A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires. I: An Overview of the Theory, Test Methods, and the European Union Regulatory Status
Abstract
:1. Introduction
- Part II: “Some examples of acid scavengers at high temperatures in the condensed phase”;
- Part III: “EN 60754-2 and focus on the species in solution affecting pH and conductivity”;
- Part IV: “The impact of acid scavengers on flame retardance and smoke emission”;
- Part V: “Comparison between EN 60754-1 and EN 60754-2: what happens to acidity with the introduction of the thermal profile of EN 60754-1 in EN 60754-2”.
2. Methods, Regulatory Status in the EU, the Pattern to Low Smoke Acidity PVC Compounds
2.1. List of Abbreviations and Acronyms
- HCl—hydrogen chloride;
- CO—carbon monoxide;
- PVC—poly(vinyl chloride);
- EU—European Union.
2.2. Smoke Acidity Test Methods
2.3. Additional Classification for Acidity in the EU
2.4. Definition of Acid Scavengers at High Temperatures
2.5. Acid Scavenger at High Temperatures in the Condensed Phase: Failures and Success
2.5.1. Where, When, and How
2.5.2. Single-Step Reaction Versus Multiple-Step Reaction
2.5.3. Main Failure Cases
The Kinetics
- The chemical nature of the acid scavenger;
- Its particle size;
- Its dispersion properties;
- The test temperature and heating regimes.
- 1.
- Chemical nature
- 2.
- Particle size
- 3.
- Dispersion
- 4.
- Temperature and heating regimes
Decomposition of Acid Scavengers and Their Reaction Products
2.6. Definition of Efficiency of the Acid Scavenger
2.7. The Driving Force of pH and Conductivity in Solution
2.8. Impact of Acid Scavengers on Flame Retardancy and Smoke Suppressant Properties
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Hull, T.R.; Stec, A.A.; Paul, K.T. Hydrogen chloride in fires. Fire Saf. Sci. 2008, 9, 665–676. [Google Scholar] [CrossRef]
- Obloj-Muzaj, M. Fire performance of PVC. Int. Polym. Sci. Technol. 2001, 28, 98–100. [Google Scholar] [CrossRef]
- Hirschler, M. Fire safety, smoke toxicity, and acidity. In Proceedings of the Flame Retardants 2006, London, UK, 14–15 February 2006. [Google Scholar]
- ISO/TR 20118:2019; Plastics—Guidance on Fire Characteristics and Fire Performance of PVC Materials Used in Building Applications 2019. ISO: Geneva, Switzerland. Available online: https://www.iso.org/standard/67071.html (accessed on 16 August 2022).
- Babrauskas, V.; Gann, R.G.; Levin, B.C.; Paabo, M.; Harris, R.H.; Peacock, R.D.; Yusa, S. A methodology for obtaining and using toxic potency data for fire hazard analysis. Fire Saf. J. 1998, 31, 345–358. [Google Scholar] [CrossRef]
