Influence of Oxygen Concentration on Combustion Kinetics and Gas Products of a Polyurethane–Coal Mixture
Abstract
:1. Introduction
2. Experimental Design and Methods
2.1. Experimental Samples
2.2. Experimental Equipment and Conditions
3. Results and Discussion
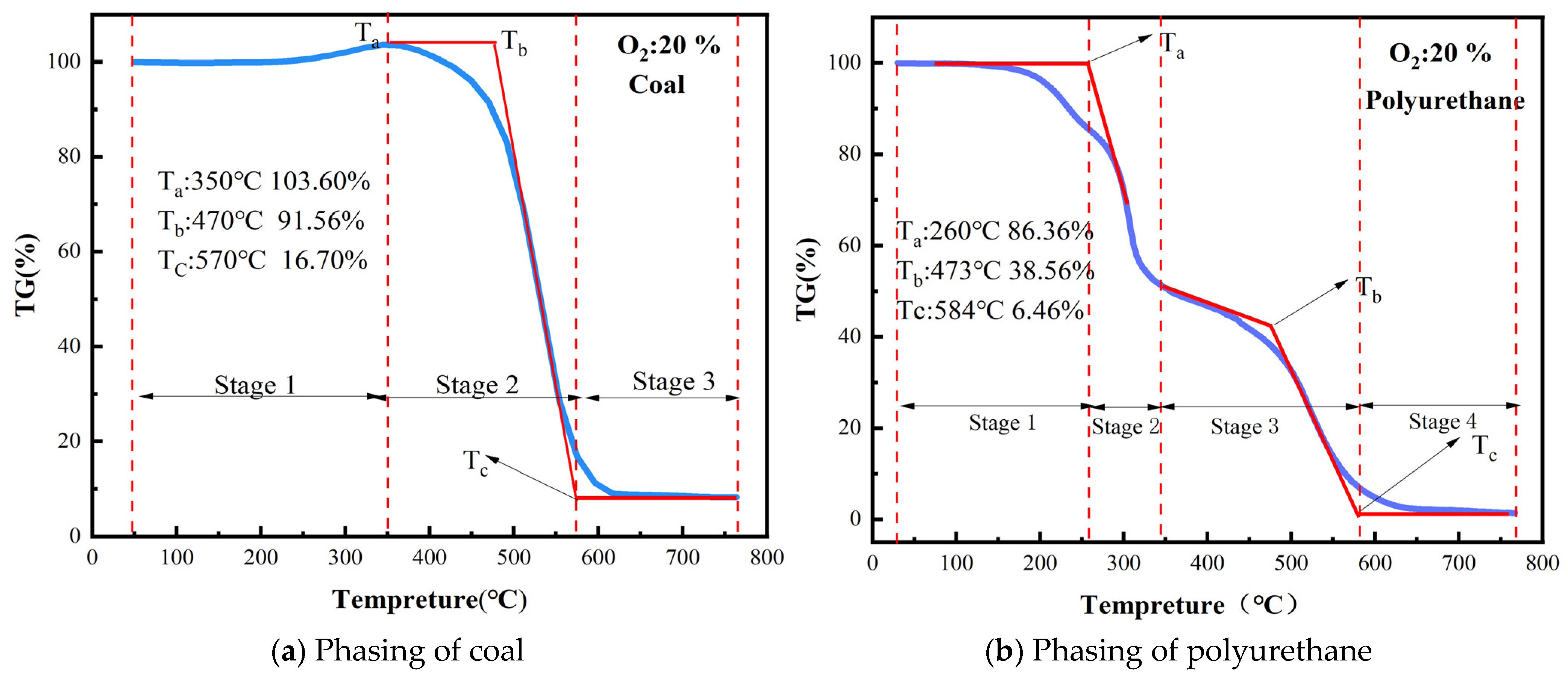
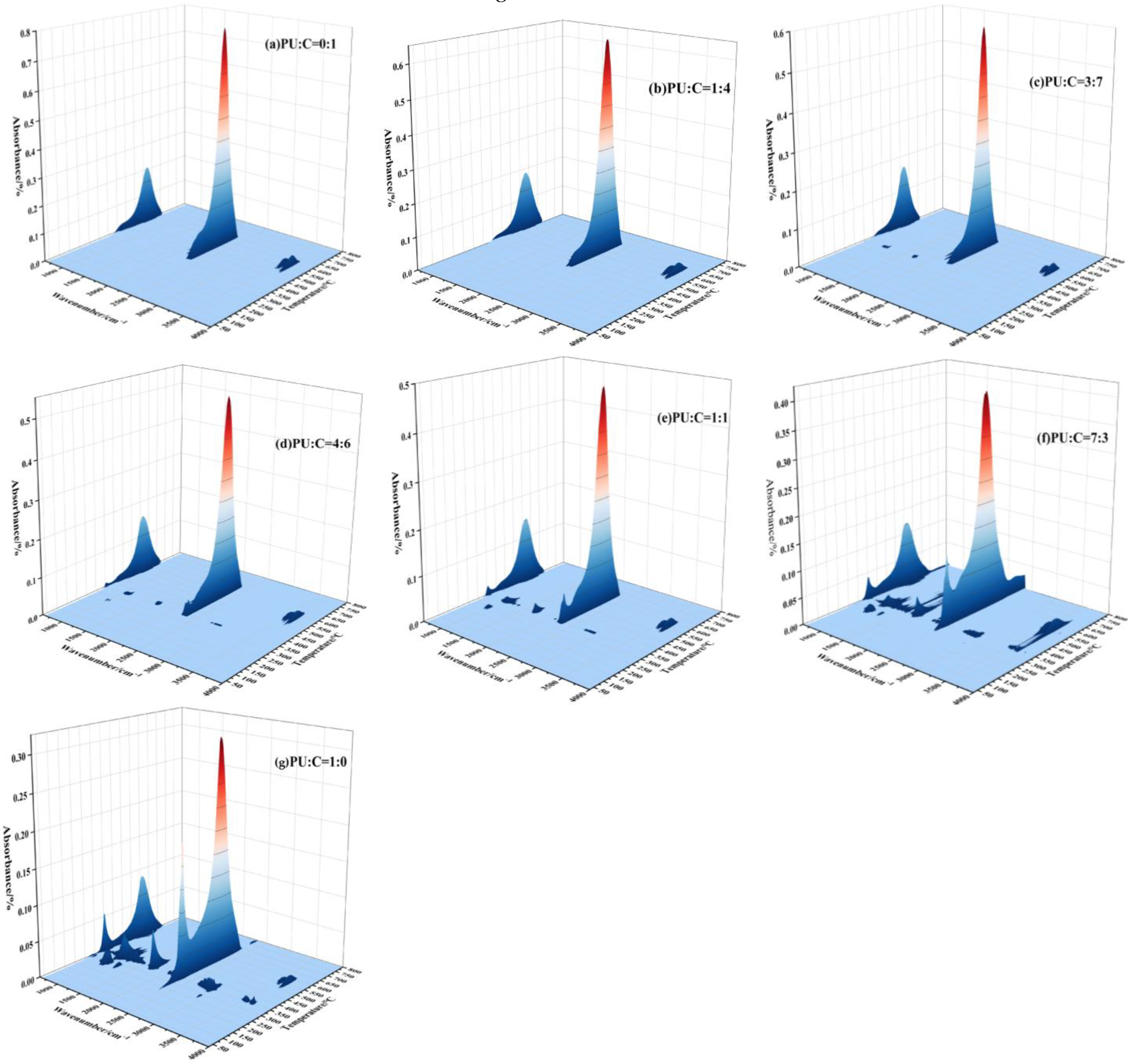
3.1. Staging
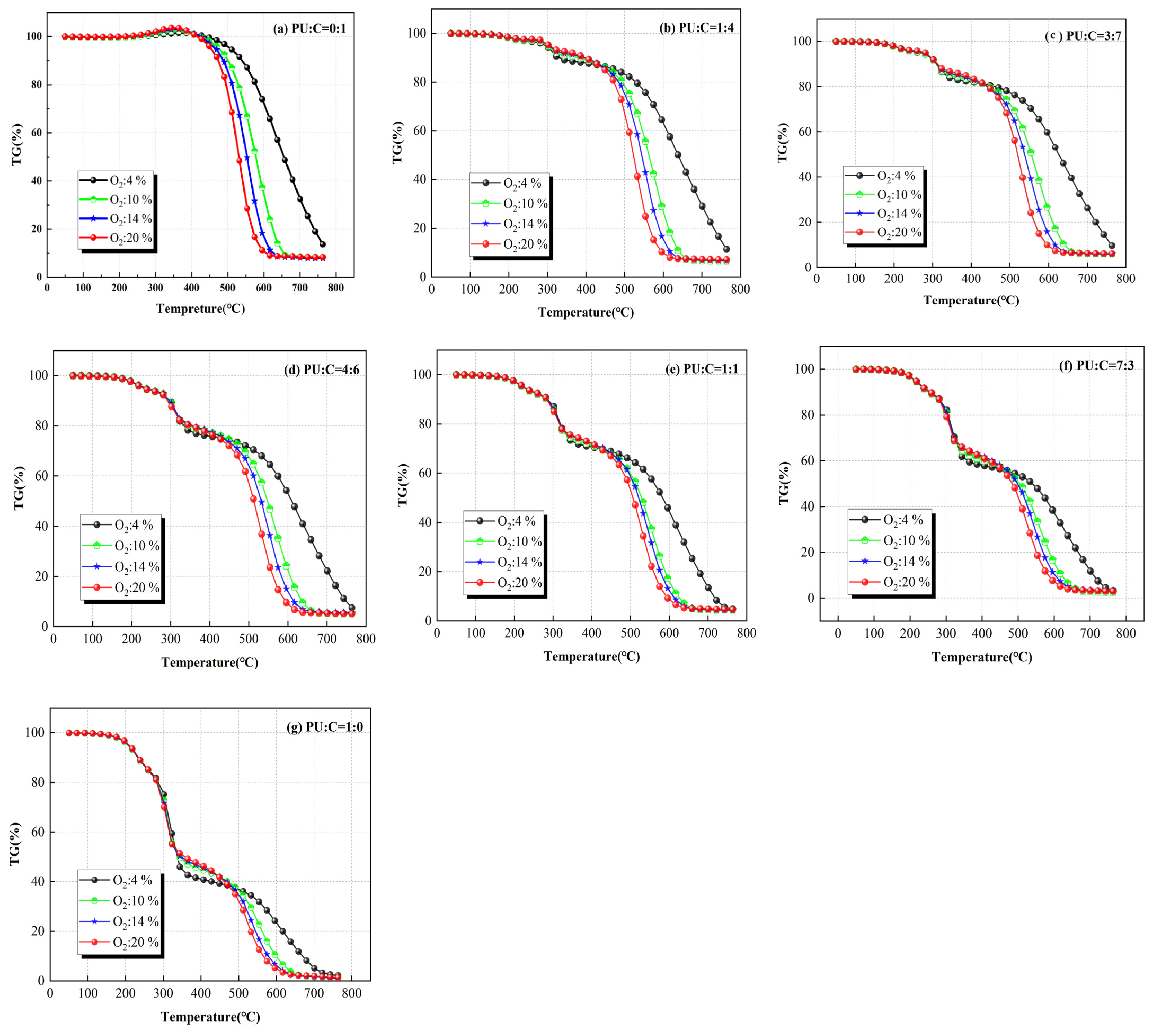
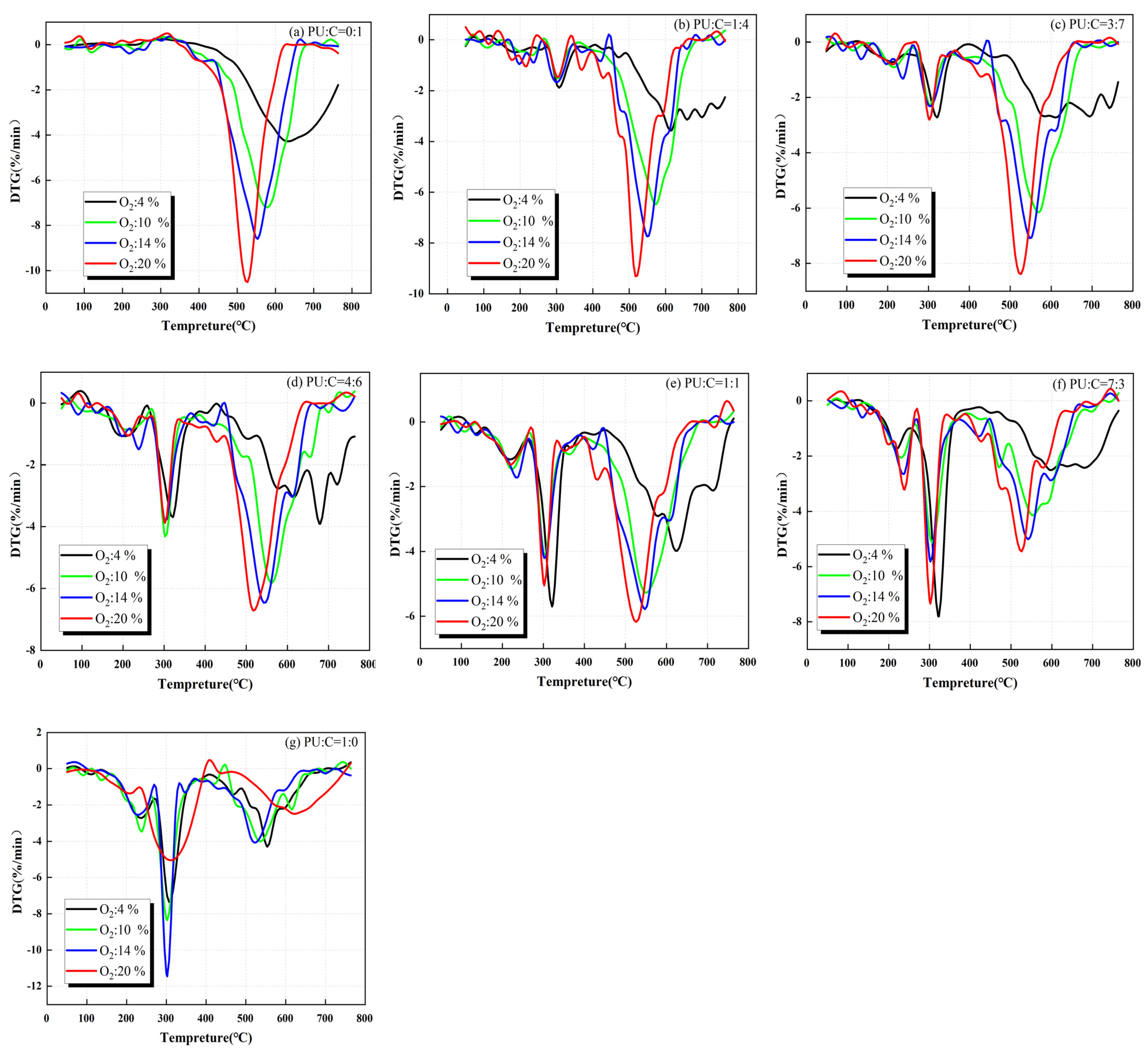
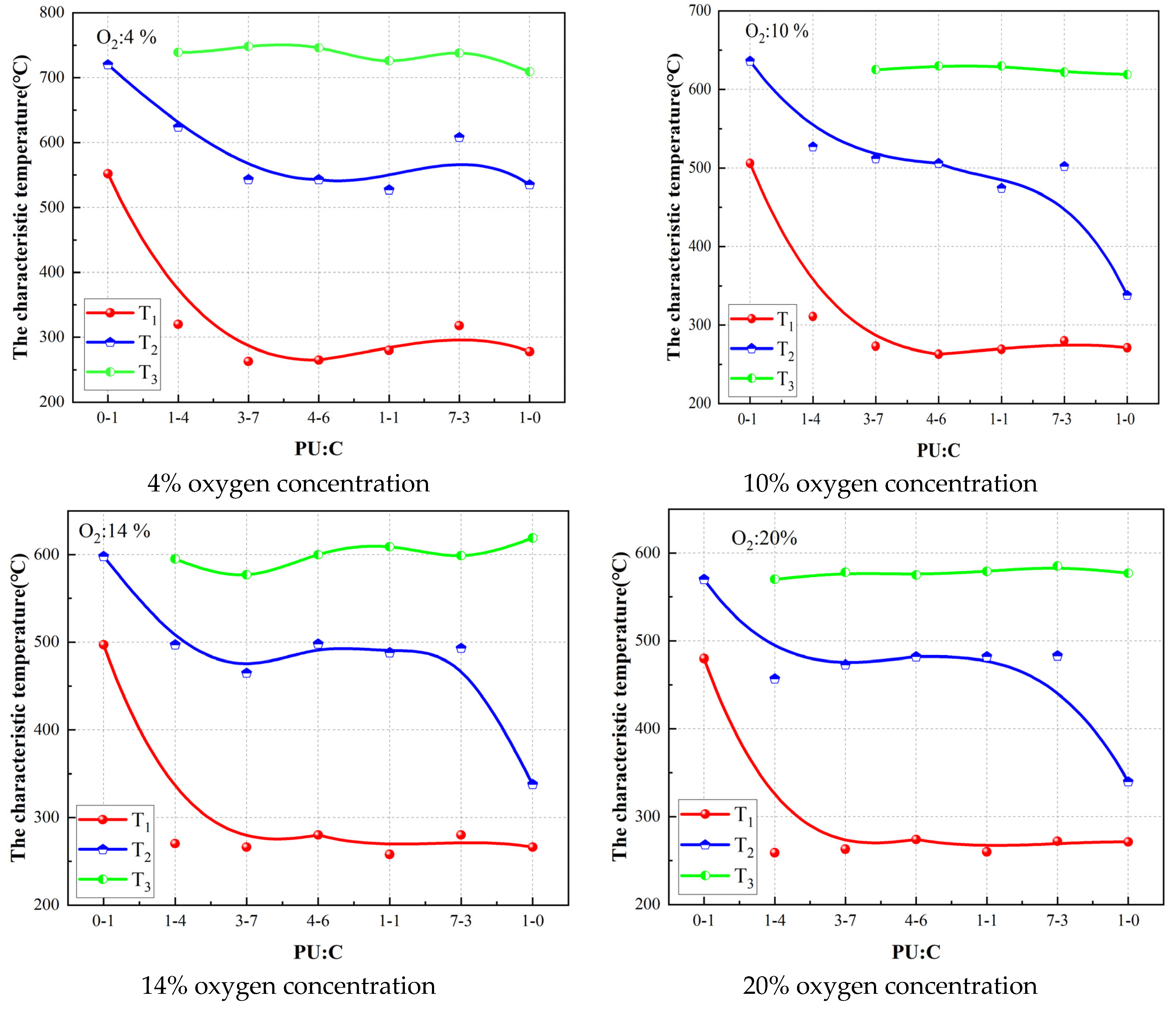
3.2. Effect of Oxygen Concentration on Mixed Combustion of Coal and Polyurethane
3.3. Kinetic Calculations
3.3.1. Kinetic Methods
3.3.2. Calculation of Activation Energy
Calculation of Activation Energy Using the Malek Method
3.4. Gas Product Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, M.; He, X.; Li, J.; Lv, D.; Huang, H.; Cao, X. Research on Application of Polyurethane Grouting Materials for Mining. Mater. Rep. 2014, 28, 96–100. [Google Scholar]
- Hou, K.; Wang, S.; Yao, S.; Niu, X.H.; Li, Z.; Wu, C.Q.; Feng, G.R. Research progress of modification for polyurethane grouting materials in coal mine. Coal Sci. Technol. 2022. Available online: http://kns.cnki.net/kcms/detail/11.2402.TD.20220816.1427.002.html (accessed on 29 September 2022).
