Compositional Analysis of SiOC(H) Powders: A Comparison of X-ray Photoelectron Spectroscopy (XPS) and Combustion Analysis
Abstract
:1. Introduction
2. Experimental Methods
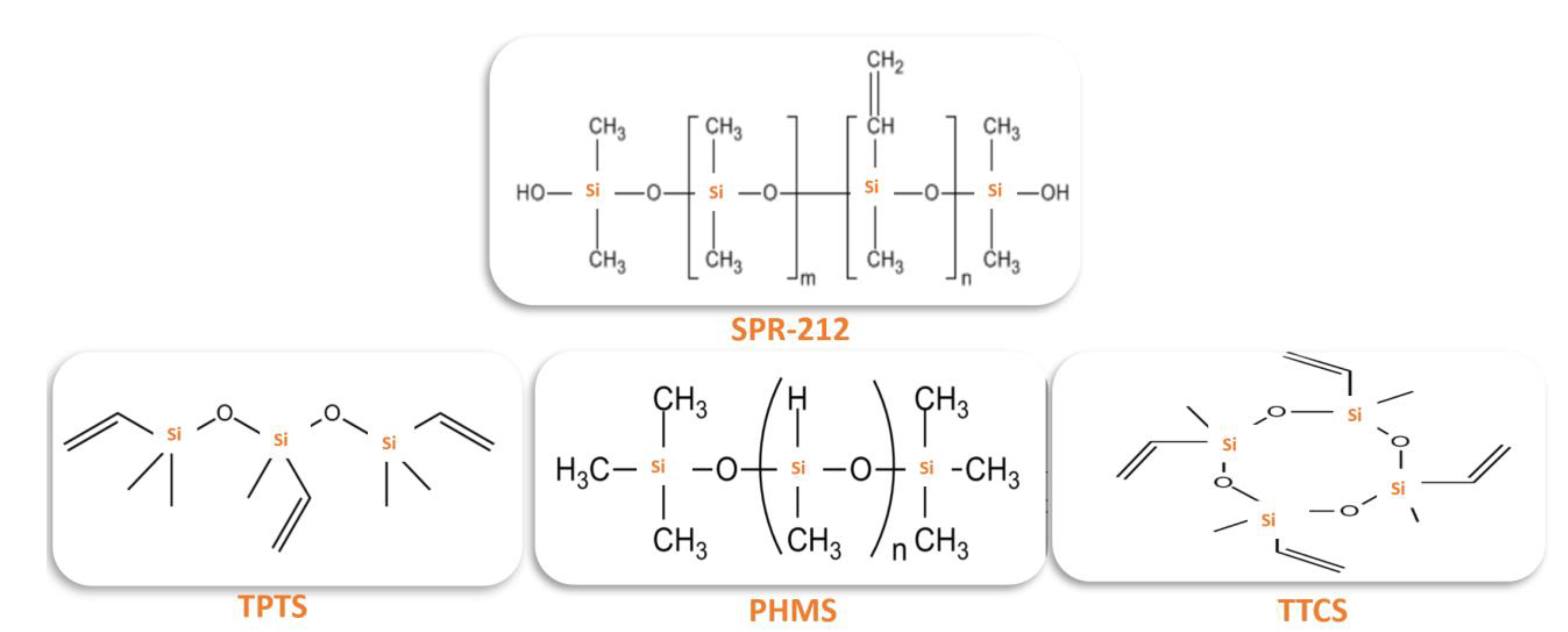
2.1. Materials
2.2. Characterization
3. Results and Discussion
3.1. Comparison of Chemical Analysis by XPS and Combustion
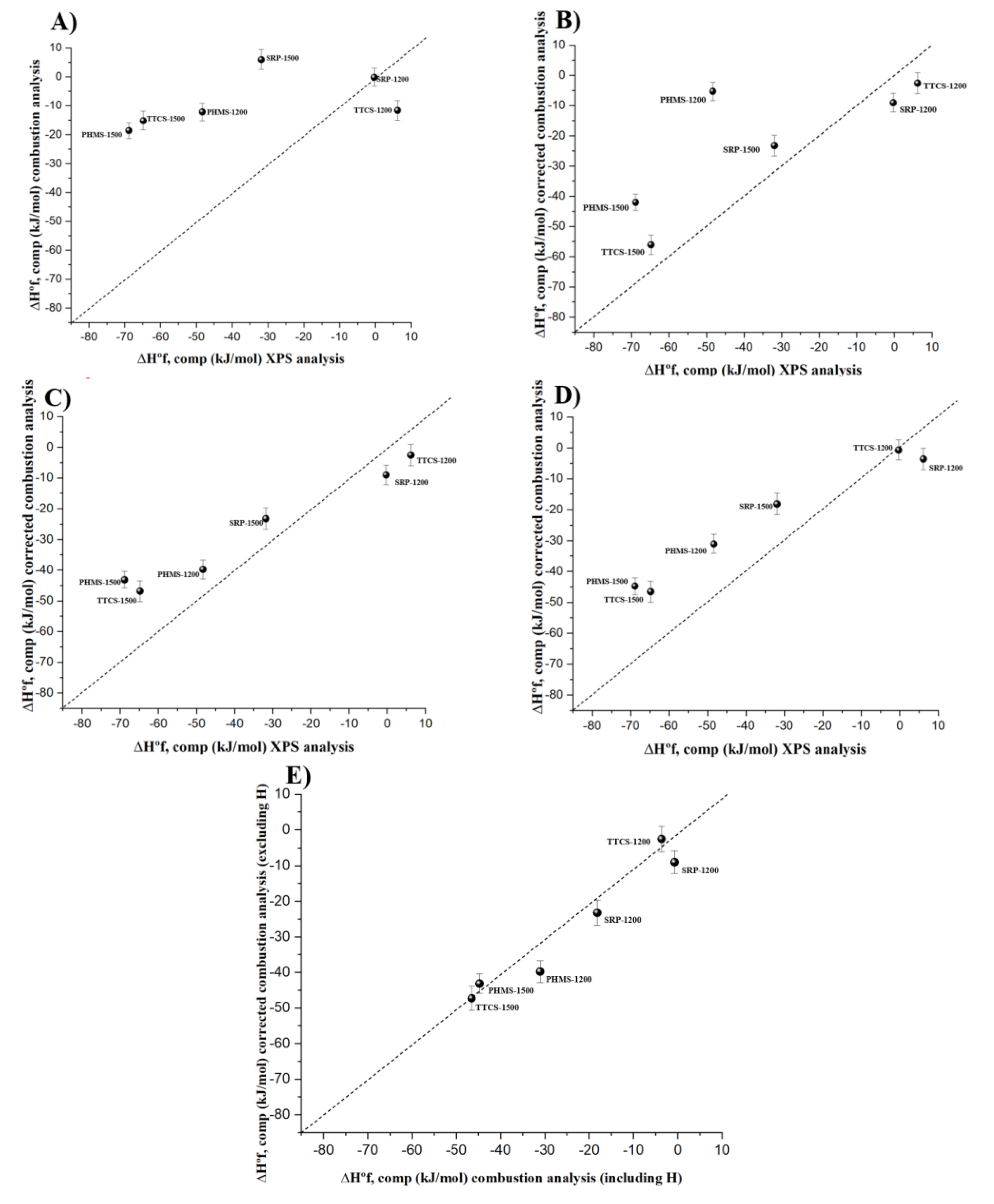
3.2. Thermochemical Calculations Using Differently Analyzed Chemical Compositions
a SiO2 (s, 800 °C) + c CO2 (g, 800 °C) + (d/2) H2O (g, 800 °C) ∆Hdis, SiOC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bozeman, T.B.; Lemon, R.R.; Eleazer, P.D. Elemental analysis of crystal precipitate from gray and white MTA. J. Endod. 2006, 32, 425–428. [Google Scholar] [CrossRef]
- Ninomiya, K.; Kubo, M.K.; Nagatomo, T.; Higemoto, W.; Ito, T.U.; Kawamura, N.; Strasser, P.; Shimomura, K.; Miyake, Y.; Suzuki, T.; et al. Nondestructive elemental depth-profiling analysis by muonic X-Ray measurement. Anal. Chem. 2015, 87, 4597–4600. [Google Scholar] [CrossRef] [PubMed]
- Nowak, S.; Winter, M. Elemental analysis of lithium ion batteries. J. Anal. At. Spectrom. 2017, 32, 1833–1847. [Google Scholar] [CrossRef]
- Atzei, D.; Fantauzzi, M.; Rossi, A.; Fermo, P.; Piazzalunga, A.; Valli, G.; Vecchi, R. Surface chemical characterization of PM10 samples by XPS. Appl. Surf. Sci. 2014, 307, 120–128. [Google Scholar] [CrossRef]
- Aquisman, A.E.; Assim, Z.B.; Wahi, R.B.; Kwabena, D.E.; Festus, W. Validation of the atomic absorption spectroscopy (AAS) for heavy metal analysis and geochemical exploration of sediment samples from the Sebangan river. Adv. Anal. Chem. 2019, 9, 23–33. [Google Scholar]
- Faubel, W.; Staub, S.; Simon, R.; Heissler, S.; Pataki, A.; Banik, G. Non-destructive analysis for the investigation of decomposition phenomena of historical manuscripts and prints. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 669–676. [Google Scholar] [CrossRef]
- Millett, E.J. Progress in the Analysis of Crystalline Solids. J. Cryst. Growth 1980, 48, 666–682. [Google Scholar] [CrossRef]
- Sujith, R.; Jothi, S.; Zimmermann, A.; Aldinger, F.; Kumar, R. Mechanical behaviour of polymer derived ceramics—A Review. Int. Mater. Rev. 2021, 66, 426–449. [Google Scholar] [CrossRef]
