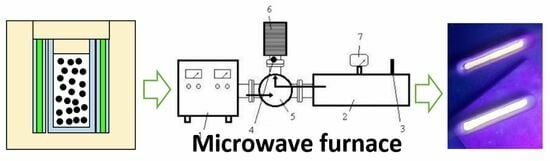
One-Step Microwave Synthesis of New Hybrid Phosphor (CSSC) for White Light-Emitting Diodes
Abstract
:1. Introduction
2. Materials and Methods
- -
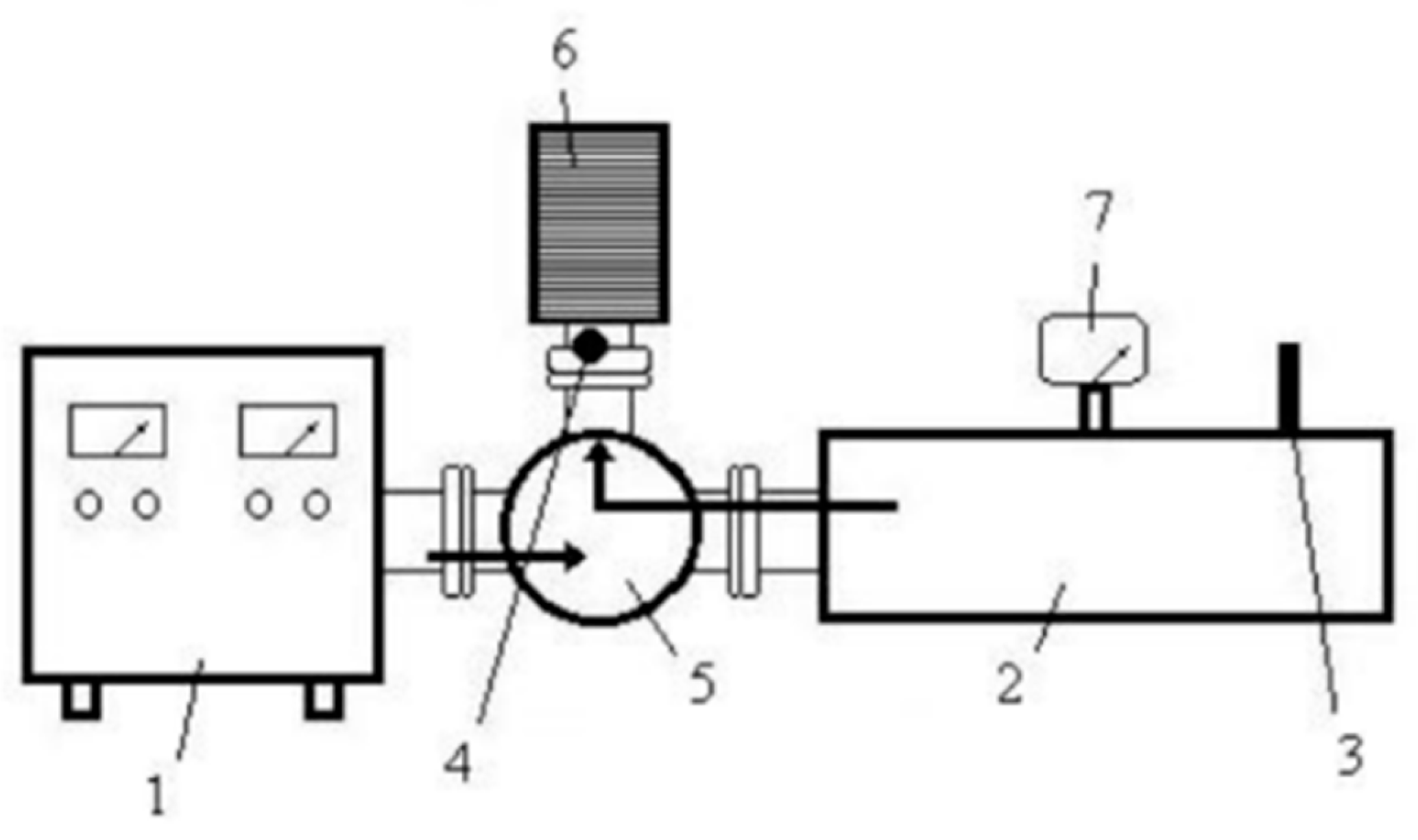
- The body of the working chamber was made of a piece of copper rectangular waveguide with a standard cross-section of 90 mm × 45 mm. This waveguide had the highest figure of merit for passing electromagnetic energy with a frequency of 2.45 GHz and power of 450 W. The length of the waveguide (235 mm) was selected to be a multiple of the length of the half-wave of the microwave radiation, ensuring that there were an integer number of zones of maximum intensity of the electromagnetic field (in this case, three bundles) formed in the chamber. The upper wall of the chamber had a loading hole in the zone of the middle beam for installing the thermostat with the object to be heated. There was also a hole with a diameter of 4 mm in the side wall of the thermostat and waveguide for temperature control using a pyrometer.
- -
- The purpose of the aperture is to allow microwave energy to enter the chamber and create a standing wave through the superposition of incoming and reflected waves from the opposite wall. The size of the aperture in the diaphragm is matched to the standing wave coefficient. However, when a dielectric load is placed in the chamber and heated, it can alter the standing wave coefficient and affect the level of matching between the chamber and the generator, causing an increase in the amount of reflected energy from the diaphragm.
- -
- The short-circuited movable wall, known as the plunger, is a control element used to adjust the length of the chamber. This adjustment can partially or completely align the phases of the waves and form energy bundles of a specific intensity.

