1. Introduction
In recent years, low temperature cofired ceramics (LTCC) have become an attractive technology for electronic components and substrates that should be compact, light, and offer high speeds and functionality for portable electronic devices. For LTCC applications, the densification temperature of the dielectric ceramics (a material having lower dielectric loss than organic materials) must, however, be lower than the melting temperatures of metallic electrodes such as Ag (~961 °C) [
1]. The sintering temperature of most ceramics is over 1200 °C and markedly exceeds the melting temperature of the electrodes. This factor precludes the co-firing.
Microwave dielectric ceramics with low permittivity and low dielectric loss are usually oxides because of their low ionic polarizabilities [
2]. In addition, the limited research on halides shows that they also exhibit low permittivity [
2]. Within the family of halides, fluorides are typically the most lightweight. The low permittivity of fluorides is attributed [
2] to the lower ionic polarizability of F
− (1.62 Å
3) than O
2− (2.00 Å
3). Lithium fluoride, LiF, has been widely applied as an effective sintering additive or flux in many ceramic substances but only recently has the interest in the synthesis of pure LiF materials via conventional sintering and their dielectric properties begun.
One serious problem with the sintering of pure lithium fluoride is the difficulty in its densification [
2]. The sintering procedure is even more problematic for LiF than for alkaline earth fluorides. The highest relative density of LiF ceramics prepared by traditional sintering was only about 90% [
2]. The reason for the low density of LiF compacts is the pore network that breaks up at the initial stage of sintering. Due to them, the air in the isolated pores balances the sintering pressure and prevents further densification.
LiF has a melting point of approximately 845 °C [
3]. In connection with this, LiF is generally treated as an effective additive in achieving highly transparent ceramics, such as Y
2O
3-MgO, Y
3Al
5O
12 (YAG), and MgAl
2O
4 [
4], as well as dielectric materials such as MgO, CaWO
4, or Li
2TiO
3. Its compatibility with silver electrodes suggests that sintered lithium fluoride has great potential as a component or an additive in LTCC formulations [
1]. Liu et al. prepared a patch antenna based on LiF dielectric film and considered its performance as excellent [
5]. The electronic structure of pure LiF shows an absence of localized defect states in the band gap that play as charge carrier trapping centers [
5,
6].
The final relative density of sintered ceramics depends on the initial relative density of green compacts (typically 50–70% of the final one). The green density can be improved, also without optimizing the particle shape and powder size distribution, by increasing the compacting pressure up to hundreds of MPa. Particles exhibiting low hardness can be easily rearranged and reshaped by the mechanical load. As a disadvantage, however, the high uniaxial pressure during the firing may result in large residual stresses. Such stresses are responsible for the subsequent cracking of the compacts [
2]. LiF has a low Mohs hardness of about 3, so the high compacting pressure not only results in the rearrangement of LiF raw particles but also leads to the deformation of the particles. This enhances the removal of pores and increases the relative density. It means that the pressure-induced plastic deformation is important for the densification and particle refinement of the LiF compacts. An analogous mechanism [
2] has also been reported in different crystals with a rather low hardness, such as NaCl. Materials like Li
2MoO
4 and NaCl have high solubilities of 36.0 g and 44.8 g in 100 g of water [
2], whereas for LiF, it is only 0.13 g.
Residual pores are considered the most deleterious factor influencing the optical transmittance of fluoride ceramics [
4]. Hence, the fabrication of fluoride-based transparent ceramics generally requires advanced techniques such as vacuum sintering, hot isostatic pressing (HIP), and spark plasma sintering (SPS) [
4]. This knowledge calls for being transferred into the field of dielectrics as well.
Low surface free energy leads to a low driving force for the densification of LiF. Given the substantial surface diffusion, the breakup of pore channels into isolated voids begins early. The pressure of trapped air in the isolated pores, however, reduces the driving force for densification. The low driving force for densification and also gas entrapment are the main causes of low final density [
3].