- Ma, B.; Cao, Y.; Gao, Y.; Wang, Y. Fabrication of a thin double-layer thermistor based on DVB-modified polymer-derived SiCN ceramics. J. Alloys Compd. 2018, 732, 491–497. [Google Scholar] [CrossRef]
- Ionescu, E.; Mera, G.; Riedel, R. Polymer-derived ceramics (PDCs): Materials design towards applications at ultrahigh-temperatures and in extreme environments. In Nanotechnology: Concepts, Methodologies, Tools, and Applications; Information Resources Management Association; Technische Universität Darmstadt: Darmstadt, Germany, 2014; pp. 1108–1139. [Google Scholar]
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Terauds, K.; Sanchez-Jimenez, P.E.; Raj, R.; Vakifahmetoglu, C.; Colombo, P. Giant Piezoresistivity of polymer-derived ceramics at high temperatures. J. Eur. Ceram. Soc. 2010, 30, 2203–2207. [Google Scholar] [CrossRef]
- Riedel, R.; Mera, G.; Hauser, R.; Klonczynski, A. Silicon-Based Ceramics Derived from Polymers-Review on Synthesis, Properties and Applications. J. Ceram. Soc. Jpn. 2006, 114, 425–444. [Google Scholar] [CrossRef] [Green Version]
- Duan, W.; Yin, X.; Li, Q.; Schlier, L.; Greil, P.; Travitzky, N. A Review of absorption properties in silicon-based polymer derived ceramics. J. Eur. Ceram. Soc. 2016, 36, 3681–3689. [Google Scholar] [CrossRef]
- Wen, Q.; Yu, Z.; Riedel, R. The fate and role of in situ formed carbon in polymer-derived ceramics. Prog. Mater. Sci. 2020, 109, 100623. [Google Scholar] [CrossRef]
- Ionescu, E.; Kleebe, H.-J.; Riedel, R. Silicon-containing polymer-derived ceramic nanocomposites (PDC-NCs): Preparative approaches and properties. Chem. Soc. Rev. 2012, 41, 5032–5052. [Google Scholar] [CrossRef]
- Ushakov, S.V.; Maram, P.S.; Kapush, D.; Pavlik, A.J.; Fyhrie, M.; Gallington, L.C.; Benmore, C.J.; Weber, R.; Neuefeind, J.C.; McMurray, J.W.; et al. Phase transformations in oxides above 2000 °C: Experimental technique development. Adv. Appl. Ceram. 2018, 117, s82–s89. [Google Scholar] [CrossRef]