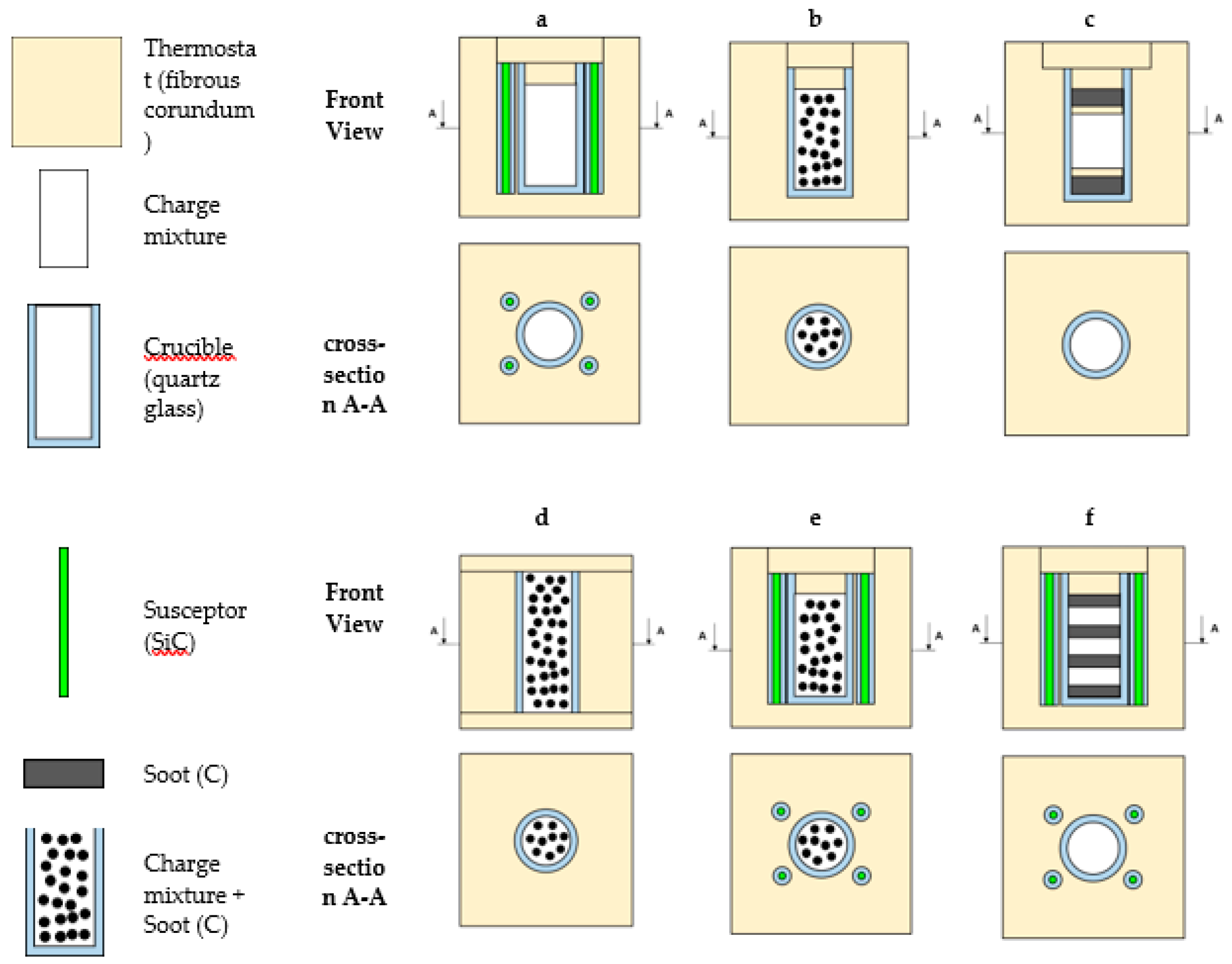
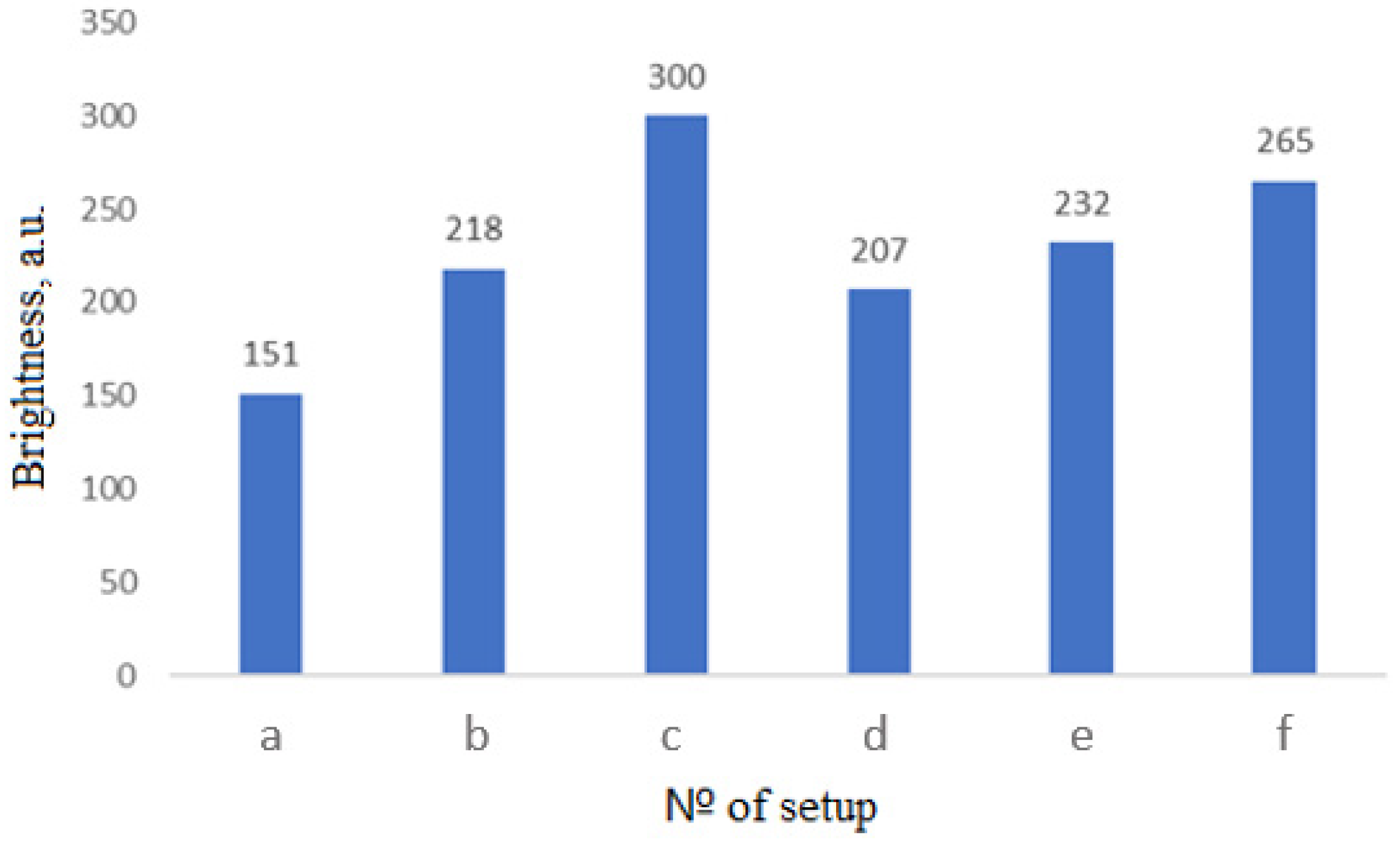
3. Results and Discussion
3.1. Selection of Thermostat Design for Synthesis in Microwave Furnace
- -
- Dielectrics (SiO2, Al2O3, MgO) have a low level of microwave absorption at room temperature, which increases as the temperature rises;
- -
3.2. Choice of Synthesis Technology (Muffle or Microwave Furnace)
3.3. Selection of Microwave Synthesis Parameters
3.4. Selection of Charge Preparation Method
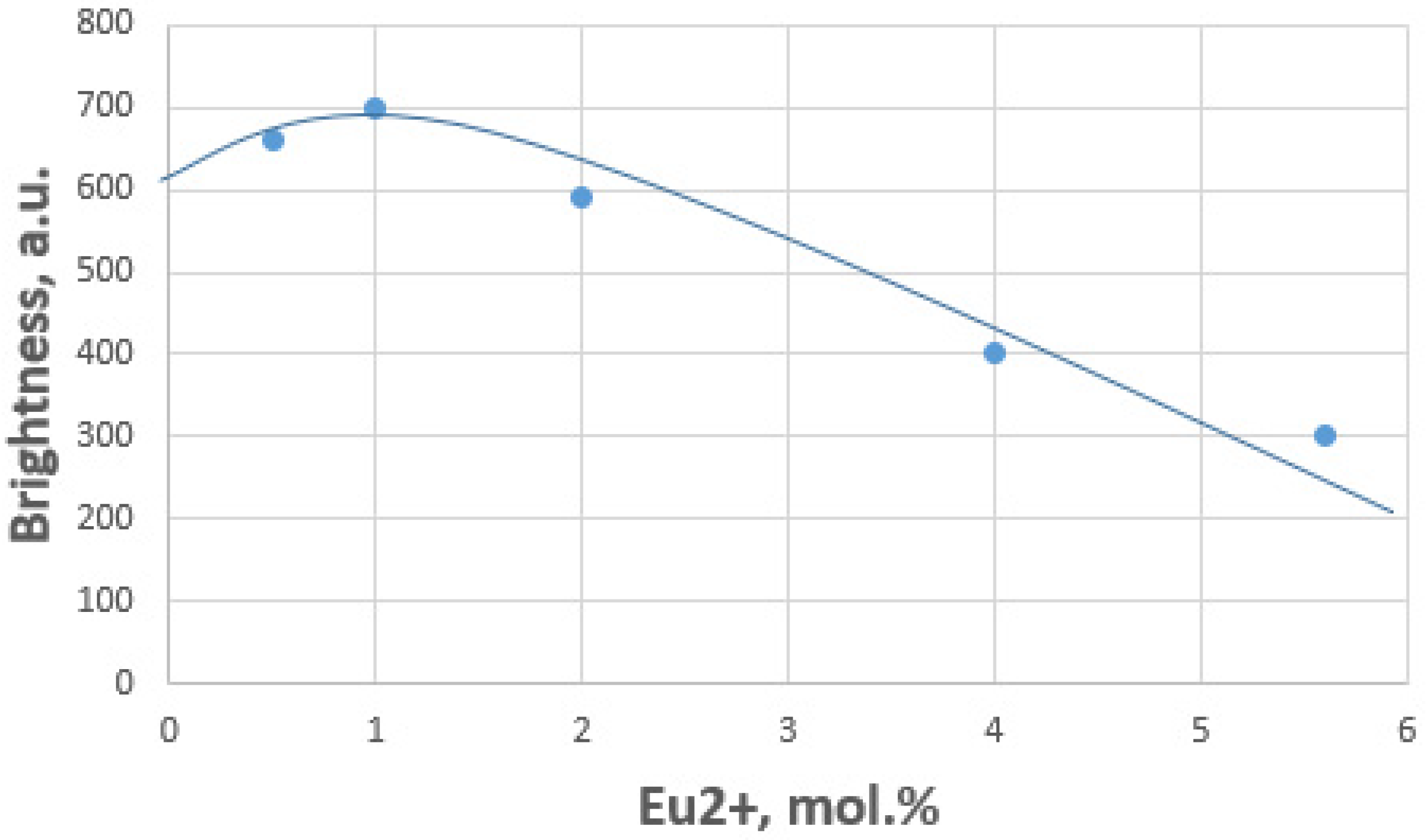
3.5. Selection of Chemical Composition in Microwave Synthesis
4. Conclusions
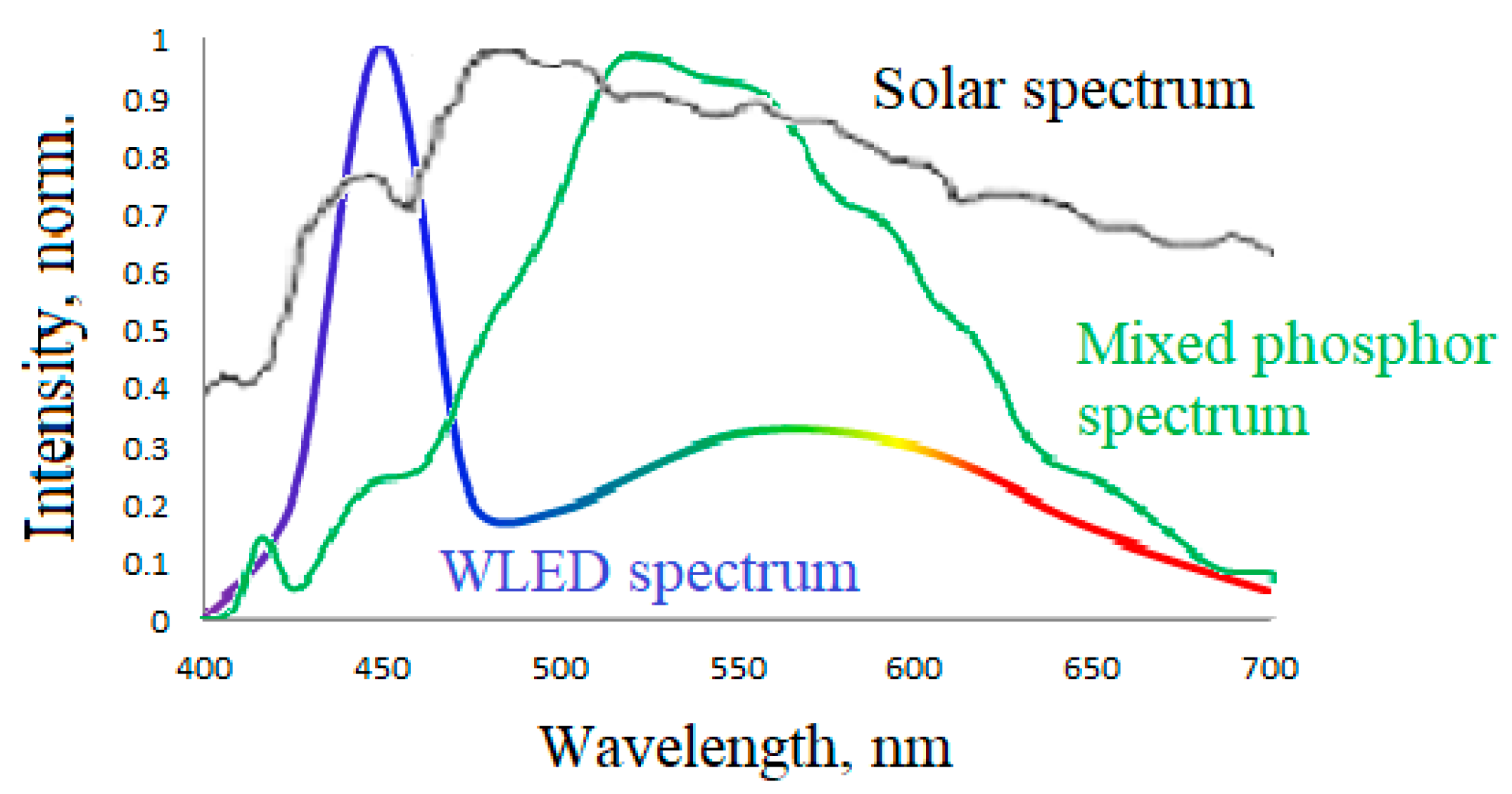
- A one-step method has been developed for synthesizing a new hybrid CSSC phosphor (0.5 CaSrSiO4:Eu2+: 0.29 Ca6Sr4Si6O21Cl2:Eu2+: 0.21 Ca10Si6O21Cl2:Eu2+) using microwave heating. The phosphor synthesized under optimal conditions exhibits a wide luminescence spectrum similar to sunlight, resulting in a high color rendering index and warm white luminescence color in WLEDs based on it.
- The sol–gel method for preparing the charge mixture for the hybrid phosphor (0.5 CaSrSiO4:Eu2+, 0.29 Ca10Si6O21Cl2:Eu2+, 0.21 Ca6Sr4(Si2O7)3Cl2:Eu2+) allows for 35% higher luminescence brightness compared to the solid-phase method, due to a more uniform distribution of the activator.
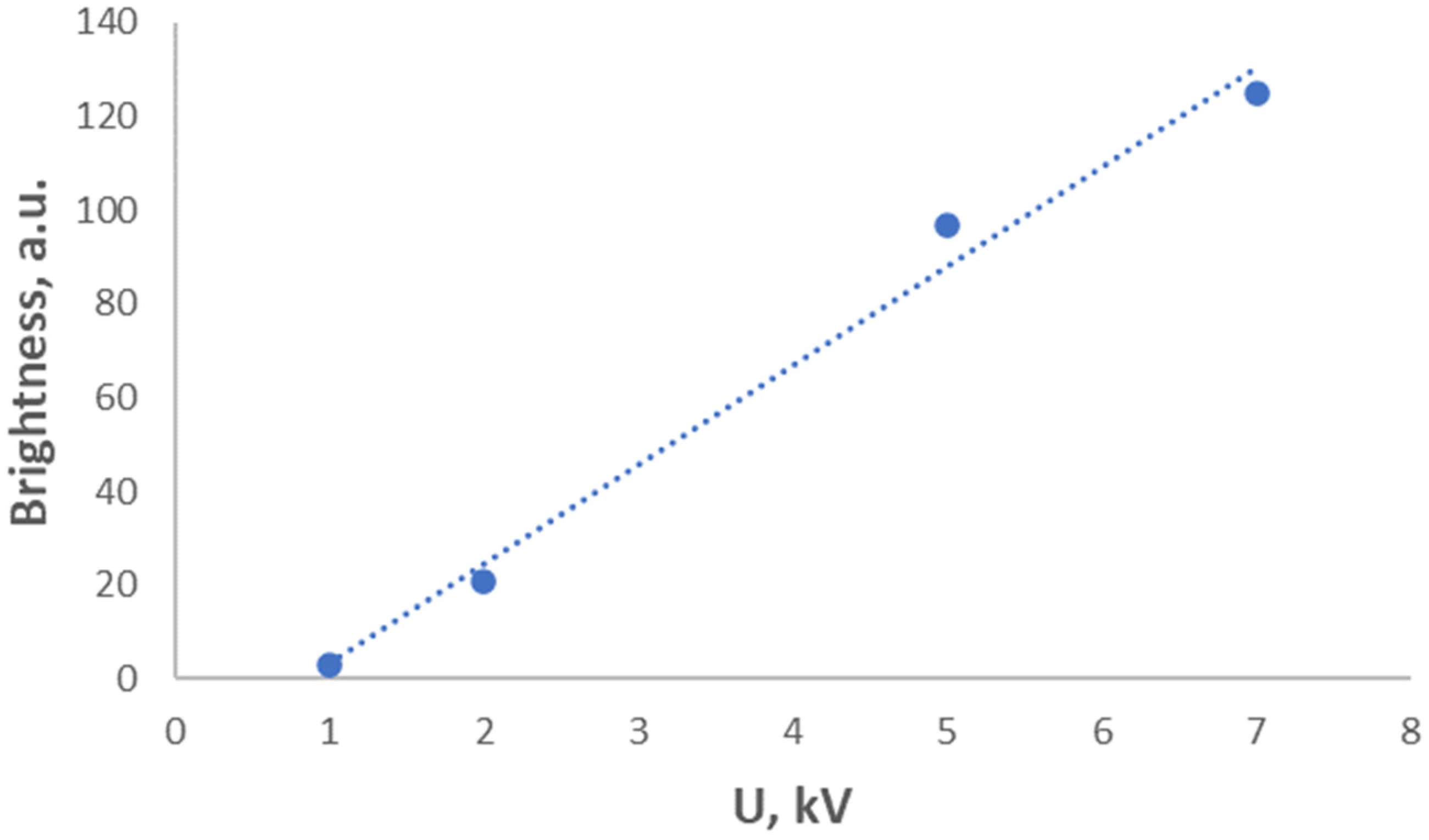
- A CSSC phosphor can be used in cathodoluminescent white light sources.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geiger, C.A. Garnet: A Key Phase in Nature, the Laboratory, and Technology. Elements 2013, 9, 447–452. [Google Scholar] [CrossRef]
