Abstract
High-entropy ceramics (HECs) represent an emerging class of materials composed of at least five different cations or anions in near-equiatomic proportions, garnering significant attention due to their extraordinary functional and structural properties. While multi-component ceramics have played a crucial role for many years, the concept of high-entropy materials was first introduced eighteen years ago with the synthesis of high-entropy alloys, and the first high-entropy nitride films were reported in 2014. These newly developed materials exhibit superior properties over traditional ceramics, such as enhanced thermal stability, hardness, and chemical resistance, making them suitable for a wide range of applications. High-entropy carbides, borides, oxides, oxi-carbides, oxi-borides, and other systems fall within the HEC category, typically occupying unique positions within phase diagrams that lead to novel properties. HECs are particularly well suited for high-temperature coatings, for tribological applications where low thermal conductivity and similar heat coefficients are critical, as well as for energy storage and dielectric uses. Computational tools like CALPHAD streamline the element selection process for designing HECs, while innovative, energy-efficient synthesis methods are being explored for producing dense specimens. This paper provides an in-depth analysis of the current state of the compositional design, the fabrication techniques, and the diverse applications of HECs, emphasizing their transformative potential in various industrial domains.
1. Introduction
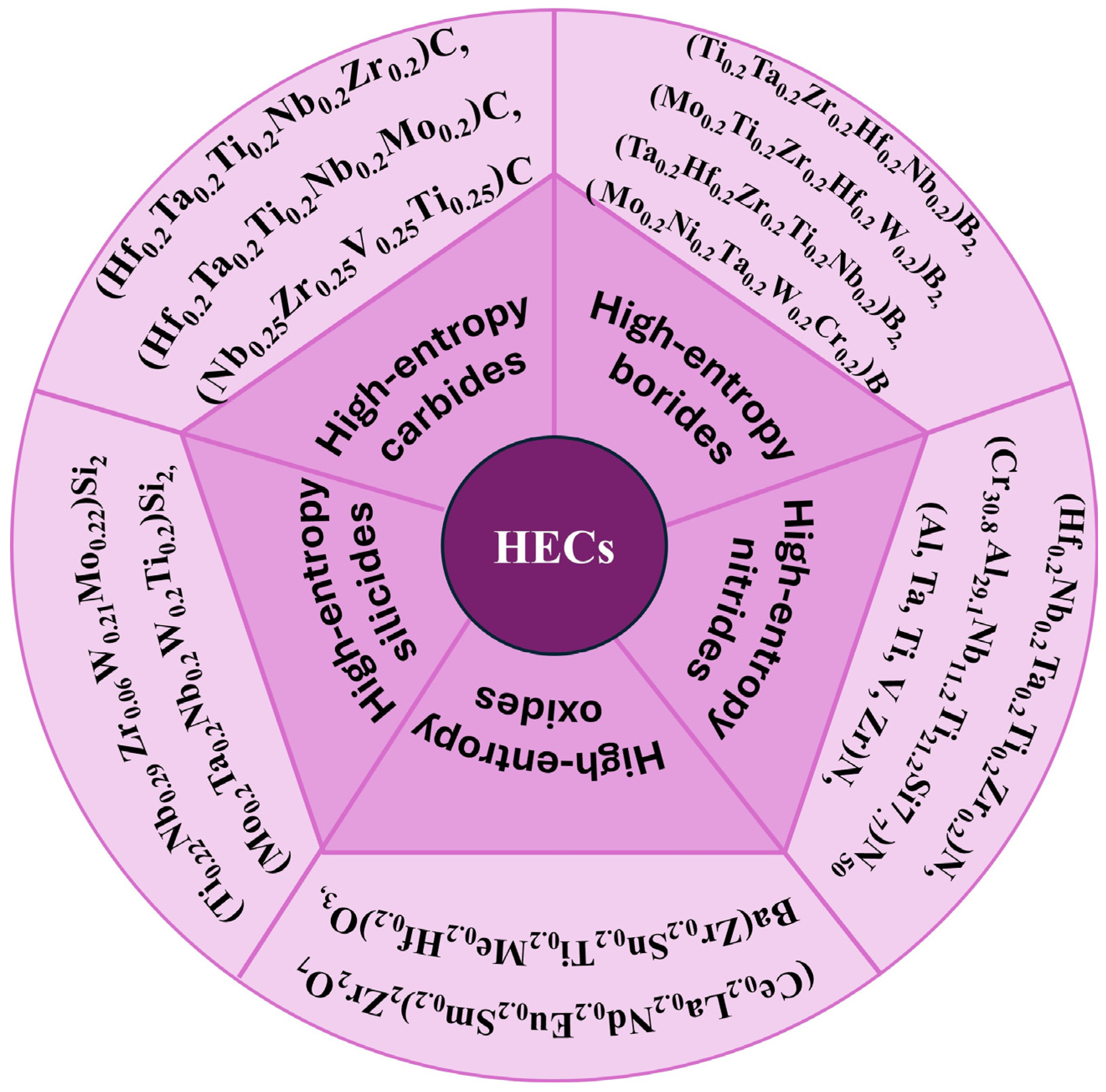
The definition of high-entropy ceramics (HECs) is still evolving; however, one characteristic feature is that they are composed of multiple elements in roughly equal atomic proportions, which creates a highly disordered crystal structure. Some researchers have defined HECs as materials consisting of five or more different cations or anions, with examples including (Zr0.2Ta0.2Hf0.2Nb0.2Ti0.2)C for high-entropy carbides and (Zr0.2Hf0.2Ta0.2Nb0.2Ti0.2)B2 for high-entropy borides. The same alloying elements can form HECs with nitrogen, carbon, boron, and oxygen, leading to compounds such as high-entropy oxides (e.g., (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O) and high-entropy nitrides (e.g., (Hf0.2Ti0.2Zr0.2Nb0.2Ta0.2)N). They remain a relatively new class of materials, with the first reports of their synthesis emerging in 2014 [1,2].
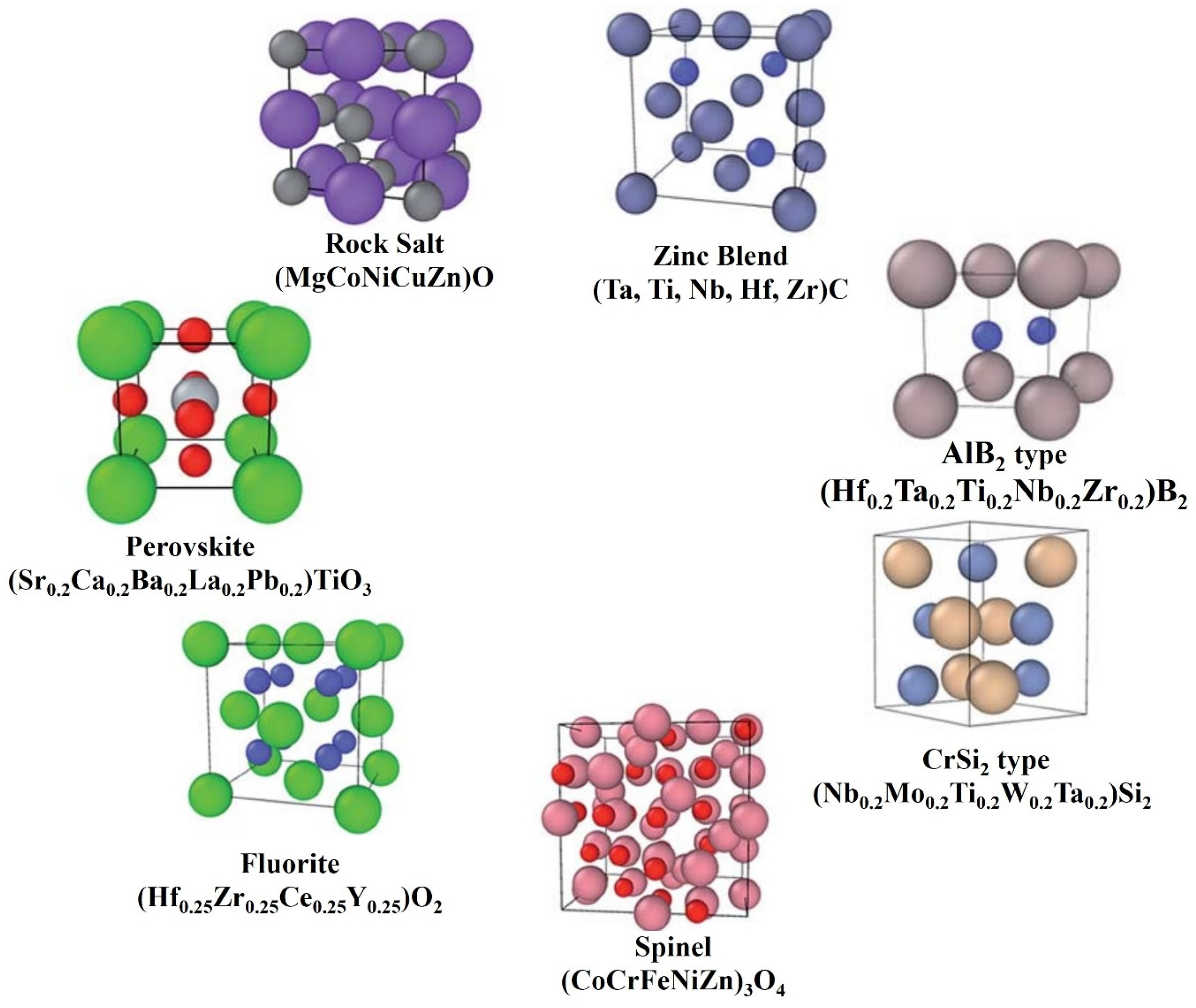
HECs are novel materials with exceptional hardness and thermal, electrical, mechanical, and oxidation properties compared to traditional ceramics. These ceramics are uniphase, with a simple lattice framework. The primary factor in producing the homogenous mixing of each element in the HEC is the atomic sizes of the constituent metallic powders. The entropic stabilization of the compact solution increases because of the rise in individual constituents [3]. The earlier reported HECs had rock salt structures in general; however, six other structures of HECs reported to date are shown in Figure 1 [4]. The first discovered structures of high-entropy ceramics (HECs) were primarily based on a rock salt structure, such as (Zr0.2Ta0.2Hf0.2Nb0.2Ti0.2)C, which involves a highly ordered arrangement of cations and anions in a face-centered cubic (FCC) lattice. There are also perovskite structures, where materials like (CaTiO3) have two cation sublattices and offer diverse properties useful for fuel cells and catalysts; fluorite structures, which are highly disordered and beneficial for thermal insulation applications due to the oxygen vacancy defects; spinel structures, characterized by the cubic packing of oxygen atoms with cations in tetrahedral and octahedral sites, known for their magnetic and electronic properties; CrSi2-type silicide structures, with hexagonal stacking and used for ultra-high-temperature devices due to their thermal stability; AlB2-type hexagonal boride structures, known for their covalent bonding and superhard properties; and finally, the ZnS (sphalerite) structure, featuring an FCC lattice and notable for its semiconductor applications. These diverse forms of HECs allow for a wide range of applications, from energy storage systems to aerospace components, due to their high-temperature stability, mechanical strength, and corrosion resistance.
Transition metal carbides, borides, and nitrides, such as those of Zr, Ta, Hf, and Nb, possess high melting points, making them excellent candidates for use in harsh environments, such as aerospace engines, nuclear reactors, and cutting tools. HECs also find applications in the energy and engineering domains. For example, HECs like (Ti0.2Hf0.2Ta0.2Nb0.2Zr0.2)C can be employed as thermal insulators in turbine engines or power plants, where thermal management is critical. Additionally, HECs could be used in battery technologies and hydrogen storage systems, owing to their excellent chemical stability and resistance to high-temperature oxidation [5,6,7]. Dual-phase carbide boride ceramics are also under experimentation by many researchers. The spark plasma sintering (SPS) route can produce a 99% compact specimen. In recent days, it has been observed that researchers and their research groups are moving from HECs to multi-cation or compositionally complex ceramics consisting of more than five elements with non-equimolar medium-entropy composition with different structures and features [8,9,10,11,12].
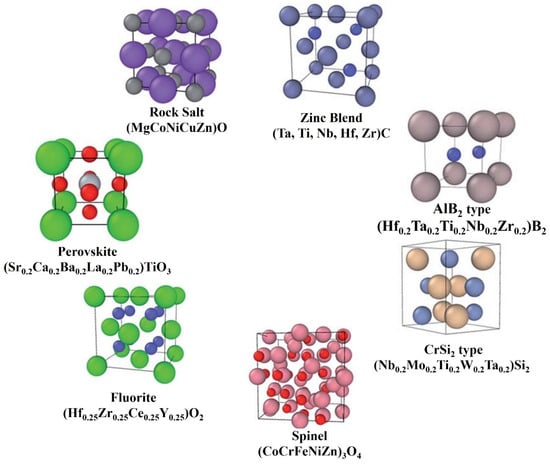
Figure 1.
Different structural types of HECs (the smaller spheres represent cations, and the larger spheres represent anions), adapted from [13], Royal Society of Chemistry, 2019.
Figure 1.
Different structural types of HECs (the smaller spheres represent cations, and the larger spheres represent anions), adapted from [13], Royal Society of Chemistry, 2019.
The rock salt (NaCl) structure is formed by repeating the face-centered cubic (FCC) unit cell. Na:Cl has a 1:1 stoichiometry ratio with a molar mass of 58.4 g/mol. Na+ ions have an atomic radius of 102 pm, and the larger Cl− ions have an atomic radius of 181 pm. The coordination numbers of Na and Cl are equal. Solid-state reaction, co-precipitation, flame spray pyrolysis, etc., have been used to synthesize rock salt-type structures and their derivative [14].
The structures of perovskite are almost similar to calcium titanium oxide (CaTiO3). They have at least two cation sublattices. Jiang et al. [3] synthesized single solution phases of different high-entropy (HE) perovskite oxides. They proclaimed that instead of cation-size difference, Goldschmidt’s tolerance factor also influences the formation and thermal stability of single cubic perovskite solid solutions. It has also been reported that perovskite oxides have diverse and excellent physical properties for uses in various areas, such as proton conductors, cathode materials for fuel cells, photocatalysts, dielectrics, and ferroelectric material [4,5,6,8,10,15]. Doping perovskite oxides can enhance their performance for applications such as in fuel cells, catalysis, oxygen sensors, photovoltaics, magnetic devices, and electrochemical devices, as demonstrated by recent studies [11,14,16]. Oxide-based ceramics could be potential thermoelectric materials because of their thermal and chemical stability at high temperatures. However, their low electrical conductivity and high heat conductivity make them unsuitable for commercial applications. The minimum thermal conductivity in high-entropy (Sr0.2Ca0.2Ba0.2La0.2Pb0.2)TiO3 bulks was found to be 1.17 W/mK at 923 K, which can be attributed to the substantial lattice distortion and significant mass fluctuation effect [17].
