Abstract
Nickel-doped iron oxide/graphene oxide powders were synthesized by the co-precipitation method varying the Ni/Fe ratio, and the activity of the materials towards the oxygen reduction reaction in a microbial fuel cell (MFC) was studied. The samples presented X-ray diffraction peaks associated with magnetite, maghemite and Ni ferrite, as well as evidence of hematite. Raman spectra confirmed the presence of maghemite (γ-Fe2O3) and NiFe2O4. Scanning electron micrographs showed exfoliated sheets decorated with nanoparticles, and transmission electron micrographs showed spherical nanoparticles about 10 nm in diameter well distributed on the individual graphene sheet. The electrocatalytic activity for the oxygen reduction reaction (ORR) was studied by cyclic voltammetry in an air-saturated electrolyte, finding that the best catalyst was the sample with a 1:2 Ni/Fe ratio, using a catalyst concentration of 15 mg·cm−2 on graphite felt. The 1:2 Ni/Fe catalyst provided an oxygen reduction potential of 397 mV and a maximum oxygen reduction current of −0.13 mA; for comparison, an electrode prepared with GO/γ-Fe2O3 showed a maximum ORR of 369 mV and a maximum current of −0.03 mA. Microbial fuel cells with a vertical proton membrane were prepared with Ni-doped Fe3O4 and Fe3O4/graphene oxide and tested for 24 h; they reached a stable OCV of +400 mV and +300 mV OCV, and an efficiency of 508 mW·m−2 and 139 mW·m−2, respectively. The better performance of Ni-doped material was attributed to the combined presence of catalytic activity between γ-Fe2O3 and NiFe2O4, coupled with lower wettability, which led to better dispersion onto the electrode.
1. Introduction
To improve the performance of alternative energy systems, nanotechnology has an important role in the development of new materials and the study of their physical and chemical properties at the nanoscale [1]. Microbial fuel cells (MFCs) have become an alternative for generating electricity and reducing organic loads in wastewater [2]. However, one of the main challenges is developing catalytic efficiency and stability at the cathode for an oxygen reduction reaction (ORR). This reaction exhibits slow kinetics, resulting in potential loss [3]. Oxygen has been widely used as an electron acceptor at the cathode of microbial fuel cells due to its high standard redox potential of 1.229 V vs. SHE, its abundance and the clean reaction products [4]. To overcome the sluggish kinetics of ORR in MFCs and to improve efficiency, several ORR catalysts have been tested [5,6,7]. Among them, metal oxides with inverse spinel structure such as NiFe2O4 have been studied as cathode material for hydrogen evolution, and energy storage in supercapacitors and other devices [8,9,10,11]; however, to the best of our knowledge, there are few reports of its use as ORR catalysts [12]. Nickel ferrites are interesting for their use in MFCs because they offer good stability and lower cost compared to platinum (Pt), the catalyst commonly used for the ORR [13,14]. The general formula for the normal spinel is AB2O4, where A indicates the presence of an M2+ ion occupying a tetrahedral site, while B refers to an M3+ ion at an octahedral site. In the case of an inverse spinel, the M2+ ions migrate towards the octahedral site, half of the M3+ ions migrate to the tetrahedral site and the rest of the M3+ ions fill the octahedral site with the M2+ ions [15]. Therefore, inverse spinels are formulated as [B3+(A2+B3+)O4]. This cationic arrangement could provide the transfer of electrons necessary to carry out the ORR [16,17]. Furthermore, spinel MFe2O4 nanostructures (M=Co, Mn, Ni) loaded on carbon-based supports can be used as a low-cost alternative to electrocatalysis with a 4e- direct path transport mechanism [18,19,20], which can be attributed to their non-stoichiometric structure and oxygen vacancies [21]. In oxides of transition elements, O2 adsorption proceeds in a bridging manner with oxidation and reduction of the electrocatalytic site [22]. To improve the ORR electrocatalytic performance of nickel ferrite, materials such as carbon, graphene, graphene oxide and carbon nanotubes have been widely used as stable supports [23,24,25,26,27]. Controlling the nanoparticle size by dispersion within the support matrix would improve the performance of catalysts due to the enlarged area for active sites and specific crystal planes that give rise to ORR performance [28]. Compared with graphene, which has limited possibilities for chemical interactions, graphene oxide (GO) can improve the activity of the catalysts because it contains reactive functional groups such as carbonyl (C=O), carboxyl (COOH), epoxy (C-O-C) and hydroxyl (-OH), which make chemical modification easier and therefore would lead to better catalyst dispersion [29,30,31]. The main reactions of GO that provide different functionalities can be classified as: (i) reduction, or removal of oxygenated groups from graphene oxide; (ii) functionalization, which consists of creating covalent bonds between graphene oxide and organic molecules; (iii) doping, when heteroatoms are introduced into the carbon rings of the sheet; and (iv) decoration, which uses the oxygenated groups of GO as reactive centers to nucleate nanoparticles of inorganic materials [32]. The application of GO decoration has been studied to manufacture electrocatalysts for fuel cells [33] and photocatalysts for hydrogen evolution [34], where the formation of MoS2-TiO2 and CeO2-x-TiO2 composites with reduced graphene oxide increased the dispersion of the active materials as well as the formation of heterostructures within the catalyst; simultaneously, the creation of reaction centers is another strategy studied to increase the efficiency of catalysts as, for example, the use of N and B doping in reduced graphene oxide [35]. During GO decoration of the oxygen groups, reduction takes place, increasing the electrode conductivity and therefore the charge transfer efficiency.
Herein, we report the synthesis of nickel-doped iron oxide decorated graphene oxide for ORR in a single-chamber microbial fuel cell configuration in a cooked clay vessel. Different amounts of nickel and different catalyst loads were tested to assess its effect on the ORR and the MFC efficiency.
2. Materials and Methods
2.1. Synthesis of Graphene Oxide by Hummers’ Method
Graphene oxide was prepared by oxidation of graphite using a modification of the Hummers’ method reported elsewhere [36]. Briefly, 2 g of synthetic graphite powder (<20 µm, 99.99 wt.%, Sigma Aldrich, St. Louis, MO, USA) was mixed with 1 g of sodium nitrate (NaNO3, Sigma Aldrich, St. Louis, MO, USA, 99.9%) in a 1000 mL flask, then 46 mL of concentrated sulfuric acid (H2SO4, Merck, Billerica, MA, USA, 98%) was added to the powder mixture while introducing the flask into an ice bath to keep the reaction at 5 °C with constant stirring for 5 min. Next, 6 g of potassium permanganate (KMnO4, Sigma Aldrich, St. Louis, MO, USA, 99.9%) was slowly added to avoid a sudden point reaction. The mixture was kept at 5 °C for 120 min under constant magnetic stirring. Subsequently, the temperature of the mixture was increased to 35 °C and held there for 30 min. Afterwards, 92 mL of deionized water was added to the mixture and the temperature was increased to 98 °C and stirred for another 30 min. The reaction was terminated by adding 280 mL of deionized water and 20 mL of hydrogen peroxide (H2O2, Merck, Billerica, MA, USA, 30%). The greenish-brown product was washed with 5 volumes of 5% hydrochloric acid (HCl) to remove the remnants of the Mn4+ and SO42− ions, followed by rinsing with 5 volumes of deionized water to remove the acid and Cl− ions. The product was recovered by centrifugation at 4000 rpm. Finally, the sample was dried in an oven at 70 °C for 24 h, producing a dark brown product.
2.2. Nickel-Doped Iron Oxide/Graphene Oxide Synthesis
Iron oxide/graphene oxide powders were synthesized via the co-precipitation method as follows: first, a mixture of iron chlorides Fe3+:Fe2+ with a 2:1 molar ratio was prepared by dissolving FeCl3·6H2O (Sigma Aldrich, St. Louis, MO, USA, 99.9%) and FeCl2·4H2O (Sigma Aldrich, St. Louis, MO, USA, 99.9%) in 25 mL of deionized (DI) water. The mixture of iron chlorides was added dropwise to a suspension of 0.9 g of GO in 250 mL of DI water, at room temperature under vigorous stirring, and under a 40 sccm flow of nitrogen gas to avoid oxidation from magnetite to hematite. The pH was adjusted to 10 using 28% NH4OH solution (Merck, Billerica, MA, USA). The mixture was heated at 80 °C; once that temperature was reached, 5 mL of hydrazine hydrate was added, and the temperature was maintained for 5 h. The product was allowed to cool freely and was washed 3 times with deionized water and separated with permanent magnets. The sample, named GOMF, was dried in an oven at 70 °C for 24 h. Despite the fact that the reported method is intended for magnetite (Fe3O4) synthesis, from previous works it is known that the phase composition of iron oxides decorating GO is dependent on the pH, reducing conditions and total iron contents; thus maghemite and magnetite, which are difficult to differentiate by X-ray diffraction, can be present in these magnetic materials [37].
Nickel-doped iron oxide/graphene oxide powders were synthesized with a procedure adapted from refs. [38,39]: a 20 mL solution of a mixture of iron chlorides Fe3+:Fe2+ with a 2:1 molar ratio was first prepared by dissolving 0.008 mol of FeCl3·6H2O and 0.004 mol of FeCl2·4H2O. The iron chloride mixture was added dropwise to the GO solution at room temperature under a 40 sccm flow of N2 under vigorous stirring. The pH was adjusted to 10 with a 28% NH4OH solution. On the other hand, a 0.1 M Ni2+ solution was prepared with NiCl2 (Sigma Aldrich, St. Louis, MO, USA, 99.9%) in DI water. Different amounts of this solution were added dropwise to the GO+Fe3+:Fe2+ mixture at room temperature under nitrogen flow to achieve different Ni/Fe molar ratios. After incorporating the Fe and Ni chlorides into the GO solution, the temperature was raised to 80 °C. Once that temperature was reached, 5 mL of hydrazine hydrate (Sigma Aldrich, St. Louis, MO, USA, 20% solution) was added, and the temperature was maintained for 5 h. The product was allowed to cool freely and was washed 3 times with deionized water and separated by permanent magnets. The sample was dried in an oven at 70 °C for 24 h. In the present work, three modifications were made in the Ni/Fe proportions as shown in Table 1 to investigate the effect of Ni contents on the electrocatalytic performance for ORR. Samples are referred to as GOMNF 1:18, GOMNF 1:6 and GOMNF 1:2, depending on their respective Ni/Fe ratio.

Table 1.
Preparation of conditions of the synthesized powders.
2.3. Characterization
The powders were characterized by X-ray diffraction (XRD) using an X’Pert PRO MPD (Multi-Purpose Diffractometer, Malvern Panalytical, Tokyo, Japan) equipped with a multiple strip X-ray detector, X’celerator. Measurements were performed using Bragg-Brentano geometry in a 2θ range 6–90° and Cu-Kα radiation of 0.15406 nm at a voltage of 40 kV and a current of 40 mA. Raman spectra were measured at room temperature with a LabRAM HR 800 micro-Raman spectrometer equipped (HORIBA, Tokyo, Japan), with notch filters, using the incident light from a 632.8 nm He-Ne laser focused on the samples through a 50× microscope objective using neutral density filters to avoid decomposition and further oxidation. Fourier transform infrared spectra (FTIR) were obtained in a Perkin Elmer Spectrum 100 spectrometer (Waltham, MA, USA), equipped with an attenuated total reflectance (ATR) accessory with a diamond pinhole window in the wave number range of 4000 to 400 cm−1. The thermogravimetric analysis (TGA) was performed on a Setaram Thermal Gravimetric Analyzer Setsys evolution 1750 (KEP Technologies, Sophia Antipolis, France), under dry air. To analyze TGA, temperature increased from room temperature to 900 °C under air of 20 mL·min−1 at a rate of 5 °C·min−1. To observe the morphology, scanning electron microscopy (SEM) was done using a Hitachi S-4800 apparatus (Hitachi, Tokyo, Japan), at 5 kV and transmission electron microscopy (TEM) was performed using a JEOL-ARM 200F microscope (JEOL Ltd., Akishima, Japan), operating at 80 kV in a bright field mode.
2.4. Fabrication of Electrodes for ORR
For the fabrication of the cathodes for ORR, a slurry was prepared by mixing 4 mL of a 10 wt.% polyvinyl alcohol (PVA) solution used as a binder and the catalyst powder of GO, GOMF, GOMNF 1:18, GOMNF 1:6 or GOMNF 1:2, impregnated by doctor blade onto a graphite felt used as a working electrode, to obtain a 15 mg·cm−2 load. After impregnation, the prepared electrodes were dried at 50 °C for 12 h.
2.5. Electrochemical Measurements
Study of the electrocatalytic activity of the prepared materials towards the oxygen reduction reaction (ORR) was carried out by cyclic voltammetry (CV) using an Autolab 302N potentiostat. A 3-electrode cell configuration was used, where an Ag/AgCl mini-electrode filled with saturated KCl was set as a reference electrode (RE), a platinum (Pt) wire as a counter electrode (CE) and 1 × 1 cm2 graphite felt impregnated with GOMNF 1:18, GOMNF 1:6 and GOMNF 1:2 as a working electrode (WE). For the CV measurements, the electrolyte was 0.1 M potassium hydroxide (KOH) saturated with air. The voltammograms were obtained at room temperature from −0.1 to 0.8 V at a scan rate of 10 mVs−1.
