Structure, Mechanical and Luminescent Properties of Solid Solution (Y0.96Eu0.01Sm0.01Tb0.01Er0.01)Nb0.7Ta0.3O4
Abstract
1. Introduction
2. Materials and Methods
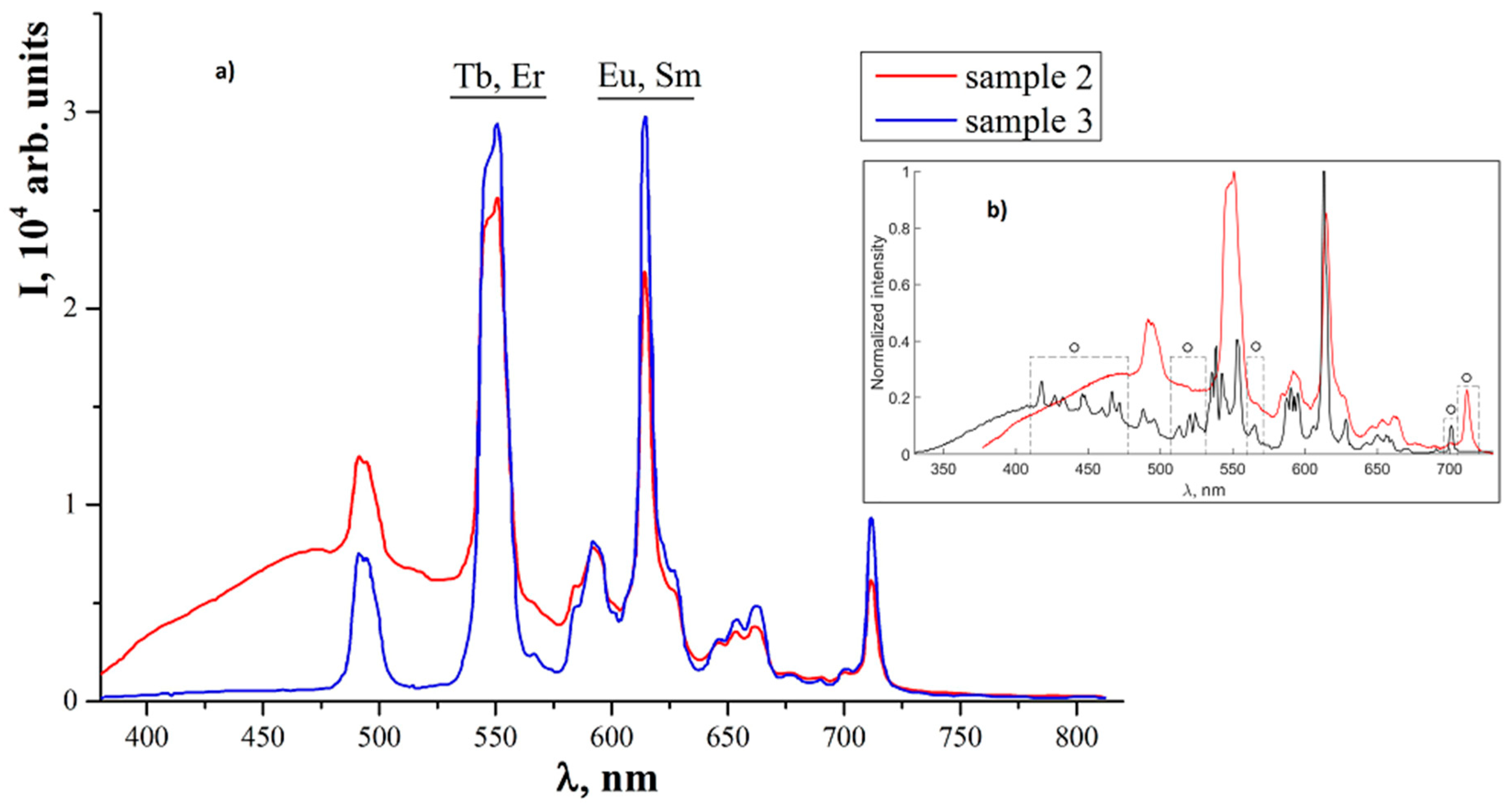
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haugsrud, R.; Norby, T. Proton conduction in rare-earth ortho-niobates and ortho-tantalates. Nat. Mater. 2006, 5, 193–196. [Google Scholar] [CrossRef]
- Forbes, T.Z.; Nyman, M.; Rodriguez, M.A.; Navrotsky, A. The energetics of lanthanum tantalate materials. J. Solid State Chem. 2010, 183, 2516–2521. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.; Wu, P.; Feng, J. Thermal expansion performance and intrinsic lattice thermal conductivity of ferroelastic RETaO4 ceramics. J. Am. Ceram. Soc. 2019, 102, 4809–4821. [Google Scholar] [CrossRef]
- Zhou, Y.; Gan, G.; Ge, Z.; Feng, J. Thermophysical properties of SmTaO4, Sm3TaO7 and SmTa3O9 ceramics. Mater. Res. Express 2020, 7, 015204. [Google Scholar] [CrossRef]
- Voloshyna, O.; Gerasymov, I.; Sidletskiy, O.; Kurtsev, D.; Gorbacheva, T.; Hubenko, K.; Boiaryntseva, I.; Ivanov, A.; Spassky, D.; Omelkov, S.; et al. Fast ultradense GdTa1-xNbxO4 scintillator crystals. Opt. Mater. 2017, 66, 332–337. [Google Scholar] [CrossRef]
- Spassky, D.; Vasil’Ev, A.; Vielhauer, S.; Sidletskiy, O.; Voloshyna, O.; Belsky, A. Composition effect in luminescence properties of Y(NbxTa1-x)O4 mixed crystals. Opt. Mater. 2018, 80, 247–252. [Google Scholar] [CrossRef]
- Nakauchi, D.; Koshimizu, M.; Okada, G.; Yanagida, T. Floating zone growth and scintillation properties of undoped and Ce-doped GdTaO4 crystals. Radiat. Meas. 2017, 106, 129–133. [Google Scholar] [CrossRef]