Fluorite is a multi-component rare earth oxide nanocrystalline powder containing seven equiatomic rare earth elements with a single-phase CaF2-type structure. The ‘F’ ions occupy the eight tetrahedral interstitial sites, whereas the ‘Ca’ ions occupy the face-centered cubic (FCC) structure’s regular spots. Further research of this type focuses on visible light absorption and oxygen vacancy. It is suggested that theoretical studies are more beneficial to model the electronic system of such HE carbides. Gild et al. [16] used high-energy ball milling to synthesize eleven fluorite oxides, followed by SPS. They found that these oxides had extraordinary configurational entropy, unlike previously reported HECs. They also observed that the electrical conductivity, hardness, and thermal conductivity were low due to phonon scattering by the strained lattices and cations. These types of fluorite oxide are even useful for thermally insulated applications, as reported by Chen et al. [12].
The spinel structure is based on the cubic packing of oxygen atoms, with cations occupying the interstitial tetrahedral and octahedral sites. Spinel oxides have a generic formula of AB2O4. There are two cation sublattices, tetrahedral (A) and octahedral (B) [18]. The majority of spinels, such as (CoCrFeMnNi)3O4, (CoCrFeNiZn)3O4, etc., have been researched and discovered recently. Some of these materials exhibited ferromagnetic behavior and the properties of these materials were enhanced by defect engineering and chemical diffusion [19,20].
Chromium-based silicides (Cr–Si) are broadly used in semiconductors, ultra-high-temperature devices, thermoelectric devices, and energy-storage systems. CrSi2 structures have outstanding stabilities. Gild et al. [21] synthesized (Nb0.2Mo0.2Ti0.2W0.2Ta0.2)Si2 and found that it possesses a CrSi2-type C40 ABC-type stack arrangement on a hexagonal structure. The AlB2 type possesses a layer-type hexagonal crystal arrangement with alternate firm two-dimensional boron webs and HE two-dimensional metal cations stratum, with covalent and mixed ionic bonds between the boron and metals. Gild et al. [22] in their other research synthesized (Hf0.2Ta0.2Ti0.2Nb0.2Zr0.2)B2, which possessed a single solid–solution phase of the boride AlB2 hexagonal type structure. The high-entropy diboride ceramics are newer types of superhard materials [23]. The ZnS or zinc blend has a unique face-centered cubic structure called sphalerite. It consists of a single bond between each atom and a 1:1 zinc-to-sulfur ratio. Only half of the tetrahedral sites are occupied [24]. At 1950 °C, equiatomic (Ti, Zr, Hf, Nb, Ta) C revealed a pure FCC-structured solid solution with metallic atoms randomly positioned in the metallic sublattice [25].
The fabrication of ceramics using the combustion route in an ultra-elevated gravity field is a significant new research area in this discipline. This method enables the development of high-performance materials by leveraging extreme conditions to enhance the material properties, especially for advanced applications [4,26]. This method has been applied to high-entropy ceramics (HECs), which include materials such as transition metal carbides, borides, and nitrides (e.g., Zr, Ta, Hf, Nb). These HECs, due to their high melting points, are particularly suitable for harsh conditions [6,7,27]. In addition, specific types of HECs, like nitrides, have shown promise in hard-coating applications, while others, such as (Hf0.2Zr0.2Ta0.2Ti0.2Nb0.2)C, can function as thermal insulators and find use in energy and engineering domains [28]. The arrival of ultra-high-temperature ceramics with high configurational entropy stabilized into a single phase has superior mechanical, oxidation, and thermal properties [29]. The ultra-high-entropy ceramic is appropriate for high elevated temperatures (≥3300 K). Such ceramics can be used in rocket nozzles, reactors, and hypersonic vehicles. In contrast to high-entropy alloys, transforming a solid solution from the metal into a binary compound makes the traditional phase rule inapplicable to HECs [30]. HE alumino-silicides (Nb0.25Mo0.25V0.25Ta0.25)(Al0.5Si0.5)2 are a novel class of HECs with a multi-anionic and cationic structure. It has a single-crystal hexagonal structure and excellent compositional uniformity. Some researchers are moving from HECs to multi-cation or compositionally complex ceramics consisting of more than five elements with a non-equimolar medium-entropy composition [31,32,33]. This study aims to explore the fabrication, design, and synthesis methods of high-entropy ceramics (HECs), focusing on how different approaches influence their properties and potential applications. The objective is to advance the knowledge of developing high-performance ceramics with superior mechanical, thermal, and oxidation resistance for use in extreme conditions.
2. Design of High-Entropy Ceramics
High-entropy ceramics possess remarkable properties such as excellent hot hardness, high-temperature thermal stability, and corrosion resistance. These characteristics make them potential candidates for applications in harsh environments, including extreme temperatures, corrosive conditions, and high-stress scenarios. The properties of these ceramics are highly influenced by the constituent elements present in the matrix, along with some of the governing factors, like the atomic size of the element, the concentration of valence electrons, etc. Some of the approaches opted for by researchers to design HECs with excellent properties are explained in following subsections.
2.1. Gibbs Free Energy Approach
The Gibbs free energy approach is a helpful tool for predicting the stability of HECs, where the high-temperature effect plays a substantial role. To apply the Gibbs free energy approach to high-entropy ceramics, the Gibbs free energy of the formation for each ceramic component must be determined experimentally or through computational modeling. Once the values for each component are known, they can be utilized to calculate the Gibbs free energy of the formation for the high-entropy ceramic. If the energy of the formation is negative, it indicates that the development of the material is favorable and will occur spontaneously under appropriate conditions. Overall, it is a powerful tool for understanding the stability of HECs and predicting their formation under different conditions. By combining experimental and computational techniques, researchers can better understand these materials’ thermodynamics and work toward developing new HECs with tailored properties. The entropy of a solid solution can be calculated from Boltzmann’s hypothesis (Equation (1)) [25].
where bi = molar % of the component. When b1 = b2 = b3 = bn, will reach the maximum.
Here, = 1.
2.2. Descriptors Approach
The descriptors approach is used to predict the properties of HECs based on their chemical composition. This approach involves identifying essential “descriptors” or parameters that can indicate specific properties of the material, such as its melting temperature or hardness. By analyzing the association between the descriptors and the material properties, models can be developed to predict the properties of new HECs without requiring extensive experimental testing. Some common descriptors used in high-entropy ceramics research include the atomic size ratio between the constituent elements, the difference in electronegativity between the elements, and the number of elements in the material. By analyzing the trends in these descriptors and how they affect the material properties, models can be developed to predict the behavior of HECs. The descriptors approach has several advantages for high-entropy ceramics research [34]. One of the main benefits is that it can significantly reduce the time and resources required for experimental testing. By using computational modeling to predict the properties of new materials, researchers can focus their efforts on the most promising candidates, reducing the time and cost involved in synthesizing and testing large numbers of materials. Another advantage of the descriptors approach is that it can be employed to design HECs with specific properties for targeted purposes. By selecting the appropriate descriptors and modeling the relationship between them and the desired properties, researchers can develop high-entropy ceramics with properties tailored for specific applications, such as high-temperature coatings or energy-storage materials. Overall, the descriptors approach is a powerful tool for high-entropy ceramics research, allowing ease of predicting the properties of new materials and designing high-entropy ceramics with specific properties for targeted applications. This methodology is based on the work of pioneering researcher Hume Rothery, who described the conditions for creating solid solutions. Many HECs are designed around these conditions [13]. Such a descriptor has been used by Liu et al. [35] to judge the atomic stability of the HEC system (Equation (2)). They proclaimed that the high solubility and low internal strain energy are associated with a small value of δ and vice versa. Averaging the numbers for quasi-binary bulk solutions yields the average weight of δ. However, many scientists believe that kinetics can predict stability because phase stability is not a material attribute [36].
where is the average active lattice constant, denotes the change in the actual lattice constant, denotes the average shear modulus and Z is the number of principle entities/unit cells.
2.3. CALPHAD, Thermodynamic Calculation, and Machine Learning Approach
CALPHAD (calculation of phase diagrams) is a phenomenological approach used for predicting thermodynamics and other properties of multi-component material systems. The Gibbs energy calculation is the most critical aspect of this type of problem-solving. The domain size, distribution, and entropy of mixing are generally considered in this approach. Zhong et al. [37] followed the CALPHAD approach based on computational thermal dynamics instead of a traditional approach. Qu et al. [38] predicted the melting temperature of high-temperature ceramics using machine learning and neural networks. Kaufmann et al. [39] also showed the usefulness of this approach for making HECs. Ye et al. [40] used thermodynamic analysis to analyze and understand the possibility of creating (Nb0.25Zr0.25V0.25Ti0.25)C. They proclaimed that the mixing enthalpy of HE carbide was 5.526 kJ/mol when studied by first-principal calculation. Moreover, they found that the entropy of mixing ranged from 0.693 R to 1.040 R. Wen et al. [41] used the deep learning potential (DLP) method to predict the thermomechanical properties of (Hf0.2Zr0.2Ta0.2Ti0.2Nb0.2)C theoretically. They found that the prediction error of DLP in force and energy was 217 meV/Å and 9.4 meV/atom, respectively. The preferential nature of HEC material oxidation has been also verified using other computational approaches.
2.4. Density-Function Theory Approach (DFT)
The DFT approach is a computational modeling approach used to analyze the electronic arrangement of HECs. However, the structure of the DFT model is very complex due to the intrinsically random nature of HECs. Additionally, the DFT models have computational limitations. Zhang et al. [42] and Ye et al. [40] used the DFT model to form (Nb0.25Zr0.25V0.25Ti0.25)C and B4(HfMo2TaTi)C HECs. They found that the enthalpy of the formation of the former was recorded as 5.526 kJ mol−1, and for the latter, the value was found to be −2.29 eV per unit cell. The thermodynamic and elastic properties of new HECs (TaHfTiZr)C and (TaHfNbZr)C were investigated using a DFT framework, revealing negative formation enthalpies, indicating stability. Despite its increased brittleness, the elevated Debye temperature of (TaHfNbZr)C showed stronger covalent connections [43].
3. Synthesis of High-Entropy Ceramics
This section shows the synthesis of some of the important HECs [27,44,45,46,47,48]. The HECs are broadly classified into high-entropy—carbide, boride, nitride, silicide, oxide ceramics, etc., based on their main constituent element. Figure 2 [7,11,19,33,49,50,51,52,53,54,55,56,57,58] shows the different types of HECs based on their principal component element. The research on HECs has theoretical implications, but it also faces synthesis issues. Various approaches, such as the solid-state reaction, HEBM, solidification, SPS, and chemical growth, have achieved the random distribution of constituent components in HECs. There is a diverse structure of HECs, with the FCC structure being the most predominant and valuable one. Various new tailored properties have been obtained while trying novel synthesis routes mentioned in the application part of HECs. Some properties were also obtained by defect engineering and doping other elements in the matrix material.
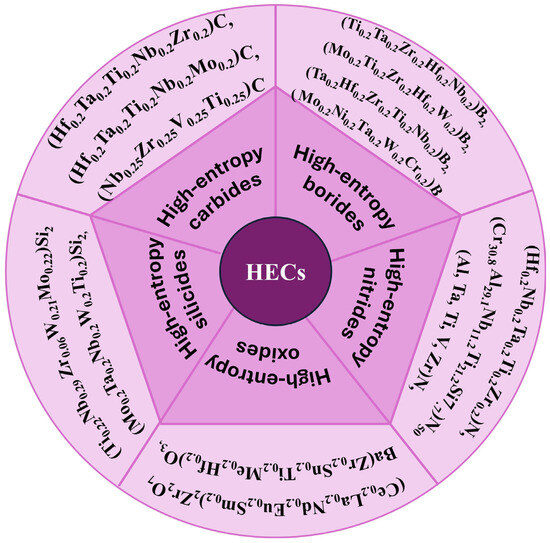
Figure 2.
Different types of high-entropy ceramics with their principal element.
3.1. High-Entropy Carbides
The carbide ceramics have shown higher hardness than the other constituent metal carbides. These HECs also reflect exceptional thermal, electrical, mechanical, and oxidation properties. Compared to traditional ceramics, HE (high-entropy) carbide ceramics exhibit specific physical properties, such as high nano hardness, good oxidation resistance, low heat conductivity, and exceptional irradiation resistance.