- Guillaume, E.; Didieux, F.; Thiry, A.; Bellivier, A. Real-scale fire tests of one-bedroom apartments with regard to tenability assessment. Fire Saf. J. 2014, 70, 81–97. [Google Scholar] [CrossRef]
- Beitel, J.J.; Bertelo, C.A.; Carroll, W.F.; Gardner, R.O.; Grand, A.F.; Hirschler, M.M.; Smith, G.F. Hydrogen chloride transport and decay in a large apparatus I. Decomposition of poly(vinyl chloride) wire insulation in a plenum by current overload. J. Fire Sci. 1986, 4, 15–41. [Google Scholar] [CrossRef]
- Babrauskas, V.; Peacock, R.D. Heat release rate: The single most important variable in fire hazard. Fire Saf. J. 1992, 18, 255–272. [Google Scholar] [CrossRef]
- Babrauskas, V.; Harris Jr, R.H.; Gann, R.G.; Levin, B.C.; Lee, B.T.; Peacock, R.D.; Paabo, M.; Twilley, W.; Yoklavich, M.F.; Clark, H.M. Fire hazard comparison of fire-retarded and non-fire-retarded products (NBS SP 749). In Special Publication (NIST SP); National Institute of Standards and Technology: Gaithersburg, MD, USA, 1988. [Google Scholar] [CrossRef]
- Sarti, G.; Piana, M. PVC in cables for building and construction. Can the “European approach” be considered a good example for other countries? Acad. Lett. 2022, 5453. [Google Scholar] [CrossRef]
- Greg, S. Quick method for determining the acid gas evolution from PVC formulations. J. Vinyl Technol. 1997, 9, 18–21. [Google Scholar] [CrossRef]
- Sumi, K.; Morwick, D.W. Acid Gas Evolution from PVC Using DBR/NRC Method; Internal Report (National Research Council of Canada. Division of Building Research), no. DBR-IR-425; National Research Council of Canada: Ottawa, ON, Canada, 1976. [CrossRef]
- Sze On Chan, H. Measurement of hydrochloric acid emission from burning PVC compounds. J. Fire Sci. 1984, 2, 106–122. [Google Scholar] [CrossRef]
- Chandler, L.A.; Hirschler Smith, G.F. A heated tube furnace test for the emission of acid gas from PVC wire coating materials: Effects of experimental procedures and mechanistic considerations. Eur. Polym. J. 1987, 23, 51–61. [Google Scholar] [CrossRef]
- Sarti, G. How formulations can influence the PVC cables fire behavior. In Proceedings of the 1st PVC4cables Conference, Lion, France, 26 October 2017. [Google Scholar] [CrossRef]
- Sarti, G.; Piana, M. New formulations and test comparison for the classification of PVC cables under EU regulation n° 305/2011 for construction products. In Proceedings of the AMI Cables 2019, Duesseldorf, Germany, 5–7 March 2019. [Google Scholar] [CrossRef]
- Sarti, G.; Piana, M. PVC cables and smoke acidity: A review comparing performances of old and new compounds. In Proceedings of the AMI Cables 2020, Duesseldorf, Germany, 3–5 March 2020. [Google Scholar] [CrossRef]