- Palatnikov, M.N.; Smirnov, M.V.; Masloboeva, S.M.; Shcherbina, O.B.; Sidorov, N.V.; Steblevskaya, N.I.; Belobeletskaya, M.V. Luminescence Properties of Sol–Gel Derived Ceramic GdNbxTa1–xO4 and YNbxTa1–xO4 Solid Solutions. Inorg. Mater. 2020, 56, 437–442. [Google Scholar] [CrossRef]
- Ayvacıklı, M.; Ege, A.; Ekdal, E.; Popovici, E.-J.; Can, N. Radioluminescence study of rare earth doped some yttrium based phosphors. Opt. Mater. 2012, 34, 1958–1961. [Google Scholar] [CrossRef]
- Siqueira, K.P.; Carmo, A.P.; Bell, M.J.V.; Dias, A. Optical properties of undoped NdTaO4, ErTaO4 and YbTaO4 ceramics. J. Lumin. 2016, 179, 146–153. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, F.; Zhang, Q.; Liu, W.; Dou, R.; Ding, S.; Luo, J.; Sun, D.; Sun, G.; Wang, X. Growth, structure and spectroscopic properties of 1 at.% Er3+: GdTaO4 laser crystal. J. Lumin. 2017, 192, 555–561. [Google Scholar] [CrossRef]
- Dou, R.; Zhang, Q.; Gao, J.; Chen, Y.; Ding, S.; Peng, F.; Liu, W.; Sun, D. Rare-Earth Tantalates and Niobates Single Crystals: Promising Scintillators and Laser Materials. Crystals 2018, 8, 55. [Google Scholar] [CrossRef]
- Karsu, E.; Popovici, E.; Ege, A.; Morar, M.; Indrea, E.; Karali, T.; Can, N. Luminescence study of some yttrium tantalate-based phosphors. J. Lumin. 2011, 131, 1052–1057. [Google Scholar] [CrossRef]
- Shcherbina, O.B.; Masloboeva, S.M.; Palatnikov, M.N.; Efremov, V.V. Synthesis, structure, luminescent and mechanical properties of YNbxTa1–xO4 solid solutions. J. Struct. Chem. 2021, 62, 1715–1722. [Google Scholar] [CrossRef]
- Blasse, G.; Bril, A. Luminescence phenomena in compounds with fergusonite structure. J. Lumin. 1970, 3, 109–131. [Google Scholar] [CrossRef]
