filipescu, L.; Anghelovici, N.; Alistarh, V.; Iliev, S.; Cotet, L. Bauxite Residue Safety Disposal and Possibilities to Further Utilization. Part 1. Acid Soils Remediation. J. Sib. Fed. Univ. Chem. 2017, 10, 6–21. [Google Scholar] [CrossRef]
- Xiao, C.; Shi, P.; Yan, W.; Chen, L.; Qian, L.; Kim, S.H. Thickness and Structure of Adsorbed Water Layer and Effects on Adhesion and Friction at Nanoasperity Contact. Colloids Interfaces 2019, 3, 55. [Google Scholar] [CrossRef] [Green Version]
- García, A.C.; Latifi, M.; Chaouki, J. Kinetics of calcination of natural carbonate minerals. Miner. Eng. 2020, 150, 106279. [Google Scholar] [CrossRef]
- Redaoui, D.; Sahnoune, F.; Heraiz, M.; Raghdi, A. Mechanism and kinetic parameters of the thermal decomposition of gibbsite Al(OH)3 by thermogravimetric analysis. Acta Phys. Pol. A 2017, 131, 562–565. [Google Scholar] [CrossRef]
- Mitsui, T.; Matsui, T.; Kikuchi, R.; Eguchi, K. Microstructural transformation with heat-treatment of aluminum hydroxide with gibbsite structure. Bull. Chem. Soc. Jpn. 2009, 82, 618–623. [Google Scholar] [CrossRef]