2.6. Construction of Single Chamber Microbial Fuel Cells (MFCs)
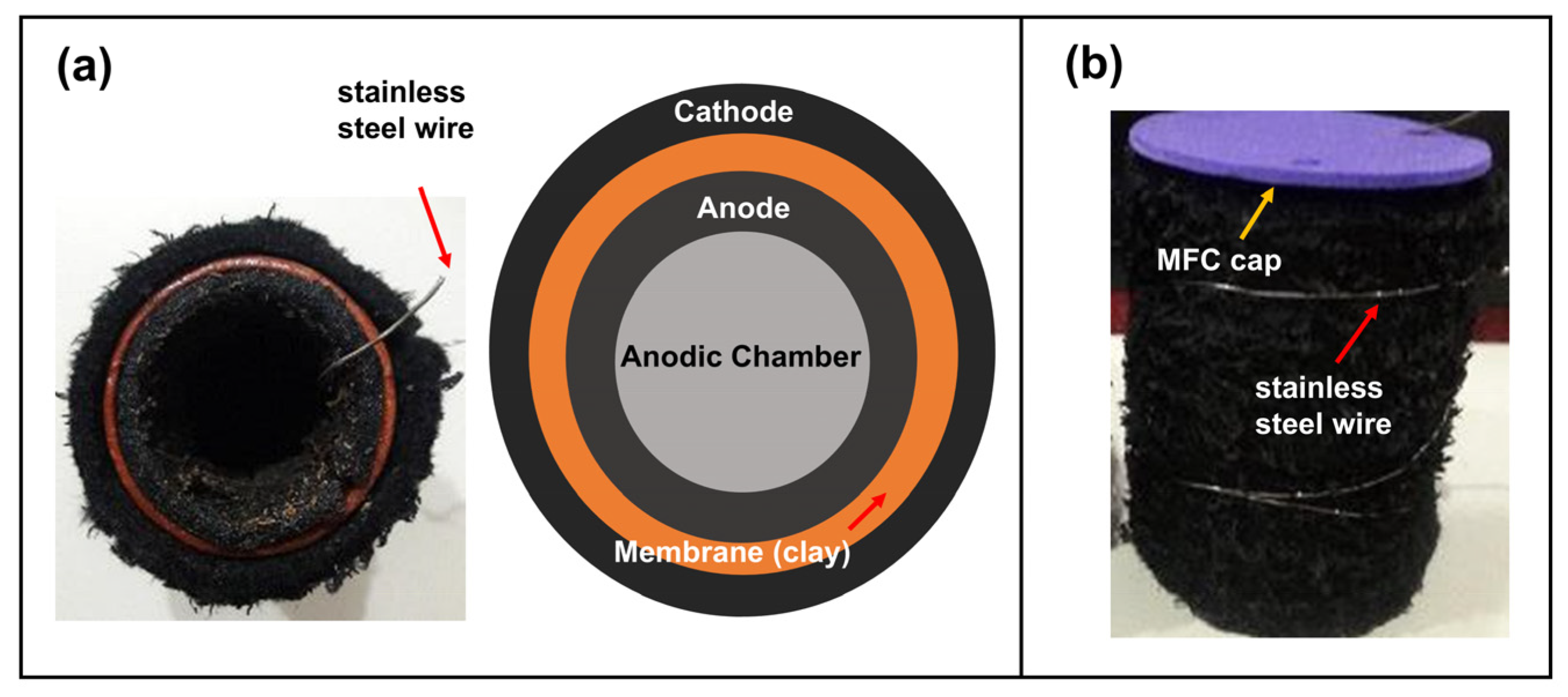
The MFCs were built with a single-chamber configuration into a cooked clay vessel with a volume of 50 mL. The cathode was fabricated from a 6 × 13 cm2 graphite felt impregnated with the catalysts as described above and placed on the outer area of the MFC with 304 stainless steel wire woven into the felt, leaving a wire tip as contact. The anode was made of graphite felt with the same area of the cathode, placed on the inner surface of the container and woven with stainless steel wire, keeping one end of the wire as the electrical contact. Figure 1 shows photographs and the top-view configuration of the MFCs.
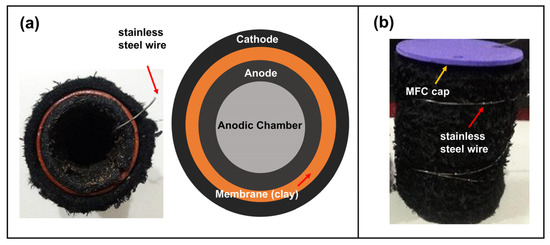
Figure 1.
Photographs and scheme of the developed single chamber MFC, (a) top view and (b) lateral view.
2.6.1. Inoculation of the Reactor
To inoculate the anode, a mixture of water with commercial, fertilized soil was prepared as follows: 5 g of soil was mixed with 50 mL of DI water to have an estimated chemical oxygen demand (COD) of 0.1 mg·mL−1. Then, to allow bacteria to start the metabolic activity, 0.05 g of sodium acetate, equivalent to a COD of 1 g·L−1, was added to the mixture [40,41]. Afterwards, the mixture was added into the MFC chamber.
2.6.2. MFC Operation
To evaluate the performance of the synthesized materials, the MFCs were put into operation to determine the maximum performance produced at open circuit voltage (OCV) with each cathode [42]. First, the inoculum prepared above was kept for a period of 12 h to allow the formation of the biofilm and remained closed without prior aeration. The OCV of the MFCs was measured every hour for 24 h with a multimeter Mul-282 (Steren, Mexico City, Mexico) at the terminals of each electrode. All measurements of cell performance were made at room temperature (27 ± 1 °C).
3. Results and Discussion
3.1. XRD Analysis
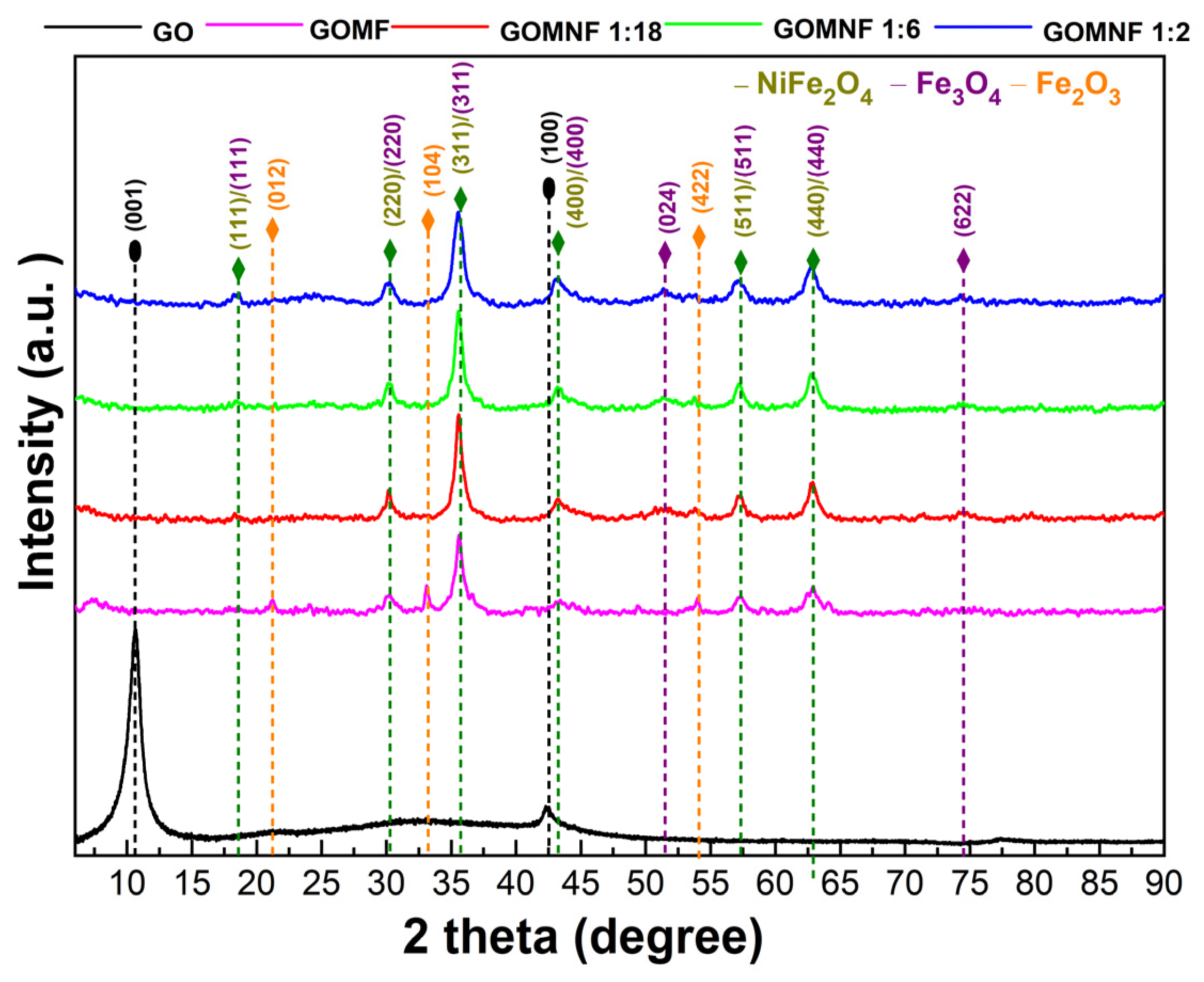
The crystal structure of the prepared GO, Fe3O4/GO and Ni-doped Fe3O4/GO powders was studied by X-ray diffraction. Figure 2 presents the X-ray diffractograms for each sample. Diffraction peaks attributed to GO were observed around 2 theta 10° corresponding to the plane (001) and 43° to the plane (100), which indicates the intercalation of water molecules and the formation of oxygenated functional groups between the graphite layers [43]. The diffraction pattern of the GOMF sample shows diffraction peaks at 2θ 30.29° (220), 35.67° (311), 43.36° (400), 53.80° (422) and 62.99° (440), which can be attributed either to magnetite (Fe3O4) or maghemite (γ-Fe2O3) [37], as well as diffraction peaks at 2θ 21.25°, 30.10° and 54° corresponding to the hematite (012), (104) and (422) planes, respectively. For the samples decorated with nickel-doped iron oxide, whose labels are GOMNF 1:2, GOMNF 1:6 and GOMNF 1:18, diffraction planes were identified at 2θ 30.29° (220), 35.67° (311), 43.36° (400), 53.80° (422) and 62.99° (440), which correspond to magnetite/maghemite and which may also correspond to crystalline planes of NiFe2O4. No crystalline planes related to nickel oxide (NiO) were found. Phases were identified with the JCPDS database as follows: NiFe2O4 (card 10-0325), Fe3O4 (card 19-0629) and hematite (card 33-0664).
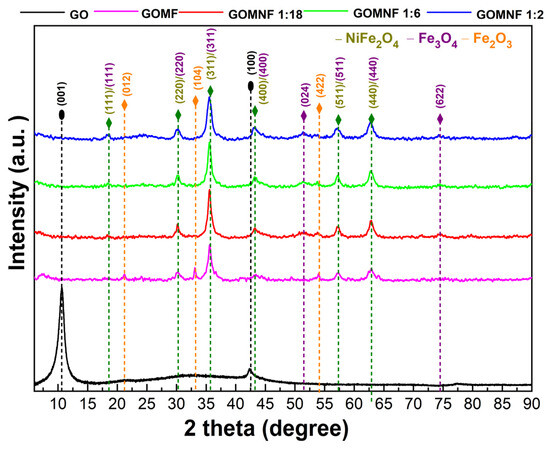
Figure 2.
Diffractograms of the GO, GOMF and GOMNF samples.
The crystallite size was calculated using the Scherrer equation [44],
where is the crystallite size, is the full width at half maximum (FWHM), is the wavelength of Cu = 1.5406 and is the Bragg angle.