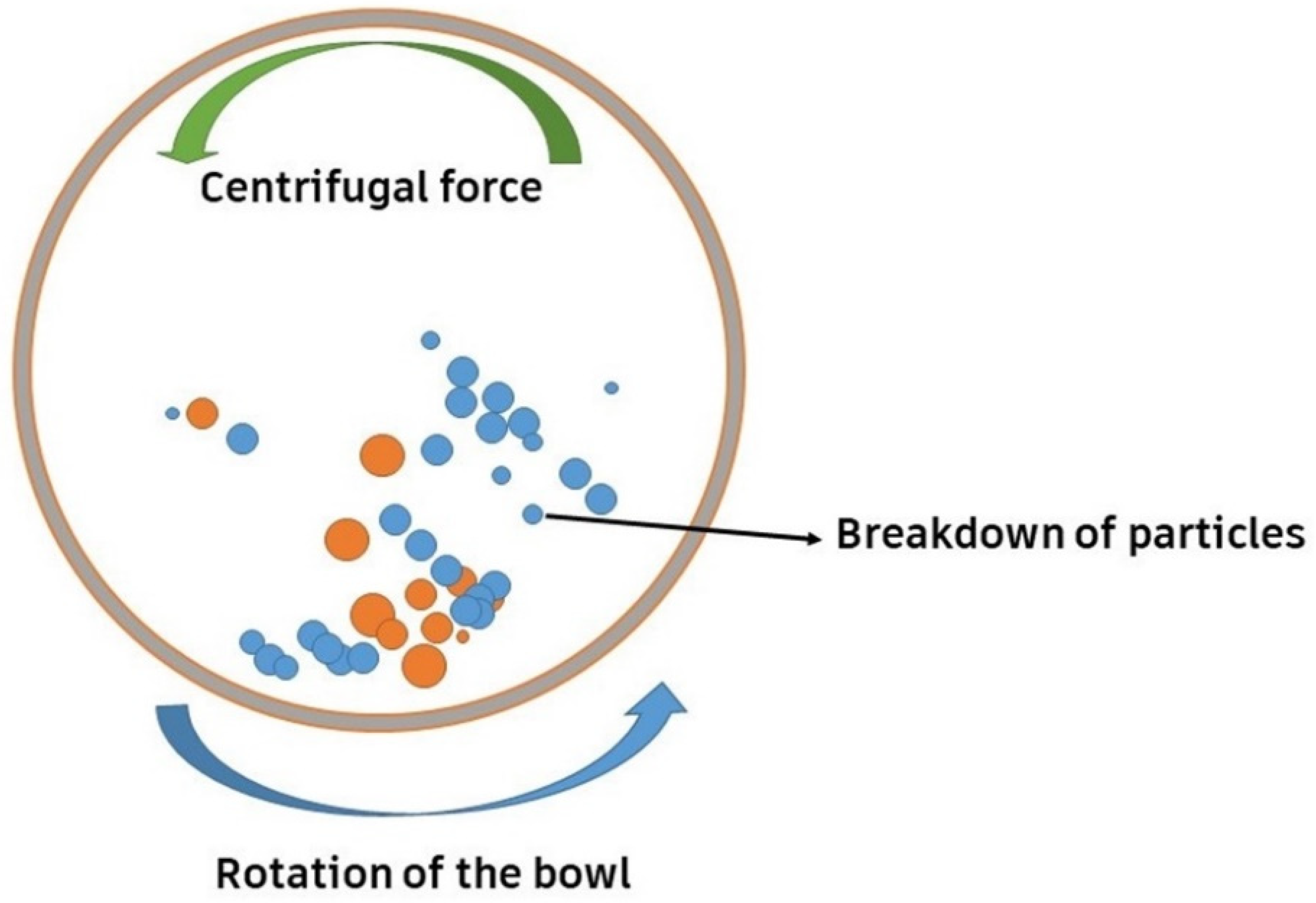
Among several synthesis techniques, conventional sintering has been a usual technique to synthesize such ceramics. However, the arrival of the flash-sintering technique has led to improved densification at lower temperatures and less time [44,46,47]. High-energy ball milling (HEBM), which involves the collision of a powder mixture with high-energy balls to produce a uniform dispersion, is also utilized for producing HE carbides, as shown in Figure 3. The balls (orange color) represent milling balls, while the smaller particles (blue color) represent the powder being milled. The relationship between the balls and the powder is crucial for the milling efficiency, as an optimal ball-to-powder ratio ensures effective energy transfer, leading to proper particle size reduction. An imbalanced ratio, either too much powder or too little, can reduce the efficiency of the milling process. The ideal ratio depends on the specific materials and desired milling outcomes, with 10:1 being a common starting point. A combination of the constituent powders is placed into a grinding jar, along with grinding balls. The grinding jar is then placed on a rotating platform, which causes the grinding balls to tumble and mix the powders. The collision and friction between the grinding balls and the powders lead to a reduction in the particle size and an increase in the homogeneity of the mixture [59]. Moskovskikh et al. [60] synthesized the HE carbides Ta0.2Nb0.2Hf0.2Ti0.2Mo0.2C (HECMo) and Ta0.2Hf0.2Nb0.2Ti0.2Zr0.2C (HECZr) by reactive high-energy ball milling. They found that 60 min of the process was sufficient to prepare the FCC solution. They also proclaimed that the bulk HECs produced using this approach have fine particle diameters. It was observed that the mechanical properties of the HECs improved when the grain size was reduced [49].
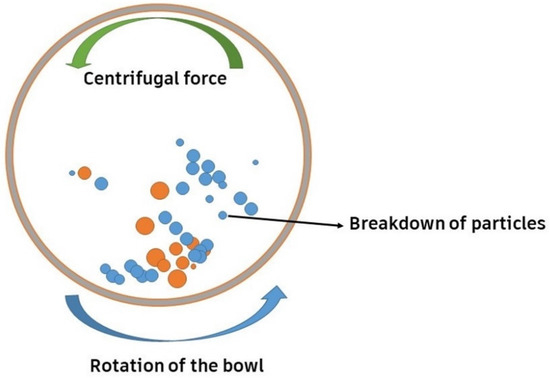
Figure 3.
High-energy ball-milling process.
The SPS (spark plasma sintering) process is the most conventional process used to synthesize HECs. This process uses elevated-intensity uniaxial pressure, pulsed current, and low voltage. This technique has been found to be very beneficial in materials that are hard to sinter, like refractory materials. It is a powder metallurgy method for rapid ceramics production and can bestow materials with exceptional properties [50,61,62]. Wei et al. [51] fabricated an HEC containing (Ta, Ti, Hf, Nb, Zr)C by employing the SPS process. They observed that the three different routes via one elemental process resulted in a salt structure, the carbide process produced homogeneous HEC, and the oxide process created a two-phase design rich with zirconium. The three products had a similar XRD pattern, with different microstructures and rock salt structures. HEC-C (employing metal carbides as starting materials, also known as HEC-C) and HEC-E (using graphite and metallic powders as starting materials, also known as HEC-E) exhibit identical two peaks; however, HEC-O has a greater 2θ (using metallic graphite and oxides as raw materials called HEC-O). This suggests that the latter has a smaller lattice parameter than the former. Additionally, it was observed that the HE carbide powders were thermally stable and oxidation resistant. Gild et al. [63] used reactive flash plasma sintering to achieve homogeneous ultra-high-temperature ceramics from commercial powders. Orru and Cao et al. [38] investigated the role of SPS in manufacturing ultra-high-temperature ceramics. It has been reported that the SPS process leads to high density and better mechanical oxidation resistance, which can be used in the aerospace and solar fields. The crystal structure of the HEC was mostly found to be similar to the rock salt structure in which the metallic atoms occupied the cation position in the lattice, while the carbon atoms inhabited the anion position. Similarly, Chicardi et al. [64] used a mechanically made carbon diffusion route to synthesize (TiZrHfVNb)C5. The novelty of their research was its preparation at room temperature by alloying (Zr, Ti, V, Hf, and Nb) and diffusing it with graphite. The innovative mechano-synthesis process for (TiZrHfVNb)C5 was carried out at room temperature. It was observed that the elevated thermal approaches centered on sintering techniques from a mix of carbides did not affect it. Additionally, it enabled the acquisition of HECs in powder form with minimal energy requirements and a nanometric character. Several research studies have shown that there is also a demonstrable benefit to using powder metallurgical methods to generate composite materials based on HECs.
At the nanoscale, there has been a high level of homogeneity for different transition metals, such as Ti, Hf, V, Nb, and Zr, validating the synthesis of the (ZrTiVHfNb)C5 HE carbide. Ye et al. [40] fabricated (Zr0.25Ti0.25Nb0.25V0.25)C through a hot press sintering technique with high compositional uniformity and a relative density of 95.1%. They calculated the ceramic’s microhardness under various applied stresses. They found that as the applied force increases, the micro-hardness of the ceramic decreases. The micro-hardness of (Nb0.25Zr0.25V0.25Ti0.25)C was observed to be 22.5 ± 0.6 GPa at 0.98 N, which further drops to 19.1 ± 0.5 GPa under applied stress of 49 N, which is mainly attributed to the porosity of the ceramic. Moreover, the microhardness of the ceramic is much lower as compared to the nanohardness due to the presence of a higher percentage of porosity.
Herrington et al. [65] achieved enhanced hardness for metallic carbides (Ta0.2 Nb0.2 V0.2 W0.2 Mo0.2)C with ultra-elevated-temperature capabilities. They found it evident that the hardness decreased with an increase in the valence electrons, whereas the mechanical properties were not found to be dependent on the entropy stabilization. In their research, the phase evolution in twelve distinct five-metallic HEC combinations was investigated. They observed that during sintering at 2473 K, nine of the twelve configurations were uniphase bulk solutions. SEM imaging of the (Hf0.2Ti0.2V0.2Ta0.2Nb0.2)C single-phase carbide and (Zr0.2Hf0.2Ta0.2Mo0.2W0.2)C, a multi-phase carbide sample, showed grain boundary phase precipitation. On the other hand, the single-phase sample revealed just an orientation contrast and no signs of a grain boundary secondary phase. From their results, it was concluded that the rock salt phase was homogeneous and mixed.
Zhou et al. [25] used SPS to fabricate equiatomic (Zr, Ti, Ta, Nb, Hf) C. They found that the HEC powder showed better oxidation resistance and possessed better thermal stability than its original component. The powder exhibited structural and chemical homogeneity, whereas metallic atoms were randomly located in the metal sublattice. Compared to its parent components, it fared better in terms of oxidative resistance. While oxidation at lower temperatures, the HEC became an indivisible entity.
Feng et al. [52] fabricated (Zr, Ta, Hf, Ti, Nb)C using carbon and metallic oxides. It involved a two-step procedure that included HEBM and carbothermal reduction. Chen et al. [66] synthesized porous (Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)C with low heat conductivity of 0.39 W/(m K). The synthesis route was partial sintering, which resulted in 80.99% porosity. The compressive strength of the resulting ceramic was found to be 3.45 MPa with a thermal diffusivity of 0.74 mm2s−1. It was found that the shrinkage of high porous entropy (Zr0.2Hf0.2Nb0.2Ta0.2Ti0.2)C occurs during preparation and the second round of heat treatment. Additionally, the sample dimensions remained unchanged during the second round of heat treatment up to 1850 °C, as can be seen. Table 1 summarizes the properties of some HE carbides synthesized through different routes [40,52,66].

Table 1.
Different types and properties of HE carbides.
3.2. High-Entropy Borides
The boride ceramics have demonstrated higher hardness than the constituent metal borides. Along with this, they exhibit exceptional thermal, electrical, mechanical, and oxidation properties. SPS is again the most common method for preparing HE borides. It is a pressure-based sintering process that yields fine particles. This technique involves the application of both pressure and electrical current to the powder compact during the sintering process. The process begins by placing a powder compact between two graphite electrodes in a die. The die is then heated to a high temperature (typically 400–1200 °C) using an electric current that is pulsed on and off. Applying the electric current causes the powder particles to become conductive, generating an electrical discharge between the powder particles. This results in localized heating of the powder particles, which further leads to rapid densification of the material. During the sintering process, a uniaxial pressure is applied to the powder compact, which helps in maintaining the material’s shape and improves the densification process. The applied pressure is typically ~10–100 MPa, although higher pressures can be used for specific materials.
Zhang et al. [67] synthesized (Zr0.2Hf0.2Mo0.2Ti0.2Nb0.2)B2, (Zr0.2Hf0.2Ta0.2Ti0.2Nb0.2)B2, and (Mo0.2Ta0.2Hf0.2Ti0.2Nb0.2)B2 from metal oxides using a boro-carbothermal reduction followed by SPS at 2000 °C, as shown in Figure 4. The entire process led to full densification with a Vickers hardness value of ~27 GPa, whereas the fracture toughness ranged from ~3.64 to 4.47 MPa.m1/2. From the research, it was proclaimed that superhard high-entropy boride ceramics could be made with no loss of fracture toughness.
Zhang et al. [68] synthesized HE diboride ceramic (Zr0.2Ti0.2Ta0.2Hf0.2Nb0.2)B2 and (Ti0.2Zr0.2Mo0.2W0.2Hf0.2)B2 by employing boro-thermal reduction followed by SPS at 2000 °C. The results of the test showed that the Vickers hardness of the former was ~16.4 GPa, and the latter had a hardness of 27.7 GPa. The ceramics showed comparatively higher densification and hardness than previously reported diboride ceramics, with a better particle size ≤ of 0.5 µm due to the better-sintered density.
Chen et al. [53] fabricated (Zr0.2Nb0.2Hf0.2Ti0.2Ta0.2)B2 ceramic with a high thermal insulation capacity by partial sintering or boron carbon reduction. The results showed that (Hf0.2Zr0.2Nb0.2Ti0.2Ta0.2)B2 possessed a low thermal conductivity of 298–500 K. This shows that designing porous UHTCs (ultra-high-temperature ceramics) is a novel strategy to change them from thermal conductors to thermal insulators. Reactive flash SPS (RFSPS) is used to synthesize such ceramics rapidly (in 2 min), with a density greater than 99%, as shown in Figure 5. RFSPS is an advanced processing technique used for the synthesis of high-performance ceramics and composites. It is a modification of the conventional SPS technique, which involves the application of both pressure and electrical current to densify a powder compact. In this process, a reactive element is added to the starting powder mixture, which reacts with one or more of the other elements during the sintering process. The reactive element is typically a metal or non-metal, and it is added in small amounts (usually less than 5% by weight) to the powder mixture. During the sintering process, the powder mixture is heated to a high temperature (usually 800–1200 °C) using a pulsed direct current. The reactive element reacts with one or more of the other elements in the mixture, producing a local temperature increase due to the reaction’s exothermic nature. This localized heating leads to the rapid and uniform densification of the powder compact, resulting in a dense and fully consolidated material. Additionally, RFSPS offers several advantages over conventional sintering techniques, including lower processing temperatures, shorter processing times, and the ability to produce complex shapes and composites. The method is particularly useful for synthesizing high-temperature materials, such as refractory metal borides and carbides, which are difficult to synthesize using other methods [49,50,51,61].
Zhao et al. [55] synthesized HE boride in which they used (MoxTaxNixCrxWx)B, where x = 0.2 = (Mo0.2Ni0.2Ta0.2Cr0.2W0.2)B, by the hot pressing technique at 30 MPa and 2000 °C for 1.5 h in an argon atmosphere. The dislocations seen in the ceramics were attributed to the increased hardness. It was found that the ring dislocations within the grains were able to considerably impact the increasing hardness. Additionally, with the applied external load, the ring dislocations did not slip up, improving the overall hardness of the (Ta0.2Mo0.2W0.2Cr0.2Ni0.2)B. Moreover, the dislocations produce imperfections at the grain boundaries, making the high-entropy ceramic hard. Table 2 summarizes the comparison of different features of some HE borides [53,54,67,68].