- Xu, Y. Causes and Control of Disaster by the Heat from Polyurethane Materials Used in Mine; Graduate School of China, Coal Research Institute: Beijing, China, 2019. [Google Scholar]
- Xu, Y.; Du, Y.F.; Du, B. Experiment to Research the Mechanism of Underground Fire due to Polyurethane for Mine. Min. Saf. Environ. Prot. 2019, 46, 29–33. [Google Scholar]
- Onifade, M.; Genc, B.; Bada, S. Spontaneous combustion liability between coal seams: A thermogravimetric study. Int. J. Min. Sci. Technol. 2020, 30, 691–698. [Google Scholar] [CrossRef]
- Wen, H.; Guo, J.; Jin, Y.F.; Wang, K.; Zhang, Y.T.; Zheng, X.Z. Experimental study on the influence of different oxygen concentrations on coal spontaneous combustion characteristic parameters. Int. J. Oil Gas Coal Technol. 2017, 16, 187–202. [Google Scholar] [CrossRef]
- Zhang, D.; Cen, X.; Wang, W.; Deng, J.; Wen, H.; Xiao, Y.; Shu, C.-M. The graded warning method of coal spontaneous combustion in Tangjiahui Mine. Fuel 2021, 288, 119635. [Google Scholar] [CrossRef]
- Yutao, Z.; Yuanbo, Z.; Yaqing, L.; Xueqiang, S.; Yujie, Z. Heat effects and kinetics of coal spontaneous combustion at various oxygen contents. Energy 2021, 234, 121299. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Chen, X.; Fan, C.; Wang, P.; Hu, L. Thermodynamic Characteristics of Oxidation and Combustion of Coal under Lean-Oxygen Conditions. ACS Omega 2021, 6, 17255–17266. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Xu, J.C.; Chen, X.K. Perspectives on spontaneous combustion mechanism and prediction theory of coal. J. Liaoning Tech. Univ. 2003, 22, 455–459. [Google Scholar]
- Yang, Y.; Deng, J.; Zhang, H.N.; Wang, W.F. Oxidation characteristics of coal by STA-FTIR experiment. J. China Coal Soc. 2018, 43, 1031–1041. [Google Scholar]
- Jia, X.; Wu, J.; Lian, C.; Rao, J. Assessment of coal spontaneous combustion index gas under different oxygen concentration environment: An experimental study. Environ. Sci. Pollut. Res. Int. 2022. [Google Scholar] [CrossRef]
- Qi, X.; Li, Q.; Zhang, H.; Xin, H. Thermodynamic characteristics of coal reaction under low oxygen concentration conditions. J. Energy Inst. 2017, 90, 544–555. [Google Scholar] [CrossRef]
- Jiang, Z.; Yuan, K.J.; Li, S.F.; Chow, W.K. Study of FTIR Spectra and Thermal Analysis of Polyurethane. Spectrosc. Spectr. Anal. 2006, 2006, 624–628. [Google Scholar]
- Yuan, K.J.; Jiang, Y.; Li, Y.F.; Chou, Y.J. The Thermal Degradation of Polyurethane. Polym. Mater. Sci. Eng. 2005, 2005, 24–28. [Google Scholar]
- Lei, Y.; Liang, D. Polyurethane Flexible Foam Materials and Yinran Properties; Sun Yat-Sen University Press: Guang Zhou, China, 2013. [Google Scholar]
- Singh, H.; Jain, A.K. Ignition, combustion, toxicity, and fire retardancy of polyurethane foams: A comprehensive review. J. Appl. Polym. Sci. 2009, 111, 1115–1143. [Google Scholar] [CrossRef]
