Investigation of Mineral Oil and CuO Mixed Synthetic Oil in Compression Ignition Engines: A Comparison of Physicochemical Attributes
Abstract
:1. Introduction
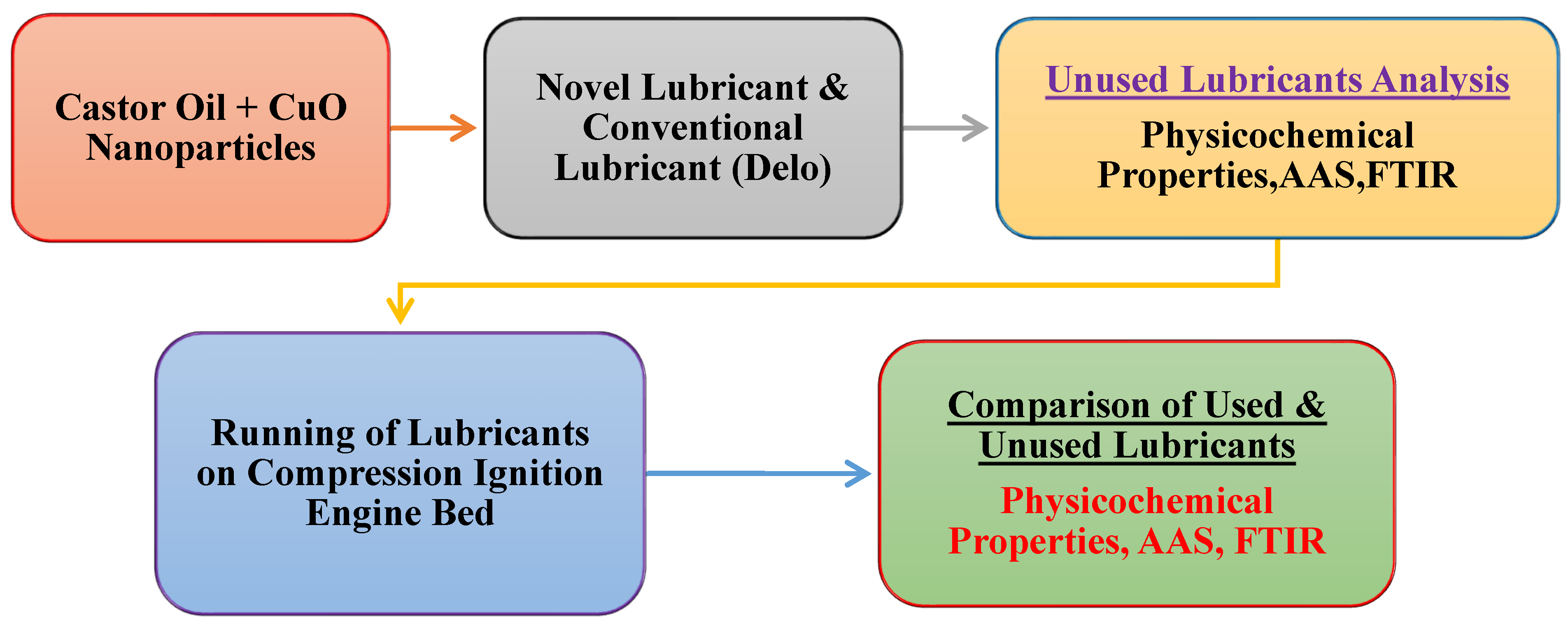
2. Materials and Methods
2.1. Lubricant Oil Synthesis
2.1.1. Base Oil
2.1.2. CuO Nanoadditives
2.1.3. Dispersant
2.2. Engine Specifications
2.3. Blend Making
2.4. Lubricant Testing
2.4.1. Fresh Novel and Conventional Lubricant Testing
2.4.2. Testing of the Used CuO-Based Novel and Delo-Based Conventional Lubricants
2.4.3. Comparison
3. Results
3.1. Physicochemical Property Analysis
3.1.1. Kinematic Viscosity
3.1.2. Pour Point (PP)
3.1.3. Flash Point (FP)
3.1.4. Total Base Number
3.1.5. Specific Gravity
3.1.6. Ash Content
3.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.3. Atomic Absorption Spectroscopy (AAS)
3.3.1. Chromium (Cr) AAS
3.3.2. Aluminum (Al) AAS
3.3.3. Calcium (Ca) AAS
3.3.4. Copper (Cu) AAS
3.3.5. Zinc (Zn) AAS
3.3.6. Iron (Fe) AAS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanchez, C.J. Tribological Characterization of Carbon Based Solid Lubricants; Texas A & M University: College Station, TX, USA, 2012. [Google Scholar]
- Woldegebriel Gebretsadik, D.; Hardell, J.; Efeoglu, I.; Prakash, B. Tribological Properties of Composite Multilayer Coatings. Tribol. Mater. Surf. Interfaces 2011, 5, 100–106. [Google Scholar] [CrossRef]
- Holmberg, K.; Andersson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Will, F. Fuel conservation and emission reduction through novel waste heat recovery for internal combustion engines. Fuel 2012, 102, 247–255. [Google Scholar] [CrossRef]
- Pinkus, O.; Wilcock, D.F. Strategy for Energy Conservation through Tribology; American Society of Mechanical Engineers: New York, NY, USA, 1977. [Google Scholar]
- Dake, L.; Russell, J.; Debrodt, D. A review of DOE ECUT tribology surveys. J. Tribol. 1986, 108, 497–501. [Google Scholar] [CrossRef]
- Ouyang, P.; Zhang, X. Regeneration of the waste lubricating oil based upon flyash adsorption/solvent extraction. Environ. Sci. Pollut. Res. 2020, 27, 37210–37217. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Masjuki, H.; Kalam, M.; Noor, F.; Farooq, M.; Ong, H.C.; Gul, M.; Soudagar, M.E.M.; Bashir, S.; Rizwanul Fattah, I. Effect of additivized biodiesel blends on diesel engine performance, emission, tribological characteristics, and lubricant tribology. Energies 2020, 13, 3375. [Google Scholar] [CrossRef]
- Singh, A.; Chauhan, P.; Mamatha, T. A review on tribological performance of lubricants with nanoparticles additives. Mater. Today Proc. 2020, 25, 586–591. [Google Scholar] [CrossRef]
- Vafadar, A.; Guzzomi, F.; Rassau, A.; Hayward, K. Advances in metal additive manufacturing: A review of common processes, industrial applications, and current challenges. Appl. Sci. 2021, 11, 1213. [Google Scholar] [CrossRef]
- Tung, S.C.; Totten, G.E. Automotive Lubricants and Testing; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Stachowiak, G.W.; Batchelor, A.W. Engineering Tribology; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Rashid, M.I.; Yaqoob, Z.; Mujtaba, M.; Fayaz, H.; Saleel, C.A. Developments in mineral carbonation for Carbon sequestration. Heliyon 2023, 9, e21796. [Google Scholar] [CrossRef]