Table 2.
Different types and properties of HE borides.
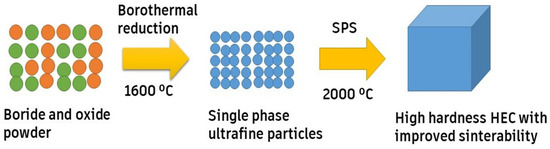
Figure 4.
SPS route for developing HE borides, adapted with permission from [69], Elsevier, 2019.
Figure 4.
SPS route for developing HE borides, adapted with permission from [69], Elsevier, 2019.
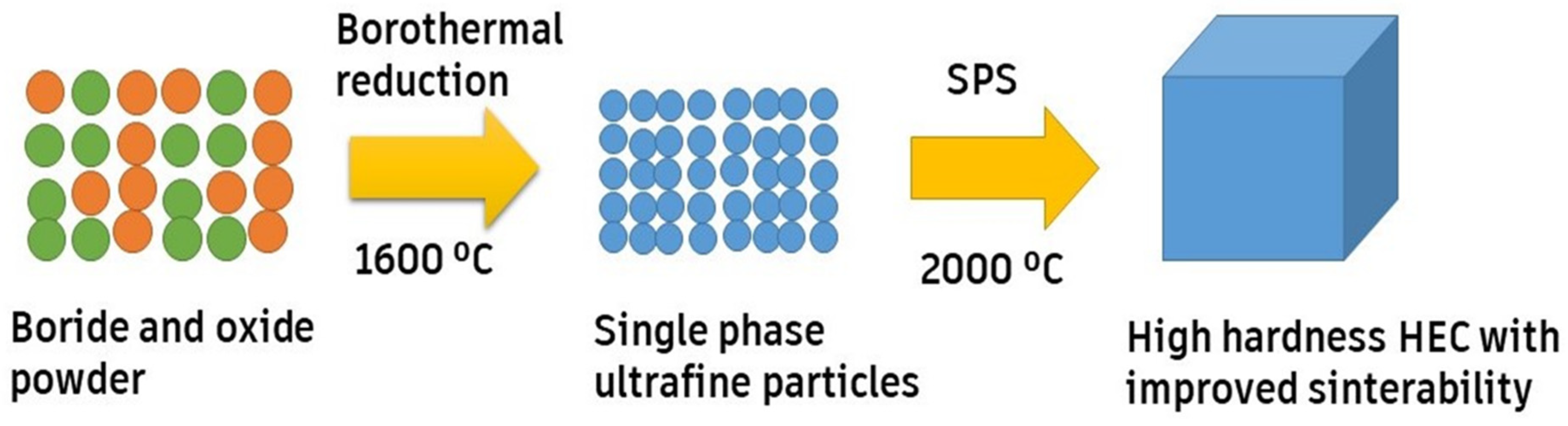
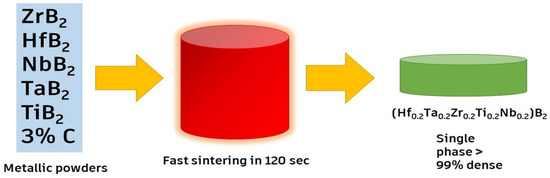
Figure 5.
Reactive flash SPS process, adapted with permission from [63], Elsevier, 2019.
Figure 5.
Reactive flash SPS process, adapted with permission from [63], Elsevier, 2019.
3.3. High-Entropy Nitrides
The synthesis of HE borides and carbides is well established and known; however, HE nitrides have only been fabricated as thin films. High-entropy nitrides, like the (Al, Ti, V, Ta, Zr)N and (Nb, Hf, Ti, Zr, Ta)N coatings, are typically synthesized as thin films using techniques like DC magnetron sputtering. These thin films are used for applications, such as ultra-hard coatings, optics, and other high-performance materials, due to their exceptional hardness and fracture toughness. Their properties vary depending on the specific elements and synthesis methods employed, including the substrate bias and deposition techniques. The HENs have excellent hardness (33 GPa) and fracture toughness (5.2 MPa∙m1/2), significantly exceeding other HECs and the estimated values from mixture rules. Magnetron sputtering is another physical vapor deposition technique that uses the magnetic field applied on a diode to deposit ceramics on the substrate. The vacuum coating process (sputtering) can deposit metals up to a thickness of 5 µm and beyond, depending on the specification of the setup. A high voltage of typically −300 V is applied to the target. The negative voltage attracts the positive ions to the target surface. This technique has many advantages in terms of the high deposition rates, extremely high adhesion, uniformity, and ease of automation [70].
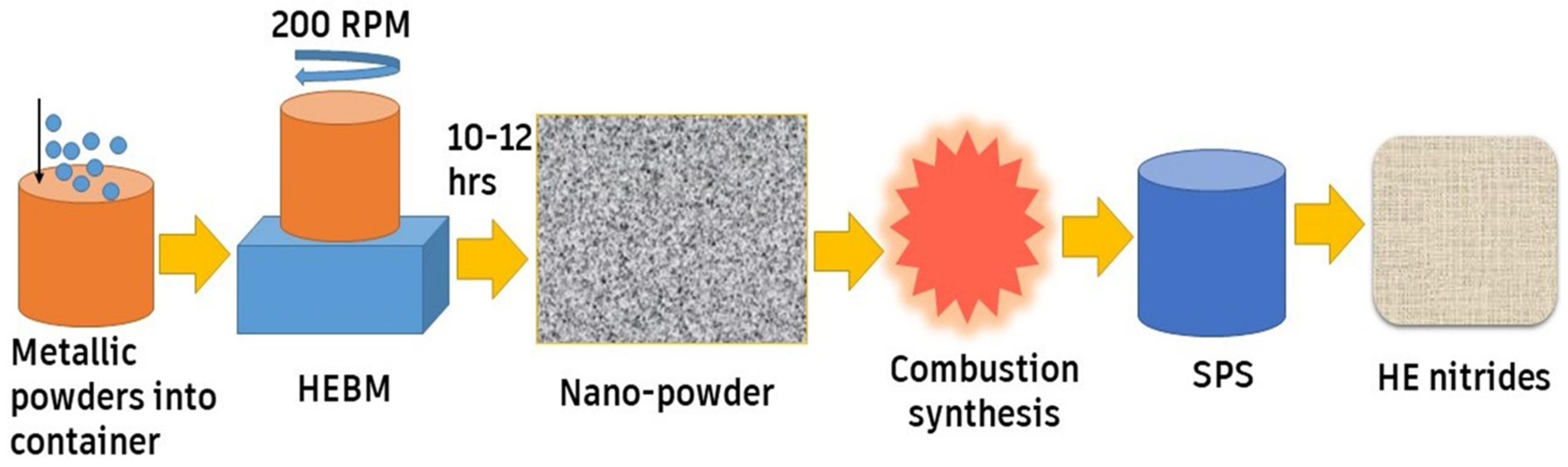
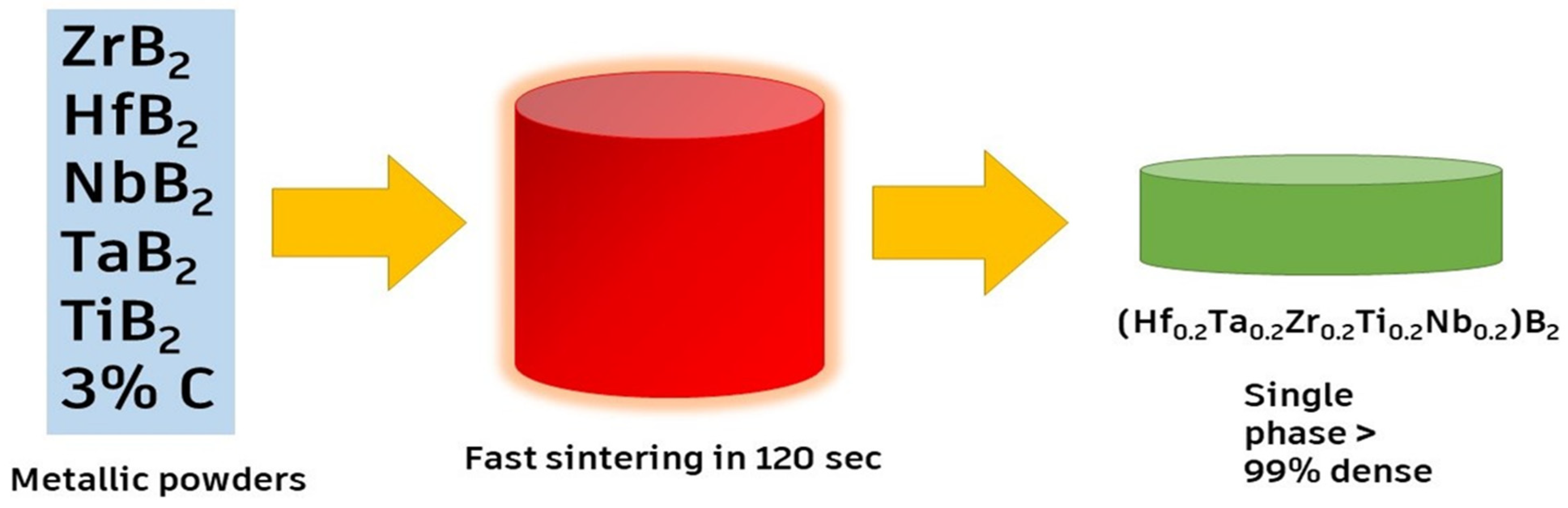
Hanh et al. [56] used the magnetron sputtering technique to manufacture HEN coatings (Al, Ti, V, Ta, Zr)N. They found that the deposited coatings demonstrated a fracture toughness of 2.4 MPa.m1/2 and a hardness of 30 GPa. Additionally, they observed that the addition of silicon leads to high damage tolerance. The synthesis route for HE nitrides (Nb0.2Hf0.2Ti0.2Zr0.2Ta0.2)N used by Moskovskikh et al. [71] utilized the HEBM technique, as shown in Figure 6. In a planetary ball-milling system, the metal powders were first stirred in HEBM for 10 h. The resulting mixture then underwent combustion synthesis in a nitrogen environment. The powders were relocated to an SPS machine, moving to the concluding build-up stage under nitrogen pressure.
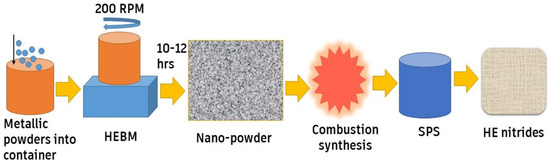
Figure 6.
HEBM fabrication process for HE nitrides, adapted from [71], Springer Nature, 2020.
Yang et al. [57] found HE nitride more stable than HE carbide with the same constituents at high temperatures. It also seems useful for coating as a cutting material due to its high thermal expansion. Hsieh et al. [40] designed the HE nitride coatings (Al29.1Nb11.2Cr30.8Ti7.7Si21.2)N50 and (Nb7.7Al23.1Cr30.8Si7.7Ti30.7)N50 with an FCC, NaCl-type structure by using reactive magnetron sputtering. The impact of the precursor bias (50 V to 150 V) on the deposited films’ microstructure and chemical content was examined. The (Al23.1Nb7.7Cr30.8 Si7.7Ti30.7)Nx coating films set at −100 V showed a maximum hardness of 36.1 GPa, whereas the (Nb11.2Cr30.8Si7.7Ti21.2Al29.1)Nx coating films at a −150 V substrate bias showed a maximum hardness of 36.7 GPa. Both the (Nb11.2Cr30.8Ti21.2Al29.1Si7.7)Nx nitride ceramic and (Al23.1Nb7.7Cr30.8Ti30.7Si7.7)Nx nitride coating films had excellent resistance to oxidation at 900 °C. Table 3 shows a comparison of the properties of some HE nitrides [40,56].

Table 3.
Comparison of the properties of different types of HE nitrides.
3.4. High-Entropy Silicides
The arrival of high-entropy silicides expanded the family of HE materials from metals, carbides, nitrides, and borides to silicides. The synthesis of bulk HE silicides is still an emerging area of research. These materials have found applications in wear resistance, diesel engine glow plugs, glass processing, and aerospace components. HEBM with SPS is the potential and successful synthesis approach for HE silicides, as detailed in the previous sections. HES was first synthesized by Gild et al. [21], where the fabricated (W0.2Ta0.2Nb0.2Mo0.2Ti0.2)Si2 with a non-cubic hexagonal arrangement demonstrated a low conductivity and high hardness. Quin et al. [58] developed HE (Ti0.22Nb0.29Zr0.06W0.21Mo0.22)Si2 doped with aluminum. They found that the Vickers hardness of the material was ~13.58 GPa due to the homogeneous distribution of Al2O3 hard particles in the matrix. Gild et al. [21] synthesized a novel non-cubic HES (Ta0.2Ti0.2Mo0.2W0.2Nb0.2)Si2 with a hexagonal-type framework. It was observed that the ABC stack framework features a CrSi2 C40-type hexagonal structure. This denoted the formation of an intricate crystal structure compared to prior studies, thus extending the scope to find newer high-entropy materials. The properties of some of the HE silicides are shown in Table 4 [21,58].

Table 4.
Properties of different HE silicides.