- Guo, P.; Tang, Y.; Wang, H.; Hu, S.; Zhou, J. Study on kinetics features of coal spontaneous combustion influenced by polyurethane. China Saf. Sci. J. 2020, 30, 68–73. [Google Scholar]
- Wang, H.; Tian, Y.; Chen, X.; Li, J. Kinetic and Synergetic Effect Analysis of the Co-Combustion of Coal Blended with Polyurethane Materials. ACS Omega 2020, 5, 26005–26014. [Google Scholar] [CrossRef]
- Wang, H.; Tian, Y.; Li, J.; Chen, X. Experimental study on thermal effect and gas release laws of coal-polyurethane cooperative spontaneous combustion. Sci. Rep. 2021, 11, 1994. [Google Scholar] [CrossRef]
- Miao, G.; Li, Z.; Meng, Q.; Li, J.; Yang, Y. Experimental research on the emission of higher molecular weight gases during coal oxidation. Fuel 2021, 300, 120906. [Google Scholar] [CrossRef]
- Liu, Z.L. Research on reaction temperature and gas release of large samples of polyurethane based reinforcement materials. China Energy Environ. Prot. 2021, 43, 156–160. [Google Scholar]
- Qi, Y.; Wang, W.; Qi, Q.; Ning, Z.; Yao, Y. Distribution of spontaneous combustion three zones and optimization of nitrogen injection location in the goaf of a fully mechanized top coal caving face. PLoS ONE 2021, 16, e0256911. [Google Scholar] [CrossRef]
- Liang, Y.T.; Luo, H.Z.; Zhou, X.Q. Coal Mine Fire Prevention Rules; Emergency Management Press: Beijing, China, 2021. [Google Scholar]
- Li, L. A Quantum Chemical Study on Dissociation Behaviour of Typical Bonds and Molecular Structural of Coal; Dalian University of Technology: Dalian, China, 2016. [Google Scholar]
- Yang, Y. Mechanism and Performance of Inhibitor Based on Oxidation Characteristic of the Spontaneous Combustion of Coal; Xi’an University of Science and Technology: Xi’an, China, 2015. [Google Scholar]
- Jiang, L. Pyrolysis Kinetics and Flame Spread Characteristics Studies of Typical Polymeric; University of Science and Technology of China: Hefei, China, 2017. [Google Scholar]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Hu, R.Z.; Gao, S.L.; Zhao, F.Q.; Shi, Q.Z.; Zhang, T.L.; Zhang, J.J. Thermal Analysis Kinetics; Science Press: Beijing, China, 2008. [Google Scholar]
- Zhang, Y.N. Application of the Thermogravimetric Analysis in the Research of the Oxidation of Coal; Xi’an University of Science and Technology: Xi’an, China, 2004. [Google Scholar]
- Scaccia, S. TG–FTIR and kinetics of devolatilization of Sulcis coal. J. Anal. Appl. Pyrolysis 2013, 104, 95–102. [Google Scholar] [CrossRef]
- Chen, X.R. Research on Quantitative Analysis of Coal Pyrolysis Products Using TG-FTIR-MS Technique; Zhejiang University: Hangzhou, China, 2016. [Google Scholar]
- Chen, L.H.; Chen, X.; Wu, Y.Y.; Zhou, H.; Cen, K.F.J. Quantitative analysis of gaseous products evolved by coal combustion using TG-FTIR-MS. J. Zhejiang Univ. Eng. Sci. 2016, 50, 961–969. [Google Scholar]

| No. | Sample | Proximate Analysis | Elemental Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mad (%) | Vad (%) | Aad (%) | FCad (%) | N (%) | C (%) | H (%) | S (%) | O (%) | ||