- Ionescu, E.; Bernard, S.; Lucas, R.; Kroll, P.; Ushakov, S.; Navrotsky, A.; Riedel, R. Polymer-derived ultra-high temperature ceramics (UHTCs) and related materials. In Ceramics, Glass and Glass-Ceramics: From Early Manufacturing Steps Towards Modern Frontiers; Baino, F., Tomalino, M., Tulyaganov, D., Eds.; PoliTO Springer Series; Springer International Publishing: Cham, Switzerland, 2021; pp. 281–323. [Google Scholar]
- David, L.; Bhandavat, R.; Barrera, U.; Singh, G. Silicon oxycarbide glass-graphene composite paper electrode for long-cycle lithium-ion batteries. Nat. Commun. 2016, 7, 10998. [Google Scholar] [CrossRef] [Green Version]
- Bhandavat, R.; Singh, G. Stable and efficient Li-ion battery anodes prepared from polymer-derived silicon oxycarbide–carbon nanotube shell/core composites. J. Phys. Chem. C 2013, 117, 11899–11905. [Google Scholar] [CrossRef] [Green Version]
- Vakifahmetoglu, C.; Zeydanli, D.; Colombo, P. Porous polymer derived ceramics. Mater. Sci. Eng. R. Rep. 2016, 106, 1–30. [Google Scholar] [CrossRef]
- Leonel, G.J.; Mujib, S.B.; Singh, G.; Navrotsky, A. Thermodynamic stabilization of crystalline silicon carbide polymer-derived ceramic fibers. Int. J. Ceram. Eng. Sci. 2022, 4, 315–326. [Google Scholar] [CrossRef]
- Sugie, C.; Navrotsky, A.; Lauterbach, S.; Kleebe, H.-J.; Mera, G. Structure and thermodynamics of silicon oxycarbide polymer-derived ceramics with and without mixed-bonding. Materials 2021, 14, 4075. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Yu, Z.; Riedel, R.; Ionescu, E. Single-source-precursor synthesis and high-temperature evolution of a boron-containing SiC/HfC ceramic nano/micro composite. J. Eur. Ceram. Soc. 2021, 41, 3002–3012. [Google Scholar] [CrossRef]
- Reinold, L.M.; Graczyk-Zajac, M.; Gao, Y.; Mera, G.; Riedel, R. Carbon-rich SiCN ceramics as high capacity/high stability anode material for lithium-ion batteries. J. Power Sources 2013, 236, 224–229. [Google Scholar] [CrossRef]
- Widgeon, S.; Mera, G.; Gao, Y.; Sen, S.; Navrotsky, A.; Riedel, R. Effect of precursor on speciation and nanostructure of SiBCN polymer-derived ceramics. J. Am. Ceram. Soc. 2013, 96, 1651–1659. [Google Scholar] [CrossRef]
- Bhandavat, R.; Singh, G. Synthesis, Characterization, and high temperature stability of Si(B)CN-coated carbon nanotubes using a boron-modified poly(ureamethylvinyl)silazane chemistry. J. Am. Ceram. Soc. 2012, 95, 1536–1543. [Google Scholar] [CrossRef]