- Santos, P.S.; Santos, H.S.; Toledo, S.P. Standard transition aluminas. Electron microscopy studies. Mater. Res. 2000, 3, 104–114. [Google Scholar] [CrossRef]
- Banerjee, S.; Dubey, S.; Gautam, R.K.; Chattopadhyaya, M.C.; Sharma, Y.C. Adsorption characteristics of alumina nanoparticles for the removal of hazardous dye, Orange G from aqueous solutions. Arab. J. Chem. 2019, 12, 5339–5354. [Google Scholar] [CrossRef]
- Zhou, S.; Antonietti, M.; Niederberger, M. Low-temperature synthesis of y-alumina nanocrystals from aluminum acetylacetonate in nonaqueous media. Small 2007, 3, 763–767. [Google Scholar] [CrossRef]
- Alnajjar, M.; Hethnawi, A.; Nafie, G.; Hassan, A.; Vitale, G.; Nassar, N.N. Silica-alumina composite as an effective adsorbent for the removal of metformin from water. J. Environ. Chem. Eng. 2019, 7, 102994. [Google Scholar] [CrossRef]
- Sayehi, M.; Tounsi, H.; Garbarino, G.; Riani, P.; Busca, G. Reutilization of silicon- and aluminum- containing wastes in the perspective of the preparation of SiO2-Al2O3 based porous materials for adsorbents and catalysts. Waste Manag. 2020, 103, 146–158. [Google Scholar] [CrossRef]