- Ning, K.; Zhang, Q.; Zhang, D.; Fan, J.; Sun, D.; Wang, X.; Hang, Y. Crystal growth, characterization of NdTaO4: A new promising stoichiometric neodymium laser material. J. Cryst. Growth 2014, 388, 83–86. [Google Scholar] [CrossRef]
- Popovici, E.-J.; Nazarov, M.; Muresan, L.; Noh, D.Y.; Morar, M.; Bica, E.; Indrea, E. Studies on terbium activated yttrium based tantalate phosphors. Radiat. Meas. 2010, 45, 300–303. [Google Scholar] [CrossRef]
- Huo, J.; Zhu, J.; Wu, S.; Zhang, H.; Wang, Q. Influence of processing parameters on the luminescence of Eu3+ activated YTa1−xNbxO4 phosphors by a molten salt method. J. Lumin. 2015, 158, 417–421. [Google Scholar] [CrossRef]
- Palatnikov, M.; Shcherbina, O.; Smirnov, M.; Masloboeva, S.; Efremov, V.; Andryushin, K. Synthesis and Optical Characteristics of Gd0.96Eu0.01Sm0.01Tb0.01Er0.01Nb0.9Ta0.1O4 Ceramic Solid Solutions Prepared under Different Temperature Conditions. Ceramics 2022, 5, 499–515. [Google Scholar] [CrossRef]
- Priya, R.S.; Chaudhary, P.; Kumar, E.R.; Balamurugan, A.; Srinivas, C.; Prasad, G.; Deepty, M.; Kumar, K.P.; Yadav, B.; Sastry, D.; et al. Effect of heat treatment on structural, morphological, dielectric and magnetic properties of Mg–Zn ferrite nanoparticles. Ceram. Int. 2022, 48, 15243–15251. [Google Scholar] [CrossRef]
- Devesa, S.; Rodrigues, J.; Teixeira, S.; Rooney, A.; Graça, M.; Cooper, D.; Monteiro, T.; Costa, L. Tuning Green to Red Color in Erbium Niobate Micro- and Nanoparticles. Nanomaterials 2021, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Wang, X.; Lv, J.; Li, Y.; Tang, B. Luminescent nanoprobes for in-vivo bioimaging. TrAC Trends Anal. Chem. 2014, 58, 112–119. [Google Scholar] [CrossRef]
- Zamoryanskaya, M.V.; Konnikov, S.G.; Zamoryanskii, A.N. A High-Sensitivity System for Cathodoluminescent Studies with the Camebax Electron Probe Microanalyzer. Instruments Exp. Tech. 2004, 47, 477–483. [Google Scholar] [CrossRef]