- Bassi, I. Characterization of PVC Compounds and Evaluation of Their Fire Performance, Focusing on the Comparison between EN 60754-1 and EN 60754-2 in the Assessment of the Smoke Acidity. Master’ Thesis, University of Bologna, Bologna, Italy, October 2021. Available online: https://www.pvc4cables.org/images/assessment_of_the_smoke_acidity.pdf (accessed on 16 August 2022).
- Sarti, G.; Piana, M. Smoke acidity and a new generation of PVC formulations for cables. In Proceedings of the AMI Formulation 2021, Cologne, Germany, 16–18 September 2021. [Google Scholar]
- Sarti, G. Developing and improving fire performance and safety in PVC. In Proceedings of the Future of PVC Compounding, Production & Recycling, online, 24–25 February 2022. [Google Scholar] [CrossRef]
- Aupetit, A. Overview of the global cable industry–markets and materials. In The Global Cable Industry: Materials, Markets, 1st ed.; Beyer, G., Ed.; John Wiley & Sons Ltd: Chichester, UK, 2021; pp. 1–20. [Google Scholar] [CrossRef]
- Starnes, W.; Xialong, G. Mechanism of autocatalysis in the thermal dehydrochlorination of poly(vinyl chloride). Macromolecules 2004, 37, 352–359. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Wu, C.H.; Wu, C.H.; Chang, C.Y.; Hor, J.L.; Shih, S.M.; Chen, L.W.; Chang, F.W. Two-stage pyrolysis model of PVC. Can. J. Chem. Eng. 1994, 72, 644–650. [Google Scholar] [CrossRef]
- O’Mara, M.M. Pyrolysis–gas chromatographic analysis of poly(vinyl chloride). II. In situ absorption of HCl during pyrolysis and combustion of PVC. J. Polym. Sci. Part A-1 Polym. Chem. 1971, 9, 1387–1400. [Google Scholar] [CrossRef]
- Brown, S.K.; Martin, K.G. Toxic gas and smoke measurement with the British Fire Propagation test. III: UPTV formulations with reduced HCl evolution. Fire Mater. 1985, 9, 95–102. [Google Scholar] [CrossRef]
- Matthews, G.; Plumper, G.S. Effects of calcium carbonate fillers on the behaviour of PVC in fires. Br. Polym. J. 1981, 13, 17–21. [Google Scholar] [CrossRef]
- Matthews, G.; Plumper, G.S. Effects of fillers on and the role of hydrogen chloride in the behaviour of PVC in fires. Br. Polym. J. 1984, 16, 34–38. [Google Scholar] [CrossRef]
- Starnes, W.H.; Wescott, L.D.; Reents, W.D.; Cais, R.E.; Villacorta, G.M.; Plitz, I.M.; Anthony, L.J. Mechanism of poly(vinyl chloride) fire retardance by molybdenum(vi) oxide. Further evidence in favor of the lewis acid theory. In Polymer Additives. Polymer Science and Technology; Kresta, J.E., Ed.; Springer: Boston, MA, USA, 2007; Volume 26, pp. 237–248. [Google Scholar] [CrossRef]