- Berends, A.C.; van de Haar, M.A.; Krames, M.R. YAG:Ce3+ Phosphor: From Micron-Sized Workhorse for General Lighting to a Bright Future on the Nanoscale. Chem. Rev. 2020, 120, 13461–13479. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, T.; Koga, S.; Toda, N.; Noguchi, H.; Yasukouchi, A. Effects of short wavelength control in polychromatic light sources on nocturnal melatonin secretion. Neurosci. Lett. 2008, 439, 256–259. [Google Scholar] [CrossRef]
- Rea, M.S.; Figueiro, M.G.; Bierman, A.; Bullough, J.D. Circadian light. J. Circadian Rhythm. 2010, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Kumar, U.; Upadhyay, S. Study of structural, electrical, and photoluminescent properties of SrCeO3 and Sr2CeO4. J. Adv. Ceram. 2019, 8, 377–388. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, L.; Devakumar, B.; Liang, J.; Wang, S.; Sun, Q.; Dhoble, S.J.; Huang, X. Novel highly luminescent double-perovskite Ca2GdSbO6:Eu3+ red phosphors with high color purity for white LEDs: Synthesis, crystal structure, and photoluminescence properties. J. Lumin. 2020, 221, 117105. [Google Scholar] [CrossRef]
- Sharma, G.; Poddar, P. Organic–Inorganic Hybrids for White-Light Phosphors. In Hybrid Phosphor Materials; Springer: Cham, Switzerland, 2022; pp. 105–118. [Google Scholar] [CrossRef]
- Xu, D.; Liu, J.; Sun, C.; Wang, L.; Han, J.; Tao, J.; Zhang, H.; Wei, T.; Su, S.; Liu, X.; et al. Copper iodide-based hybrid phosphors for eco-friendly white-light-emitting diodes. J. Lumin. 2022, 244, 118733. [Google Scholar] [CrossRef]