- Tung, S.; Totten, G. Gear Oil Screen Testing with FZG Back-to-Back Rig. In Automotive Lubricants and Testing; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Shafi, W.K.; Raina, A.; Ul Haq, M.I. Friction and wear characteristics of vegetable oils using nanoparticles for sustainable lubrication. Tribol. Mater. Surf. Interfaces 2018, 12, 27–43. [Google Scholar] [CrossRef]
- Jeevan, T.; Jayaram, S.; Afzal, A.; Ashrith, H.; Soudagar, M.E.M.; Mujtaba, M. Machinability of AA6061 aluminum alloy and AISI 304L stainless steel using nonedible vegetable oils applied as minimum quantity lubrication. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 159. [Google Scholar] [CrossRef]
- Hamrock, B.J.; Schmid, S.R.; Jacobson, B.O. Fundamentals of Fluid Film Lubrication; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- McNutt, J. Development of biolubricants from vegetable oils via chemical modification. J. Ind. Eng. Chem. 2016, 36, 1–12. [Google Scholar] [CrossRef]
- Noori, A.A.S.; Hussein, H.A.; Namer, N.S. Influence of adding CuO and MoS2 nano-particles to castor oil and moulding oil on tribological properties. Proc. IOP Conf. Ser. Mater. Sci. Eng. 2019, 518, 032040. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Industrial development and applications of plant oils and their biobased oleochemicals. Arab. J. Chem. 2012, 5, 135–145. [Google Scholar] [CrossRef]
- Syahir, A.; Zulkifli, N.; Masjuki, H.; Kalam, M.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.; Harith, M.H. A review on bio-based lubricants and their applications. J. Clean. Prod. 2017, 168, 997–1016. [Google Scholar] [CrossRef]
- Singh, A.K. Castor oil-based lubricant reduces smoke emission in two-stroke engines. Ind. Crops Prod. 2011, 33, 287–295. [Google Scholar] [CrossRef]
- Bongfa, B.; Peter, A.A.; Barnabas, A.; Adeotic, M.O. Comparison of lubricant properties of castor oil and commercial engine oil. J. Tribol. 2015, 5, 1–11. [Google Scholar]
- Gulzar, M.; Masjuki, H.; Varman, M.; Kalam, M.; Mufti, R.; Zulkifli, N.; Yunus, R.; Zahid, R. Improving the AW/EP ability of chemically modified palm oil by adding CuO and MoS2 nanoparticles. Tribol. Int. 2015, 88, 271–279. [Google Scholar] [CrossRef]
- Akhtar, N.; Adnan, Q.; Ahmad, M.; Mehmood, A.; Farzana, K. Rheological studies and characterization of different oils. J. Chem. Soc. Pak. 2009, 31, 201–206. [Google Scholar]
- Imran, S.; Gul, M.; Kalam, M.; Zulkifli, N.; Mujtaba, M.; Yusoff, M.; Awang, M. Effect of various nanoparticle biodiesel blends on thermal efficiency and exhaust pollutants. Int. J. Energy Environ. Eng. 2023, 14, 937–948. [Google Scholar] [CrossRef]
- Abdel-Rehim, A.A.; Akl, S.; Elsoudy, S. Investigation of the tribological behavior of mineral lubricant using copper oxide nano additives. Lubricants 2021, 9, 16. [Google Scholar] [CrossRef]
- Namer, N.S.; Nama, S.A.; Mezher, M.T. The influence of nano particles additive on tribological properties of aa2024-t4 coated with tin or sin thin films. J. Mech. Eng. Res. Dev. 2019, 42, 30–34. [Google Scholar] [CrossRef]
- Raina, A.; Anand, A. Tribological investigation of diamond nanoparticles for steel/steel contacts in boundary lubrication regime. Appl. Nanosci. 2017, 7, 371–388. [Google Scholar] [CrossRef]