Concerning the dielectric parameters received in earlier works with a pure LiF material, conventionally sintered LiF exhibited [
3] a relative permittivity of 9, a loss tangent of 0.078, and a temperature coefficient of capacitance of −118 ppm/K (measured between 12 and 15 GHz). Another team used a very innovative “cold sintering” approach to produce LiF [
1] with a permittivity of 8.2, a loss tangent of 0.135, and a temperature coefficient of −135 ppm/K. Relative permittivity speaks about polarization and the ability of materials to store the energy of the AC field if is it charged as a capacitor. Loss tangent is a parameter characterizing the quantity of energy dissipated into heat. The temperature coefficient describes how the capacitance is stable with the temperature. In applications of such low-loss ceramics, as LiF in general is, we need low permittivity and low loss (the material serves as a perfect insulator without energy dissipation or parasitic phenomena at a signal transfer, and moreover, all these parameters should be as thermally stable as possible to ensure the constant behavior of the circuit equipped with the LiF-based components versus the unavoidable temperature fluctuations in the environment.
We will show in the presented research that the relative density can be improved to values over 90% T.D. with the aid of a high-pressure forming (HPF) approach. The low sintering temperature is beneficial for the possible practical applications of LiF. The combination of its low permittivity and low loss tangent, both of them very stable with frequency, and also with the high DC resistivity, makes LiF a very prospective material. The temperature evolution of the dielectric properties of pure LiF ceramics was, however, until now, outside the focus of researchers. In our actual work, these investigations are conducted in up to 300 °C.
2. Experimental Section
2.1. Sample Preparation
With a motivation to produce high-quality LiF dielectrics, we applied high-pressure forming (HPF) [
7] before conventional sintering. This approach enabled us to avoid the addition of a plasticizer before the green body formation. A mechanical force corresponding to an uniaxial pressure of 300 MPa was applied for 1 min (ramps up and down 1 MPa per second) with the spark plasma sintering (SPS) apparatus without any heating. Stainless steel die and punches were used and the compressed LiF bodies were removed from the punch/die assembly with slow movements in a manual press. The compressed discs (20 mm diameter) were placed into a laboratory furnace and sintered in air. The procedure consisted of a heating rate of 7 °C/min, dwelling at maximum temperature, and a cooling rate of 7 °C/min down to room temperature. Because of the pronounced shrinkage, the produced samples were cylinders with 18.5 mm in diameter and 2–3 mm in height. The samples were classified according to the maximum temperature and dwell time, see
Table 1. For several tests, only the sample 700-2 as the extremely “low-sintered” and the sample 750-8 as a representative of “high-sintered” products were selected.
2.2. Structure Characterization
The phase composition was evaluated by means of X-ray diffraction (XRD), carried out by the powder diffractometer D8 Discover (Bruker, Germany). The Bragg–Brentano geometry with a 1D detector and Cu-Kα radiation was used. The scanned region started from 20 to 130° 2θ with a 0.03° 2θ step size and a 192 s counting time per step. The obtained diffraction patterns were subjected to TOPAS 5 software treatment.
Apparent density and open porosity were measured by the Archimedean (i.e., water immersion) method. The precision of these measurements is ±0.002 g/cm3 for the apparent density and about ±0.1% for the open porosity.
The cross-sectional optical micrographs were taken with a digital camera and analyzed using Lucia G software (Laboratory Imaging, Prague, Czech Republic). The cross-sections were observed also via scanning electron microscopy (SEM) using a Phenom-Pro microscope (Thermo Fisher Sci., Eindhoven, The Netherlands) equipped with a CeB6 thermionic cathode and working in backscattered electron (BSE) mode. The images were collected at 5 kV electron beam tension.
The microhardness of the samples was measured using a Hanemann microhardness head (Zeiss, Germany) mounted on an optical microscope with a fixed load of 1 N and a Vickers indenter. Twenty indentations made on randomly selected areas on the cross-section of each sample were analyzed.