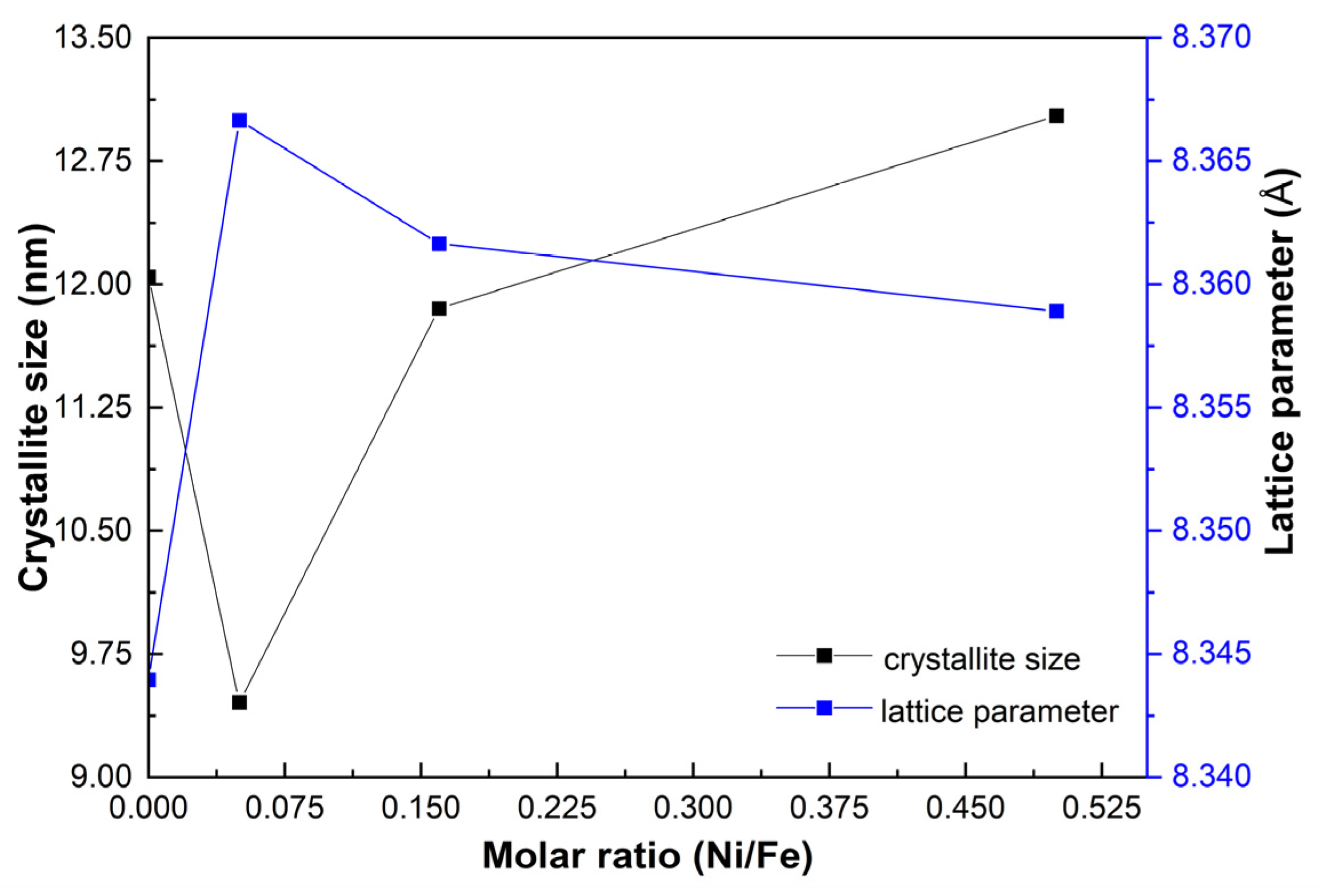
Figure 3 shows the crystallite size and lattice parameter evolution of the prepared samples with the Ni/Fe ratio. The lattice parameter in the GOMF sample is ca. 8.341 Å, closer to the maghemite (8.34 Å), than to magnetite (8.396 Å) as reported in the JCPDS cards. When adding a small concentration of nickel (GOMNF 1:18), the lattice parameter a increases to ca. 8.366 Å, due to lattice distortion at the octahedral coordination when the larger Ni2+ ion (0.078 nm) is positioned at the interstitial sites of Fe3+ vacancies for the case of maghemite (ca. 0.0645 nm). In correspondence, due to this distortion, the crystallite size decreases. However, as the nickel content increases, the lattice parameter tends to stabilize around 8.36 Å, suggesting increasing formation of NiFe2O4 [45]. Correspondingly, a better ordering leads to a slight increase in the crystallite size.
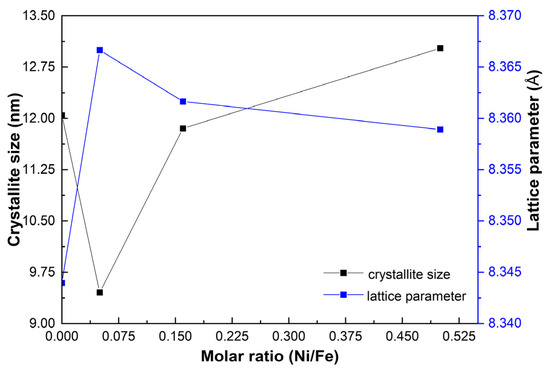
Figure 3.
Variation of crystallite size and lattice parameter with respect to the Ni/Fe ratio in GOMNF samples.
3.2. TGA/DTG Analysis
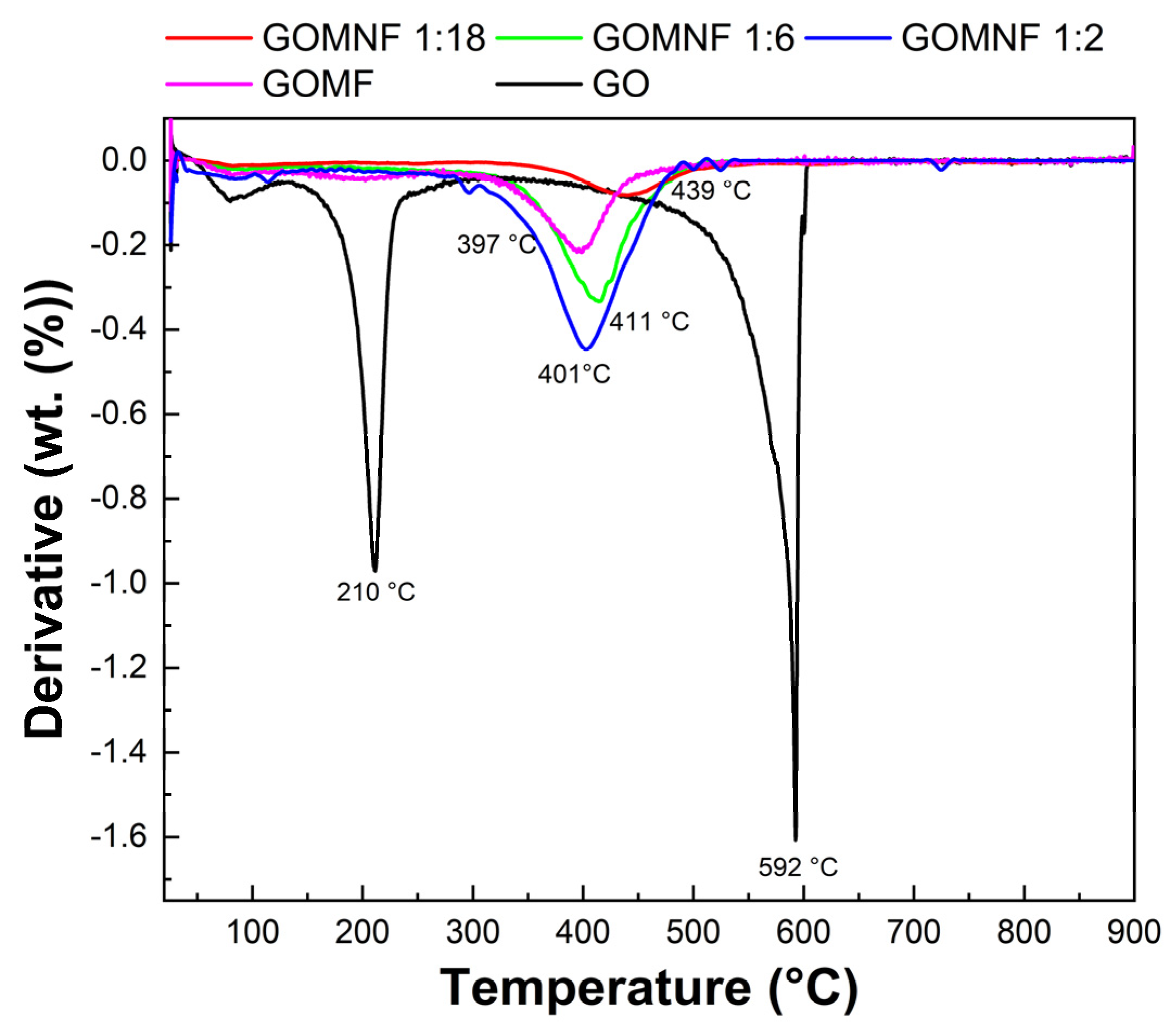
The thermal behavior of prepared samples was investigated by thermogravimetry; Figure 4 shows the derivative thermogravimetric (DTG) curves. The first peak of GO at 210 °C is caused by the loss of functional groups and oxygen, while the second peak at 592 °C is attributed to carbon combustion. However, for the decorated samples, there is no peak related to the oxygen functional group loss, in correspondence to the decoration of the oxygen moieties of GO, which reduces the GO sheet. For the doped samples, a peak at around 405–450 °C (Tmax) corresponds to the complete oxidation of the iron and iron/nickel to Fe2O3 and NiFe2O4, respectively. It was found that the Tmax shifted to a lower temperature with the following trend: GOMF (439 °C) > GOMNF 1:18 (411 °C) > GOMNF (401 °C) > 1:6 GOMNF 1:2 (397 °C), respectively. The shift in Tmax is related to the formation enthalpies of NiFe2O4 −1000 kJ/mol and Fe2O3 = −825.5 kJ/mol, although the Tmax values are slightly higher than those reported, possibly because of the graphene matrix, which adds stability [46,47,48].
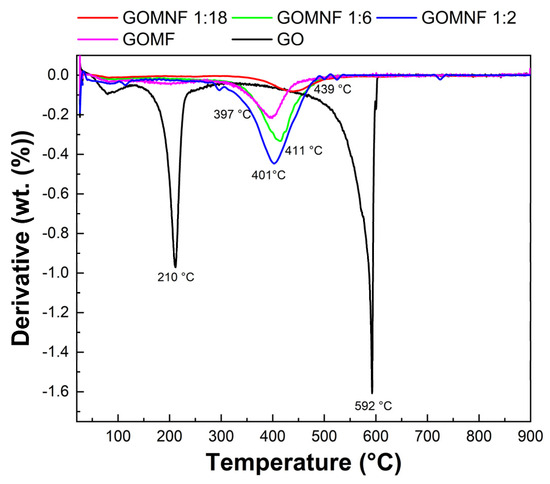
Figure 4.
DTG curve of the Ni-doped Fe3O4/powders.
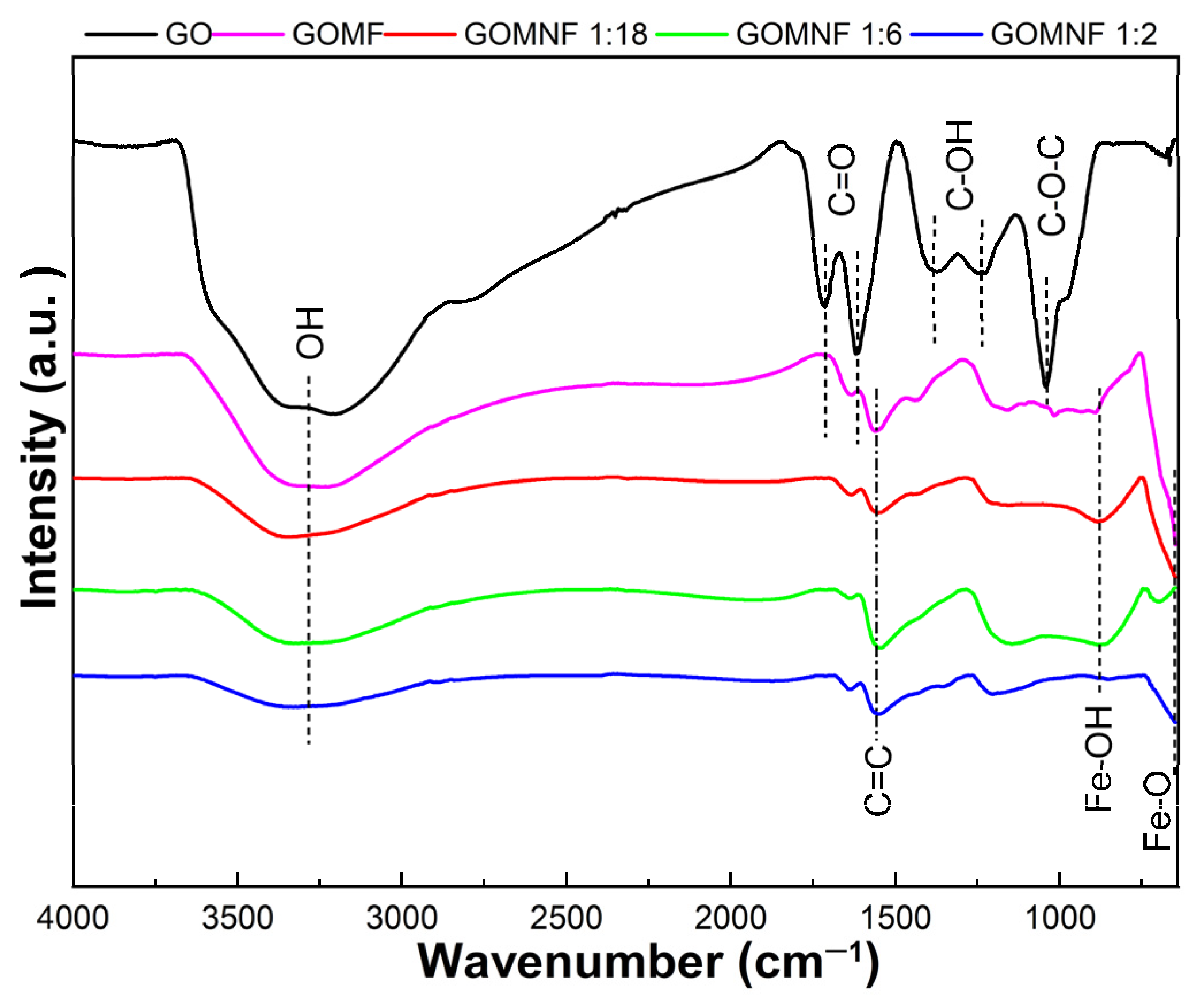
3.3. FTIR Analysis
Figure 5 presents the FTIR spectra of the synthesized samples. For GO, an intense and wide band around 3260 cm−1 is attributed to the O-H bond stretch mode [49]. The water molecules intercalated between the graphene sheets participate in the widening of the band. The band located at 1730 cm−1 is attributed to the vibration of the C=O stretch in carboxylic acids that are located on the edges of the graphene oxide sheets; the band at 1626 cm−1 is attributed to the C=O stretch in carbonyl groups and the band at 1560 cm−1 to the vibration of graphitic domains of the non-oxidized carbon skeleton (C=C). The bands at 1248 cm−1 and 1370 cm−1 are attributed to the vibrations of the stretching of hydroxyl groups (C-OH) on the basal plane of the GO, and the band at 1045 cm−1 corresponds to the mode of stretching of the epoxy ring (C-O-C) [50]. In the GOMF and GOMNF samples, the band at 3260 cm−1 corresponding to OH bonds reduces with the Ni contents, indicating a lower wettability, which would have a consequence in reduced nanoparticle agglomeration as discussed later. Also, with iron and nickel doping, the bands corresponding to carboxyl, carbonyl and epoxy groups disappear, indicating the reduction of graphene oxide. In the FTIR spectra of GOMNF 1:2, 1:6 and 1:18, the band at 867 cm−1 is attributed to the flex and stretch vibrations of O-H bound with iron ions and moisture adsorbed onto the nanoparticles [51]; this band also reduces with the increased Ni contents. The band at around 587 cm−1 corresponds to the stretching vibrations due to Fe-O tetrahedral, and it is observed that as nickel contents increase, this band decreases, corresponding to the suggested formation of NiFe2O4.