- Najan, A.; Navthar, R.; Gitay, M. Experimental Investigation of tribological properties using nanoparticles as modifiers in lubricating oil. Int. Res. J. Eng. Technol. 2017, 4, 1125–1129. [Google Scholar]
- Popoola, C.A.; Ogundola, J.; Kamtu, P. Tribological properties of copper (II) oxide nanoparticle-enriched sandbox bio-lubricant. Glob. J. Eng. Technol. Adv. 2021, 8, 8–19. [Google Scholar] [CrossRef]
- Baskar, S.; Sriram, G.; Arumugam, S. Tribological analysis of a hydrodynamic journal bearing under the influence of synthetic and biolubricants. Tribol. Trans. 2017, 60, 428–436. [Google Scholar] [CrossRef]
- Sukkar, K.A.; Karamalluh, A.A.; Jaber, T.N. Rheological and thermal properties of lubricating oil enhanced by the effect of CuO and TiO2 nano-additives. Al-Khwarizmi Eng. J. 2019, 15, 24–33. [Google Scholar] [CrossRef]
- Fayad, M.A.; AL-Ogaidi, B.R.; Abood, M.K.; AL-Salihi, H.A. Influence of post-injection strategies and CeO2 nanoparticles additives in the C30D blends and diesel on engine performance, NOX emissions, and PM characteristics in diesel engine. Part. Sci. Technol. 2022, 40, 824–837. [Google Scholar] [CrossRef]
- Esfe, M.H.; Kamyab, M.H.; Ardeshiri, E.M.; Toghraie, D. Study of MWCNT (40%)–CuO (60%)/10W40 hybrid nanofluid for improving laboratory oil performance by laboratory method and statistical response surface methodology. Alex. Eng. J. 2023, 63, 115–125. [Google Scholar] [CrossRef]
- Al-Tabbakh, B.A.A.; Jaed, D.M.; Qubian, N.A.; Kareem, S. Preparation of CuO Nanoparticles for Improving Base Oil Properties. J. Pet. Res. Stud. 2022, 12, 191–205. [Google Scholar]
- Navada, M.K.; Rai, R.; Ganesha, A.; Patil, S. Synthesis and characterization of size controlled nano copper oxide structures for antioxidant study and as eco-friendly lubricant additive for bio-oils. Ceram. Int. 2023, 49, 10402–10410. [Google Scholar] [CrossRef]
- Kumar, D.S.; Garg, H.; Kumar, G. Tribological analysis of blended vegetable oils containing CuO nanoparticles as an additive. Mater. Today Proc. 2022, 51, 1259–1265. [Google Scholar] [CrossRef]
- Gupta, H.; Rai, S.K.; Satya Krishna, N.; Anand, G. The effect of copper oxide nanoparticle additives on the rheological and tribological properties of engine oil. J. Dispers. Sci. Technol. 2021, 42, 622–632. [Google Scholar] [CrossRef]
- Subedi, B.R.; Trital, H.M.; Rajbhandari, A. Characterization of CuO-Nanoadditive Blended Engine Oil. J. Inst. Sci. Technol. 2017, 22, 152–158. [Google Scholar] [CrossRef]
- Ghaednia, H.; Jackson, R.L.; Khodadadi, J.M. Experimental analysis of stable CuO nanoparticle enhanced lubricants. J. Exp. Nanosci. 2015, 10, 1–18. [Google Scholar] [CrossRef]
- Shah, A.K.; Vineesh, K.; Joy, M. Study of the lubricating properties of hybrid liquid paraffin with TiO2 and CuO as nano-additives for engine oil application. Proc. IOP Conf. Ser. Mater. Sci. Eng. 2019, 624, 012006. [Google Scholar] [CrossRef]
- Hassan, M.U.; Usman, M.; Bashir, R.; Naeem Shah, A.; Ijaz Malik, M.A.; Mujtaba, M.; Elkhatib, S.E.; Kalam, M.A. Tribological Analysis of Molybdenum Disulfide (MOS2) Additivated in the Castor and Mineral Oil Used in Diesel Engine. Sustainability 2022, 14, 10485. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Tabatabaei, M.; Aghbashlo, M.; Khanali, M.; Demirbas, A. A comprehensive review on the environmental impacts of diesel/biodiesel additives. Energy Convers. Manag. 2018, 174, 579–614. [Google Scholar] [CrossRef]