- Ahn, Y.N.; Kim, K.D.; Anoop, G.; Kim, G.S.; Yoo, J.S. Design of highly efficient phosphor-converted white light-emitting diodes with color rendering indices (R1 − R15) ≥ 95 for artificial lighting. Sci. Rep. 2019, 9, 16848. [Google Scholar] [CrossRef]
- Vasina, O.U. Sol-Gel Phosphors on the Basis of Silicates of Second Group Element. Ph.D. Thesis, D. Mendeleyev University of Chemical Technology of Russia, Moscow, Russia, 2003. [Google Scholar]
- Park, J.K.; Choi, K.J.; Kim, C.H.; Park, H.D.; Choi, S.Y. Optical Properties of Eu2+-Activated Sr2SiO4 Phosphor for Light-Emitting Diodes. Electrochem. Solid-State Lett. 2004, 7, H15–H17. [Google Scholar] [CrossRef]
- Kim, J.S.; Jeon, P.E.; Choi, J.C.; Park, H.L. Emission color variation of M2SiO4: Eu2+ (M = Ba, Sr, Ca) phosphors for light-emitting diode. Solid State Commun. 2005, 133, 187–190. [Google Scholar] [CrossRef]
- Barry, T.L. Fluorescence of Eu2+ activated phase in binary alkaline earth orthosilicate systems. J. Electrochem. 1968, 115, 1181–1184. [Google Scholar] [CrossRef]
- Wang, J.; Qin, L.; Huang, Y.; Wei, D.; Seo, H.J. Color centers and dynamic emission of Eu2+ ion doped halosilicate Ca10Si6O21Cl2. Appl. Phys. 2014, 115, 1215–1221. [Google Scholar] [CrossRef]
- Li, Z.; Gao, S.; Chen, X.; Yu, Q. Synthesis and luminescence properties of green phosphors Ca6Sr4(Si2O7)3Cl2:Eu2+ for white light emitting diodes. J. Lumin. 2012, 132, 1497–1500. [Google Scholar] [CrossRef]
- Xia, Z.; Sun, J.; Laamanen, T. Luminescence of Eu2+ in alkali earth chlorosilicate phosphor and their color-tunable properties. Opt. Mater. 2006, 28, 524–529. [Google Scholar] [CrossRef]