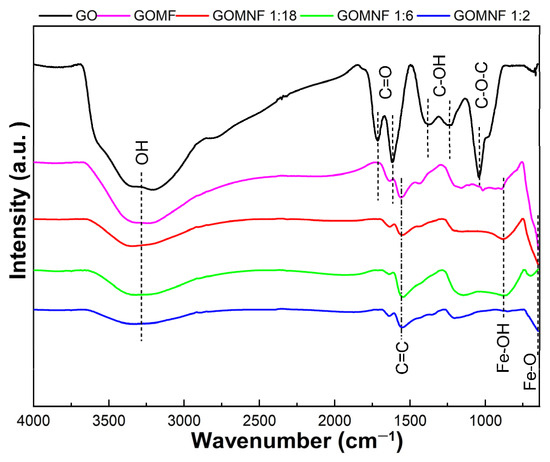
Figure 5.
FTIR spectra of the GO, GOMF and GOMNF samples in different ratios of Ni/Fe.
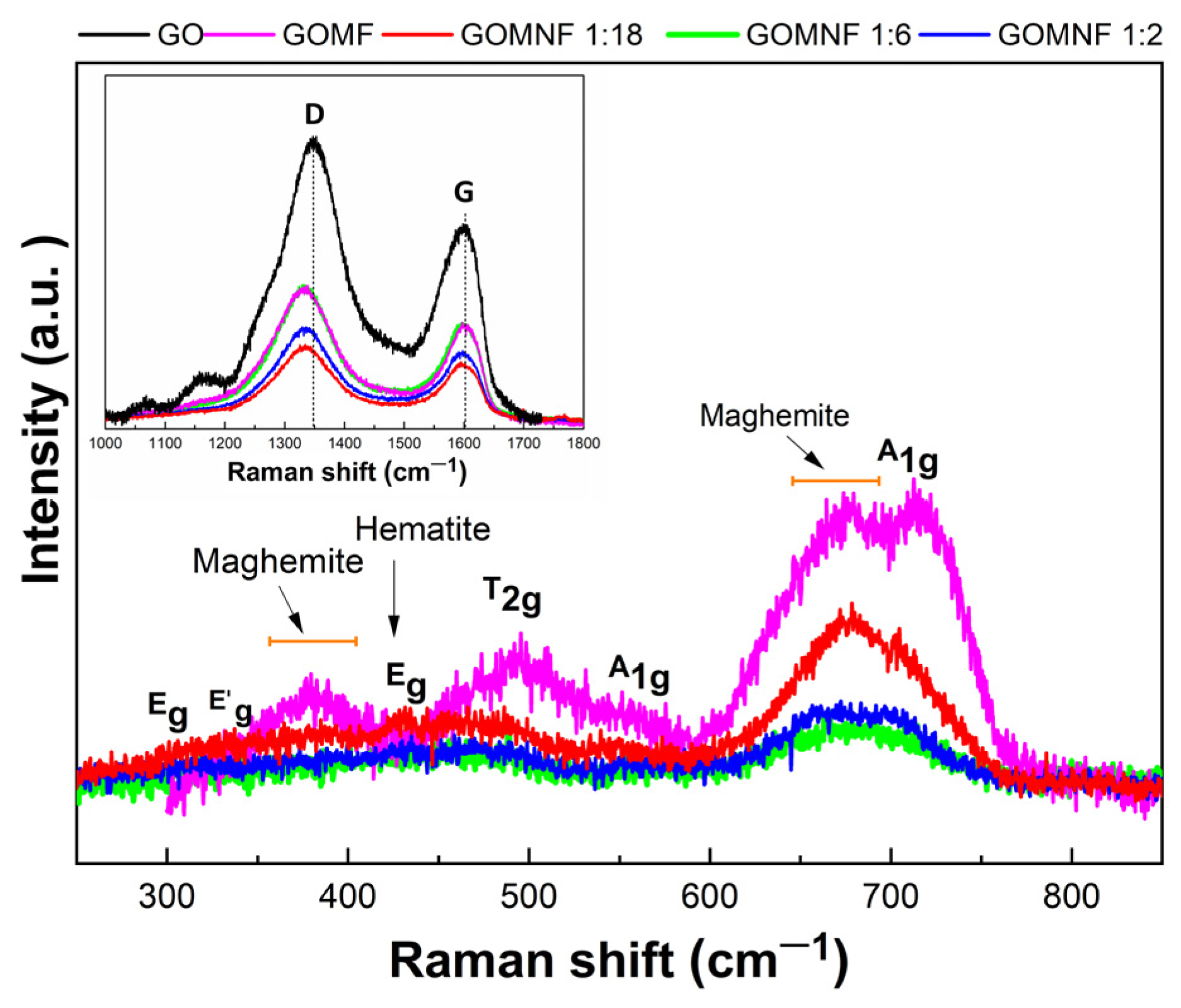
3.4. Raman Analysis
Figure 6 presents the Raman spectra of GO and those of the GOMF and GONMF samples. The Raman spectrum at the Figure 6 inset displays the characteristic D and G bands mentioned in the literature for GO [52]. The G band at 1598 cm−1 is caused by the E2g mode of first order dispersion of the sp2 domains, i.e., due to the vibration of the carbon atoms in the plane. The D band, located at 1345 cm−1, corresponds to out-of-the-plane vibrations, associated to the increase of disordered regions containing sp3 carbon, because of the oxidation of the graphene sheets [53]. In the Raman spectra for the GOMNF 1:2, 1:6 and 1:18 samples, bands appeared between 292 cm−1 and 704 cm−1, which were assigned to the vibrational modes Eg at 292.17 cm−1, T2g (2) at 489.79 cm−1 and A1g at 704.33 cm−1, respectively, of the symmetry of the (Fd3m) space group, corresponding to NiFe2O4 [54]. In addition, broad bands between 490 and 680 cm−1 are observed, confirming the presence of the maghemite phase of iron oxide [55].
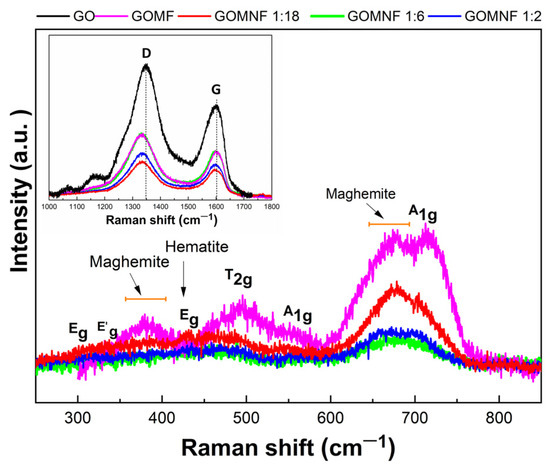
Figure 6.
Raman spectra and vibrational modes of GO, GOMF and GOMNF samples.
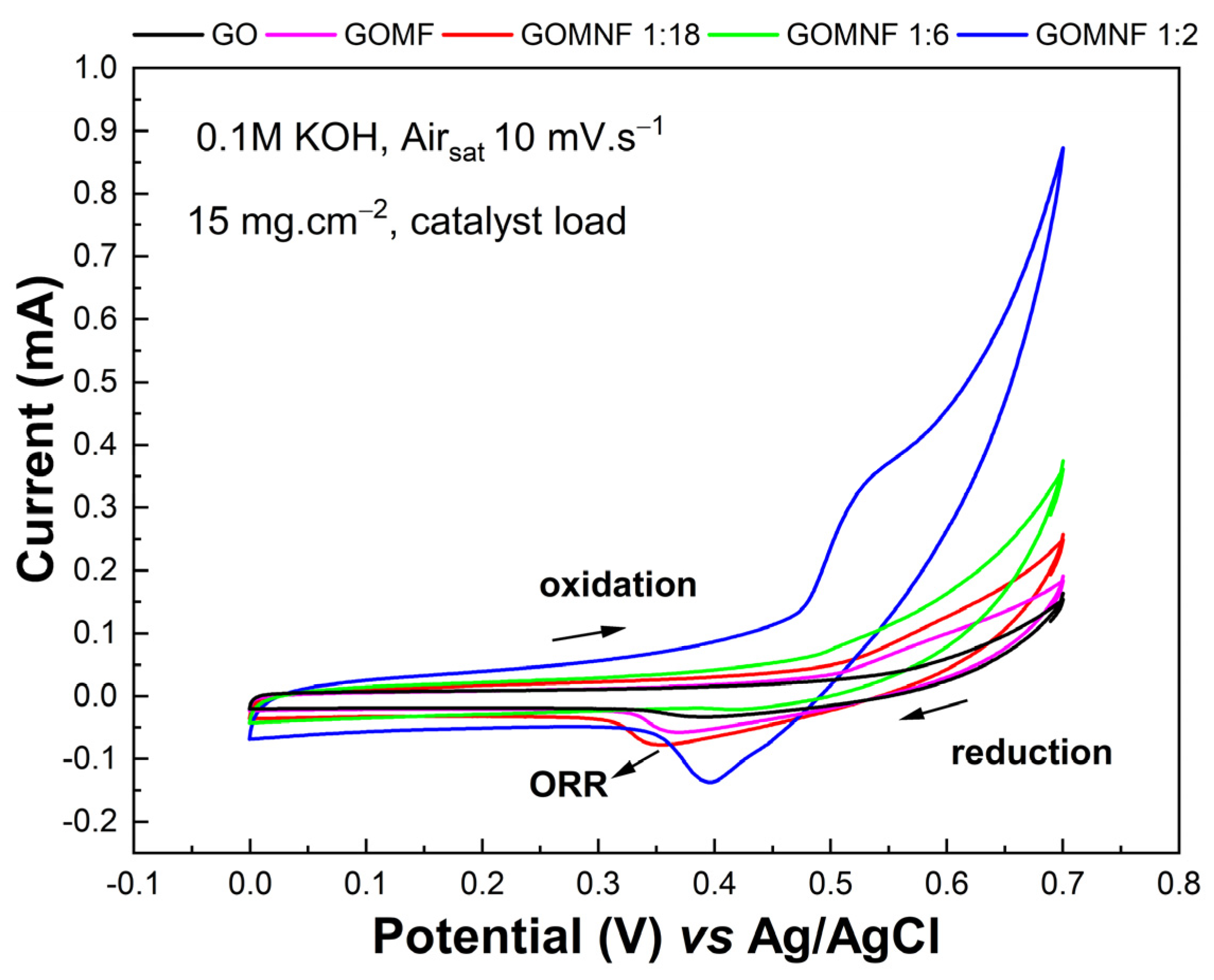
3.5. Electrochemical Characterization of Air Cathodes
The catalytic activity of the cathodes towards ORR was studied by cyclic voltammetry in air-saturated KOH. Figure 7 shows the voltammograms of GO, GOMF and Ni-doped samples (GOMNF 1:18, GOMNF 1:6, GOMNF 1:2) with a catalyst loading of 15 mg/cm2. The samples GOMF, GOMNF 1:18 and GOMNF 1:2 display a reduction peak around 0.3–0.4 V as the potential excursion goes towards cathodic direction, which evidences catalytic activity towards ORR. Then the potential excursion goes towards anodic direction, an oxidation peak at ca. 0.5–0.6 V, indicating a reversible process. The potential and intensity of the reduction peak varies with the nickel contents. Similar responses with catalyst loads of 7 mg/cm2 and 22 mg/cm2 were found, with the maximum at 15 mg/cm2, which was chosen as the best load for further studies.
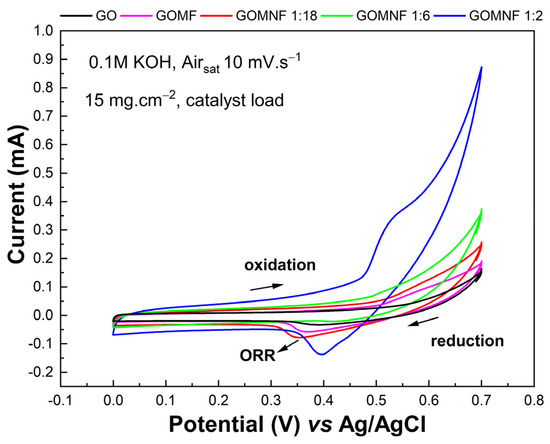
Figure 7.