- Poerschke, D.L.; Braithwaite, A.; Park, D.; Lauten, F. Crystallization behavior of polymer-derived Si-O-C for ceramic matrix composite processing. Acta Mater. 2018, 147, 329–341. [Google Scholar] [CrossRef]
- Daccà, A.; Gemme, G.; Mattera, L.; Parodi, R. XPS Analysis of the Surface composition of niobium for superconducting RF cavities. Appl. Surf. Sci. 1998, 126, 219–230. [Google Scholar] [CrossRef]
- Hooshmand, T.; Daw, R.; van Noort, R.; Short, R.D. XPS Analysis of the surface of leucite-reinforced feldspathic ceramics. Dent. Mater. 2001, 17, 1–6. [Google Scholar] [CrossRef]
- Sarkar, S.; Chunder, A.; Fei, W.; An, L.; Zhai, L. Superhydrophobic mats of polymer-derived ceramic fibers. J. Am. Ceram. Soc. 2008, 91, 2751–2755. [Google Scholar] [CrossRef]
- Stojilovic, N. Why can’t we see hydrogen in X-ray photoelectron spectroscopy? J. Chem. Educ. 2012, 89, 1331–1332. [Google Scholar] [CrossRef]
- Guo, X.; Szenknect, S.; Mesbah, A.; Labs, S.; Clavier, N.; Poinssot, C.; Ushakov, S.V.; Curtius, H.; Bosbach, D.; Ewing, R.C.; et al. Thermodynamics of formation of coffinite, USiO4. Proc. Natl. Acad. Sci. USA 2015, 112, 6551–6555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakoli, A.H.; Armentrout, M.M.; Narisawa, M.; Sen, S.; Navrotsky, A. White Si–O–C ceramic: Structure and thermodynamic stability. J. Am. Ceram. Soc. 2015, 98, 242–246. [Google Scholar] [CrossRef]
- Tavakoli, A.H.; Golczewski, J.A.; Bill, J.; Navrotsky, A. Effect of boron on the thermodynamic stability of amorphous polymer-derived Si(B)CN ceramics. Acta Mater. 2012, 60, 4514–4522. [Google Scholar] [CrossRef]
- Niu, M.; Wang, H.; Chen, J.; Su, L.; Wu, D.; Navrotsky, A. Structure and energetics of SiOC and SiOC-modified carbon-bonded carbon fiber composites. J. Am. Ceram. Soc. 2017, 100, 3693–3702. [Google Scholar] [CrossRef]
- Tavakoli, A.H.; Campostrini, R.; Gervais, C.; Babonneau, F.; Bill, J.; Sorarù, G.D.; Navrotsky, A. Energetics and structure of polymer-derived Si–(B–)O–C glasses: Effect of the boron content and pyrolysis temperature. J. Am. Ceram. Soc. 2014, 97, 303–309. [Google Scholar] [CrossRef]