- Sasaki, Y.; Daicho, H.; Auyagi, S.; Sawa, H. Phosphor and Light-Emitting Device. U.S. Patent No. 0256222, 11 October 2012. [Google Scholar]
- Keskinova, M.V.; Ogurtsov, K.A.; Sychov, M.M.; Kolobkova, E.V.; Turkin, I.A.; Nakanishi, Y.; Hara, K. Synthesis of chlorine-silicate phosphors for white light-emitting diodes. Adv. Mater. Res. 2015, 1117, 48–51. [Google Scholar] [CrossRef]
- Turkin, I.A.; Keskinova, M.V.; Sychov, M.M.; Ogurtsov, K.A.; Hara, K.; Nakanishi, Y.; Shilova, O.A. Microwave Synthesis of Eu-doped Silicate Phosphors. In Proceedings of the JJAP Conference Proceedings 14th International Conference on Global Research and Education, Inter-Academia 2015, Hamamatsu, Japan, 28–30 September 2015; Volume 4, pp. 011108–011114. [Google Scholar]
- Turkin, I.A.; Keskinova, M.V.; Sychov, M.M.; Ogurtsov, K.A.; Hara, K.; Nakanishi, Y.; Shilova, O.A. Cathodoluminescent Properties and Particle Morphology of Eu-Doped Silicate Phosphors Synthesized in Microwave Furnace. Adv. Intell. Syst. Comput. 2017, 519, 339–346. [Google Scholar]
- Keskinova, M.V.; Eremeeva, M.A.; Sychev, M.M. Single-Stage Microwave Synthesis of Mixed Luminophor for Light Sources. Glass Phys. Chem. 2021, 47, 360–365. [Google Scholar] [CrossRef]
- Keskinova, M.V.; Verzunov, P.P.; Turkin, I.A.; Sychev, M.M. Activation of Zr0.95−xY0.05O2:Eu3+x by heat treatment in an electromagnetic field of the microwave range. Glass Phys. Chem. 2019, 45, 501–505. [Google Scholar] [CrossRef]