3.5. High-Entropy Oxides
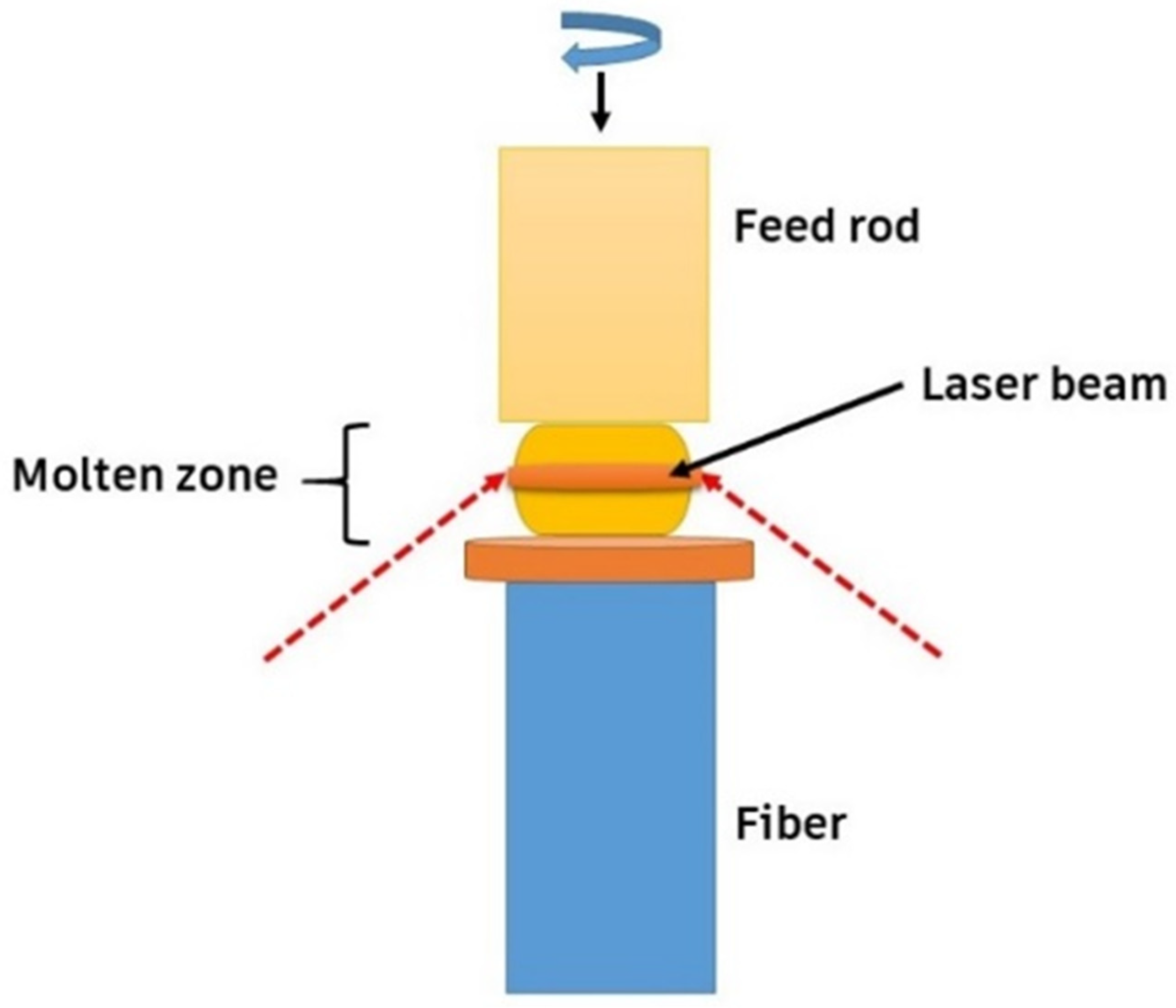
The elastic modulus-to-heat conductivity ratios of high-entropy oxides at room temperature are among the highest values ever recorded in the literature, surpassing those of well-known thermal barrier coatings. Novel laser floating, HEBM (high-energy ball-milling), and solid-state reaction methods have been employed to fabricate such HECs. These ceramics have been fabricated by a novel laser floating route, HEBM solid-state reaction method, etc. The laser floating approach was created as a quick, easy, and crucible-free way to develop high-quality crystalline materials. It requires precursor powder materials in cylindrical rod forms as feed, as shown in Figure 7. The laser floating zone (LFZ) method is a material processing technique that can produce high-quality single crystals, including high-entropy oxides. This technique uses a laser beam to heat and melt a small material section while the rest remains solid. As the laser beam moves across the material, the molten zone moves with it, creating a narrow zone of molten material that floats on the solid material below it. The molten zone acts as a filter, pushing impurities to the edges of the molten zone and away from the crystal. This technique has been used to produce high-entropy oxide single crystals [72].
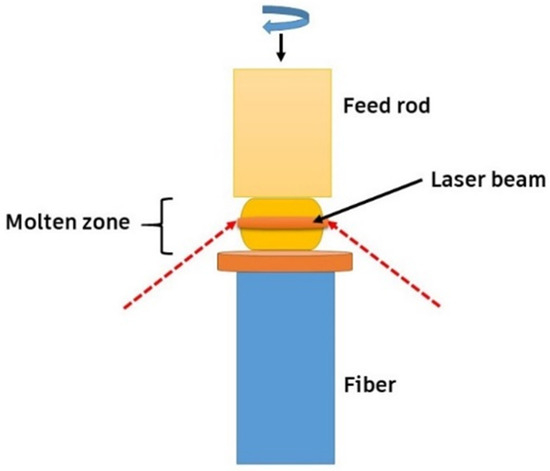
Figure 7.
Novel laser floating route material processing technique, adapted from [72], MDPI, 2020.
Mesa et al. [73] studied the Al2O3-Er3Al5O12-ZrO2 ceramic using a novel laser floating technique. The use of altered growth rates of 25 mm/h, 350 mm/h, and 1200 mm/h in the study resulted in the formation of different structures. The most significant phase was found in the 25 mm/h growth rate. The coarsening of the microstructure was found for other growth rates. Zhou et al. synthesized Ba(Me0.2Sn0.2Ti0.2Hf0.2Zr0.2)O3 by the solid-state reaction method. They found that the ceramic showed a permittivity of 25–200, with a breakdown strength in the 290–370 kV/cm range, and the dielectric loss was less than 0.002 Hz. Zhao et al. developed the (Eu0.2Sm0.2Y0.2Er0.2Nd0.2)AlO3 rare earth aluminate as a thermally insulating material, where they observed that the thermal conductivity of the ceramic was 4.1 W/mK. Zhao et al. [74] fabricated the rare earth (Nd0.2Ce0.2La0.2Sm0.2Eu0.2)2Zr2O7 ceramic with sluggish grain growth, with heat conductivity of 0.76 W/mK, to be used as a coating material. Zhang et al. [62] synthesized (Yb0.2Sm0.2Nd0.2Gd0.2La0.2)2Zr2O7 using combustion-synthesized nanopowder. It was the first transparent HEC developed with a pyrochlore structure, which showed an in-line transmittance measured value of 69.06% at 2108 nm. In another research study, Zifan et al. [75] fabricated (Yb0.2Lu0.2Y0.2Er0.2Eu0.2)3Al5O12, as a thermal barrier ceramic using a solid-state reaction method followed by SPS. It was observed that the ceramic, with an average grain size of 1.56 µm to 2.27 µm, showed a conductivity of 3.81 W/mK. Additionally, it showed a thermal expansion coefficient of (8.54 ± 0.29) × 10−6 K−1.
HEBCN powder (Hf0.2Nb0.2Ta0.2W0.2Zr0.2)BCN, (Ta0.2Zr0.2Hf0.2Ti0.2Nb0.2)BCN, and (Ti0.2Ta0.2Nb0.2Zr0.2W0.2)BCN were synthesized by the mechanical alloying method at room temperature. A single FCC cubic rock salt solution was observed after twenty-four hours of HEBM. The solid-state reaction method synthesized the HE (Yb0.25Y0.25Lu0.25Er0.25)2SiO5 rare earth mono-silicate with anisotropic thermal expansion. The three crystallographic directions showed the thermal expansion coefficient of la = (2.57 ± 0.07) × 10−6/K, lb = (8.07 ± 0.13) × 10−6/K, lc = (9.98 ± 0.10) × 10−6/K. A new equimolar solid solution (TiZrHf)P2O7 was synthesized, showing high thermal stability. It was found that its thermal conductivity was poor at 0.78 W/mK [76,77]. A unique HE (Ce0.2La0.2Eu0.2Sm0.2Nd0.2)PO4 rare earth phosphate monazite ceramic was prepared by Zhao et al. [74], where it was found that the ceramic exhibited low heat conductivity of 2.08 W/mK. Table 5 shows the properties of such types of HE oxides.

Table 5.
Comparative results of different HE oxides.
4. Properties and Behavior of High-Entropy Ceramics
HECs have the potential to outperform typical ceramics in terms of the mechanical characteristics. HE nitrides are particularly appealing for their elevated thermal properties and hardness. The eventual property of an HEC is unpredictable. The synergetic combination of constituents produces an HEC with high functional properties as energy storage and conversion materials [46].
HECs possess enhanced creep properties, which shows the solid material’s tendency to deform under the effect of persistent mechanical pressures. It can occur due to prolonged exposure to high stress below the material’s yield strength. Han et al. [78] noted an improved creep response of (Ta-Zr-Hf-Nb)C and found it comparatively lower than monocarbides. They found that the steady-state creep value lay between 2 × 10−9/s and 8 × 10−8/s due to the lattice dislocation and thermal stability at high temperatures (1400–1600 °C). The compressive stress in a vacuum was found to be in the range of 150 to 300 MPa. They proclaimed that the dislocation climb and grain boundary sliding were two creep modes controlled by diffusion. The typical initiation energy was 212 kJ/mol, and the stress exponent n ranged between 2.34 and 2.89. The creep response improvement has been a critical parameter for HECs, as this property was first reported in only high-entropy alloys [79,80].
Ultra-high-temperature HEC like (Zr0.2Ta0.2Hf0.2Ti0.2Nb0.2)B2 exhibits high heat insulation [53]. The porous HEB possesses low heat conductivity and heat diffusivity of 0.51 W/mK and 0.74 mm2/s, respectively. Porous (Hf0.2Zr0.2Ti0.2Ta0.2Nb0.2)C exhibits a homogeneous microstructure with a small heat conductivity of 0.39 W/mK and a low heat diffusivity of 0.74 mm2/s. This makes HEC a lightweight ultra-high-temperature thermal insulation material [66].
HE (Zr0.2Ti0.2Hf0.2Ta0.2Nb0.2)C has shown superior electromagnetic wave absorption (EMW) properties. Descent thermal conductivity and splitting of the d orbitals into lower and higher levels were the mechanisms for the improved EMW absorption capabilities, i.e., dielectric loss and magnetic loss coupling. HE carbide has an RLmin (minimum reflection loss) of 38.5 dB at 9.5 GHz, with a width of 1.9 mm, and an EAB (effective absorption bandwidth) of 2.3 GHz (11.3–13.6 GHz), with a width of 1.5 mm [81]. (Lu0.25Er0.25Yb0.25Y0.25)2SiO5 possesses outstanding anisotropy and phase stability in terms of the heat expansion. In this case, if the preferred orientation of the HEC is used, the robust anisotropy in the heat expansion minimizes the precursor incompatibility. The heat expansion coefficient of HE (Sm0.2Eu0.2Nd0.2Yb0.2Y0.2)4Al2O9 is similar to mullite (6.96 × 10−6 K−1 at 300–1473 K). Anisotropic heat expansion is caused by anisotropic chemical bonding, aided by the large size of rare earth cations [77].
Magnetic correlations in HEOs are altered by an enormous diversity of nearby ionic configurations, which depend on the valence electrons, coordination geometry, spin state, and type of metal cations that are hybridized with the associating oxygen. Under these conditions, HEOs’ complicated magneto-electronic free-energy landscape is envisaged, stabilizing the unconventional spin-electronic states. HEOs exhibit exceptional magnetic capabilities and the opportunity for more research and discovery of potential utility [82]. A long-range magnetic order was observed in (Co0.2Mg0.2Ni0.2Zn0.2Cu0.2)O, an HE oxide with a rock salt structure below 120 K, compatible with the previous detection of exchange bias in HEOx-based heterostructures. The magnetism found was 2.95 (µB/f.u.). The other rock salt-type high-entropy oxides with varied chemical substitutions host either an antiferromagnetic order or spin-glass state based on the magnetic ions. The magnetic order in such a disordered material could allow new magnetic characteristics and functionalities to be discovered [83].
In addition to these enhanced properties, HECs have a unique way of behaving to process modifications when a process change is made. The doping of elements in the matrix material also changes the properties and thus the microstructure. The elastic deformation behavior of HE nitride increases with the addition of silicon. It is reported that the elastic behavior was drastically reduced by adding 5% Si to this high-entropy nitride. The oxidation resistance of HE carbide at elevated temperatures could be enhanced by adding SiC. This is because of the oxidation of SiC. The addition of SiC to (Nb0.25Ta0.25Zr0.25Hf0.25)C suppressed micro-cracks at elevated temperatures. A small quantity of Sn substitution in Sr0.05Ba0.95(Zr0.05Ti0.95)1−x SnxO3 (x = 0; 0.025) led to the reduction of the “Curie temperature” from 392 K to 364 K for the examined compositions; characteristic hysteresis loops were obtained, confirming the ferroelectric-to-paraelectric transition [84,85,86]. At elevated temperatures, it is also reported that the oxidation of HECs can track the parabolic rate, where the oxidation rate increased at first at higher temperatures, then decreased due to the synthesis of the distinct oxidation yields at 1073–1473K. The oxidation behavior of (Ta0.2Zr0.2Hf0.2Ti0.2Nb0.2)C HECs was investigated in air. According to the findings, the oxidation rate charted a parabolic rate trend, with the oxidation rate initially increasing with an increasing temperature and then reducing with ab increasing temperature. All the oxide layers have a layered structure except the thick oxide layer generated at 1173 K [87]. There are several factors reported in the literature that influence the properties of the HECs, among which irradiation is one of the important factors.
Wang et al. [88] in their research found that (Ta0.25Ti0.25Nb0.25Zr0.25)C HEC demonstrated no phase change when exposed to 3 MeV zirconium ions. There was no void formation, which could be due to carbide ceramics’ high vacancy migration barriers. They also did not observe radiation-induced segregation in the defect boundaries. It is reported that the defect clusters possessed two dislodgment rings: perfect loops with Burgers vectors and faulty Frank loops. The substantial local lattice distortion may prevent the formation of dislocation loops. It was found that due to the dislodgment rings and internal strain, there was an increase in the irradiation-induced hardness in nano-indentation testing. HEC is a viable structural material for advanced reactor designs due to its strong irradiation resistance and excellent physical features.
In the case of entropy, it was found that HECs outperform their low-entropy relatives in terms of the fracture toughness and thermal stability. In HE diborides, the hardness of the ZrB2 layers may be enhanced from 43.2 GPa to 47.2 GPa. Furthermore, in HECs, one sublattice can remain constant; therefore, the core effects, such as slow diffusion, are mainly focused on the HE sublattice. However, due to the distortion of the metallic sublattice, the research reveals that the non-metal sublattice may have higher diffusivities [89].