Voltammograms of GOMNF samples with different Ni/Fe ratios with a 15 mg/cm2 load of catalyst.
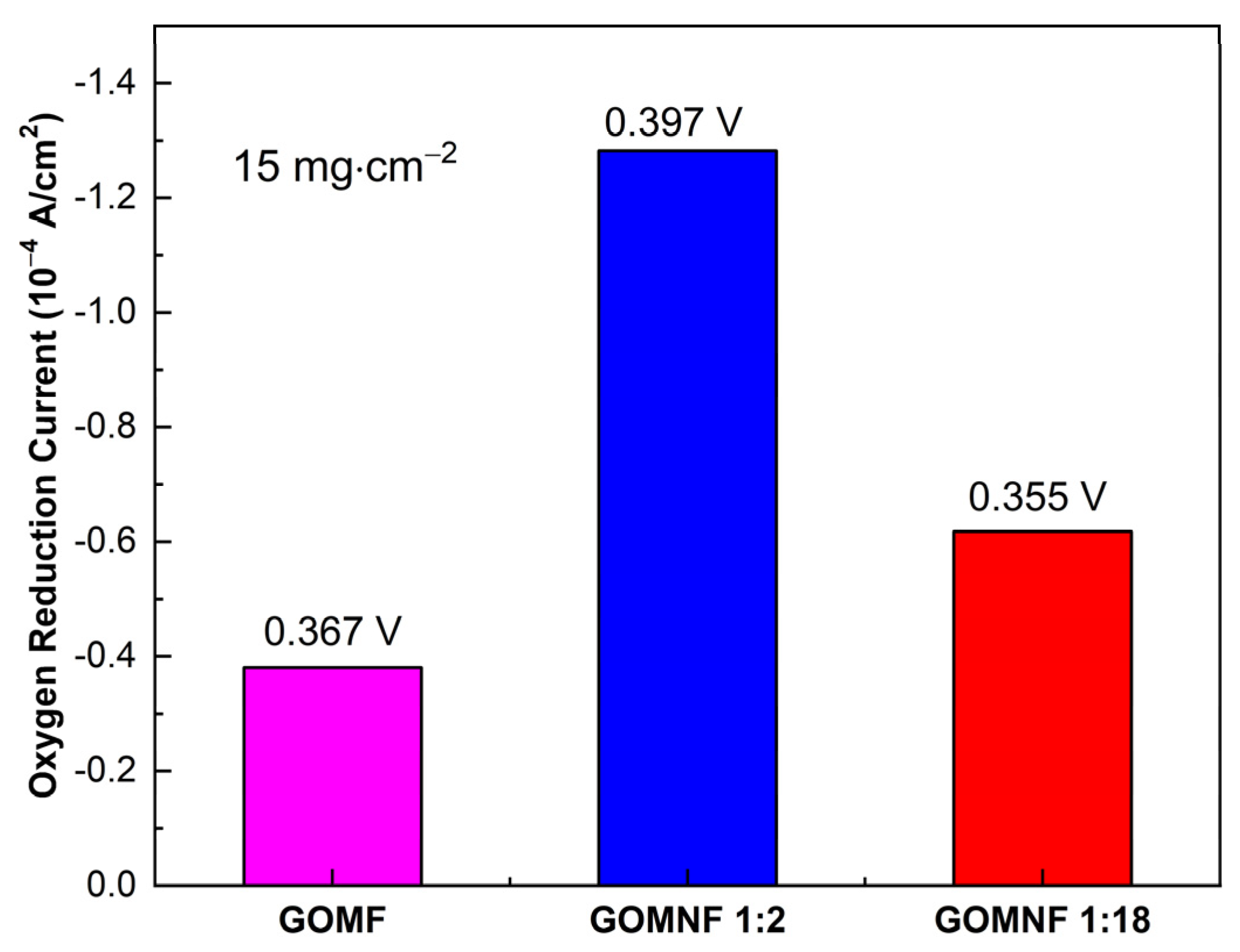
A summary of the catalytic performance, obtained by cyclic voltammetry of the decorated graphene materials, is shown in Figure 8. The reduction potentials are very similar, indicating that all catalysts have similar activities. On the other hand, according to the reduction current, the GOMNF 1:2 sample is the most efficient. This behavior could be due to the presence of crystalline phases of nickel ferrite (NiFe2O4) and maghemite (γ-Fe2O3), which give it an increase in electronic transfer, as the maghemite phase containing Fe2+ could be reversibly oxidized to magnetite [56,57]. A study of the catalyst stability and spent catalysts will be subject of a future work.
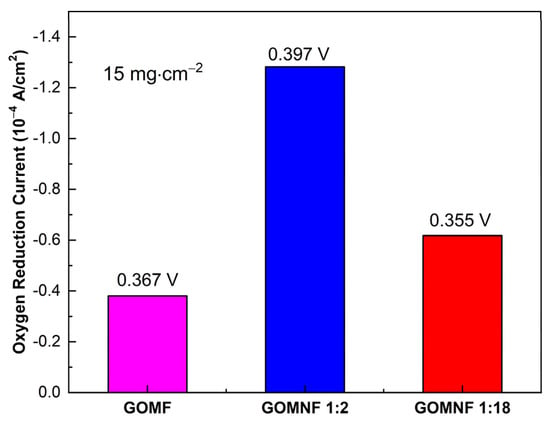
Figure 8.
Electrocatalytic activity for ORR for samples GOMF, GOMNF 1:2 and GOMNF 1:18.
MFC Performance with Different Electrocatalysts
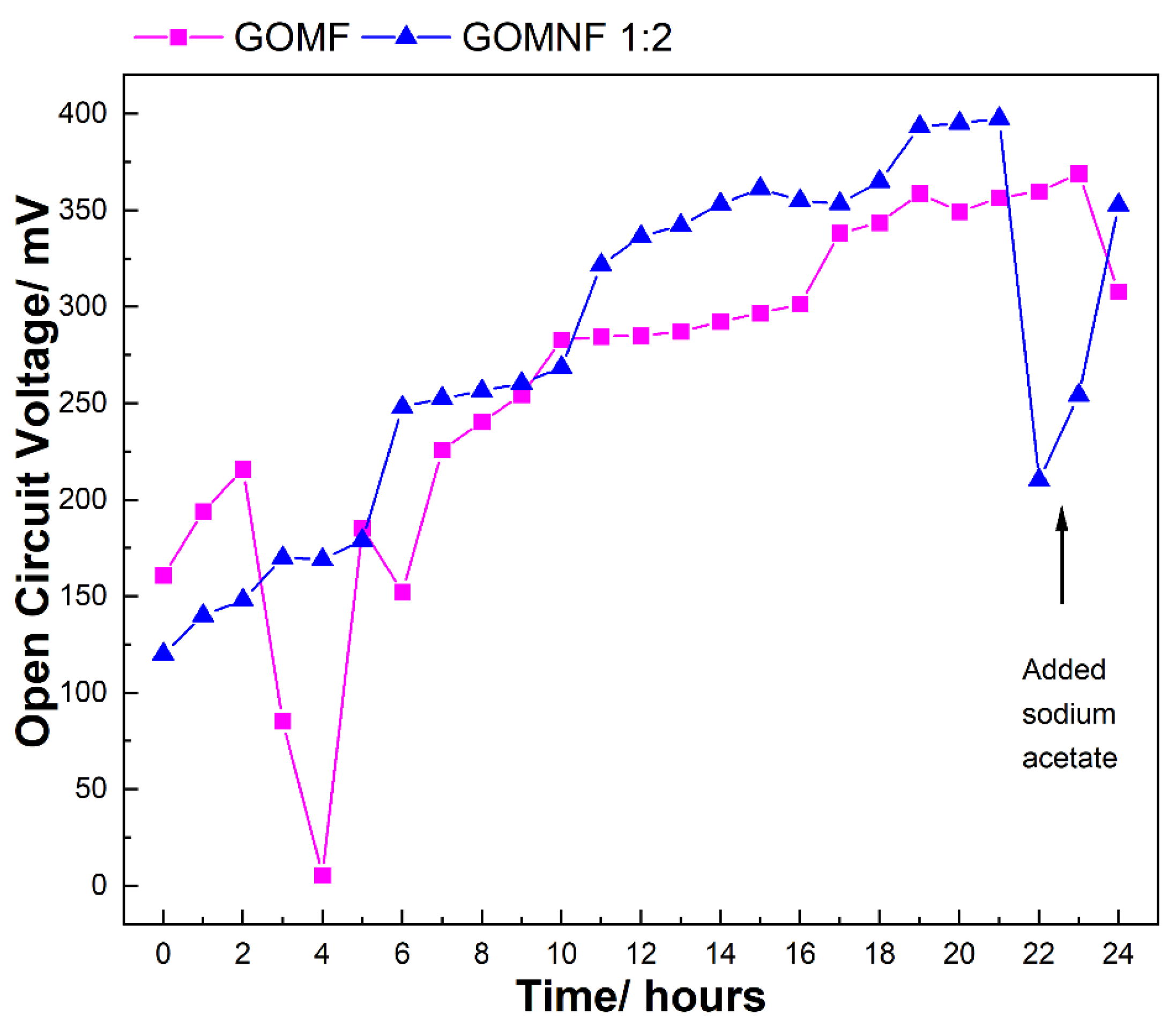
The catalysts GOMF and GOMNF 1:2 that showed ORR activity were used to make air electrodes evaluated in laboratory scale microbial fuel cells with a volume of 50 mL. The evolution of the open circuit voltage (OCV) in the cells built with the GOMNF 1:2 and GOMF electrocatalysts are presented in Figure 9. In the case of the GOMNF 1:2 electrocatalyst that showed the highest OCV in cell performance and the highest oxygen reduction reaction, the voltage gradually increases until reaching a maximum potential of 397.7 mV after 21 h. From this moment on, a sharp decline in the OCV is observed; by adding sodium acetate, the voltage increases again, indicating that the drop is due to starvation of the microbiological population in the anode chamber when COD is reduced [58,59]. The cell with the GOMF catalyst showed a continuous increase in voltage in the 24 h of operation, until reaching 350 mV. The respective power densities were 508 mW·m2 for GONMF 1:2 and 139 mW·m2 for GOMF. Table 2 presents a comparison of MFCs prepared using different ORR catalysts with those prepared in this work.
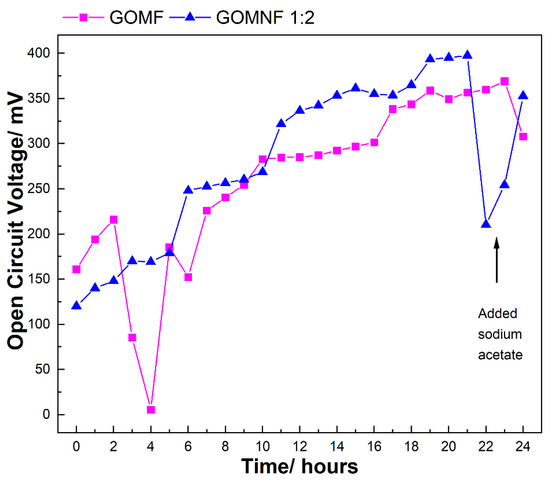
Figure 9.
Evolution of the OCV in 24 h in a MFC built with the ORR electrocatalysts.

Table 2.
Comparison between ORR catalysts.
The results suggest that the efficiency increase upon increasing the addition of Ni to the ORR catalyst can be attributed to the formation of a mixture of NiFe2O4 and phases of iron oxide, mainly maghemite, which can act as cocatalysts [68,69]. As observed in Table 2, the power density values of the catalyst are in the order of magnitude of other reported materials, with the advantage of a single pot synthesis. A study of the catalyst stability after several operation cycles, as well as the characteristics of the spent material, will be subject of a future work.