- Rubakov, K.I.; Semenov, V.E. A non-thermal vacancy drift mechanism of plastic deformation of grains in ceramics microwave sintering. MRS Online Proc. Libr. 1994, 347, 661–666. [Google Scholar] [CrossRef]
- Bonifas, C.J.; Marconnet, J. Perry; Microwave–induced mass transport enhancement in nano-porous aluminum oxide membranes. J. Microw. Power Electromagn. Energy 2008, 42, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Janne, M.A.; Kimrey, H.D.; Kiggins, J.O. Microwave proceedings of ceramics: Guide-lines used of the Oak Ridge Laboratory. MRS Online Proc. Libr. 1992, 269, 173–185. [Google Scholar] [CrossRef]
- Unifantowicz, P.; Vaucher, S.; Lewandowska, M.; Kurzydłowski, K.J. Mechanism of SiC crystals growth on {100} and {111} diamond surfaces upon microwave heating. Mater. Charact. 2010, 61, 648–652. [Google Scholar] [CrossRef]
- Ayyappadas, C.; Annamalai, A.R.; Agrawal, D.K.; Muthuchamy, A. Conventional and microwave assisted sintering of copper-silicon carbide metal matrix composites: A comparison. Metall. Res. Technol. 2017, 114, 506–516. [Google Scholar] [CrossRef]
- Madhan, M.; Prabhakaran, G. Microwave versus conventional sintering: Microstructure and mechanical properties of Al2O3–SiC ceramic composites. Boletín Soc. Española Cerámica Vidr. 2018, 58, 14–22. [Google Scholar] [CrossRef]
- Sutton, W.H. Microwave Firing of High–Alumina Ceramics. MRS Online Proc. Libr. 1992, 269, 3–20. [Google Scholar] [CrossRef]
- Spotz, M.S.; Skamser, D.J.; Jonson, D.L. Thermal stability of ceramic materials in microwave heating. J. Am. Ceram. Soc. 1995, 78, 1041–1048. [Google Scholar] [CrossRef]