The importance of the gas flow parameter comes into the picture when sputtering is used in general. In a related study, Xing et al. [90] fabricated NbTiAlSiZrNx HEC films using magnetron sputtering under various nitrogen flow rates. After annealing for 24 h at 700 °C, the films also remained amorphous, with no significant crystallization, while their hardness declined. However, at nitrogen flow rates of 4 sccm and 8 sccm, nanocrystallization occurred after annealing at 900 °C for 30 min, leading to the formation of an FCC structure. The increased nitrogen flow rate resulted in a noticeable decrease in hardness. With an increased nitrogen inflow rate, the heat stability of the amorphous phase deteriorated, and at high temperatures, it formed a nitride.
Berardan et al. [91] showed that aliovalent elements with a charge compensation mechanism could substitute HE oxides, widening their phase space. They found that the ionic conductivity increases dramatically after Li+ replaces a +2 element in a superionic conductor like HE (CoMgNiZnCu)O. When the proportion of lithium is increased, the ionic conductivity increases considerably. By expanding the complicated phase space of novel materials, this capability greatly improves their potential development. The HEOx composition has colossal dielectric constants, implying that it might be employed as a large-k dielectric material [92].
It has been reported that in HEO (La0.2Ce0.2Pr0.2Y0.2Sm0.2)O2, the presence of Pr and Ce (in 4+/3+states) causes the creation of intermediary 4f energy, which adjusts the bandgap energy in HEOs. The bandgap energy of the HEO is reversibly changed from 1.9 eV to 2.5 eV after heat treatment. The observation aligns with Pr’s reversible oxidation state shifts and the associated f-orbital occupancy. These intermediate levels are altered by heat treatment in an oxidizing/reducing environment, allowing the bandgap energy in HEOs to be modified. Though concrete applications for the above have yet to emerge, it may be advantageous for mixed electronic–ionic conduction, catalysis, and other applications [93]. However, HE carbides within a host steel matrix, mainly (Zr, Ti, Hf, Ta, Nb)C particles, can be precipitated. This method provides an alternate manufacturing procedure for HEC powders by leeching away the steel matrix. The product is fundamentally a steel matrix composite reinforced with an HE carbide phase, making this a viable engineering material in and of itself [94].
It has been reported that the high-entropy carbides have shown modest particle growth kinetics in (Zr0.2Hf0.2Ta0.2Ti0.2Nb0.2)C at 1300 °C and 1600 °C, indicating good thermal stability, which could be attributed to the slow diffusion and compositional intricacy in the HE carbide. It was also observed that the bending strength and fracture toughness of fine-grained HEC were 25% and 20% greater, respectively, than coarse-grained HE carbide with a particle diameter of 16.5 µm. Micro-mechanistic phenomena such as fracture deflection may further enhance the physical characteristics [95].
In the reported research, it is observed that the incorporation of lithium changed the structure of the HEO. When monovalent Li+ ions were included, the phase change from spinel to rock salt was seen, along with partial oxidation of some elements in the lattice. In particular, HEOs with a lithiated rock salt structure have shown promise as electrodes in rechargeable Li-ion batteries. The Li concentration gradually increased while the other metal ratios remained stable. The patterns of the as-prepared materials showed the switch from a spinel toa rock salt structure with increased Li content [96].
5. Application of High-Entropy Ceramics
The emergence of the HEC group has opened up new possibilities for material fabrication and property modification. Ceramics, unlike metals, have a wide range of crystal and electronic structures. This allows for extensive property modification via the band structure and phonon mechanism. HECs have shown hardening, strengthening, and low heat conductivity. Some of the major applications of HECs are demonstrated in Figure 8.
Figure 8.
Major application areas of HECs.
HECs have found tremendous applications in thermal and environmental barrier coatings. Chen et al. [77] synthesized high-temperature thermal barrier ceramic (Yb0.2Y0.2Lu0.2Eu0.2Er0.2)3Al5O12 at 673–1273 K. They found that the thermal expansion coefficient of HEC was (8.54 ± 0.29) × 10−6 K−1, 9% more than Yb3Al5O12. At 300 K, the thermal conductivity of HEC was found to be 3.81 W/mK, 18% lower than Yb3Al5O12. The (Nd0.2Y0.2Eu0.2Sm0.2Er0.2)AlO3 rare earth aluminate could be used as a heat insulator. Additionally, it had a linear heat expansion coefficient of 9.02 × 10−6/°C, similar to Al2O3, making it a good candidate material for heat insulators or thermal barrier coatings in high-temperature environments [75,97]. The electrochemical behavior of the HE oxides is demonstrated to rely on each metal cation available, allowing the electrochemical attributes to be designed by changing the elemental composition. The energy storage and dielectric properties of (Ba0.2K0.2Na0.2Bi0.2Ca0.2)TiO3 were also reported. Under a 129 kV/cm electric field, it had an efficiency of 87.5% and a discharge energy density of 0.684 J/cm3, which marked it as a potential candidate for use in energy storage and dielectrics [98,99].
Moreover, HE oxides have recently received much attention as a lithium-ion battery anode material. (Ni, Cu, Mg, Zn, Co)O HEO could be used for lithium-ion battery applications. Remarkable lithium storage properties were found at 1 A/g after 1000 cycles [100]. An HE oxide with Mg, Ni, Cu, Zn, and Co was used to provide a chemical anchor for lithium-sulfur batteries. After 600 cycles, it showed a low decline of 0.077% each cycle [101]. The electrochemical performances of (MgCoNiZn)1−xLixO (x = 0.05, 0.15, 0.25, and 0.35) HEOs as anode materials in lithium-ion batteries were investigated. The investigations revealed that increasing the lithium cation concentration results in more oxygen vacancies, impacting the electrochemical performance of the anodes. The higher the anode’s oxygen vacancy concentration, the greater the battery discharge behavior. At a current density of 1000 mAhg−1, the anode had an initial discharge capacity of 1930 mAhg−1 and a stable discharge capacity of 610 mAhg−1 after 130 cycles [102].
It is well known that ceramic materials are potential choices for application in supercapacitor (SC) electrodes. It has been investigated that the ceramic materials’ SC performance is significantly hampered by their low specific surface area and poor surface activity [103]. (FeCoCrMnZn)3O4 performed exceptionally well as an electrode material for the supercapacitor with a specific mass capacitance of 340.3 Fg−1. (CoCrFeMnMg)3O4 also demonstrated supercapacitor capabilities, with 193.7 Fg−1 specific mass capacitance [104].
HECS can also be used in thermoelectric areas. The thermoelectric effect is a phenomenon in which an electric potential causes a temperature gradient. While all materials have some degree of thermoelectricity, it is usually insufficient to be helpful. Beyond the current methodologies and approaches, entropy engineering employing multi-component crystal structures or other conceivable techniques offers a new route for improving thermoelectric performance [35]. (Sr0.2Ca0.2Ba0.2La0.2Pb0.2)TiO3 showed 1.17 W/mK at 923 K due to the considerable mass fluctuation effect and the significant lattice distortion. It was thermally and chemically stable at elevated temperatures, with low thermal conductivity. Oxide-based ceramics could be potential thermoelectric materials because of their thermochemical stability at high temperatures [17]. Moreover, entropy is important in catalysis, and researchers have spent a lot of time trying to figure out how the enthalpy–entropy connection specifies the reaction paths of chemical species. On the other hand, entropic effects on the surface of catalysts have been studied infrequently. Even at temperatures as high as 900 °C, (NiMgCuZnCo)Ox can be operated as a CO oxidation catalyst. The catalyst could be reused for at least 40 h. In the hydrogenation of ambient CO2 to CO, (NiMgCuZnCo)O showed decent stability at elevated temperatures [105].
HEC films refer to the boride, carbide, oxide, or nitride films, which offer potential applications under high thermal conditions when deposited in a thin film state. Using DC magnetron reactive sputtering, several HECs have been produced, like the NbTiAlSiZrNx HEC films that were effectively produced with varied nitrogen concentrations. These have the potential to be employed as protective coverings in high-temperature environments. It was observed that after 24 h of annealing at 700 °C, the hardness was 8.812 GPa [89,90]. HECs that are both hard and durable open up new possibilities for a wide range of innovative applications. (Zr0.2Hf0.2Ta0.2Ti0.2Cr0.2)B2 showed a hardness of 28.3 GPa, much more significant than the previously stated standards. The hardness of (NbHfTaZrTi)C5 and (TaHfTiZrW)C5 was measured to be 32 GPa and 33 GPa, respectively, indicating a novel avenue for superhard material design. (Nb0.2Hf0.2Ti0.2Ta0.2Zr0.2)N has shown an exceptional fracture toughness (5.2 MPa.m1/2), far exceeding the expected values from mixture rules and other reported binary and HECs [71]. Some of the HECs have also shown their candidacy for their use in superionic conductivity. The specific conductivity of superionic conductors, also known as solid electrolytes, is typically in the range of 10−3 to 10 S/cm, while metals have representative values varying from 10 to 105 S/cm. HEC can be a good substitute for Li+ and Na+ as a superionic conductor. The charge compensation occurs when Li+ replaces a +2 element in the high-entropy superionic conductor (CoMgNiZnCu)O, leading to even higher Li mobility. When the proportion of lithium increases, the ionic conductivity increases considerably, above 1 mS/cm at ambient temperature and 20 mS/cm at 100 °C [92,106]. Moreover, they can also be used in dielectric materials. A dielectric material is a substance that conducts electricity poorly but effectively supports electrostatic fields. HE Ba(Sn0.2Hf0.2Zr0.2Me0.2Ti0.2)O3 was fabricated as a dielectric material. The HEO had decent thermal stability of permittivity from 25 °C to 200 °C, small dielectric loss (0.002) from 20 Hz to 2 MHz, and sensible breakdown strength (290 kV/cm, 370 kV/cm). The dielectric constants of an HEO produced by Berardan et al. [19] were also colossal, making it suitable as a dielectric material. Li(Ho0.2Gd0.2Er0.2Lu0.2Yb0.2)GeO4 was investigated as a microwave dielectric ceramic. An olivine structure was formed in the sintering temperature range of 1020–1100 °C. After 4 h at 1080 °C, ideal microwave dielectric properties were achieved, with a relative density of 94.9% [107].
HECs can also be used in the area of electrolytic water splitting. Electrocatalytic water splitting uses have been required to meet environmental and economic objectives. On the other hand, the conventional fabrication routes limit the development of extraordinary performance catalysts. HECs, which result from a material design idea based on many elements, offer a method to break away from compositional design constraints in materials science [108]. A novel approach was implied for continuous hydrogen regeneration based on two-step water splitting. The heat-reduction and water-splitting processes occurred at the same microwave irradiation power. At 700 W microwave intensity, the HE (FeMgCoNi)O1.2@SiC could produce a maximum hydrogen generation rate of 13.89 mL/min/g, with a maximum hydrogen yield of 122 mL/g. This finding was higher than the thermodynamic limits of current materials like spinel ferrites and ceria. Table 6 shows several other applications of HECs [17,35,38,43,50,56,57,71,77,78,107,109].

Table 6.
Various applications of different HECs.
6. Conclusions and Future Prospect
High-entropy compounds (HECs) represent an emerging class of materials with exceptional potential across a range of applications due to their unique structural, mechanical, and thermal properties. Recent advancements in synthesis techniques—such as solid-state reactions, combustion synthesis, and mechanical alloying—have enabled the development of HECs with promising features like high hardness, toughness, and thermal stability. These properties make them suitable for various high-demand applications, including energy storage, thermal barrier coatings, and composite materials. However, significant challenges remain in the field of HEC fabrication and optimization. A deeper understanding of the relationship between the structure and properties of HECs is essential to unlocking their full potential. Issues like the phase stability, the microstructural evolution, and the effects of multi-element compositions on properties need further exploration. Additionally, tailoring synthesis techniques to achieve precise control over HECs’ properties remains a key hurdle. Addressing these challenges is crucial for enhancing the material performance and expanding the practical applications of HECs in engineering.
In terms of future applications, HECs hold great promise, particularly in energy storage and thermal management. Their unique electrochemical properties make them ideal for next-generation energy storage devices, while their superior thermal stability could lead to breakthroughs in thermal barrier coatings for high-temperature equipment. Moreover, reinforcing composites with HECs could significantly improve the strength and durability of structural materials.
In conclusion, while the field of HEC research is still in the developmental stage, its potential for advancing materials science and engineering applications is substantial. Continued research to overcome the current limitations and improve the synthesis methods will be pivotal in realizing the broad potential of HECs, ultimately leading to widespread use in fields ranging from energy to aerospace engineering.
Author Contributions
Conceptualization, P.C.V.; methodology, A.S.; validation, P.C.V.; formal analysis, A.M.; investigation, S.K.T.; data curation, S.K.T. and A.S.; writing—original draft preparation, P.C.V., S.K.T., A.S. and A.M.; writing—review and editing, P.C.V. and A.M.; visualization, S.K.T. and A.M.; project administration, P.C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare that there have been no potential conflicts of interest concerning the research, authorship, and publication.