| 1 | Coal | 0.57 | 16.27 | 4.38 | 78.77 | 1.37 | 87.70 | 5.68 | 1.35 | 3.902 |
| 2 | Polyurethane | 3.66 | 88.72 | 1.33 | 6.29 | 6.32 | 60.50 | 6.03 | 0.00 | 27.15 |
| No. | Sample | PU:C | Proportion of Polyurethane | Heating Rate (℃/min) | Oxygen Concentration |
|---|---|---|---|---|---|
| 1 | C | 0:1 | 0% | 5, 10, 15 | 4%, 10%, 14%, 20% |
| 2 | PU:C | 1:4 | 20% | 5, 10, 15 | 4%, 10%, 14%, 20% |
| 3 | PU:C | 3:7 | 30% | 5, 10, 15 | 4%, 10%, 14%, 20% |
| 4 | PU:C | 4:6 | 40% | 5. 10, 15 | 4%, 10%, 14%, 20% |
| 5 | PU:C | 1:1 | 50% | 5, 10, 15 | 4%, 10%, 14%, 20% |
| 6 | PU:C | 7:3 | 70% | 5, 10, 15 | 4%, 10%, 14%, 20% |
| 7 | PU | 1:0 | 100% | 5, 10, 15 | 4%, 10%, 14%, 20% |
| PU:C | Activation Energy (KJ/mol) | |||
|---|---|---|---|---|
| Oxygen Concentration | ||||
| 4% | 10% | 14% | 20% | |
| 0:1 | E1 = 27.676 E2 = 246.634 E3 = 657.732 | E1 = 24.214 E2 = 298.722 E3 = 445.098 | E1 = 20.045 E2 = 371.644 E3 = 283.214 | E1 = 36.341 E2 = 403.212 E3 = 229.108 |
| 1:4 | E1 = 15.839 E2 = 17.388 E3 = 193.125 | E1 = 31.318 E2 = 10.937 E3 = 260.263 | E1 = 47.202 E2 = 82.261 E3 = 361.759 E4 = 316.655 | E1 = 52.959 E2 = 92.800 E3 = 334.988 E4 = 245.138 |
| 3:7 | E1 = 40.917 E2 = 68.948 E3 = 103.804 E4 = 264.760 | E1 = 44.364 E2 = 312.582 E3 = 336.077 E4 = 432.114 | E1 = 42.196 E2 = 104.898 E3 = 311.102 E4 = 329.891 | E1 = 47.289 E2 = 204.624 E3 = 362.956 E4 = 263.870 |
| 4:6 | E1 = 46.742 E2 = 70.672 E3 = 243.564 E4 = 503.642 | E1 = 47.148 E2 = 83.439 E3 = 312.806 E4 = 458.459 | E1 = 32.598 E2 = 61.189 E3 = 373.473 E4 = 321.453 | E1 = 39.581 E2 = 80.003 E3 = 383.042 E4 = 240.050 |
| 1:1 | E1 = 21.161 E2 = 63.125 E3 = 214.776 E4 = 530.458 | E1 = 43.541 E2 = 87.305 E3 = 272.981 E4 = 343.260 | E1 = 43.560 E2 = 89.209 E3 = 149.419 E4 = 34.355 | E1 = 27.007 E2 = 30.990 E3 = 143.533 E4 = 21.330 |
| 7:3 | E1 = 66.463 E2 = 44.873 E3 = 299.062 E4 = 907.59 | E1 = 12.643 E2 = 69.017 E3 = 318.451 E4 = 406.875 | E1 = 44.440 E2 = 66.862 E3 = 352.197 E4 = 296.294 | E1 = 42.736 E2 = 68.360 E3 = 350.16 E4 = 253.959 |
| 1:0 | E1 = 52.722 E2 = 75.071 E3 = 243.110 E4 = 407.590 | E1 = 44.388 E2 = 314.635 E3 = 63.083 E4 = 266.879 | E1 = 32.972 E2 = 71.750 E3 = 57.120 E4 = 258.823 | E1 = 40.869 E2 = 335.636 E3 = 105.080 E4 = 192.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Chen, X.; Wang, Z.; Xiang, L. Influence of Oxygen Concentration on Combustion Kinetics and Gas Products of a Polyurethane–Coal Mixture. Fire 2022, 5, 181. https://doi.org/10.3390/fire5060181
Wang H, Chen X, Wang Z, Xiang L. Influence of Oxygen Concentration on Combustion Kinetics and Gas Products of a Polyurethane–Coal Mixture. Fire. 2022; 5(6):181. https://doi.org/10.3390/fire5060181
Chicago/Turabian StyleWang, Haiyan, Xiao Chen, Zhuo Wang, and Linchuan Xiang. 2022. "Influence of Oxygen Concentration on Combustion Kinetics and Gas Products of a Polyurethane–Coal Mixture" Fire 5, no. 6: 181. https://doi.org/10.3390/fire5060181