- Mardkhe, M.K.; Huang, B.; Bartholomew, C.H.; Alam, T.M.; Woodfield, B.F. Synthesis and characterization of silica doped alumina catalyst support with superior thermal stability and unique pore properties. J. Porous Mater. 2016, 23, 475–487. [Google Scholar] [CrossRef]
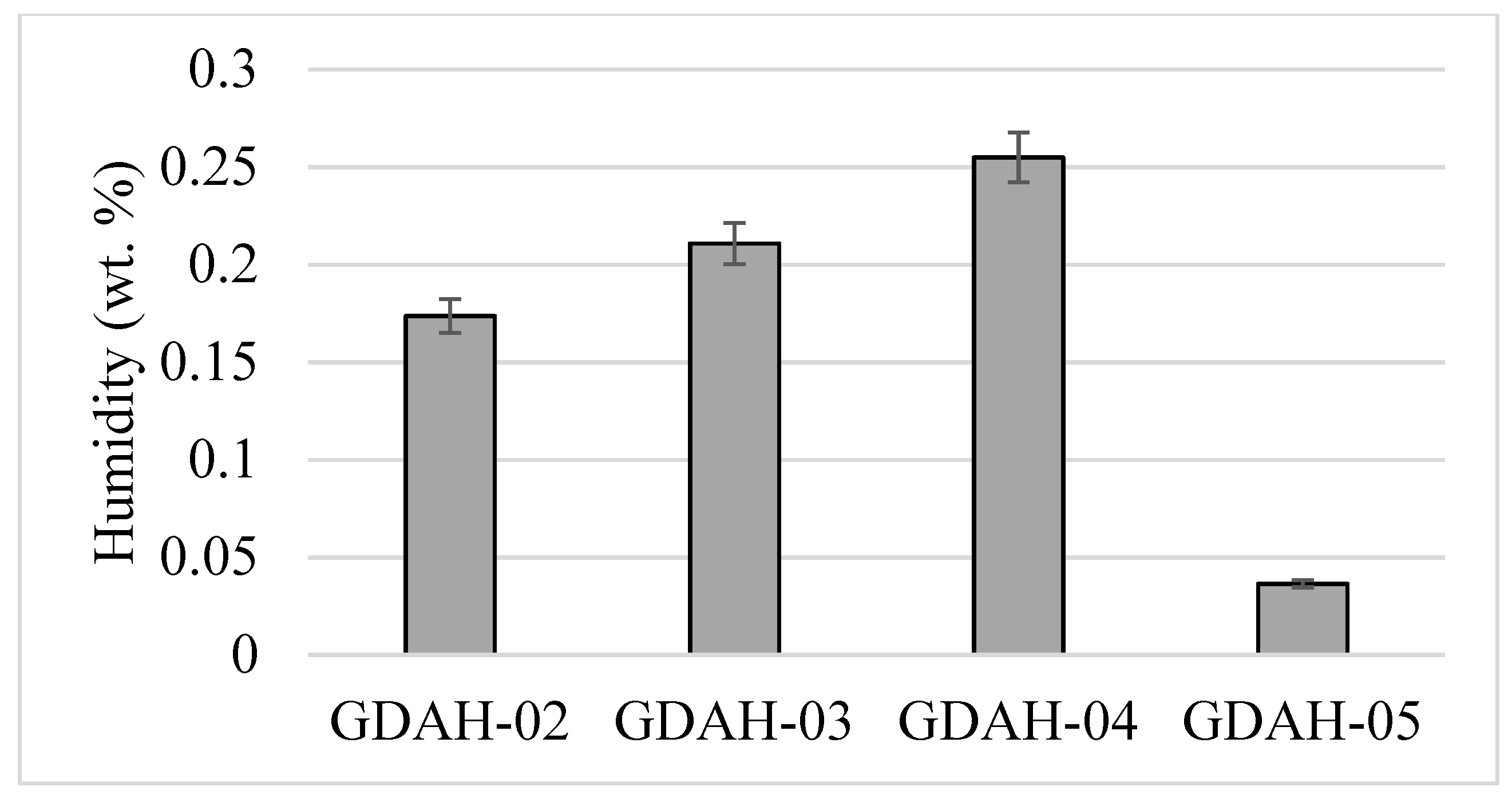
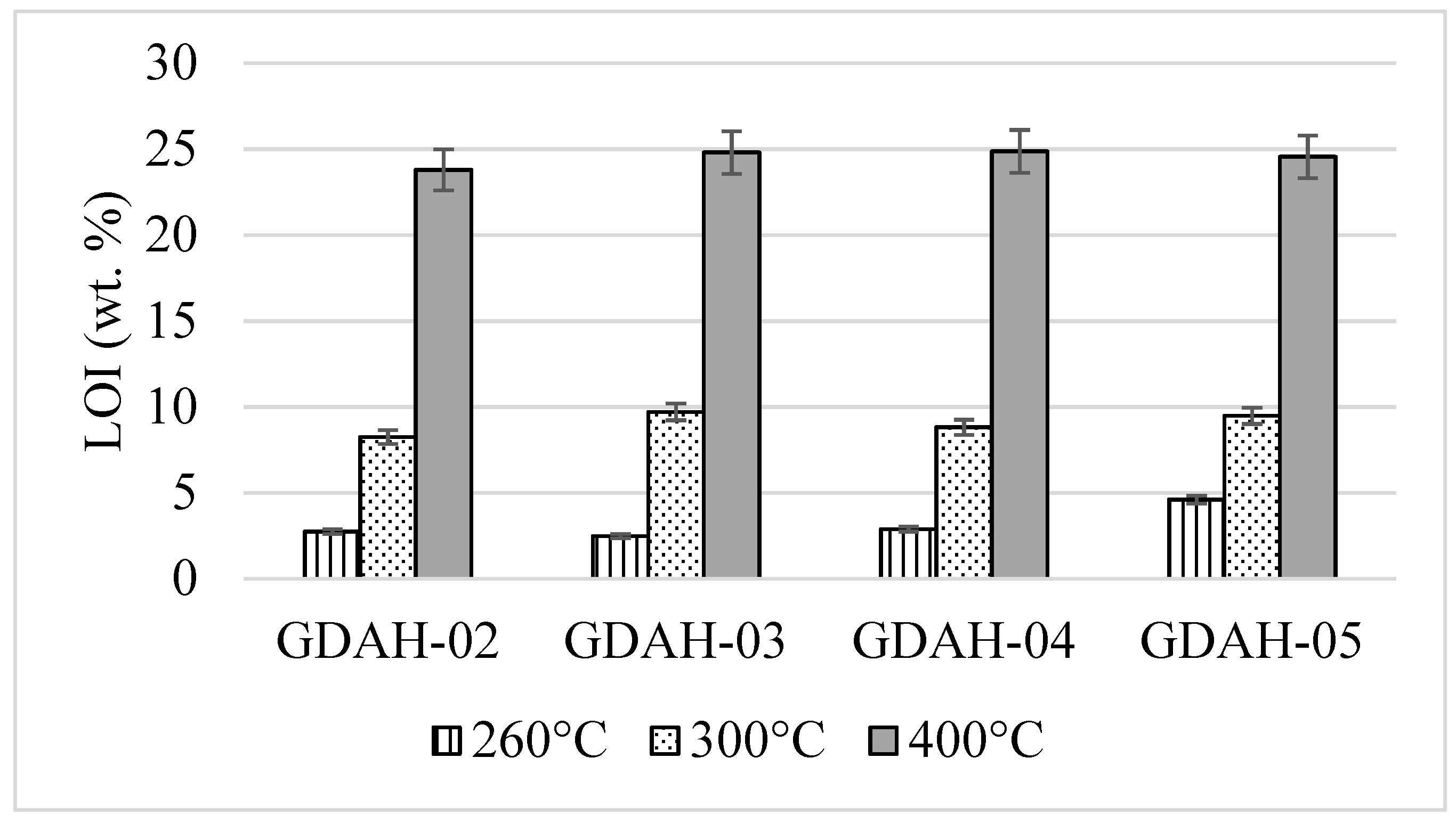



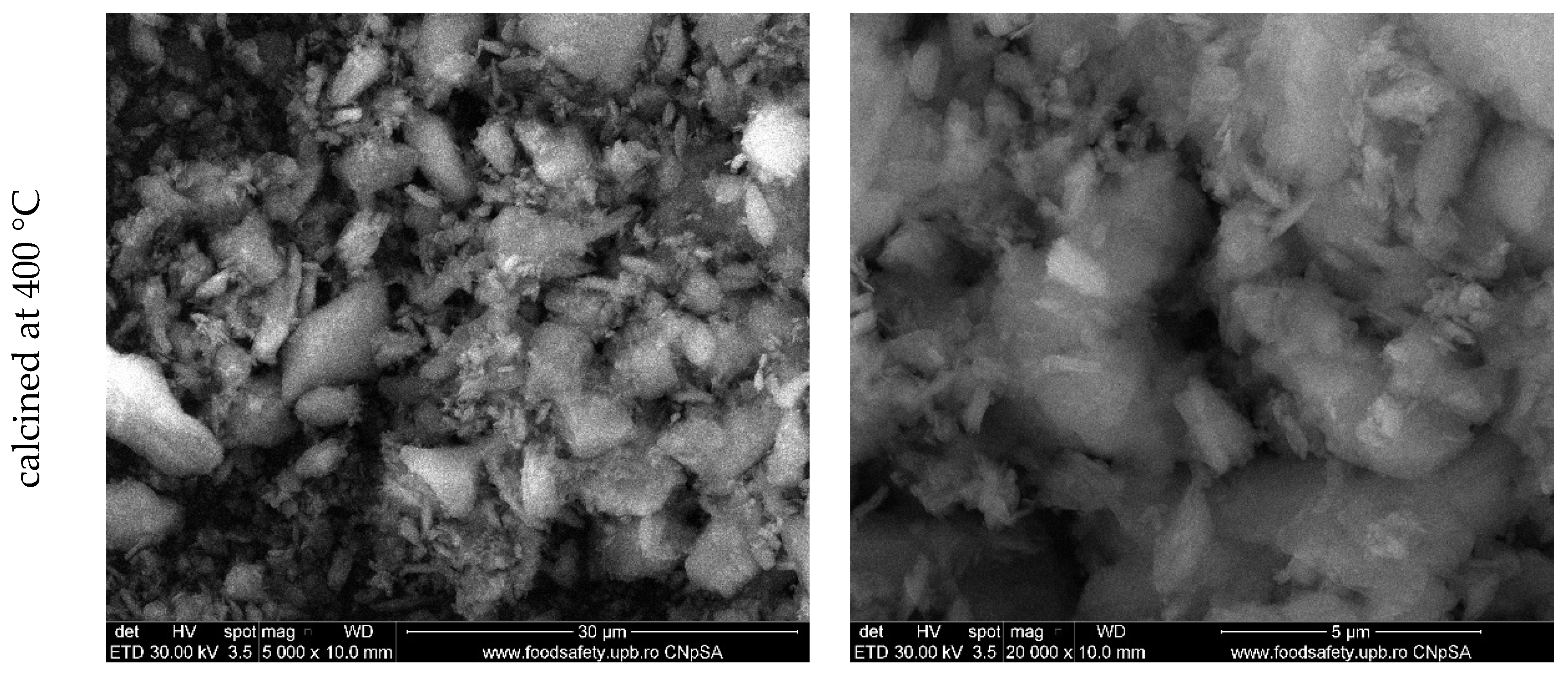
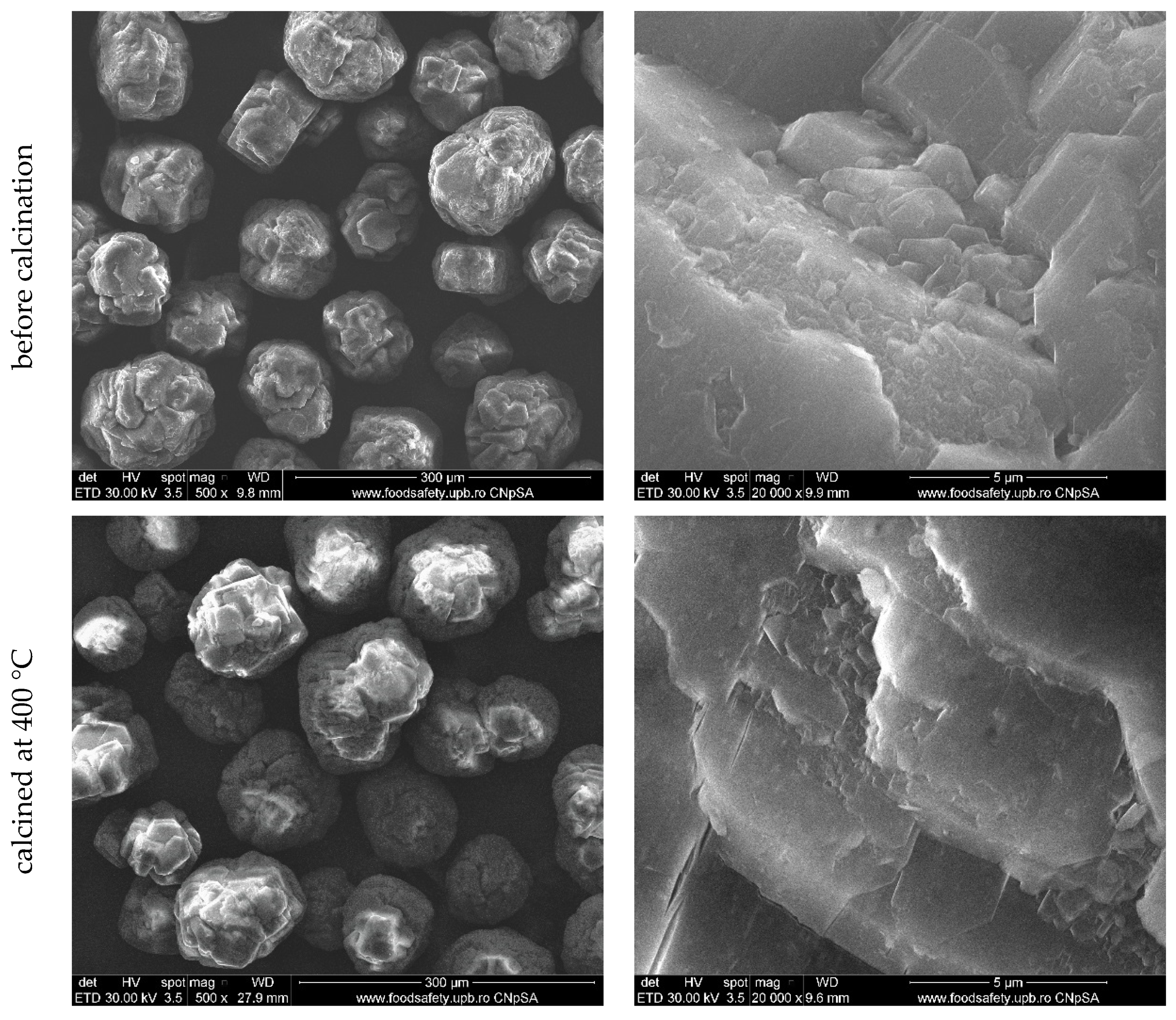

| Sample Name | Details of the Dimension Classes after Milling |
|---|---|
| GDAH-02 | <45 µm = 98.29% |
| GDAH-03 | <20 µm = 92.13% |
| GDAH-04 | <10 µm = 76.28% |
| GDAH-05 | <45 µm = 0.001%; >150 µm = 6.54% |
| Sample | GDAH-02 | GDAH-03 | GDAH-04 | GDAH-05 |
|---|---|---|---|---|
| Compound | wt.% | wt.% | wt.% | wt.% |
| Al2O3 3H2O | 63.90 | 64.26 | 64.11 | 64.24 |
| Na2O | 0.0775 | 0.0989 | 0.0885 | 0.376 |
| SiO2 | 0.0619 | 0.0537 | 0.0591 | 0.0881 |