- Kumar, U.; Upadhyay, S.; Alvi, P.A. Study of reaction mechanism, structural, optical and oxygen vacancy-controlled luminescence properties of Eu-modified Sr2SnO4 Ruddlesden popper oxide. Phys. B Condens. Matter 2020, 604, 412708. [Google Scholar] [CrossRef]
- Tezuka, S.; Sato, Y.; Komukai, T.; Takatsuka, Y.; Kato, H.; Kakihana, M. Eu2+-Activated CaSrSiO4: A New Red-Emitting Oxide Phosphor for White-Light-Emitting Diodes. Appl. Phys. Express 2013, 6, 072101. [Google Scholar] [CrossRef]

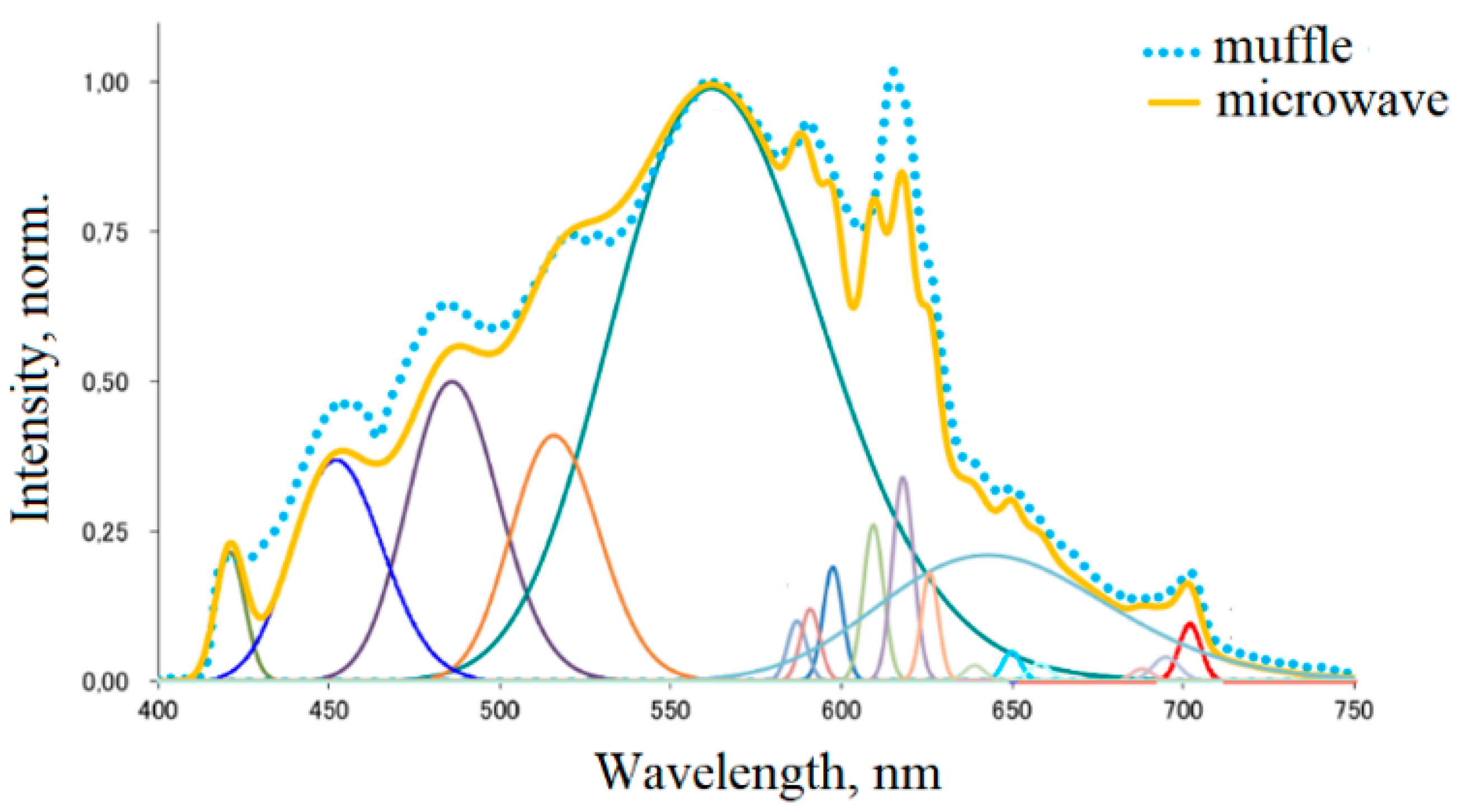

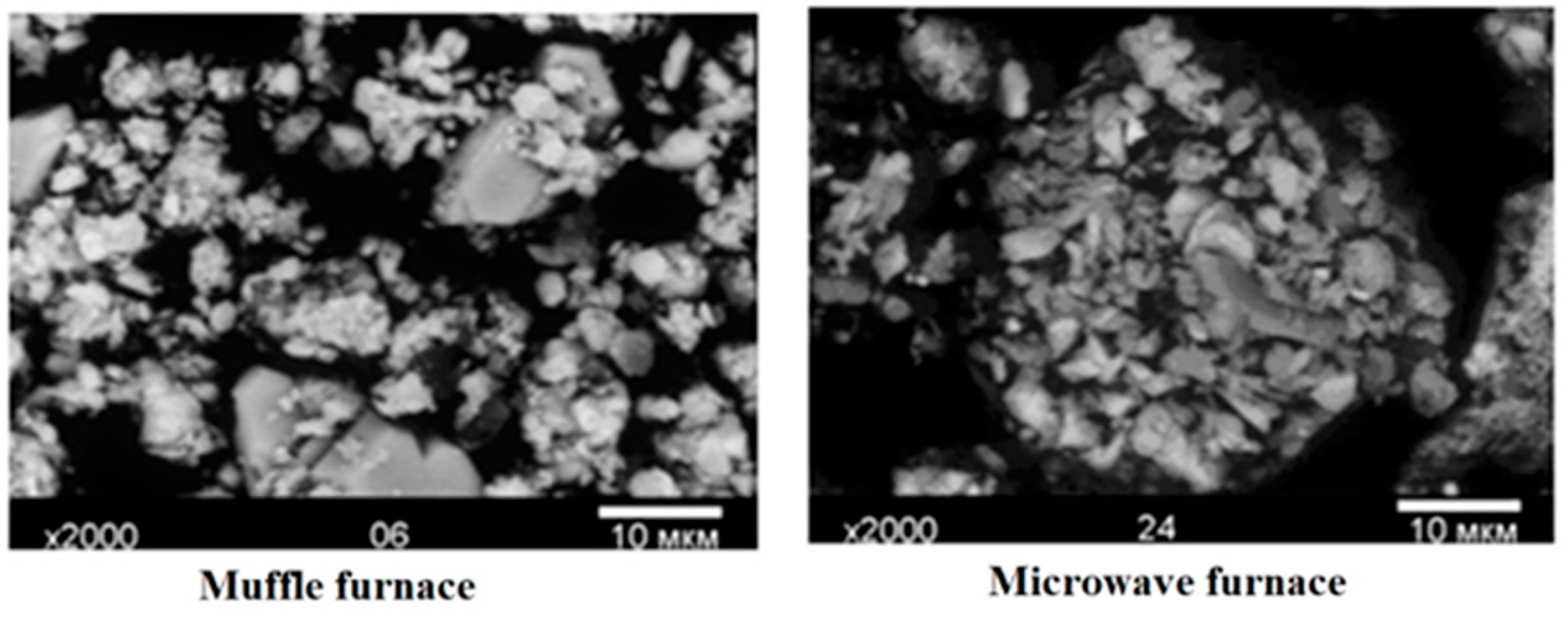
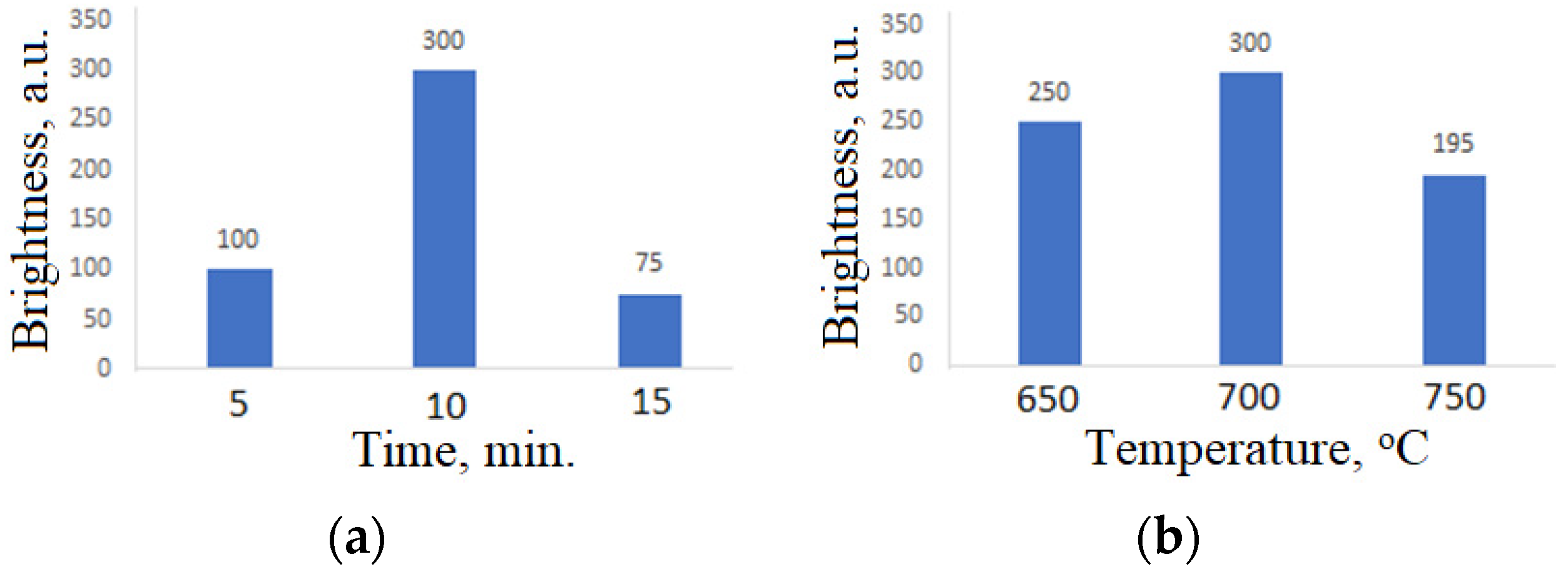
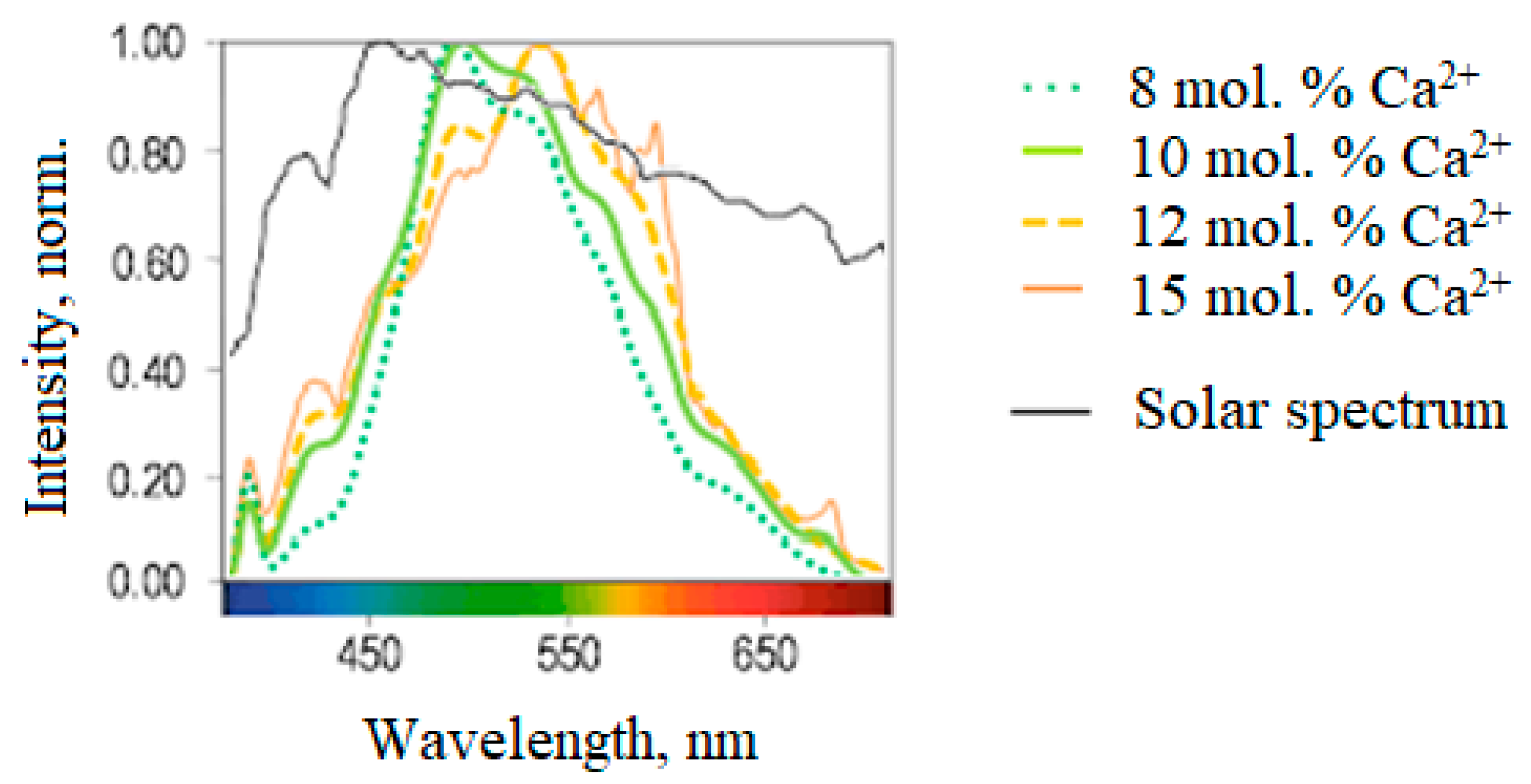
| № of the Band | Wavelength, nm | Electron Transitions | Phases |
|---|---|---|---|
| 1 | 421 | 5d-4f Eu2+ | SrCl2 |
| 2 | 452 | 5d-4f Eu2+ | Ca10Si6O21Cl2 |
| 3 | 486 | 5d-4f Eu2+ | Ca6Sr4Si6O21Cl2 |
| 4 | 516 | 5d-4f Eu2+ | CaSrSiO4 |
| 5 | 562 | 5d-4f Eu2+ | Ca6Sr4Si6O21Cl2 |
| 6 | 643 | 5d-4f Eu2+ | CaSrSiO4 |
| 7–18 | 597–702 | 4f-4f Eu3+ | CaSrSiO4 |
| Characteristics | Synthesis Conditions | |
|---|---|---|
| Microwave Furnace, 10 min, 750 °C | Muffle Furnace, N2:H2 2.5 h, 950 °C | |
| Phase composition (CaSrSiO4:Eu2+, Ca6Sr4Si6O21Cl2:Eu2+, Ca10Si6O21Cl2:Eu2+), % | 40, 27, 33 | 45, 26, 29 |
| Particle size, µm | 1–5 | 1–10 |
| FWHM, nm | 155 | 160 |
| Intensity, a.u. | 3010 | 2135 |
| Correlated color temperature, K | 3700 | 3440 |
| Color coordinates | x = 0.401 | x = 0.417 |
| Color rendering index | y = 0.408 | y = 0.418 |