- Rastogi, P.; Kumar, N.; Sharma, A.; Vyas, D.; Gajbhiye, A. Sustainability of aluminium oxide nanoparticles blended mahua biodiesel to the direct injection diesel engine performance and emission analysis. Pollution 2020, 6, 25–33. [Google Scholar]
- D’Silva, R.; Vinoothan, K.; Binu, K.; Thirumaleshwara, B.; Raju, K. Effect of titanium dioxide and calcium carbonate nanoadditives on the performance and emission characteristics of CI engine. J. Mech. Eng. Autom. 2016, 6, 28–31. [Google Scholar]
- Mello, V.; Faria, E.; Alves, S.; Scandian, C. Enhancing Cuo nanolubricant performance using dispersing agents. Tribol. Int. 2020, 150, 106338. [Google Scholar] [CrossRef]
- Pisal, A.S.; Chavan, D. Experimental investigation of tribological properties of engine oil with CuO nanoparticles. Res. Mech. Eng. 2014, 2014, 49. [Google Scholar]
- Diegelmann, S.R.; Parks, B.W. TBN and Performance Booster. 2017. Available online: https://patentimages.storage.googleapis.com/26/f4/f2/b17895edad4930/US20170292083A1.pdf (accessed on 15 September 2023).
- Huang, Y.; Li, F.; Bao, G.; Wang, W.; Wang, H. Estimation of kinematic viscosity of biodiesel fuels from fatty acid methyl ester composition and temperature. J. Chem. Eng. Data 2020, 65, 2476–2485. [Google Scholar] [CrossRef]
- Curtis, M.; Aduse-Opoku, J.; Slaney, J.; Rangarajan, M.; Booth, V.; Cridland, J.; Shepherd, P. Characterization of an adherence and antigenic determinant of the ArgI protease of Porphyromonas gingivalis which is present on multiple gene products. Infect. Immun. 1996, 64, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, I. Some Methods for Determining the Viscosity Index of Hydraulic Oil. Indian J. Sci. Technol. 2023, 16, 254–258. [Google Scholar] [CrossRef]
- Jamil, M.K.; Akhtar, M.; Farooq, M.; Abbas, M.M.; Saad; Khuzaima, M.; Ahmad, K.; Kalam, M.A.; Abdelrahman, A. Analysis of the Impact of Propanol-Gasoline Blends on Lubricant Oil Degradation and Spark-Ignition Engine Characteristics. Energies 2022, 15, 5757. [Google Scholar] [CrossRef]
- Malik, M.A.I.; Kalam, M.; Mujtaba, M.; Almomani, F. A review of recent advances in the synthesis of environmentally friendly, sustainable, and nontoxic bio-lubricants: Recommendations for the future implementations. Environ. Technol. Innov. 2023, 32, 103366. [Google Scholar] [CrossRef]
- Kaminski, P. Experimental investigation into the effects of fuel dilution on the change in chemical properties of lubricating oil used in fuel injection pump of pielstick PA4 V185 marine diesel engine. Lubricants 2022, 10, 162. [Google Scholar] [CrossRef]
- Abdelkhalik, A.; Elsayed, H.; Hassan, M.; Nour, M.; Shehata, A.; Helmy, M. Using thermal analysis techniques for identifying the flash point temperatures of some lubricant and base oils. Egypt. J. Pet. 2018, 27, 131–136. [Google Scholar] [CrossRef]
- Mujtaba, M.; Kalam, M.; Masjuki, H.; Gul, M.; Soudagar, M.E.M.; Ong, H.C.; Ahmed, W.; Atabani, A.; Razzaq, L.; Yusoff, M. Comparative study of nanoparticles and alcoholic fuel additives-biodiesel-diesel blend for performance and emission improvements. Fuel 2020, 279, 118434. [Google Scholar] [CrossRef]