| CaO | 0.0408 | 0.0377 | 0.0391 | 0.0546 |
| Fe2O3 | 0.0126 | 0.0117 | 0.0117 | 0.0149 |
| Sample | BET Specific Area (m2/g) | Langmuir Specific Area (m2/g) | Average Pore Width (nm) |
|---|---|---|---|
| GDAH-02 | 5.9596 | 8.9199 | 10.9876 |
| GDAH-02_260 °C | 10.6208 | 15.5593 | 5.7378 |
| GDAH-02_300 °C | 36.3853 | 52.7869 | 3.7164 |
| GDAH-02_400 °C | 234.4518 | 345.6202 | 3.2249 |
| GDAH-03 | 10.3375 | 16.0231 | 7.1186 |
| GDAH-03_260 °C | 10.3094 | 15.5083 | 5.9023 |
| GDAH-03_300 °C | 65.5179 | 95.2101 | 3.1337 |
| GDAH-03_400 °C | 241.9623 | 356.6276 | 3.3867 |
| GDAH-04 | 9.4725 | 14.0771 | 7.4804 |
| GDAH-04_260 °C | 19.4569 | 28.2606 | 5.4720 |
| GDAH-04_300 °C | 6.9195 | 10.0146 | 3.7566 |
| GDAH-04_400 °C | 238.6443 | 350.5961 | 3.2303 |
| GDAH-05 | 2.2240 | 7.3240 | 4.3304 |
| GDAH-05_260 °C | 2.2964 | 3.7623 | 6.1816 |
| GDAH-05_300 °C | 20.8556 | 30.3694 | 3.8894 |
| GDAH-05_400 °C | 181.5672 | 267.8954 | 3.5443 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasile, B.S.; Dobra, G.; Iliev, S.; Cotet, L.; Neacsu, I.A.; Nicoara, A.I.; Surdu, V.A.; Boiangiu, A.; Filipescu, L. Thermally