- Belikov, M.L.; Sedneva, T.A.; Lokshin, E.P. Synthesis, Properties, and Visible Light Photocatalytic Activity of Nonstoichiometric Titanium Dioxide-Based Composites. Inorg. Mater. 2020, 56, 723–733. [Google Scholar] [CrossRef]
- Jehng, J.M.; Wachs, I.E. Structural chemistry and Raman spectra of niobium oxides. Chem. Mater. 1991, 3, 100–107. [Google Scholar] [CrossRef]
- Ferguson, R.B. The crystallography of synthetic YTaO4 and fused fergusonite. Can. Mineral. 1957, 6, 72–77. [Google Scholar]
- Stubican, V.S. High-Temperature Transitions in Rare-Earth Niobates and TantaIates. J. Am. Ceram. Soc. 1964, 47, 55–58. [Google Scholar] [CrossRef]
- Mather, S.A.; Davies, P.K. Nonequilibrium Phase Formation in Oxides Prepared at Low Temperature: Fergusonite-Related Phases. J. Am. Ceram. Soc. 1995, 78, 2737–2745. [Google Scholar] [CrossRef]
- Brixner, L.H.; Chen, H. On the Structural and Luminescent Properties of the M ′ LnTaO4 Rare Earth Tantalates. J. Electrochem. Soc. 1983, 130, 2435–2443. [Google Scholar] [CrossRef]
- Keller, C. Über ternäre Oxide des Niobs und Tantals vom Typ ABO4. Z. Anorg. Allg. Chem. 1962, 318, 89–106. [Google Scholar] [CrossRef]
- Nishio-Hamane, D.; Minakawa, T.; Ohgoshi, Y. Takanawaite-(Y), a new mineral of the M-type polymorph with Y(Ta, Nb)O4 from Takanawa Mountain, Ehime Prefecture, Japan. J. Miner. Pet. Sci. 2013, 108, 335–344. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, G.; Yang, L.; Zhou, Y.; Du, Y. Thermodynamic modeling of YO1.5-TaO2.5 system and the effects of elastic strain energy and diffusion on phase transformation of YTaO4. J. Eur. Ceram. Soc. 2019, 39, 5036–5047. [Google Scholar] [CrossRef]