References
- Dupuy, A.D.; Wang, X.; Schoenung, J.M. Entropic phase transformation in nanocrystalline high entropy oxides. Mater. Res. Lett. 2019, 7, 60–67. [Google Scholar] [CrossRef]
- Albedwawi, S.H.; AlJaberi, A.; Haidemenopoulos, G.N.; Polychronopoulou, K. High entropy oxides-exploring a paradigm of promising catalysts: A review. Mater. Des. 2021, 202, 109534. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, T.; Gild, J.; Zhou, N.; Nie, J.; Qin, M.; Harrington, T.; Vecchio, K.; Luo, J. A new class of high-entropy perovskite oxides. Scr. Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Kroutvar, M.; Ducommun, Y.; Heiss, D.; Bichler, M.; Schuh, D.; Abstreiter, G.; Finley, J.J. Optically programmable electron spin memory using semiconductor quantum dots. Nature 2004, 432, 81–84. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, M.; Zhang, Q.; Hao, H.; Yao, Z.; Wang, Z.; Song, Z.; Zhang, Y.; Hu, W.; Liu, H. Dielectric relaxation in Zr-doped SrTiO3 ceramics sintered in N2 with giant permittivity and low dielectric loss. J. Am. Ceram. Soc. 2015, 98, 476–482. [Google Scholar] [CrossRef]
- Eerenstein, W.; Mathur, N.D.; Scott, J.F. Multiferroic and magnetoelectric materials. Nature 2006, 442, 759–765. [Google Scholar] [CrossRef]
- Karczewski, J.; Riegel, B.; Gazda, M.; Jasinski, P.; Kusz, B. Electrical and structural properties of Nb-doped SrTiO3 ceramics. J. Electroceramics 2010, 24, 326–330. [Google Scholar] [CrossRef]
- Wang, N.; Cao, M.; He, Z.; Diao, C.; Zhang, Q.; Zhang, Y.; Dai, J.; Zeng, F.; Hao, H.; Yao, Z.; et al. Structural and dielectric behavior of giant permittivity SrNbxTi1−xO3 ceramics sintered in nitrogen atmosphere. Ceram. Int. 2016, 42, 13593–13600. [Google Scholar] [CrossRef]
- Bokov, A.A.; Ye, Z.G. Recent progress in relaxor ferroelectrics with perovskite structure. J. Mater. Sci. 2006, 41, 31–52. [Google Scholar] [CrossRef]
- Oses, C.; Toher, C.; Curtarolo, S. High-entropy ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Duan, C.; Tong, J.; Shang, M.; Nikodemski, S.; Sanders, M.; Ricote, S.; Almansoori, A.; O’Hayre, R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Pei, X.; Tang, L.; Cheng, H.; Li, Z.; Li, C.; Zhang, X.; An, L. A five-component entropy-stabilized fluorite oxide. J. Eur. Ceram. Soc. 2018, 38, 4161–4164. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Reece, M.J. Review of high entropy ceramics: Design, synthesis, structure and properties. J. Mater. Chem. A 2019, 7, 22148–22162. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.; Blinn, K.; Liu, M.; Liu, Z.; Cheng, Z.; Liu, M. Enhanced Sulfur and Coking tolerance of a MIEC for SOFC: BCZYYb. Science 2009, 326, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Farizah, N.H.; Shamsuddin, S. Electrical Transport Analysis using Two Different Hopping Models on Pr0.75Na0.25Mn1−xCrxO3 Manganite. Enhanc. Knowl. Sci. Technol. 2022, 2, 274–281. [Google Scholar]
- Gorte, R.J. Cooling down ceramic fuel cells. Science 2015, 349, 1290. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zou, M.; Zhang, W.; Yi, D.; Lan, J.; Nan, C.W.; Lin, Y.H. Electrical and thermal transport behaviours of high-entropy perovskite thermoelectric oxides. J. Adv. Ceram. 2021, 10, 377–384. [Google Scholar] [CrossRef]
- Vozniuk, O.; Tanchoux, N.; Millet, J.M.; Albonetti, S.; Di Renzo, F.; Cavani, F. Spinel Mixed Oxides for Chemical-Loop Reforming: From Solid State to Potential Application. Stud. Surf. Sci. Catal. 2019, 178, 281–302. [Google Scholar] [CrossRef]
- Grzesik, Z.; Smoła, G.; Miszczak, M.; Stygar, M.; Dąbrowa, J.; Zajusz, M.; Świerczek, K.; Danielewski, M. Defect structure and transport properties of (Co,Cr,Fe,Mn,Ni)3O4 spinel-structured high entropy oxide. J. Eur. Ceram. Soc. 2020, 40, 835–839. [Google Scholar] [CrossRef]
- Mao, A.; Xiang, H.Z.; Zhang, Z.G.; Kuramoto, K.; Zhang, H.; Jia, Y. A new class of spinel high-entropy oxides with controllable magnetic properties. J. Magn. Magn. Mater. 2020, 497, 165884. [Google Scholar] [CrossRef]
- Gild, J.; Braun, J.; Kaufmann, K.; Marin, E.; Harrington, T.; Hopkins, P.; Vecchio, K.; Luo, J. A high-entropy silicide: (Mo0.2Nb0.2Ta0.2Ti0.2W0.2)Si2. J. Mater. 2019, 5, 337–343. [Google Scholar] [CrossRef]
- Gild, J.; Zhang, Y.; Harrington, T.; Jiang, S.; Hu, T.; Quinn, M.C.; Mellor, W.M.; Zhou, N.; Vecchio, K.; Luo, J. High-Entropy Metal Diborides: A New Class of High-Entropy Materials and a New Type of Ultrahigh Temperature Ceramics. Sci. Rep. 2016, 6, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Monteverde, F.; Fahrenholtz, W.G.; Hilmas, G.E. Superhard high-entropy AlB2-type diboride ceramics. Scr. Mater. 2021, 199, 113855. [Google Scholar] [CrossRef]
- Sarwan, M.; Singh, S. Structural, elastic and mechanical properties of group III-nitrides in zinc-blend structure. J. Alloys Compd. 2013, 550, 150–158. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Zhang, F.; Niu, B.; Lei, L.; Wang, W. High-entropy carbide: A novel class of multicomponent ceramics. Ceram. Int. 2018, 44, 22014–22018. [Google Scholar] [CrossRef]
- Zhou, N.; Jiang, S.; Huang, T.; Qin, M.; Hu, T.; Luo, J. Single-phase high-entropy intermetallic compounds (HEICs): Bridging high-entropy alloys and ceramics. Sci. Bull. 2019, 64, 856–864. [Google Scholar] [CrossRef]
- Xiang, H.; Xing, Y.; Dai, F.Z.; Wang, H.; Su, L.; Miao, L.; Zhang, G.; Wang, Y.; Qi, X.; Yao, L.; et al. High-entropy ceramics: Present status, challenges, and a look forward. J. Adv. Ceram. 2021, 10, 385–441. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Y. Functional properties and promising applications of high entropy alloys. Scr. Mater. 2020, 187, 188–193. [Google Scholar] [CrossRef]
- Nisar, A.; Zhang, C.; Boesl, B.; Agarwal, A. A Perspective on Challenges and Opportunities in Developing High Entropy-Ultra High Temperature Ceramics. Ceram. Int. 2020, 46, 25845–25853. [Google Scholar] [CrossRef]
- Qin, M.; Gild, J.; Hu, C.; Wang, H.; Hoque, M.S.B.; Braun, J.L.; Harrington, T.J.; Hopkins, P.E.; Vecchio, K.S.; Luo, J. Dual-phase high-entropy ultra-high temperature ceramics. J. Eur. Ceram. Soc. 2020, 40, 5037–5050. [Google Scholar] [CrossRef]
- Page, A.; Poudeu, P.F.P.; Uher, C. A first-principles approach to half-Heusler thermoelectrics: Accelerated prediction and understanding of material properties. J. Mater. 2016, 2, 104–113. [Google Scholar] [CrossRef]
- Haché, M.J.R.; Cheng, C.; Zou, Y. Nanostructured high-entropy materials. J. Mater. Res. 2020, 35, 1051–1075. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.; Huang, C.; Nieto, A.; Chen, R.; Luo, J. From high-entropy ceramics to compositionally-complex ceramics: A case study of fluorite oxides. J. Eur. Ceram. Soc. 2020, 40, 2120–2129. [Google Scholar] [CrossRef]
- Fan, Q.C.; Li, B.S.; Zhang, Y. The microstructure and properties of (FeCrNiCo)AlxCuy high-entropy alloys and their TiC-reinforced composites. Mater. Sci. Eng. A 2014, 598, 244–250. [Google Scholar] [CrossRef]
- Liu, R.; Chen, H.; Zhao, K.; Qin, Y.; Jiang, B.; Zhang, T.; Sha, G.; Shi, X.; Uher, C.; Zhang, W.; et al. Entropy as a Gene-Like Performance Indicator Promoting Thermoelectric Materials. Adv. Mater. 2017, 29, 1702712. [Google Scholar] [CrossRef] [PubMed]
- Abu-Odeh, A.; Galvan, E.; Kirk, T.; Mao, H.; Chen, Q.; Mason, P.; Malak, R.; Arróyave, R. Efficient exploration of the High Entropy Alloy composition-phase space. Acta Mater. 2018, 152, 41–57. [Google Scholar] [CrossRef]
- Zhong, Y.; Sabarou, H.; Yan, X.; Yang, M.; Gao, M.C.; Liu, X.; Sisson, R.D. Exploration of high entropy ceramics (HECs) with computational thermodynamics—A case study with LaMnO3±δ. Mater. Des. 2019, 182, 108060. [Google Scholar] [CrossRef]
- Orrù, R.; Cao, G. Ultra-High Temperature Ceramics by Spark Plasma Sintering; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Kaufmann, K.; Maryanovsky, D.; Mellor, W.M.; Zhu, C.; Rosengarten, A.S.; Harrington, T.J.; Oses, C.; Toher, C.; Curtarolo, S.; Vecchio, K.S. Discovery of high-entropy ceramics via machine learning. NPJ Comput. Mater. 2020, 6, 42. [Google Scholar] [CrossRef]
- Ye, B.; Wen, T.; Nguyen, M.C.; Hao, L.; Wang, C.; Chu, Y. First-principles study, fabrication, and characterization of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramic. J. Am. Ceram. Soc. 2019, 102, 4344–4352. [Google Scholar] [CrossRef]
- Dai, F.Z.; Wen, B.; Sun, Y.; Xiang, H.; Zhou, Y. Theoretical prediction on thermal and mechanical properties of high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)C by deep learning potential. J. Mater. Sci. Technol. 2020, 43, 168–174. [Google Scholar] [CrossRef]
- Zhang, H.; Hedman, D.; Feng, P.; Han, G.; Akhtar, F. A high-entropy B4(HfMo2TaTi)C and SiC ceramic composite. Dalton Trans. 2019, 48, 5161–5167. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Shao, L.; Fan, T.W.; Duan, J.M.; Chen, X.T.; Tang, B.Y. Elastic and thermodynamic properties of high entropy carbide (HfTaZrTi)C and (HfTaZrNb)C from ab initio investigation. Ceram. Int. 2020, 46, 15104–15112. [Google Scholar] [CrossRef]
- Guan, J.; Li, D.; Yang, Z.; Wang, B.; Cai, D.; Duan, X.; He, P.; Jia, D.; Zhou, Y. Synthesis and thermal stability of novel high-entropy metal boron carbonitride ceramic powders. Ceram. Int. 2020, 46, 26581–26589. [Google Scholar] [CrossRef]
- Cologna, M.; Rashkova, B.; Raj, R. Flash sintering of nanograin zirconia in <5 s at 850 °C. J. Am. Ceram. Soc. 2010, 93, 3556–3559. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef]
- Musicó, B.L.; Gilbert, D.; Ward, T.Z.; Page, K.; George, E.; Yan, J.; Mandrus, D.; Keppens, V. The emergent field of high entropy oxides: Design, prospects, challenges, and opportunities for tailoring material properties. APL Mater. 2020, 8, 040912. [Google Scholar] [CrossRef]
- Dong, Y.; Ren, K.; Lu, Y.; Wang, Q.; Liu, J.; Wang, Y. High-entropy environmental barrier coating for the ceramic matrix composites. J. Eur. Ceram. Soc. 2019, 39, 2574–2579. [Google Scholar] [CrossRef]
- Faraji, G.; Torabzadeh, H. An overview on the continuous severe plastic deformation methods. Mater. Trans. 2019, 60, 1316–1330. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Zhang, Z.H.; Cheng, X.W.; Wang, F.C.; Zhang, Y.F.; Li, S.L. A review of multi-physical fields induced phenomena and effects in spark plasma sintering: Fundamentals and applications. Mater. Des. 2020, 191, 108662. [Google Scholar] [CrossRef]
- Wei, X.F.; Qin, Y.; Liu, J.X.; Li, F.; Liang, Y.C.; Zhang, G.J. Gradient microstructure development and grain growth inhibition in high-entropy carbide ceramics prepared by reactive spark plasma sintering. J. Eur. Ceram. Soc. 2020, 40, 935–941. [Google Scholar] [CrossRef]
- Feng, L.; Fahrenholtz, W.G.; Hilmas, G.E.; Zhou, Y. Synthesis of single-phase high-entropy carbide powders. Scr. Mater. 2019, 162, 90–93. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, H.; Dai, F.Z.; Liu, J.; Zhou, Y. Porous high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)B2: A novel strategy towards making ultrahigh temperature ceramics thermal insulating. J. Mater. Sci. Technol. 2019, 35, 2404–2408. [Google Scholar] [CrossRef]
- Hsieh, M.; Tsai, M.; Shen, W.; Yeh, J. Structure and properties of two Al—Cr—Nb—Si—Ti high-entropy nitride coatings. Surf. Coat. Technol. 2013, 221, 118–123. [Google Scholar]
- Zhao, P.; Zhu, J.; Zhang, Y.; Shao, G.; Wang, H.; Li, M.; Liu, W.; Fan, B.; Xu, H.; Lu, H.; et al. A novel high-entropy monoboride (Mo0.2Ta0.2Ni0.2Cr0.2W0.2)B with superhardness and low thermal conductivity. Ceram. Int. 2020, 46, 26626–26631. [Google Scholar] [CrossRef]
- Hahn, R.; Kirnbauer, A.; Bartosik, M.; Kolozsvári, S.; Mayrhofer, P.H. Toughness of Si alloyed high-entropy nitride coatings. Mater. Lett. 2019, 251, 238–240. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, L.; Gan, G.Y.; Wang, W.; Tang, B.Y. Investigation of thermodynamic properties of high entropy (TaNbHfTiZr)C and (TaNbHfTiZr)N. J. Alloys Compd. 2019, 788, 1076–1083. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, J.C.; Liu, J.X.; Wei, X.F.; Li, F.; Zhang, G.J.; Jing, C.; Zhao, J.; Wu, H. High-entropy silicide ceramics developed from (TiZrNbMoW)Si2 formulation doped with aluminum. J. Eur. Ceram. Soc. 2020, 40, 2752–2759. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S. Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Moskovskikh, D.O.; Vorotilo, S.; Sedegov, A.S.; Kuskov, K.V.; Bardasova, K.V.; Kiryukhantsev-korneev, P.V.; Zhukovskyi, M.; Mukasyan, A.S. High-entropy (HfTaTiNbZr)C and (HfTaTiNbMo)C carbides fabricated through reactive high-energy ball milling and spark plasma sintering. Ceram. Int. 2020, 46, 19008–19014. [Google Scholar] [CrossRef]