Activated Al(OH)3: Part I—Morphology and Porosity Evaluation. Ceramics 2021, 4, 265-277. https://doi.org/10.3390/ceramics4020021
Vasile BS, Dobra G, Iliev S, Cotet L, Neacsu IA, Nicoara AI, Surdu VA, Boiangiu A, Filipescu L. Thermally Activated Al(OH)3: Part I—Morphology and Porosity Evaluation. Ceramics. 2021; 4(2):265-277. https://doi.org/10.3390/ceramics4020021
Chicago/Turabian StyleVasile, Bogdan Stefan, Gheorghe Dobra, Sorin Iliev, Lucian Cotet, Ionela Andreea Neacsu, Adrian Ionut Nicoara, Vasile Adrian Surdu, Alina Boiangiu, and Laurențiu Filipescu. 2021. "Thermally Activated Al(OH)3: Part I—Morphology and Porosity Evaluation" Ceramics 4, no. 2: 265-277. https://doi.org/10.3390/ceramics4020021
APA StyleVasile, B. S., Dobra, G., Iliev, S., Cotet, L., Neacsu, I. A., Nicoara, A. I., Surdu, V. A., Boiangiu, A., & Filipescu, L. (2021). Thermally Activated Al(OH)3: Part I—Morphology and Porosity Evaluation. Ceramics, 4(2), 265-277. https://doi.org/10.3390/ceramics4020021