3.6. SEM Analysis
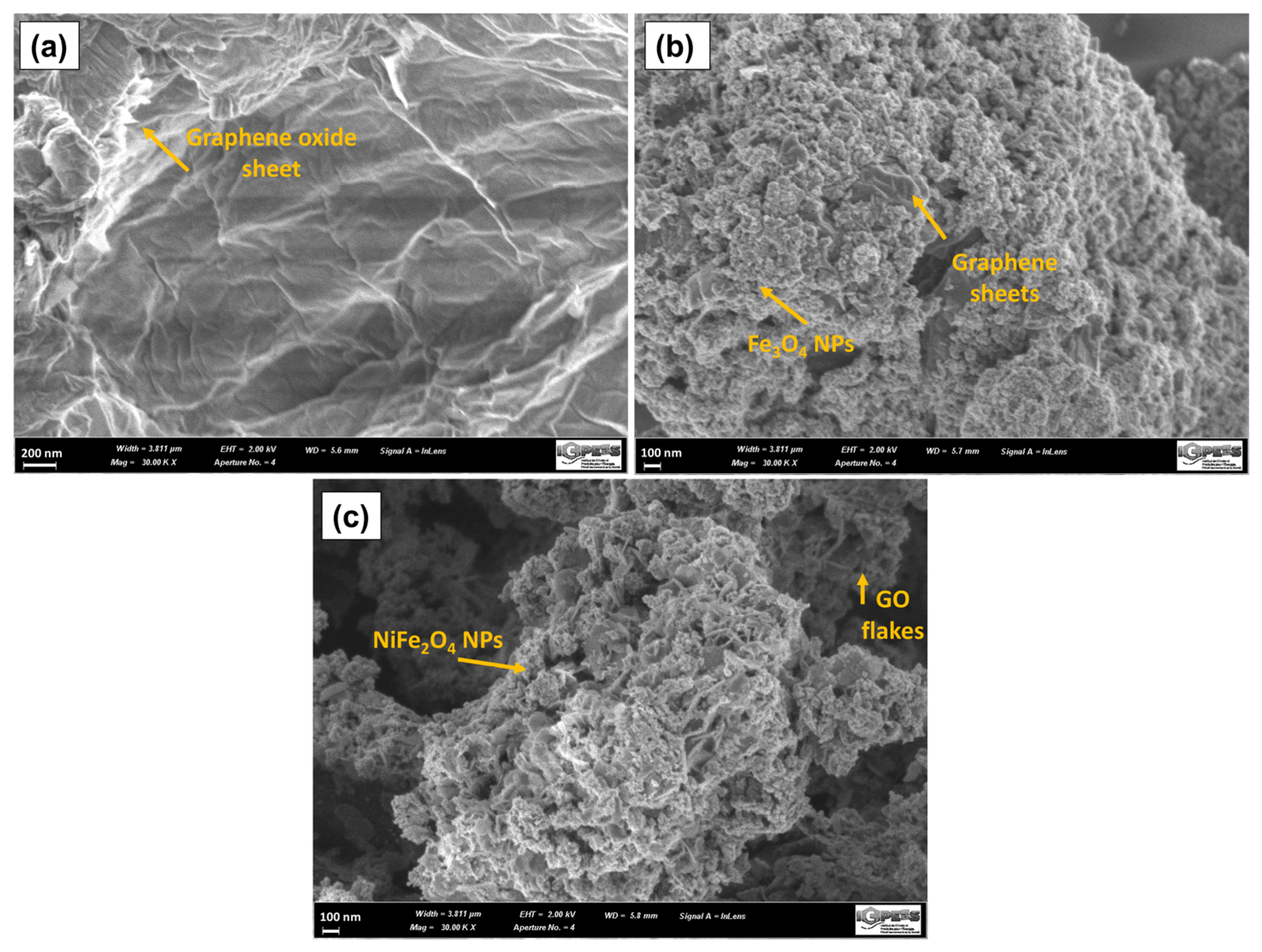
The morphology of the samples was studied by scanning electron microscopy. Figure 10a presents micrographs of graphene oxide. A wrinkled surface is observed in the GO layers because of the deformation by exfoliation and the re-stacking of the sheets when drying [70]. Furthermore, it is possible to observe the edges of individual sheets of graphene, which have a wavy and folded shape. In Figure 10b, the micrograph of the GOMF is observed where the magnetite nanoparticles cover the graphene oxide sheet. Figure 10c shows the SEM micrograph of GOMNF 1:2, where exfoliated flakes covered with nanoparticles (NPs) are observed.
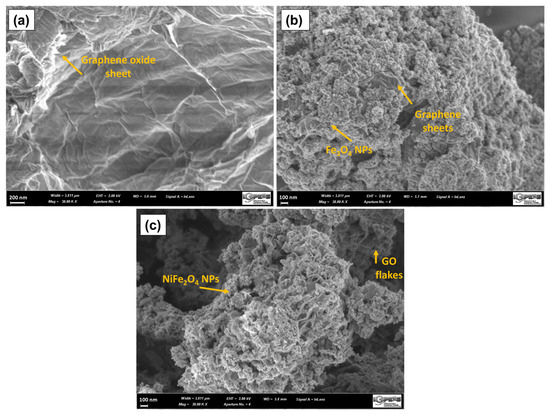
Figure 10.
SEM micrograph of (a) GO, (b) GOMF and (c) GOMNF 1:2 samples.
3.7. TEM Analysis
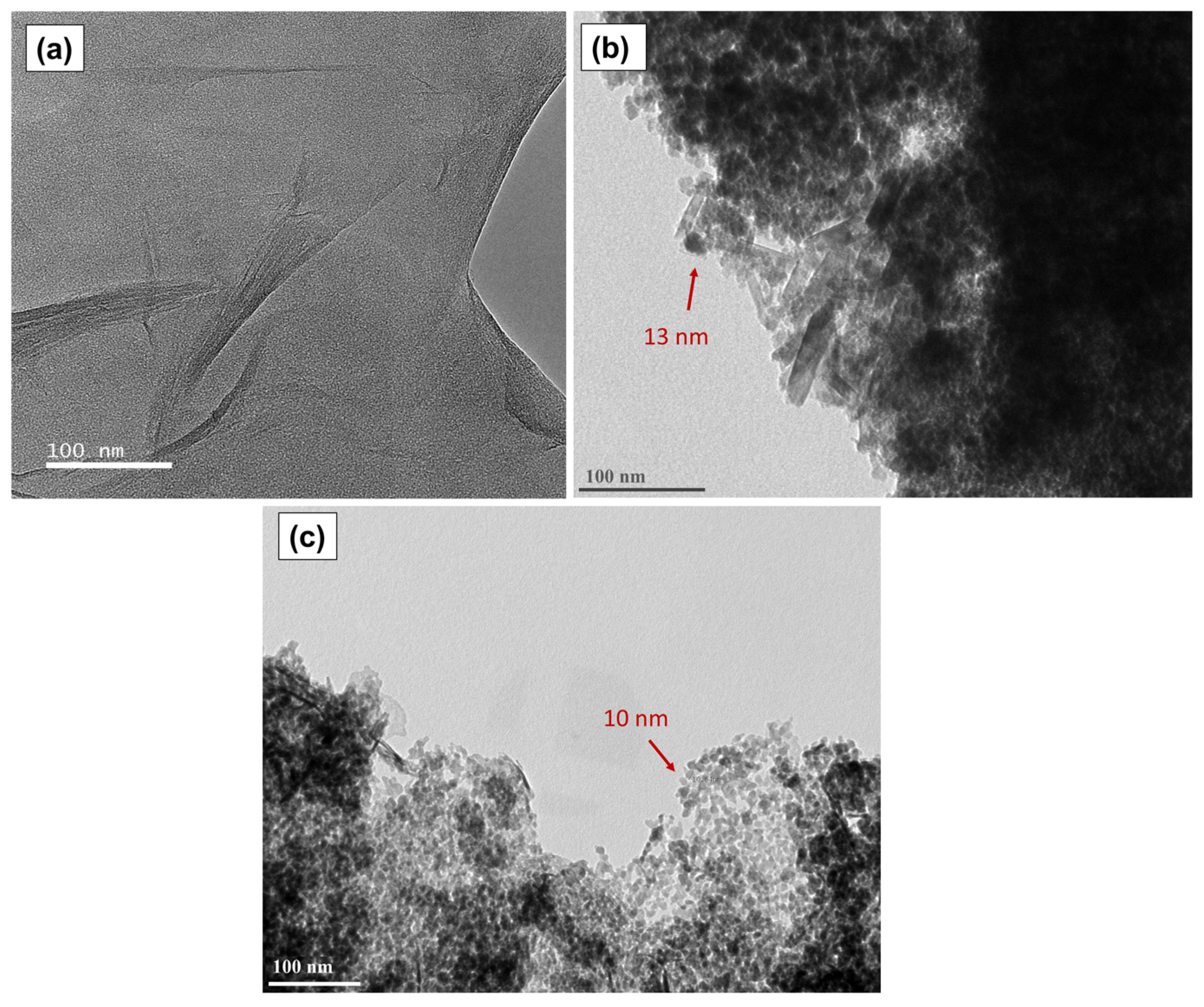
To have further insight into the catalyst morphology, Figure 11 presents the transmission electron micrographs of the graphene oxide, GOMF and GOMNF 1:2 samples. Figure 11a presents the microscopic characteristics of the GO, which consist of 8 to 10 stacked sheets; the folding is attributed to the functional groups incorporated onto the edges of the sheet during oxidation, in which the carbon adopts the sp3 configuration. The darker regions in the micrographs are due to the increased number of layers stacked in the area. In addition, the corrugated sheet is observed, attributed to the formation of hydrogen bonds in the oxygenated groups between the basal plane of graphene [71,72]. Figure 11b belongs to the GOMF sample where agglomerated nanoparticles around 13 nm are observed. Some tubes are seen that could be graphene sheets rolled up on themselves upon the terminal group reduction [73]. In Figure 11c, corresponding to the 1:2 GOMNF sample, nanoparticles around 10 nm are observed decorating the edge of the GO sheet, reducing the carboxyl and carbonyl groups as shown in FTIR. The observed particle sizes in both samples are close to the crystallite sizes determined by XRD.
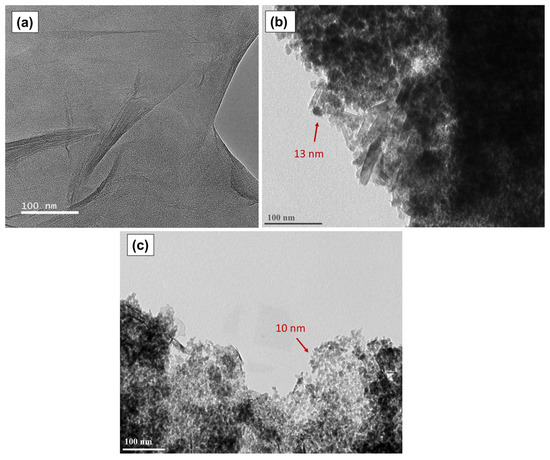
Figure 11.
TEM images of (a) graphene oxide, (b) GOMF sample, (c) GOMNF 1:2 sample.
3.8. SEM/EDS Analysis of the MFC Electrodes
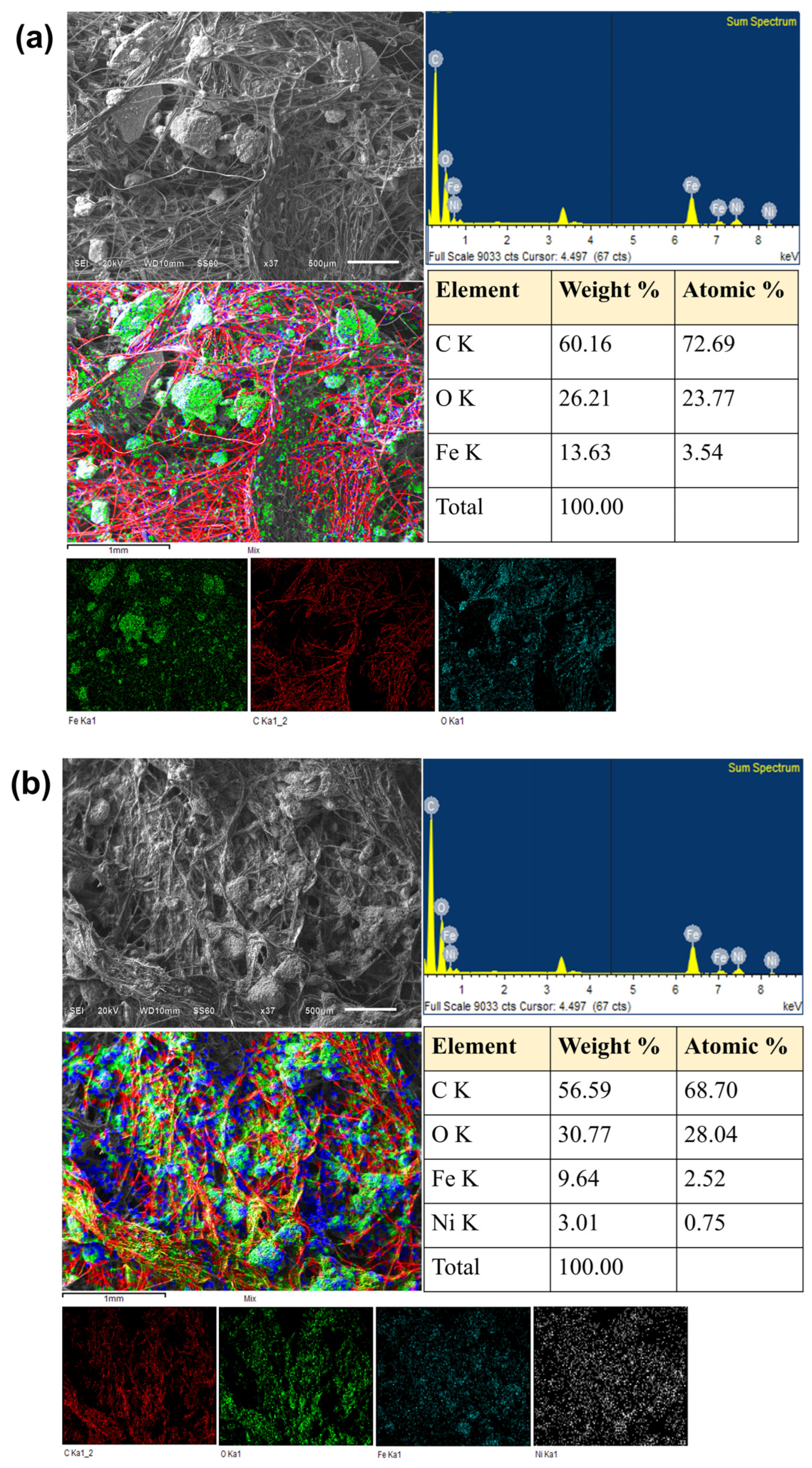
Figure 12 shows the SEM micrographs, the EDS spectra and the elemental and composite EDS maps, as well as the elemental composition tables of the GOMF and GOMNF 1:2 samples deposited onto the graphite felt used in the MFCs. Figure 12a shows the SEM image and the composition of the GOMF/graphite felt sample, where large Fe-rich particles (green in color on the individual EDS map) are evenly distributed onto the graphite fibers. The individual elemental maps show that the particles are mostly Fe and O, with almost any of them attached to the graphite fibers. In FTIR, there is a noticeable large peak of OH stretching in GOMF with respect to GOMNF 1:2, which is evidence of larger moisture trapping in the former, which shall be behind the observed agglomeration of the particles. Thus, the large agglomeration in GOMF can be associated with the reduced electrochemical performance. The SEM image and the EDS composite map of the GOMNF 1:2/graphite sample in Figure 12b show some particle agglomeration, but in the elemental maps, a uniform distribution of Ni, Fe and O can be seen along the electrode fibers, which indicates a more uniform dispersion of the material and, therefore, a higher catalytic surface. In the respective composition tables, GOMF displays an iron content of ca. 3.5%, while GOMNF 1:2 has 2.52% Fe and 0.75% Ni contents, fairly corresponding to 1 mol NiFe2O4 + 0.5 mol γ-Fe2O3.