| Mixing Method | Intensity, a.u. | Full Width at Half Maximum, nm | Correlated Color Temperature, K |
|---|---|---|---|
| Mechanical mixing | 700 | 111 | 3800 |
| Sol–gel synthesis | 945 | 111 | 4200 |
| Hybrid Phosphor Characteristics | Ca2+ Concentration in Charge Mixture | |||
|---|---|---|---|---|
| 8 mol. % | 10 mol. % | 12 mol. % | 15 mol. % | |
| Phase composition (CaSrSiO4:Eu2+, Ca6Sr4Si6O21Cl2:Eu2+, Ca10Si6O21Cl2:Eu2+), % | 57, 33, 10 | 50, 29, 21 | 42, 28, 30 | 40, 27, 33 |
| Color rendering index | 87 | 93 | 85 | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sychov, M.; Keskinova, M.; Dolgin, A.; Turkin, I.; Hara, K.; Kominami, H. One-Step Microwave Synthesis of New Hybrid Phosphor (CSSC) for White Light-Emitting Diodes. Ceramics 2023, 6, 2086-2097. https://doi.org/10.3390/ceramics6040128
Sychov M, Keskinova M, Dolgin A, Turkin I, Hara K, Kominami H. One-Step Microwave Synthesis of New Hybrid Phosphor (CSSC) for White Light-Emitting Diodes. Ceramics. 2023; 6(4):2086-2097. https://doi.org/10.3390/ceramics6040128
Chicago/Turabian StyleSychov, Maxim, Mariia Keskinova, Andrey Dolgin, Igor Turkin, Kazuhiko Hara, and Hiroko Kominami. 2023. "One-Step Microwave Synthesis of New Hybrid Phosphor (CSSC) for White Light-Emitting Diodes" Ceramics 6, no. 4: 2086-2097. https://doi.org/10.3390/ceramics6040128
APA StyleSychov, M., Keskinova, M., Dolgin, A., Turkin, I., Hara, K., & Kominami, H. (2023). One-Step Microwave Synthesis of New Hybrid Phosphor (CSSC) for White Light-Emitting Diodes. Ceramics, 6(4), 2086-2097. https://doi.org/10.3390/ceramics6040128