2.3. Dielectric Parameters
Before placing the electrodes, the samples were ground with SiC papers to eliminate surface unevenness. The opposite faces of the cylindrical LiF samples were covered with an Al film in an evaporating apparatus using a mask. An assembly of three electrodes was applied to diminish the stray current effect. The bottom side was entirely coated, while the top side was equipped with an internal circle electrode of 12 mm in diameter and an external ring electrode (earth-connected during the measurements). This one was separated from the internal ring with a 1 mm gap. The electric field was applied along the same direction as the pressure before sintering (i.e., perpendicular to the cylinder face). The capacitance was measured using a programmable impedance analyzer, model 4284A (Agilent, Santa Clara, CA, USA), and a high-precision sample fixture 16451B (Agilent, Santa Clara, CA, USA). The relative permittivity
εr was calculated from the measured capacitance and LiF cylinder dimensions using the equation:
where
C (F) stands for the electrical capacitance of the sample,
d (m) is the sample thickness,
A (m
2) is the area of the measuring electrode, and
ε0 is the permittivity of vacuum (8.854 × 10
−12 F/m). The same setup was also used for the simultaneous loss tangent (Tan δ) measurement. The applied voltage was set to 1 ± 0.02 V. Measurements at elevated temperatures were carried out using a Novotherm Heating Unit 2108 (Novocontrol, Montabaur, Germany).
Electric resistance was examined at room temperature with a special adapter—Keithley model 6105. The DC electric field was applied from a regulated high-voltage source and the values were recorded with a multi-purpose electrometer (617C, Keithley Instruments, Solon, OH, USA). The magnitude of the applied voltage was 100 ± 0.05 V. Volume resistivity calculation was based on the measured resistance and specimen dimensions.
2.4. Reflectance and Band Gap
The optical diffuse reflectance spectra were collected using a UV-vis-NIR scanning spectrophotometer MPC 3100 (Shimadzu, Japan) with a multi-purpose large sample compartment. The reflectance curves were recorded between 250 nm and 1350 nm. The reflectance standard was applied, which is a barium sulfate (BaSO4) mirror with 100% reflection in the corresponding range.
The optical band gap energy was calculated using the Tauc relationship [
8]:
with the absorption coefficient
α, the photon energy
hν, a constant
A, and the optical band gap
Eg. The exponent
n value is equal to 2 for direct allowed transition and 1/2 for indirect allowed transition. Since LiF has a direct transition [
9], the used
n value is 2:
Correspondingly,
α is related to the Kubelka–Munk function
F:
where
R is the reflectance and
S is the scattering factor.
Eg [eV] is determined from the extrapolation of the linear part of the Tauc plot up to the intercept of the x-axis.
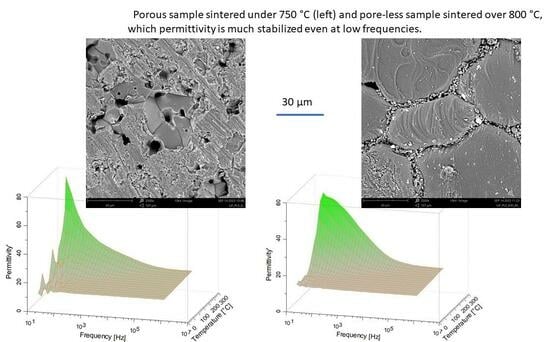
4. Conclusions
Lithium fluoride (LiF) processed via so-called high-pressure forming (HPF) plus subsequent furnace sintering is a good quality dielectric material. Its optimum sintering temperature was found to be slightly over 730 °C, and from this standpoint, it could be processed in the future in a solid phase together with silver electrodes without destroying their arrangement. At room temperature, LiF was fully comparable with oxide ceramics fired at temperatures over 1300 °C, for example, aluminum oxide or YAG. When we would like to shift the stability limit of LiF electronic components to higher temperatures, such as over 150 °C, we need to develop LiF ceramics with an even more intensively sintered microstructure. For this purpose, firing at 750 °C for 8 h after the HPF of the initial powder was recognized as the most suitable. The final product had a relative permittivity of 12.1, a loss tangent of 0.0005, and a DC resistivity of 27.4 × 1012 Ωm. Its permittivity and loss tangent were frequency-independent and temperature-independent up to at least 150 °C. The optical band gap was markedly larger than the electrical band gap. The optical band gap of the LiF samples was in the range 3.20–3.65 eV depending on the sintering conditions. With increasing sintering temperature and time, the microstructure improves (and could be improved even beyond the frames studied here) but the lattice-level polarization and charging phenomena seem to have a threshold at about 750 °C at the sintering step, above which the physical behavior of the samples is not markedly modified. Production programs based on a pure LiF material could dramatically shift the energy demand for the elaboration of low-loss dielectrics down in many application fields.