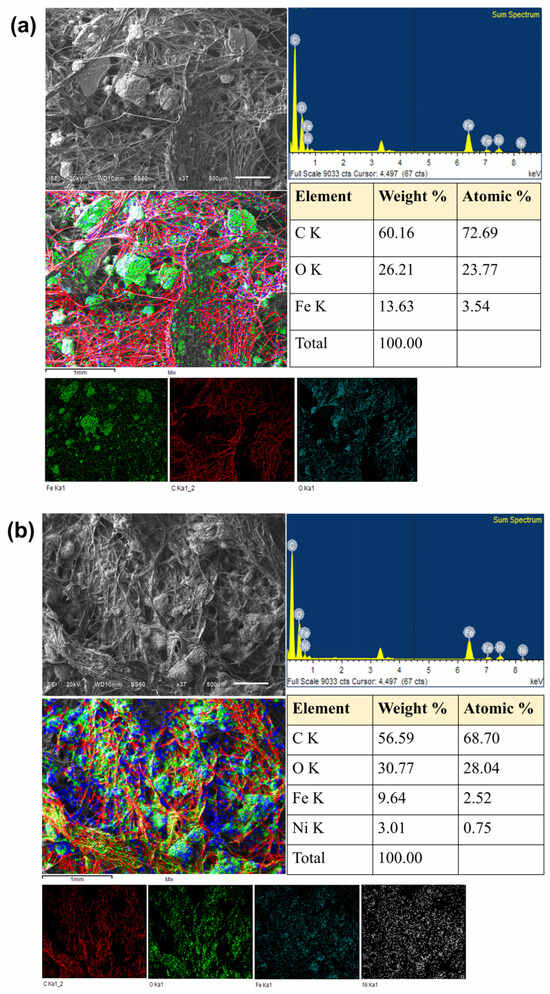
Figure 12.
SEM/EDS analysis of the graphite electrode impregnated with 15 mg·cm−2 of (a) GOMF and (b) GOMNF 1:2 as catalyst. Figure displays the general topographic image of the electrode, the topography superimposed with the elemental mapping, the elemental mapping of each element, the EDS spectrum with the peaks of the detected elements and the respective composition tables.
4. Conclusions
In this work, graphene oxide decorated with iron oxide and nickel-doped iron oxide nanoparticles were obtained using the co-precipitation synthesis method. While varying the Ni/Fe ratios, graphene oxide decorated with maghemite and nickel ferrite (NiFe2O4) nanoparticles were obtained. The materials were applied at different loads onto graphite felt electrodes and tested as electrocatalysts for the oxygen reduction reaction in small-scale microbial fuel cells over 24 h. The sample without nickel (GOMF) showed a large particle agglomeration at the electrode surface, due to water uptake, which led to reduced ORR activity. The sample GOMNF 1:2 with a 1:2 Ni/Fe ratio had good dispersion onto the electrode and was the better variant for ORR with an open-circuit output potential of 397 mV, compared with 367 mV for the GOMF sample and a reduction current an order of magnitude higher. The increased efficiency of Ni-doped materials was attributed to better dispersibility onto the graphite electrode due to decreasing wettability with Ni contents, and to the presence of nickel ferrite and maghemite, which act as co-catalysts. The tested materials had a power density of 508 mW·m−2 (GOMNF 1:2) and 139 mW·m−2 (GOMNF), respectively, which make GOMNF 1:2 a suitable ORR catalyst with respect to those reported in the literature, prepared by a simple method.
Author Contributions
Conceptualization, S.E.B.-S. and F.C.-B.; methodology, S.E.B.-S.; formal analysis, F.C.-B., B.V. and D.B.; investigation, S.E.B.-S.; resources, F.C.-B. and B.V.; data curation, J.G., D.B. and S.E.B.-S.; writing—original draft preparation, S.E.B.-S. and F.C.-B.; writing—review and editing, F.C.-B. and B.V.; supervision, F.C.-B. and S.-K.K.; project administration, F.C.-B.; funding acquisition, F.C.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by CONACYT 40798 Science of Frontier Grant and SIP Project 20240907.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
S.E.B.-S. acknowledges SIP-IPN as well as the Institute Jean Lamour for financial and material support for an academic stay in France.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| MFC | Microbial Fuel Cell |
| ORR | Oxygen Reduction Reaction |
| GO | Graphene Oxide |
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| OCV | Open Circuit Voltage |
| TGA | Thermogravimetric Analysis |
| DTG | Derivative Thermogravimetric Analysis |
| EDS | Energy Dispersive Spectroscopy |
References
- Bunyanidhi, P.; Phattharasupakun, N.; Duangdangchote, S.; Prempluem, S.; Joraleechanchai, N.; Sawangphruk, M. Exploring the impact of metal oxide coating and metal atom doping on the electrochemical performance of Ni-rich cathode materials. J. Mater. Chem. A Mater. 2023, 11, 23223–23227. [Google Scholar] [CrossRef]
- Bazina, N.; Ahmed, T.G.; Almdaaf, M.; Jibia, S.; Sarker, M. Power generation from wastewater using microbial fuel cells: A review. J. Biotechnol. 2023, 374, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, H.; Guo, S.; Li, C. Metal-based cathode catalysts for electrocatalytic ORR in microbial fuel cells: A review. Chin. Chem. Lett. 2024, 35, 109418. [Google Scholar] [CrossRef]
- Pollet, B.G.; Kalanur, S.S. Applications of Ferric Oxide in Water Splitting by Electrolysis: A Comprehensive Review. Molecules 2024, 29, 4990. [Google Scholar] [CrossRef]
- Han, J.; Bian, J.; Sun, C. Recent Advances in Single-Atom Electrocatalysts for Oxygen Reduction Reaction. Research 2020, 2020, 9512763. [Google Scholar] [CrossRef] [PubMed]
- Matseke, M.S.; Luo, H.; Wen, L.; Zheng, H. The upgraded performance of the NiFe2O4 /C electrocatalyst using Co substitution for the oxygen reduction reaction in an alkaline solution. J. Phys. Chem. Solids 2022, 165, 110644. [Google Scholar] [CrossRef]
- Kumar, N.; Ansari, M.R.; Khaladkar, S.; Maurya, O.; Peta, K.R.; Kalekar, A.; Singha, M.K.; Dash, J.K. NiFe2O4 nanoparticles as highly efficient catalyst for oxygen reduction reaction and energy storage in supercapacitor. Mater. Chem. Phys. 2024, 316, 129072. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Li, C.; Yan, X.; Hu, S.; Yin, R.; Wei, Y.; Gao, K.; Gao, H. Research progress of NiFe2O4 electrode materials in supercapacitors: Preparation, modification, structural regulation, and future challenges. Coord. Chem. Rev. 2024, 519, 216103. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, Q.; Li, K.; Zhang, S.; Ma, X. First-row transition metal oxide oxygen evolution electrocatalysts: Regulation strategies and mechanistic understandings. Sustain. Energy Fuels 2020, 4, 5417–5432. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, P.; Cheng, Y.; Mo, Y.; Luo, X.; Liu, P.; Guo, R.; Liu, X. Recent progress on NiFe2O4 spinels as electrocatalysts for the oxygen evolution reaction. J. Electroanal. Chem. 2023, 946, 117703. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P. Binary nickel ferrite oxide (NiFe2O4) nanoparticles coated on reduced graphene oxide as stable and high-performance asymmetric supercapacitor electrode material. Int. J. Hydrogen Energy 2020, 45, 27482–27491. [Google Scholar] [CrossRef]
- Yano, H.; Iwasaki, K. Size-Dependence of the Electrochemical Activity of Platinum Particles in the 1 to 2 Nanometer Range. Surfaces 2024, 7, 472–481. [Google Scholar] [CrossRef]
- Ghasemi, S.; Gheshlaghi, R.; Mahdavi, M.A.; Abazarian, E. Evaluation of low-cost carbon/metal electrodes as cathodes and anodes in sediment microbial fuel cells. Fuel 2024, 373, 132349. [Google Scholar] [CrossRef]
- Alves, C.T.; Onwudili, J.A. Screening of Nickel and Platinum Catalysts for Glycerol Conversion to Gas Products in Hydrothermal Media. Energies 2022, 15, 7571. [Google Scholar] [CrossRef]
- Soufi, A.; Hajjaoui, H.; Elmoubarki, R.; Abdennouri, M.; Qourzal, S.; Barka, N. Spinel ferrites nanoparticles: Synthesis methods and application in heterogeneous Fenton oxidation of organic pollutants—A review. Appl. Surf. Sci. Adv. 2021, 6, 100145. [Google Scholar] [CrossRef]
- Fan, Y.; Li, R.; Zhao, C.; Hu, A.; Zhou, B.; Pan, Y.; Chen, J.; Yan, Z.; Liu, M.; He, M.; et al. Chromium-doped inverse spinel electrocatalysts with optimal orbital occupancy for facilitating reaction kinetics of lithium-oxygen batteries. J. Colloid. Interface Sci. 2023, 645, 439–447. [Google Scholar] [CrossRef]
- Janani, G.; Chae, Y.; Surendran, S.; Sim, Y.; Park, W.; Kim, J.K.; Sim, U. Rational Design of Spinel Oxide Nanocomposites with Tailored Electrochemical Oxygen Evolution and Reduction Reactions for ZincAir Batteries. Appl. Sci. 2020, 10, 3165. [Google Scholar] [CrossRef]
- Sabaa, H.M.; El-Khatib, K.M.; El-Kady, M.Y.; Mahmoud, S.A. Spinel structure of activated carbon supported MFe2O4 composites as an economic and efficient electrocatalyst for oxygen reduction reaction in neutral media. J. Solid. State Electrochem. 2022, 26, 2749–2763. [Google Scholar] [CrossRef]
- Buaki-Sogó, M.; Zubizarreta, L.; García-Pellicer, M.; Quijano-López, A. Sustainable Carbon as Efficient Support for Metal-Based Nanocatalyst: Applications in Energy Harvesting and Storage. Molecules 2020, 25, 3123. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Q.; Qiu, J.; Guo, R.; Liu, X. Research progress of spinel CoFe2O4 as an electrocatalyst for the oxygen evolution reaction. Catal. Sci. Technol. 2023, 13, 6102–6125. [Google Scholar] [CrossRef]
- Eppstein, R.; Toroker, M.C. On the Interplay Between Oxygen Vacancies and Small Polarons in Manganese Iron Spinel Oxides. ACS Mater. Au 2022, 2, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Mladenović, D.; Mladenović, A.; Santos, D.M.F.; Yurtcan, A.B.; Miljanić, Š.; Mentus, S.; Šljukić, B. Transition metal oxides for bifunctional ORR/OER electrocatalysis in unitized regenerative fuel cells. J. Electroanal. Chem. 2023, 946, 117709. [Google Scholar] [CrossRef]
- Ma, W.; Wu, F.; Yu, P.; Mao, L. Carbon support tuned electrocatalytic activity of a single-site metal–organic framework toward the oxygen reduction reaction. Chem. Sci. 2021, 12, 7908–7917. [Google Scholar] [CrossRef]
- Gothandapani, K.; Jeniffer, R.S.; Selvi, G.T.; Velmurugan, V.; Assaifan, A.K.; Alzahrani, K.E.; Albrithen, H.; Muthuramamoorthy, M.; Pandiaraj, S.; Pitchaimuthu, S.; et al. Nickel nanoparticles supported on carbon surface as an electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 52, 1137–1146. [Google Scholar] [CrossRef]
- Abaft, E.; Taleghani, H.G.; Lashkenari, M.S. 3D graphene oxide/nickel ferrite aerogel for high-performance supercapacitor application. J. Energy Storage 2024, 98, 112797. [Google Scholar] [CrossRef]
- Qavami, A.; Ghasemi, S. Nickel-cobalt manganate supported on reduced graphene oxide/carbon nanotube for improving air cathode performance in single chamber microbial fuel cell. Mater. Sci. Eng. B 2022, 275, 115492. [Google Scholar] [CrossRef]
- Almansoori, A.; Balázsi, K.; Balázsi, C. Advances, Challenges, and Applications of Graphene and Carbon Nanotube-Reinforced Engineering Ceramics. Nanomaterials 2024, 14, 1881. [Google Scholar] [CrossRef]
- Kang, S.; Kim, H.; Chung, Y.-H. Recent developments of nano-structured materials as the catalysts for oxygen reduction reaction. Nano Converg. 2018, 5, 13. [Google Scholar] [CrossRef]
- Burkholder, M.B.; Rahman, F.B.A.; Chandler, E.H.; Regalbuto, J.R.; Gupton, B.F.; Tengco, J.M.M. Metal supported graphene catalysis: A review on the benefits of nanoparticular supported specialty sp2 carbon catalysts on enhancing the activities of multiple chemical transformations. Carbon. Trends 2022, 9, 100196. [Google Scholar] [CrossRef]
- Qamar, S.; Ramzan, N.; Aleem, W. Graphene dispersion, functionalization techniques and applications: A review. Synth. Met. 2024, 307, 117697. [Google Scholar] [CrossRef]
- Ghulam, A.N.; Santos, O.A.L.D.; Hazeem, L.; Backx, B.P.; Bououdina, M.; Bellucci, S. Graphene Oxide (GO) Materials—Applications and Toxicity on Living Organisms and Environment. J. Funct. Biomater. 2022, 13, 77. [Google Scholar] [CrossRef]