- Starnes, W.H., Jr.; Edelson, D. Mechanistic aspects of the behavior of molybdenum (VI) oxide as a fire-retardant additive for poly (vinyl chloride). An interpretive review. Macromolecules 1979, 12, 797–802. [Google Scholar] [CrossRef]
- Li, B.; Wang, J. A cone calorimetric study of flame retardance and smoke emission of PVC. I. The effect of cuprous and molybdic oxides. J. Fire Sci. 1997, 15, 341–357. [Google Scholar] [CrossRef]
- Li, B. A study of the thermal decomposition and smoke suppression of poly(vinyl chloride) treated with metal oxides using a cone calorimeter at a high incident heat flux. Polym. Degrad. Stab. 2002, 78, 349–356. [Google Scholar] [CrossRef]
- Rodolfo, A.; Innocentini Mei, L.H. Metallic oxides as fire retardants and smoke suppressants in flexible poly(vinyl chloride). J. Appl. Polym. Sci. 2010, 118, 2613–2623. [Google Scholar] [CrossRef]
- O’ Mara, M.M. Combustion of PVC. Pure Appl. Chem. 1977, 49, 649–660. [Google Scholar] [CrossRef]
- Zestos, A.G.; Grinnell, C.L.; Vinh, L.J.; Pike, R.D.; Starnes, W.H., Jr. Metal-exchanged clay and zeolite additives as smoke suppressants and fire retardants for poly(vinyl chloride). J. Vinyl Addit. Technol. 2009, 15, 87–91. [Google Scholar] [CrossRef]
- Starnes, W.H., Jr.; Pike, R.D.; Cole, J.R.; Doyal, A.S.; Kimlin, E.J.; Lee, J.T.; Murray, P.J.; Quinlan, R.A.; Zhang, J. Cone calorimetric study of copper-promoted smoke suppression and fire retardance of poly(vinyl chloride). Polym. Degrad. Stab. 2003, 82, 15–24. [Google Scholar] [CrossRef]
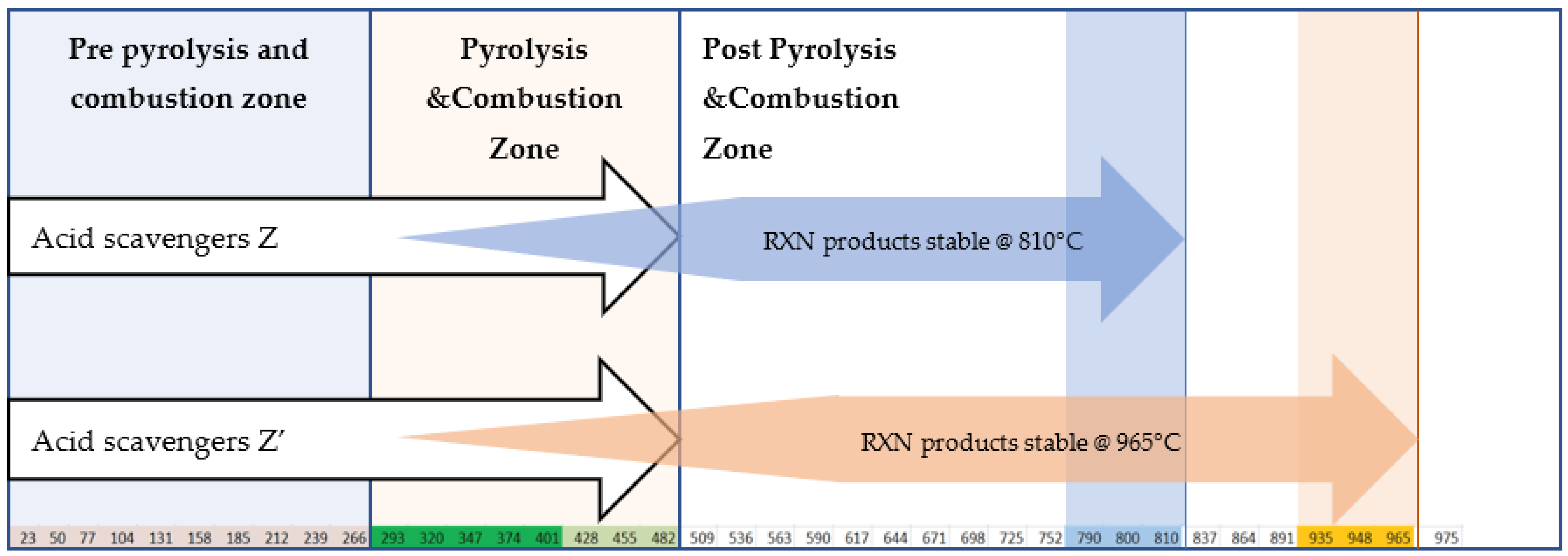
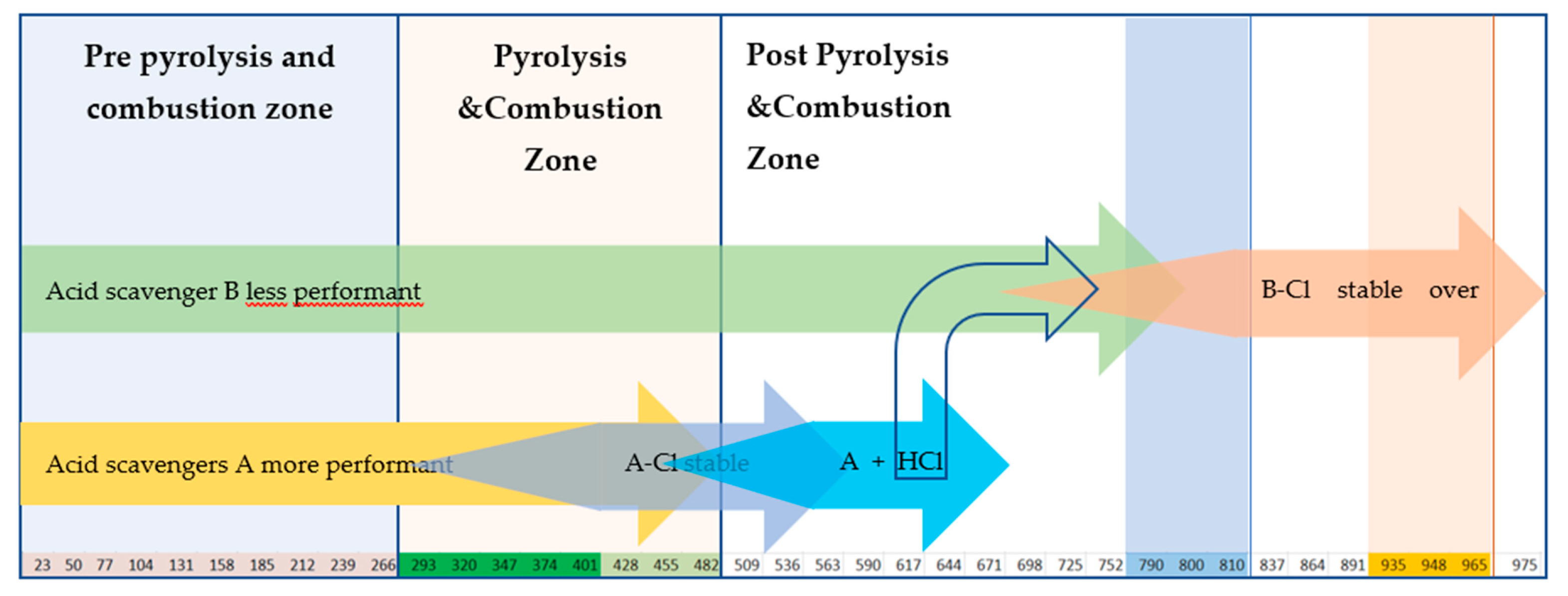

| Class | Requirement Performing EN 60754-2 |
|---|---|
| Class a1 | pH > 4.3, Conductivity < 2.5 (µS/mm) |
| Class a2 | pH > 4.3, Conductivity < 10 (µS/mm) |
| Class a3 | not a1 or a2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarti, G. A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires. I: An Overview of the Theory, Test Methods, and the European Union Regulatory Status. Fire 2022, 5, 127. https://doi.org/10.3390/fire5050127
Sarti G. A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires. I: An Overview of the Theory, Test Methods, and the European Union Regulatory Status. Fire. 2022; 5(5):127. https://doi.org/10.3390/fire5050127
Chicago/Turabian StyleSarti, Gianluca. 2022. "A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires. I: An Overview of the Theory, Test Methods, and the European Union Regulatory Status" Fire 5, no. 5: 127. https://doi.org/10.3390/fire5050127