- Moskovskikh, D.O.; Lin, Y.C.; Rogachev, A.S.; McGinn, P.J.; Mukasyan, A.S. Spark plasma sintering of SiC powders produced by different combustion synthesis routes. J. Eur. Ceram. Soc. 2015, 35, 477–486. [Google Scholar] [CrossRef]
- Zhang, K.; Li, W.; Zeng, J.; Deng, T.; Luo, B.; Zhang, H.; Huang, X. Preparation of (La0.2Nd0.2Sm0.2Gd0.2Yb0.2)2Zr2O7 high-entropy transparent ceramic using combustion synthesized nanopowder. J. Alloys Compd. 2020, 817, 153328. [Google Scholar] [CrossRef]
- Gild, J.; Kaufmann, K.; Vecchio, K.; Luo, J. Reactive flash spark plasma sintering of high-entropy ultrahigh temperature ceramics. Scr. Mater. 2019, 170, 106–110. [Google Scholar] [CrossRef]
- Chicardi, E.; García-Garrido, C.; Gotor, F.J. Low temperature synthesis of an equiatomic (TiZrHfVNb)C5 high entropy carbide by a mechanically-induced carbon diffusion route. Ceram. Int. 2019, 45, 21858–21863. [Google Scholar] [CrossRef]
- Harrington, T.J.; Gild, J.; Sarker, P.; Toher, C.; Rost, C.M.; Dippo, O.F.; McElfresh, C.; Kaufmann, K.; Marin, E.; Borowski, L.; et al. Phase stability and mechanical properties of novel high entropy transition metal carbides. Acta Mater. 2019, 166, 271–280. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, H.; Dai, F.Z.; Liu, J.; Lei, Y.; Zhang, J.; Zhou, Y. High porosity and low thermal conductivity high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)C. J. Mater. Sci. Technol. 2019, 35, 1700–1705. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.B.; Sun, S.K.; Guo, W.M.; Chen, Q.S.; Qiu, J.X.; Plucknett, K.; Lin, H.T. Microstructure and mechanical properties of high-entropy borides derived from boro/carbothermal reduction. J. Eur. Ceram. Soc. 2019, 39, 3920–3924. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S.K.; Zhang, W.; You, Y.; Guo, W.M.; Chen, Z.W.; Yuan, J.H.; Lin, H.T. Improved densification and hardness of high-entropy diboride ceramics from fine powders synthesized via borothermal reduction process. Ceram. Int. 2020, 46, 14299–14303. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, W.M.; Jiang, Z.B.; Zhu, Q.Q.; Sun, S.K.; You, Y.; Plucknett, K.; Lin, H.T. Dense high-entropy boride ceramics with ultra-high hardness. Scr. Mater. 2019, 164, 135–139. [Google Scholar] [CrossRef]
- Tudose, I.V.; Comanescu, F.; Pascariu, P.; Bucur, S.; Rusen, L.; Iacomi, F.; Koudoumas, E.; Suchea, M.P. Chemical and Physical Methods for Multifunctional Nanostructured Interface Fabrication; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Moskovskikh, D.; Vorotilo, S.; Buinevich, V.; Sedegov, A.; Kuskov, K.; Khort, A.; Shuck, C.; Zhukovskyi, M.; Mukasyan, A. Extremely hard and tough high entropy nitride ceramics. Sci. Rep. 2020, 10, 19874. [Google Scholar] [CrossRef]
- Rey-García, F.; Ibáñez, R.; Angurel, L.A.; Costa, F.M.; de la Fuente, G.F. Laser floating zone growth: Overview, singular materials, broad applications, and future perspectives. Crystals 2021, 11, 38. [Google Scholar] [CrossRef]
- Mesa, M.C.; Serrano-Zabaleta, S.; Oliete, P.B.; Larrea, A. Microstructural stability and orientation relationships of directionally solidified Al2O3-Er3Al5O12-ZrO2 eutectic ceramics up to 1600 °C. J. Eur. Ceram. Soc. 2014, 34, 2071–2080. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Xiang, H.; Dai, F.Z.; Wang, X.; Peng, Z.; Zhou, Y. (La0.2Ce0.2Nd0.2Sm0.2Eu0.2)PO4: A high-entropy rare-earth phosphate monazite ceramic with low thermal conductivity and good compatibility with Al2O3. J. Mater. Sci. Technol. 2019, 35, 2892–2896. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Xiang, H.; Dai, F.Z.; Wang, X.; Xu, W.; Sun, K.; Peng, Z.; Zhou, Y. High-entropy (Y0.2Nd0.2Sm0.2Eu0.2Er0.2)AlO3: A promising thermal/environmental barrier material for oxide/oxide composites. J. Mater. Sci. Technol. 2020, 47, 45–51. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiang, H.; Dai, F.Z.; Peng, Z.; Zhou, Y. (TiZrHf)P2O7: An equimolar multicomponent or high entropy ceramic with good thermal stability and low thermal conductivity. J. Mater. Sci. Technol. 2019, 35, 2227–2231. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, H.; Dai, F.Z.; Liu, J.; Zhou, Y. High entropy (Yb0.25Y0.25Lu0.25Er0.25)2SiO5 with strong anisotropy in thermal expansion. J. Mater. Sci. Technol. 2020, 36, 134–139. [Google Scholar] [CrossRef]
- Han, X.; Girman, V.; Sedlak, R.; Dusza, J.; Castle, E.G.; Wang, Y.; Reece, M.; Zhang, C. Improved creep resistance of high entropy transition metal carbides. J. Eur. Ceram. Soc. 2020, 40, 2709–2715. [Google Scholar] [CrossRef]
- Cao, C.; Fu, J.; Tong, T.; Hao, Y.; Gu, P.; Hao, H.; Peng, L. Intermediate-temperature creep deformation and microstructural evolution of an equiatomic FCC-structured CoCrFeNiMn high-entropy alloy. Entropy 2018, 20, 960. [Google Scholar] [CrossRef]
- Kang, Y.B.; Shim, S.H.; Lee, K.H.; Hong, S.I. Dislocation creep behavior of CoCrFeMnNi high entropy alloy at intermediate temperatures. Mater. Res. Lett. 2018, 6, 689–695. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, B.; Chen, H.; Xiang, H.; Dai, F.Z.; Wu, S.; Xu, W. Electromagnetic wave absorbing properties of TMCs (TM = Ti, Zr, Hf, Nb and Ta) and high entropy (Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)C. J. Mater. Sci. Technol. 2021, 74, 105–118. [Google Scholar] [CrossRef]
- Sarkar, A.; Kruk, R.; Hahn, H. Magnetic properties of high entropy oxides. Dalton Trans. 2021, 50, 1973–1982. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K. High-entropy ceramics: Review of principles, production and applications. Mater. Sci. Eng. R Rep. 2021, 146, 100644. [Google Scholar] [CrossRef]
- Wang, H.; Cao, Y.; Liu, W.; Wang, Y. Oxidation behavior of (Hf0.2Ta0.2Zr0.2Ti0.2Nb0.2)C-xSiC ceramics at high temperature. Ceram. Int. 2020, 46, 11160–11168. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Cao, Y.; Liu, W.; Wang, Y. Oxidation behaviors of (Hf0.25Zr0.25Ta0.25Nb0.25)C and (Hf0.25Zr0.25Ta0.25Nb0.25)C-SiC at 1300–1500 °C. J. Mater. Sci. Technol. 2021, 60, 147–155. [Google Scholar] [CrossRef]
- Djemel, I.; Kriaa, I.; Abdelmoula, N.; Khemakhem, H. The effect of low Sn doping on the dielectric and electrocaloric properties of ferroelectric ceramics Ba0.95Sr0.05Ti0.95Zr0.05O3. J. Alloys Compd. 2017, 720, 284–288. [Google Scholar] [CrossRef]
- Ye, B.; Wen, T.; Liu, D.; Chu, Y. Oxidation behavior of (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C high-entropy ceramics at 1073–1473 K in air. Corros. Sci. 2019, 153, 327–332. [Google Scholar] [CrossRef]
- Wang, F.; Yan, X.; Wang, T.; Wu, Y.; Shao, L.; Nastasi, M.; Lu, Y.; Cui, B. Irradiation damage in (Zr0.25Ta0.25Nb0.25Ti0.25)C high-entropy carbide ceramics. Acta Mater. 2020, 195, 739–749. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Kirnbauer, A.; Ertelthaler, P.; Koller, C.M. High-entropy ceramic thin films; A case study on transition metal diborides. Scr. Mater. 2018, 149, 93–97. [Google Scholar] [CrossRef]
- Xing, Q.W.; Xia, S.Q.; Yan, X.H.; Zhang, Y. Mechanical properties and thermal stability of (NbTiAlSiZr)Nx high-entropy ceramic films at high temperatures. J. Mater. Res. 2018, 33, 3347–3354. [Google Scholar] [CrossRef]
- Bérardan, D.; Franger, S.; Dragoe, D.; Meena, A.K.; Dragoe, N. Colossal dielectric constant in high entropy oxides. Phys. Status Solidi—Rapid Res. Lett. 2016, 10, 328–333. [Google Scholar] [CrossRef]
- Osenciat, N.; Bérardan, D.; Dragoe, D.; Léridon, B.; Holé, S.; Meena, A.K.; Franger, S.; Dragoe, N. Charge compensation mechanisms in Li-substituted high-entropy oxides and influence on Li superionic conductivity. J. Am. Ceram. Soc. 2019, 102, 6156–6162. [Google Scholar] [CrossRef]
- Sarkar, A.; Eggert, B.; Velasco, L.; Mu, X.; Lill, J.; Ollefs, K.; Bhattacharya, S.S.; Wende, H.; Kruk, R.; Brand, R.A.; et al. Role of intermediate 4 f states in tuning the band structure of high entropy oxides. APL Mater. 2020, 8, 051111. [Google Scholar] [CrossRef]
- Kan, W.H.; Zhang, Y.; Tang, X.; Lucey, T.; Proust, G.; Gan, Y.; Cairney, J. Precipitation of (Ti, Zr, Nb, Ta, Hf)C high entropy carbides in a steel matrix. Materialia 2020, 9, 100540. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yan, X.; Lu, Y.; Nastasi, M.; Chen, Y.; Cui, B. The effect of submicron grain size on thermal stability and mechanical properties of high-entropy carbide ceramics. J. Am. Ceram. Soc. 2020, 103, 4463–4472. [Google Scholar] [CrossRef]
- Yan, J.; Wang, D.; Zhang, X.; Li, J.; Du, Q.; Liu, X.; Zhang, J.; Qi, X. A high-entropy perovskite titanate lithium-ion battery anode. J. Mater. Sci. 2020, 55, 6942–6951. [Google Scholar] [CrossRef]
- Dudnik, O.V.; Lakiza, S.M.; Grechanyuk, I.M.; Red’ko, V.P.; Glabay, M.S.; Shmibelsky, V.B.; Marek, I.O.; Ruban, A.K.; Grechanyuk, M.I. High-Entropy Ceramics for Thermal Barrier Coatings Produced from ZrO2 Doped with Rare-Earth Metal Oxides. Powder Metall. Met. Ceram. 2021, 59, 556–563. [Google Scholar] [CrossRef]
- Sarkar, A.; Velasco, L.; Wang, D.; Wang, Q.; Talasila, G.; de Biasi, L.; Kübel, C.; Brezesinski, T.; Bhattacharya, S.S.; Hahn, H.; et al. High entropy oxides for reversible energy storage. Nat. Commun. 2018, 9, 3400. [Google Scholar] [CrossRef]
- Liu, J.; Ren, K.; Ma, C.; Du, H.; Wang, Y. Dielectric and energy storage properties of flash-sintered high-entropy (Bi0.2Na0.2K0.2Ba0.2Ca0.2)TiO3 ceramic. Ceram. Int. 2020, 46, 20576–20581. [Google Scholar] [CrossRef]
- Kheradmandfard, M.; Minouei, H.; Tsvetkov, N.; Vayghan, A.K.; Kashani-Bozorg, S.F.; Kim, G.; Hong, S.I.; Kim, D.E. Ultrafast green microwave-assisted synthesis of high-entropy oxide nanoparticles for Li-ion battery applications. Mater. Chem. Phys. 2021, 262, 124265. [Google Scholar] [CrossRef]
- Zheng, Y.; Yi, Y.; Fan, M.; Liu, H.; Li, X.; Zhang, R.; Li, M.; Qiao, Z.A. A high-entropy metal oxide as chemical anchor of polysulfide for lithium-sulfur batteries. Energy Storage Mater. 2019, 23, 678–683. [Google Scholar] [CrossRef]
- Lökçü, E.; Toparli, Ç.; Anik, M. Electrochemical Performance of (MgCoNiZn)1−xLixO High-Entropy Oxides in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 23860–23866. [Google Scholar] [CrossRef]
- Zeng, X.; Song, H.; Shen, Z.Y.; Moskovits, M. Progress and challenges of ceramics for supercapacitors. J. Mater. 2021, 7, 1198–1224. [Google Scholar] [CrossRef]
- Liang, B.; Ai, Y.; Wang, Y.; Liu, C.; Ouyang, S.; Liu, M. Spinel-type (FeCoCrMnZn)3O4 high-entropy oxide: Facile preparation and supercapacitor performance. Materials 2020, 13, 5798. [Google Scholar] [CrossRef]
- Sun, Y.; Dai, S. High-entropy materials for catalysis: A new frontier. Sci. Adv. 2021, 7, eabg1600. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, D.; Liu, R.; Li, D. Designing high-entropy ceramics via incorporation of the bond-mechanical behavior correlation with the machine-learning methodology. Cell Rep. Phys. Sci. 2021, 2, 100640. [Google Scholar] [CrossRef]
- Xiang, H.; Yao, L.; Chen, J.; Yang, A.; Yang, H.; Fang, L. Microwave dielectric high-entropy ceramic Li(Gd0.2Ho0.2Er0.2Yb0.2Lu0.2)GeO4 with stable temperature coefficient for low-temperature cofired ceramic technologies. J. Mater. Sci. Technol. 2021, 93, 28–32. [Google Scholar] [CrossRef]
- Huo, W.Y.; Wang, S.Q.; Zhu, W.H.; Zhang, Z.L.; Fang, F.; Xie, Z.H.; Jiang, J.Q. Recent progress on high-entropy materials for electrocatalytic water splitting applications. Tungsten 2021, 3, 161–180. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Z.; Xiang, H.; Dai, F.Z.; Xu, W.; Sun, K.; Liu, J.; Zhou, Y. High entropy (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12: A novel high temperature stable thermal barrier material. J. Mater. Sci. Technol. 2020, 48, 57–62. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).