- Navrotsky, A. Progress and new directions in calorimetry: A 2014 perspective. J. Am. Ceram. Soc. 2014, 97, 3349–3359. [Google Scholar] [CrossRef]
- Widgeon, S.; Mera, G.; Gao, Y.; Stoyanov, E.; Sen, S.; Navrotsky, A.; Riedel, R. Nanostructure and energetics of carbon-rich SiCN ceramics derived from polysilylcarbodiimides: Role of the nanodomain interfaces. Chem. Mater. 2012, 24, 1181–1191. [Google Scholar] [CrossRef]
- Shen, C.; Barrios, E.; Zhai, L. Bulk polymer-derived ceramic composites of graphene oxide. ACS Omega 2018, 3, 4006–4016. [Google Scholar] [CrossRef]
- Sreeja, R.; Jayalatha, T.; Devapal, D. Silicon oxycarbide (SiOC) foam from methylphenylpoly(silsesquioxane)(PS) by direct foaming technique. J. Porous Mater. 2022. [Google Scholar] [CrossRef]
- Sun, X.; Yang, G.; Tian, Z.; Zhu, W.; Su, D. In-Situ Formation of titanium carbide in carbon-rich silicon oxycarbide ceramic for enhanced thermal stability. J. Eur. Ceram. Soc. 2022, 42, 6935–6941. [Google Scholar] [CrossRef]
- Seo, W.-S.; Koumoto, K. Stacking Faults in β-SiC Formed during Carbothermal Reduction of SiO2. J. Am. Ceram. Soc. 1996, 79, 1777–1782. [Google Scholar] [CrossRef]
- Berger, L.-M.; Gruner, W.; Langholf, E.; Stolle, S. On the mechanism of carbothermal reduction processes of TiO2 and ZrO2. Int. J. Refract. Met. Hard Mater. 1999, 17, 235–243. [Google Scholar] [CrossRef]


| Elemental Composition by Combustion Analysis | ||||
|---|---|---|---|---|
| Elements (at.%) | ||||
| SiOC Sample | C | O | Si | H |
| SRP-1200 | 36.54 ± 0.35 | 30.0 ± 0.17 | 29.52 | 3.94 ± 0.02 |
| SRP-1500 | 37.96 ± 0.34 | 30.40 ± 0.30 | 30.79 | 0.85 ± 0.01 |
| TTCS-1200 | 50.45 ± 0.48 | 23.09 ± 0.155 | 24.45 | 2.01 ± 0.02 |
| TTCS-1500 | 50.97 ± 0.49 | 23.47 ± 0.18 | 24.75 | 0.81 ± 0.08 |
| PHMS-1200 | 31.75 ± 0.30 | 33.24 ± 0.30 | 29.37 | 5.68 + 0.05 |
| PHMS-1500 | 33.54 ± 0.34 | 34.88 ± 0.34 | 30.94 | 0.67 ± 0.07 |
| Elemental Composition by XPS | |||
|---|---|---|---|
| Elements (at.%) | |||
| SiOC Sample | C1s | O1s | Si2p |
| SRP-1200 | 35.47 ± 0.55 | 30.56 ± 0.60 | 33.97 ± 0.05 |
| SRP-1500 | 37.12 ± 0.02 | 27.32 ± 0.74 | 35.56 ± 0.71 |
| TTCS-1200 | 50.28 ± 0.22 | 25.29 ± 0.62 | 24.43 ± 0.39 |
| TTCS-1500 | 48.76 ± 0.52 | 19.37 ± 0.05 | 31.87 ± 0.47 |