- Feng, J.; Shian, S.; Xiao, B.; Clarke, D.R. First-principles calculations of the high-temperature phase transformation in yttrium tantalate. Phys. Rev. B 2014, 90, 094102. [Google Scholar] [CrossRef]
- Xiao, W.; Yang, Y.; Pi, Z.; Zhang, F. Phase Stability and Mechanical Properties of the Monoclinic, Monoclinic-Prime and Tetragonal REMO4 (M = Ta, Nb) from First-Principles Calculations. Coatings 2022, 12, 73. [Google Scholar] [CrossRef]
- Wu, F.; Wu, P.; Zhou, Y.; Chong, X.; Feng, J. The thermo-mechanical properties and ferroelastic phase transition of RENbO4 (RE = Y, La, Nd, Sm, Gd, Dy, Yb) ceramics. J. Am. Ceram. Soc. 2019, 103, 2727–2740. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, J.; Zhang, P.; Meng, X.; Cao, S.; Wu, J.; Wei, M.; Shi, Y.; Reece, M.J.; Gao, F. Enhanced mechanical and thermal properties of ferroelastic high-entropy rare-earth-niobates. Scr. Mater. 2021, 200, 113912. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, B.; Zhao, Z. Microscopic theory of hardness and design of novel superhard crystals. Int. J. Refract. Met. Hard Mater. 2012, 33, 93–106. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Q.; Lü, M.; Qiu, Z.; Zhang, A. Combustion Synthesis and Photoluminescence Properties of YNbO4-Based Nanophosphors. J. Phys. Chem. C 2008, 112, 19901–19907. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Q.; Zhou, W.; Gu, C.; Yin, S. Growth and Luminescence of M-Type GdTaO4 and Tb:GdTaO4 Scintillation Single Crystals. IEEE Trans. Nucl. Sci. 2010, 57, 1287–1290. [Google Scholar] [CrossRef]
- Xiao, X.; Yan, B. Synthesis and luminescent properties of novel RENbO4:Ln3+ (RE = Y, Gd, Lu; Ln = Eu, Tb) micro-crystalline phosphors. J. Non-Cryst. Solids 2005, 351, 3634–3639. [Google Scholar] [CrossRef]