- Narayan, J.; Bezborah, K. Recent advances in the functionalization, substitutional doping and applications of graphene/graphene composite nanomaterials. RSC Adv. 2024, 14, 13413–13444. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Wu, J.; Liu, Z.; Yan, D.; Wu, Z.; Zhong, C. Advanced electrocatalysts for fuel cells: Evolution of active sites and synergistic properties of catalysts and carrier materials. Exploration 2025, 5, 20230052. [Google Scholar] [CrossRef]
- Gnanaseelan, N.; Latha, M.; Mantilla, A.; Sathish-Kumar, K.; Caballero-Briones, F. The role of redox states and junctions in photocatalytic hydrogen generation of MoS2-TiO2-rGO and CeO2-Ce2Ti3O8.7-TiO2-rGO composites. Mater. Sci. Semicond. Process 2020, 118, 105185. [Google Scholar] [CrossRef]
- Gnanaseelan, N.; Marasamy, L.; Mantilla, A.; Kamaraj, S.K.; Espinosa-Faller, F.J.; Caballero-Briones, F. Exploring the impact of doping and co-doping with B and N on the properties of graphene oxide and its photocatalytic generation of hydrogen. Int. J. Hydrogen Energy 2022, 47, 40905–40919. [Google Scholar] [CrossRef]
- Guerrero-Contreras, J.; Caballero-Briones, F. Graphene oxide powders with different oxidation degree. prepared by synthesis variations of the Hummers method. Mater. Chem. Phys. 2015, 153, 209–220. [Google Scholar] [CrossRef]
- Espinosa-Faller, F.J.; Hoy-Benítez, J.; Colina-Ruiz, R.A.; Contreras, J.G.; de León, J.M.; Lezama-Pacheco, J.S.; Llamazares, J.L.S.; Caballero-Briones, F. Magnetic properties of nano(iron oxide)-decorated graphene oxide. Mater. Chem. Phys. 2024, 317, 129173. [Google Scholar] [CrossRef]
- Yao, Y.; Miao, S.; Liu, S.; Ma, L.P.; Sun, H.; Wang, S. Synthesis, characterization, and adsorption properties of magnetic Fe3O4@graphene nanocomposite. Chem. Eng. J. 2012, 184, 326–332. [Google Scholar] [CrossRef]
- Kazi, S.; Inamdar, S.; Sarnikar, Y.; Kamble, D.; Tigote, R. Simple Co-precipitation synthesis and characterization of magnetic spinel NiFe2O4 nanoparticles. Mater. Today Proc. 2023, 73, 448–454. [Google Scholar] [CrossRef]
- Li, L.; Liao, Q.; Liu, C.; Zhang, T.; Liu, C.; Chen, Z.; Gao, R.; He, Q. Enhanced biological wastewater treatment supplemented with anaerobic fermentation liquid of primary sludge. J. Env. Manag. 2023, 347, 119086. [Google Scholar] [CrossRef]
- Liang, H.; Han, J.; Yang, X.; Qiao, Z.; Yin, T. Performance improvement of microbial fuel cells through assembling anodes modified with nanoscale materials. Nanomater. Nanotechnol. 2022, 12, 184798042211329. [Google Scholar] [CrossRef]
- Shirkosh, M.; Hojjat, Y.; Mardanpour, M.M. Boosting microfluidic microbial fuel cells performance via investigating electron transfer mechanisms. metal-based electrodes, and magnetic field effect. Sci. Rep. 2022, 12, 7417. [Google Scholar] [CrossRef] [PubMed]
- Ikram, R.; Jan, B.M.; Ahmad, W. An overview of industrial scalable production of graphene oxide and analytical approaches for synthesis and characterization. J. Mater. Res. Technol. 2020, 9, 11587–11610. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.A. Precise calculation of crystallite size of nanomaterials: A review. J. Alloys Compd. 2023, 968, 171914. [Google Scholar] [CrossRef]
- La, G.H.; Lee, S.H.; Min, D.J. Fundamental study on preferential reduction of Ni in NiFe2O4. Miner. Eng. 2021, 164, 106829. [Google Scholar] [CrossRef]
- Belotcerkovtceva, D.; Maciel, R.P.; Berggren, E.; Maddu, R.; Sarkar, T.; Kvashnin, Y.O.; Thonig, D.; Lindblad, A.; Eriksson, O.; Kamalakar, M.V. Insights and Implications of Intricate Surface Charge Transfer and sp3-Defects in Graphene/Metal Oxide Interfaces. ACS Appl. Mater. Interfaces 2022, 14, 36209–36216. [Google Scholar] [CrossRef] [PubMed]
- Sivagurunathan, P.; Gibin, S.R. Preparation and characterization of nickel ferrite nano particles by co-precipitation method with citrate as chelating agent. J. Mater. Sci. Mater. Electron. 2016, 27, 2601–2607. [Google Scholar] [CrossRef]
- da Guarda Souza, M.O.; Santos, M.V.R.D.; Castro, L.M.F.; da Silva, C.P. Production and in situ transformation of hematite into magnetite from the thermal decomposition of iron nitrate or goethite mixed with biomass. J. Therm. Anal. Calorim. 2020, 139, 1731–1739. [Google Scholar] [CrossRef]
- Farzan, A.; Borandeh, S.; Seppälä, J. Conductive polyurethane/PEGylated graphene oxide composite for 3D-printed nerve guidance conduits. Eur. Polym. J. 2022, 167, 111068. [Google Scholar] [CrossRef]
- Brusko, V.; Khannanov, A.; Rakhmatullin, A.; Dimiev, A.M. Unraveling the infrared spectrum of graphene oxide. Carbon 2024, 229, 119507. [Google Scholar] [CrossRef]
- Flores-Cano, D.A.; Checca-Huaman, N.-R.; Castro-Merino, I.-L.; Pinotti, C.N.; Passamani, E.C.; Litterst, F.J.; Ramos-Guivar, J.A. Progress toward Room-Temperature Synthesis and Functionalization of Iron-Oxide Nanoparticles. Int. J. Mol. Sci. 2022, 23, 8279. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, A.; Bin-Shuwaish, M.S.; Al-Hamdan, R.S.; Almohareb, T.; Maawadh, A.M.; Al Deeb, M.; Alhenaki, A.M.; Abduljabbar, T.; Vohra, F. Graphene oxide nano-filler based experimental dentine adhesive. A SEM/EDX, Micro-Raman and microtensile bond strength analysis. J. Appl. Biomater. Funct. Mater. 2020, 18, 228080002096693. [Google Scholar] [CrossRef]
- Khalil, K.A.; Barakat, N.A.M.; Motlak, M.; Al-Mubaddel, F.S. A novel graphene oxide-based ceramic composite as an efficient electrode for capacitive deionization. Sci. Rep. 2020, 10, 9676. [Google Scholar] [CrossRef]
- Kumar, S.; Ahmed, F.; Shaalan, N.M.; Arshi, N.; Dalela, S.; Chae, K.H. Investigations of Structural, Magnetic, and Electrochemical Properties of NiFe2O4 Nanoparticles as Electrode Materials for Supercapacitor Applications. Materials 2023, 16, 4328. [Google Scholar] [CrossRef]
- More, S.; Raut, S.; Premkumar, S.; Bhopale, S.; Bhoraskar, S.; More, M.; Mathe, V. Structural and morphological tuning of iron oxide polymorphs by ECR plasma-assisted thermal oxidation. RSC Adv. 2020, 10, 32088–32101. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Hunter, T.N.; Xu, H.; Harbottle, D.; Kale, G.M.; Tillotson, M.R. Synergistic effect of Fe and Ni on carbon aerogel for enhanced oxygen reduction and H2O2 activation in electro-Fenton process. Sep. Purif. Technol. 2025, 353, 128436. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, H.; Ai, L.; Ding, J.; Zhu, P.; Li, Z.; Li, B.; Jiang, H.; Yu, F.; Duan, X.; et al. Synthesis, Electronic Structure. and Electrochemical Properties of the Cubic Mg2MnO4 Spinel with Porous-Spongy Structure. Nanomaterials 2021, 11, 1122. [Google Scholar] [CrossRef]
- Blatter, M.; Delabays, L.; Furrer, C.; Huguenin, G.; Cachelin, C.P.; Fischer, F. Stretched 1000-L microbial fuel cell. J. Power Sources 2021, 483, 229130. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, L.; Zhang, Y.; Yu, Y. Microbial Fuel Cell-Based Biosensor for Simultaneous Test of Sodium Acetate and Glucose in a Mixed Solution. Int. J. Env. Res. Public Health 2022, 19, 12297. [Google Scholar] [CrossRef]
- Wang, H.; Wei, L.; Yang, C.; Liu, J.; Shen, J. A pyridine-Fe gel with an ultralow-loading Pt derivative as ORR catalyst in microbial fuel cells with long-term stability and high output voltage. Bioelectrochemistry 2020, 131, 107370. [Google Scholar] [CrossRef]
- Huang, Q.; Zhou, P.; Yang, H.; Zhu, L.; Wu, H. In situ generation of inverse spinel CoFe2O4 nanoparticles onto nitrogen-doped activated carbon for an effective cathode electrocatalyst of microbial fuel cells. Chem. Eng. J. 2017, 325, 466–473. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, X.; Lu, Y.; Jv, R.; Liu, Y.; Li, N.; Chen, S. CoMn2O4 doped reduced graphene oxide as an effective cathodic electrocatalyst for ORR in microbial fuel cells. Electrochim. Acta 2019, 296, 214–223. [Google Scholar] [CrossRef]
- Wu, X.-T.; Li, J.-C.; Pan, Q.-R.; Li, N.; Liu, Z.-Q. Gallic acid-assisted synthesis of Pd uniformly anchored on porous N-rGO as efficient electrocatalyst for microbial fuel cells. Dalton Trans. 2018, 47, 1442–1450. [Google Scholar] [CrossRef]
- Harshiny, M.; Samsudeen, N.; Kameswara, R.J.; Matheswaran, M. Biosynthesized FeO nanoparticles coated carbon anode for improving the performance of microbial fuel cell. Int. J. Hydrogen Energy 2017, 42, 26488–26495. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S. α-Fe2O3/polyaniline nanocomposites as an effective catalyst for improving the electrochemical performance of microbial fuel cell. Chem. Eng. J. 2018, 339, 539–546. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Mohamed, H.O.; Park, S.-G.; Al Mayyahi, R.B.; Al-Dhaifallah, M.; Rezk, H.; Ren, X.; Yu, H.; Chae, K.-J. Electrophoretically fabricated nickel/nickel oxides as cost effective nanocatalysts for the oxygen reduction reaction in air-cathode microbial fuel cell. Int. J. Hydrogen Energy 2020, 45, 5960–5970. [Google Scholar] [CrossRef]
- Xia, W.-Y.; Tan, L.; Li, N.; Li, J.-C.; Lai, S.-H. Nickel cobaltite@nanocarbon hybrid materials as efficient cathode catalyst for oxygen reduction in microbial fuel cells. J. Mater. Sci. 2017, 52, 7539–7545. [Google Scholar] [CrossRef]
- Laobuthee, A.; Khankhuean, A.; Panith, P.; Veranitisagul, C.; Laosiripojana, N. Ni–Fe Cocatalysts on Magnesium Silicate Supports for the Depolymerization of Kraft Lignin. ACS Omega 2023, 8, 8675–8682. [Google Scholar] [CrossRef]
- Fang, L.J.; Wang, X.L.; Li, Y.H.; Liu, P.F.; Wang, Y.L.; Zeng, H.D.; Yang, H.G. Nickel nanoparticles coated with graphene layers as efficient co-catalyst for photocatalytic hydrogen evolution. Appl. Catal. B 2017, 200, 578–584. [Google Scholar] [CrossRef]
- Deng, S.; Berry, V. Wrinkled, rippled and crumpled graphene: An overview of formation mechanism, electronic properties, and applications. Mater. Today 2016, 19, 197–212. [Google Scholar] [CrossRef]
- Kueh, T.C.; Chew, S.H.; Soh, A.K.; Hung, Y.M. Investigation of the influence of the location of oxygenated functional groups in graphene nanostructures on water permeation via molecular dynamics simulations. Appl. Surf. Sci. 2023, 609, 155414. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef] [PubMed]
- Briones, F.C.; Guerrero-Contreras, J.; Benito-Santiago, S.E.; Llamazares, J.L.S.; Espinosa-Faller, F.J. Formation of magnetic nanoscrolls and nanoribbons in iron oxide-decorated graphene oxide. Mater. Lett. 2022, 309, 131321. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).