| PHMS-1200 | 38.16 ± 0.30 | 30.25 ± 1.00 | 31.62 ± 0.70 |
| PHMS-1500 | 40.35 ± 1.09 | 28.57 ± 0.03 | 31.15 ± 0.09 |
| Sample | Composition SixOyCz (x + y + z = 1) | ∆Hdis (kJ.mol−1) | ∆H°f, elem (kJ.mol−1) | ∆H°f, comp (kJ.mol−1) |
|---|---|---|---|---|
| SRP-1200 | Si0.34O0.31C0.35 | −275.41 ± 2.40 | −154.40 ± 3.19 | −0.31 ± 3.23 |
| TTCS-1200 | Si0.25O0.25C0.50 | −291.94 ± 2.77 | −116.35 ± 3.48 | +6.16 ± 3.51 |
| PHMS-1200 | Si0.32O0.30C0.38 | −226.92 ± 2.21 | −197.55 ± 3.05 | −48.34 ± 3.09 |
| SRP-1500 | Si0.36O0.27C0.37 | −284.91 ± 2.78 | −170.12 ± 3.50 | −31.88 ± 3.52 |
| TTCS-1500 | Si0.32O0.19C0.49 | −299.76 ± 2.60 | −167.29 ± 3.35 | −64.80 ± 3.38 |
| PHMS-1500 | Si0.31O0.29C0.40 | −210.21 ± 1.70 | −212.43 ± 2.71 | −68.87 ± 2.74 |
| Sample | Composition SiwOxCyHz (w + x + y + z = 1) | ∆Hdis (kJ.mol−1) | ∆H°f, elem (kJ.mol−1) | ∆H°f, comp (kJ.mol−1) |
|---|---|---|---|---|
| SRP-1200 | Si0.295O0.300C0.365H0.04 | −256.23 ± 2.23 | −144.18 ± 3.07 | −0.173 ± 3.10 |
| TTCS-1200 | Si0.244O0.231C0.504H0.021 | −283.70 ± 2.70 | −123.43 ± 3.42 | −11.65 ± 3.45 |
| PHMS-1200 | Si0.294O0.332C0.317H0.057 | −214.20 ± 2.09 | −168.54 ± 2.97 | −12.16 ± 3.0 |
| SRP-1500 | Si0.308O0.304C0.380H0.008 | −271.47 ± 2.65 | −142.50 ± 3.50 | +5.9828 ± 3.42 |
| TTCS-1500 | Si0.247O0.235C0.510H0.008 | −280.33 ± 2.43 | −130.23 ± 2.62 | −15.14 ± 3.24 |
| PHMS-1500 | Si0.309O0.349C0.335H0.007 | −210.51 ± 1.70 | −186.51 ± 2.70 | −18.61 ± 2.74 |
| Sample | Composition SixOyCz (x + y + z = 1) | ∆Hdis (kJ.mol−1) | ∆H°f, elem (kJ.mol−1) | ∆H°f, comp (kJ.mol−1) |
|---|---|---|---|---|
| SRP-1200 | Si0.34O0.30C0.36 | −274.82 ± 2.39 | −158.93 ± 3.18 | −9.03 ± 3.22 |
| TTCS-1200 | Si0.25O0.24C0.51 | −291.31 ± 2.76 | −120.95 ± 3.47 | −2.59 ± 3.50 |
| PHMS-1200 | Si0.32O0.35C0.33 | −229.37 ± 2.23 | −174.64 ± 3.07 | −5.29 ± 3.1 |
| SRP-1500 | Si0.36O0.28C0.36 | −285.43 ± 2.78 | −167.31 ± 3.49 | −23.25 ± 3.51 |
| TTCS-1500 | Si0.32O0.20C0.48 | −300.37 ± 2.60 | −162.74 ± 3.35 | −56.07 ± 3.37 |
| PHMS-1500 | Si0.31O0.31C0.34 | −205.57 ± 1.66 | −193.93 ± 2.68 | −42.02 ± 2.72 |
| Sample | Composition SixOyCz (x + y + z = 1) | ∆Hdis (kJ.mol−1) | ∆H°f, elem (kJ.mol−1) | ∆H°f, comp (kJ.mol−1) |
|---|---|---|---|---|