- Si, J.; Yang, N.; Xu, M.; Li, G.; Cai, G.; Yi, W.; Zhang, J. Structure and tunable luminescence in Sm3+/Er3+ doped host-sensitized LaNbO4 phosphor by energy transfer. Ceram. Int. 2020, 46, 28373–28381. [Google Scholar] [CrossRef]
- Voloshyna, O.; Boiaryntseva, I.; Spassky, D.; Sidletskiy, O. Luminescence Properties of the Yttrium and Gadolinium Tantalo-Niobates. Sol. St. Phenom. 2015, 230, 172–177. [Google Scholar] [CrossRef]
- Xie, M.; Li, Y.; Li, R. Na2CaSiO4:Eu3+-deep red-emitting phosphors with intense 5D0→7F4 transition. J. Lumin. 2013, 136, 303–306. [Google Scholar] [CrossRef]
- Voloshyna, O.V.; Boiaryntseva, I.A.; Baumer, V.N.; Ivanov, A.I.; Korjik, M.V.; Sidletskiy, O.T. New, dense, and fast scintillators based on rare-earth tantalo-niobates. Nucl. Instruments Methods Phys. Res. Sect. A 2014, 764, 227–231. [Google Scholar] [CrossRef]

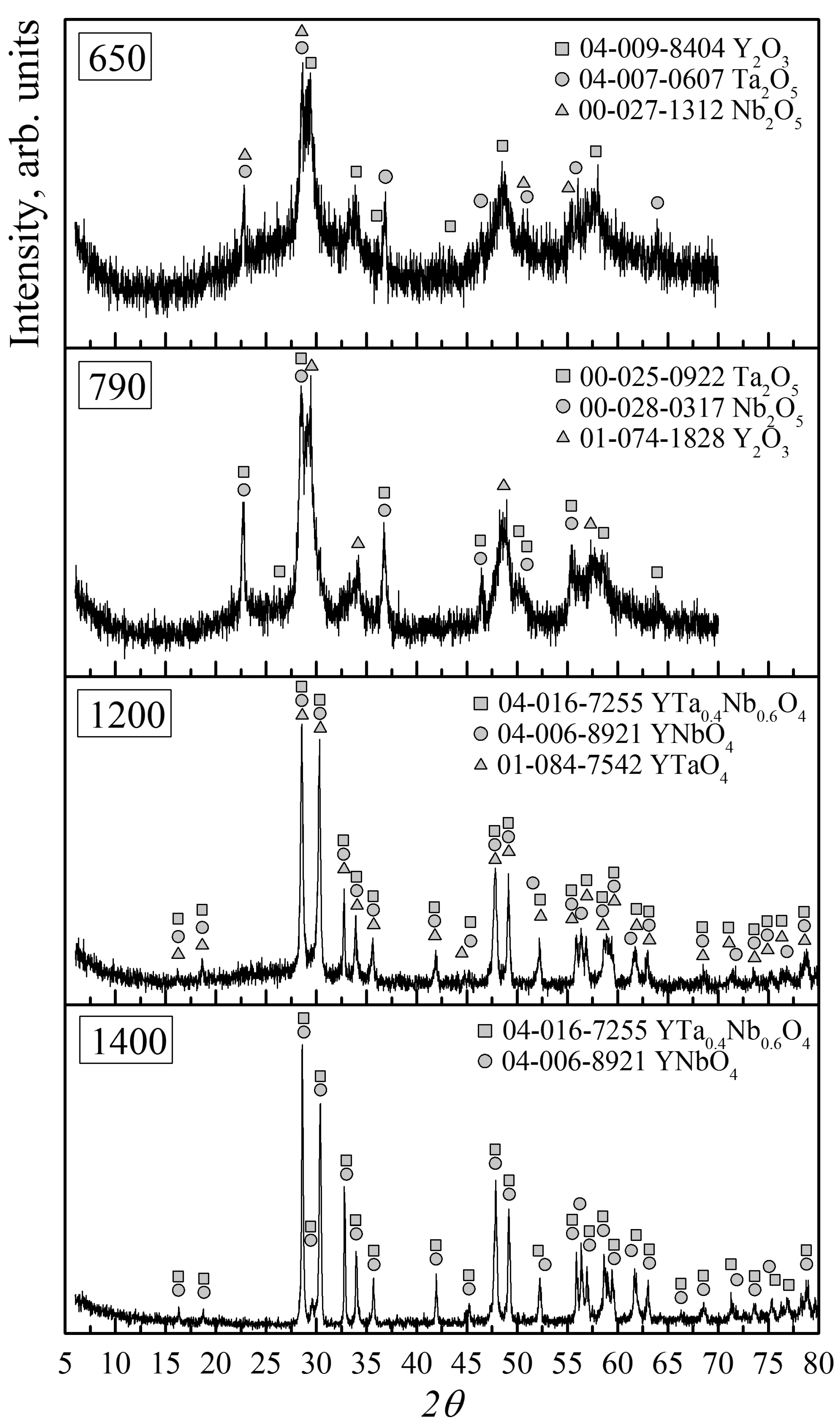
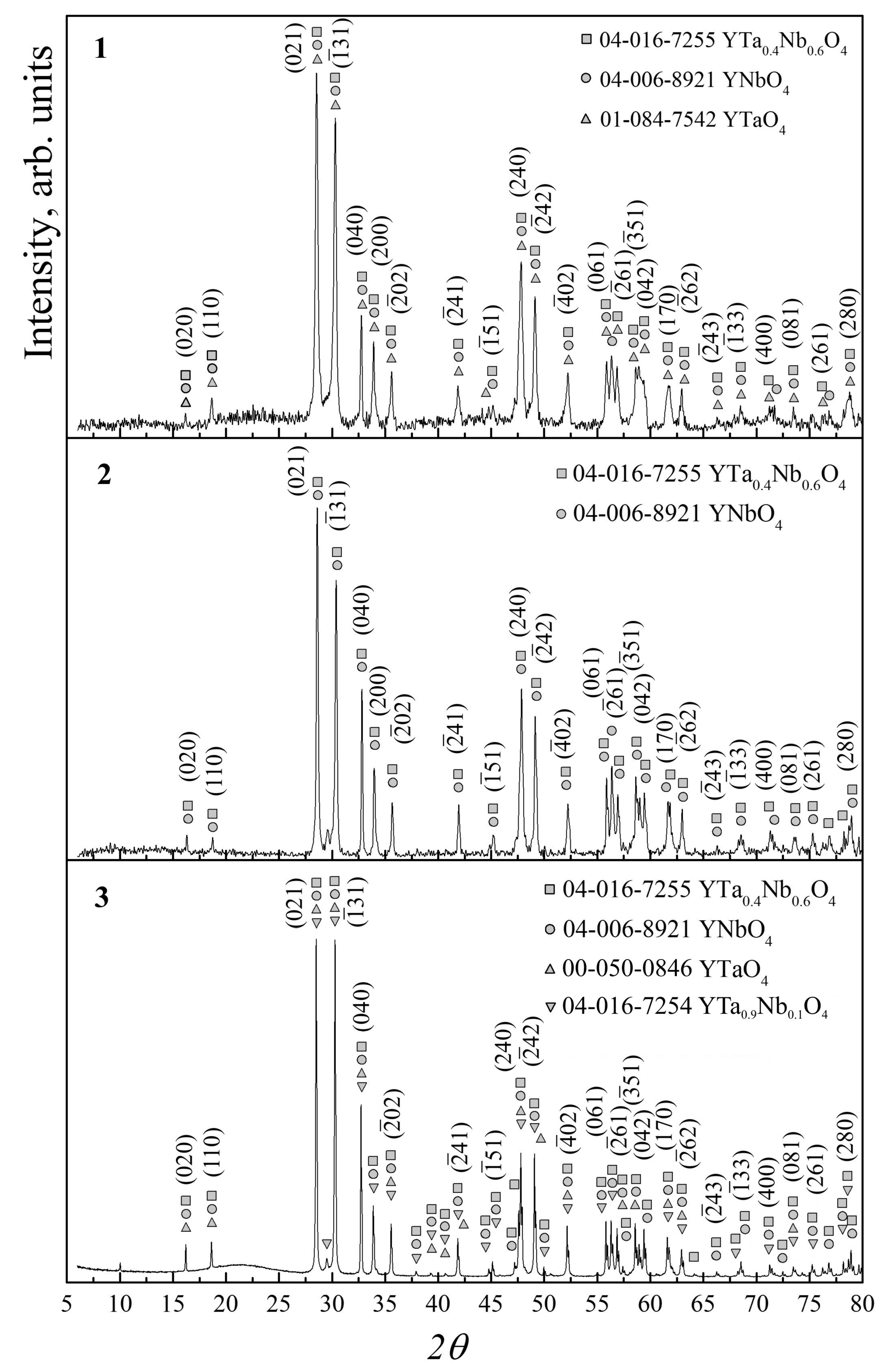
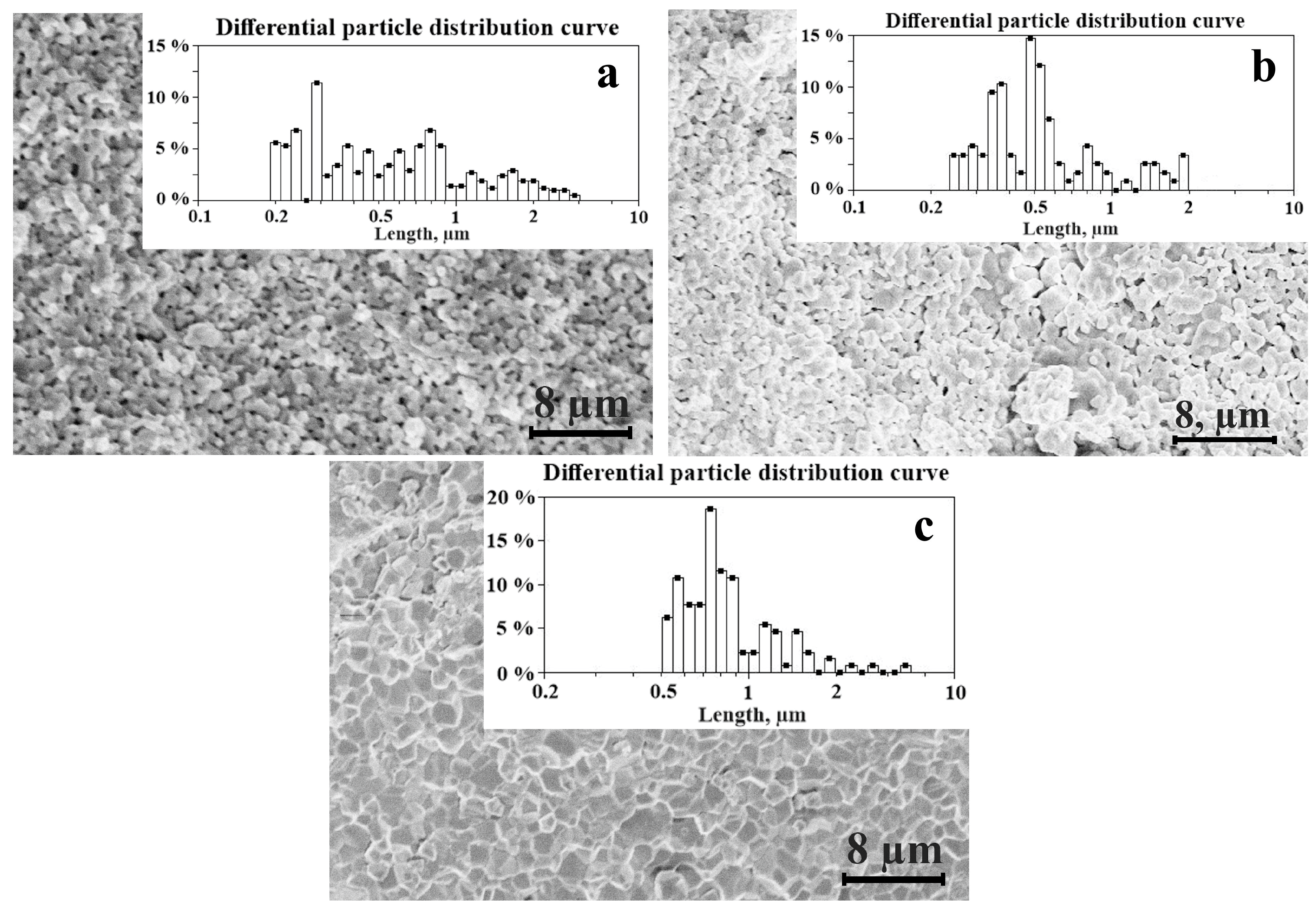

| Sample Number | Powder Calcination Temperature, Tc °С | Ceramic Sintering Temperature, Ts °С | Сeramics Sintering Time, h |
|---|---|---|---|
| 1 | 1200 | 1400 | 3 |
| 2 | 1200 | 1500 | 2 |
| 3 | 1400 | 1500 | 2 |
| Sample | Nb | Ta | Eu | Sm | Tb | Er |
|---|---|---|---|---|---|---|
| (Y0.96Eu0.01Sm0.01Tb0.01Er0.01)Nb0.7Ta0.3O4 | 0.69 ± 0.02 | 0.31 ± 0.020 | 0.0072 ± 0.0010 | 0.0056 ± 0.0020 | 0.0075 ± 0.0010 | 0.0068 ± 0.0005 |
| Sample 1 | YTa0.4Nb0.6O4 with SPGR C2/с (ICDD Card 04-016-7255) | ||||||
|---|---|---|---|---|---|---|---|
| a = 7.0175(9), b = 10.9421(2), c = 5.3060(1) Å, β = 134.01(5)° | a = 7.037, b = 10.945, c = 5.298 Å, β = 134.07° | ||||||
| atom | G | x/a | y/b | z/c | x/a | y/b | z/c |
| O1 | 1.0 | 0.2452(1) | 0.0409(1) | 0.3399(2) | 0.2442 | 0.0418 | 0.3374 |