| SRP-1200 | Si0.34O0.30C0.36 | −274.82 ± 2.39 | −158.93 ± 3.18 | −9.03 ± 3.22 |
| TTCS-1200 | Si0.25O0.24C0.51 | −291.31 ± 2.76 | −120.95 ± 3.47 | −2.59 ± 3.50 |
| PHMS-1200 | Si0.32O0.31C0.37 | −227.39 ± 2.22 | −192.38 ± 3.06 | −39.78 ± 3.07 |
| SRP-1500 | Si0.36O0.28C0.36 | −285.43 ± 2.78 | −166.23 ± 3.49 | −23.25 ± 3.51 |
| TTCS-1500 | Si0.32O0.21C0.47 | −301.05 ± 2.61 | −158.12 ± 3.35 | −46.82 ± 3.36 |
| PHMS-1500 | Si0.31O0.32C0.37 | −211.59 ± 1.71 | −247.58 ± 2.71 | −43.13 ± 2.73 |
| Sample | Composition SixOyCz (x + y + z = 1) | ∆Hdis (kJ.mol−1) | ∆H°f, elem (kJ.mol−1) | ∆H°f, comp (kJ.mol−1) |
|---|---|---|---|---|
| SRP-1200 | Si0.34O0.305C0.315H0.04 | −270.97 ± 4.06 | −149.61 ± 4.57 | −0.70 ± 4.60 |
| TTCS-1200 | Si0.25O0.240C0.489H0.021 | −286.0 ± 2.76 | −120.59 ± 3.47 | −3.64 ± 3.50 |
| PHMS-1200 | Si0.32O0.316C0.307H0.057 | −219.61 ± 2.13 | −182.78 ± 3.0 | −31.09 ± 3.03 |
| SRP-1500 | Si0.36O0.287C0.345H0.008 | −283.17 ± 2.76 | −163.59 ± 3.47 | −18.16 ± 3.50 |
| TTCS-1500 | Si0.32O0.212C0.46H0.008 | −298.54 ± 2.59 | −157.72 ± 3.34 | −46.54 ± 3.37 |
| PHMS-1500 | Si0.31O0.319C0.364H0.007 | −209.388 ± 1.67 | −182.78 ± 2.69 | −44.73 ± 2.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonel, G.J.; Guo, X.; Singh, G.; Navrotsky, A. Compositional Analysis of SiOC(H) Powders: A Comparison of X-ray Photoelectron Spectroscopy (XPS) and Combustion Analysis. Ceramics 2023, 6, 74-85. https://doi.org/10.3390/ceramics6010006
Leonel GJ, Guo X, Singh G, Navrotsky A. Compositional Analysis of SiOC(H) Powders: A Comparison of X-ray Photoelectron Spectroscopy (XPS) and Combustion Analysis. Ceramics. 2023; 6(1):74-85. https://doi.org/10.3390/ceramics6010006
Chicago/Turabian StyleLeonel, Gerson J., Xin Guo, Gurpreet Singh, and Alexandra Navrotsky. 2023. "Compositional Analysis of SiOC(H) Powders: A Comparison of X-ray Photoelectron Spectroscopy (XPS) and Combustion Analysis" Ceramics 6, no. 1: 74-85. https://doi.org/10.3390/ceramics6010006
APA StyleLeonel, G. J., Guo, X., Singh, G., & Navrotsky, A. (2023). Compositional Analysis of SiOC(H) Powders: A Comparison of X-ray Photoelectron Spectroscopy (XPS) and Combustion Analysis. Ceramics, 6(1), 74-85. https://doi.org/10.3390/ceramics6010006