| O2 | 1.0 | 0.2839(2) | 0.2847(7) | 0.2983(5) | 0.2919 | 0.2819 | 0.2968 |
| Nb | 0.7 | 0.0 | 0.1458(7) | 0.25 | 0.0 | 0.1445 | 0.25 |
| Ta(Nb) | 0.3 | 0.0 | 0.1437(3) | 0.25 | |||
| Y | 0.93 | 0.0 | 0.6202(1) | 0.25 | 0.0 | 0.6212 | 0.25 |
| Tb(Y) | 0.01 | 0.0 | 0.6333(5) | 0.25 | |||
| Er(Y) | 0.007 | 0.0 | 0.6035(5) | ||||
| Eu(Y) | 0.01 | 0.0 | 0.6212(5) | 0.25 | |||
| Sm(Y) | 0.005 | 0.0 | 0.6297(5) | 0.25 | |||
| Fomula | YTa0.4Nb0.6O4 | YNbO4 | ||
|---|---|---|---|---|
| ICDD Card | 04-016-7255 | 04-006-8921 | ||
| Syngony | Monoclinic | Monoclinic | ||
| SPGR | C2/c, M´-type | Sample 2 | C2/c, M´-type | Sample 2 |
| a, Å | 7.0089 | 7.01484 | 7.0297 | 7.03387 |
| b, Å | 10.9394 | 10.54867 | 10.937 | 10.94350 |
| c, Å | 5.0628 | 5.06709 | 5.069 | 5.07201 |
| α, ° | 90.000 | 90.000 | 90.000 | 90.000 |
| β, ° | 130.991 | 130.991 | 131.374 | 131.374 |
| γ, ° | 90.000 | 90.000 | 90.000 | 90.000 |
| V, Å3 | 293.00 | 293.02 | 292.45 | 292.45 |
| Deformation, % | 0.0(3) | 0.0(2) | ||
| Weight fraction in the sample, wt% | 91.1(3) | 8.88(19) | ||
| Formula | YTa0.4Nb0.6O4 | YNbO4 | YTa0.9 Nb0.1O4 | YTaO4 | ||
|---|---|---|---|---|---|---|
| ICDD Card | 04-016-7255 | 04-006-8921 | 04-016-7254 | 00-050-0846 | ||
| Syngony SPGR | Monoclinic C2/c | Monoclinic C2/c | Monoclinic P2/c (13) M-type | Tetragonal P42/mmc (131) | ||
| Sample 3 | Sample 3 | Sample 3 | Card | Sample 3 | Card | |
| a, Å | 7.02074(13) | 6.81(4) | 5.26(3) | 5.1107 | 3.654(8) | 3.648(5) |
| b, Å | 10.9490(3) | 10.06(6) | 5.47(4) | 5.4469 | 3.654(8) | 3.648(5 |
| c, Å | 5.06794(10) | 4.96(3) | 5.11 (14) | 5.2989 | 5.478(7) | 5.466(9) |
| α, ° | 90.000 | 90.000 | 90.000 | 90.000 | 90.000 | 90.000 |
| β, ° | 131.0853(10) | 120.0(4) | 96.54(1) | 96.43 | 90.20(11 | 90.000 |
| γ, ° | 90.000 | 90.000 | 90.000 | 90.000 | 90.000 | 90.000 |
| V, Å3 | 293.633 | 294.350 | 146.62 | 146.58 | 73.18 | 72.74 |
| Deformation, % | 0.0(3) | 2.58(18) | 0.0(3) | 0.0(14) | ||
| Weight fraction in the sample, wt% | 84.6(7) | 6.6(4) | 7.4(2) | 2.3(7) | ||
| Sample | ρexp | ρrel, % | Microhardness, H, GPa | Young’s Modulus, E, GPa | Crack Resistance KIC, MPa m0.5 |
|---|---|---|---|---|---|
| 1 | 4.06 | 69.82 | 4.04 ± 0.6 | 176.8 ± 2.5 | 0.88 ± 0.1 |
| 2 | 4.35 | 74.87 | 4.85 ± 0.5 | 269.9 ± 1.9 | 1.0 ± 0.8 |
| 3 | 4.68 | 80.55 | 7.2 ± 1.0 | 306.0 ± 5.0 | 1.15 ± 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palatnikov, M.; Shcherbina, O.; Smirnov, M.; Masloboeva, S.; Efremov, V. Structure, Mechanical and Luminescent Properties of Solid Solution (Y0.96Eu0.01Sm0.01Tb0.01Er0.01)Nb0.7Ta0.3O4. Ceramics 2023, 6, 86-101. https://doi.org/10.3390/ceramics6010007
Palatnikov M, Shcherbina O, Smirnov M, Masloboeva S, Efremov V. Structure, Mechanical and Luminescent Properties of Solid Solution (Y0.96Eu0.01Sm0.01Tb0.01Er0.01)Nb0.7Ta0.3O4. Ceramics. 2023; 6(1):86-101. https://doi.org/10.3390/ceramics6010007
Chicago/Turabian StylePalatnikov, Mikhail, Olga Shcherbina, Maxim Smirnov, Sofja Masloboeva, and Vadim Efremov. 2023. "Structure, Mechanical and Luminescent Properties of Solid Solution (Y0.96Eu0.01Sm0.01Tb0.01Er0.01)Nb0.7Ta0.3O4" Ceramics 6, no. 1: 86-101. https://doi.org/10.3390/ceramics6010007
APA StylePalatnikov, M., Shcherbina, O., Smirnov, M., Masloboeva, S., & Efremov, V. (2023). Structure, Mechanical and Luminescent Properties of Solid Solution (Y0.96Eu0.01Sm0.01Tb0.01Er0.01)Nb0.7Ta0.3O4. Ceramics, 6(1), 86-101. https://doi.org/10.3390/ceramics6010007