- Hettiarachchi, S.J.; Kellici, S.; Kershaw, M.; Bowen, J. Enhancing physicochemical properties of coconut oil for the application of engine lubrication. Tribol. Int. 2023, 190, 109060. [Google Scholar] [CrossRef]
- Niu, Y.; Pang, X.; Yue, S.; Shangguan, B.; Zhang, Y. The friction and wear behavior of laser textured surfaces in non-conformal contact under starved lubrication. Wear 2021, 476, 203723. [Google Scholar] [CrossRef]
- Abro, R.; Chen, X.; Harijan, K.; Dhakan, Z.A.; Ammar, M. A Comparative study of recycling of used engine oil using extraction by composite solvent, single solvent, and acid treatment methods. ISRN Chem. Eng. 2013, 2013, 952589. [Google Scholar] [CrossRef]
- Ijaz Malik, M.A.; Usman, M.; Akhtar, M.; Farooq, M.; Saleem Iqbal, H.M.; Irshad, M.; Shah, M.H. Response surface methodology application on lubricant oil degradation, performance, and emissions in SI engine: A novel optimization of alcoholic fuel blends. Sci. Prog. 2023, 106, 00368504221148342. [Google Scholar] [CrossRef] [PubMed]
- Wolak, A.; Molenda, J.; Zając, G.; Janocha, P. Identifying and modelling changes in chemical properties of engine oils by use of infrared spectroscopy. Measurement 2021, 186, 110141. [Google Scholar] [CrossRef]
- Patel, N.; Shadangi, K.P.; Kar, P.K. Characterization of waste engine oil derived pyrolytic char (WEOPC): SEM, EDX and FTIR analysis. Mater. Today Proc. 2021, 38, 2866–2870. [Google Scholar] [CrossRef]
- Abdul-Munaim, A.M.; Holland, T.; Sivakumar, P.; Watson, D.G. Absorption wavebands for discriminating oxidation time of engine oil as detected by FT-IR spectroscopy. Lubricants 2019, 7, 24. [Google Scholar] [CrossRef]
- Liu, Y.; Bao, K.; Wang, Q.; Zio, E. Application of FTIR method to monitor the service condition of used diesel engine lubricant oil. In Proceedings of the 2019 4th International Conference on System Reliability and Safety (ICSRS), Rome, Italy, 20–22 November 2019; pp. 175–180. [Google Scholar]
- van de Voort, F.R. FTIR Condition Monitoring of In-Service Lubricants: Analytical Role and Quantitative Evolution. Tribol. Online 2022, 17, 144–161. [Google Scholar] [CrossRef]
- Dos Santos, H.S.; De Jesus, A.; Laroque, D.O.; Piatnicki, C.M.; Da Silva, M.M. Multi-element determination of trace elements in B7-diesel Oil by high-resolution continuum source flame atomic absorption spectrometry. Braz. J. Anal. Chem. 2021, 8, 59–70. [Google Scholar] [CrossRef]
- Kurre, S.; Pandey, S.; Khatri, N.; Bhurat, S.; Kumawat, S.; Saxena, S.; Kumar, S. Study of lubricating oil degradation of CI engine fueled with diesel-ethanol blend. Tribol. Ind. 2021, 43, 222. [Google Scholar] [CrossRef]
- Manjusha, R.; Shekhar, R.; Kumar, S.J. Ultrasound-assisted extraction of Pb, Cd, Cr, Mn, Fe, Cu, Zn from edible oils with tetramethylammonium hydroxide and EDTA followed by determination using graphite furnace atomic absorption spectrometer. Food Chem. 2019, 294, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Widowati, A.; Wakid, M. Study of piston from the natural science perspective. AIP Conf. Proc. 2023, 2671, 020013. [Google Scholar]
- Usman, M.; Khan, T.; Riaz, F.; Ijaz Malik, M.A.; Amjad, M.T.; Shah, M.H.; Ashraf, W.M.; Krzywanski, J.; Nowak, W. Acetone–Gasoline Blend as an Alternative Fuel in SI Engines: A Novel Comparison of Performance, Emission, and Lube Oil Degradation. ACS Omega 2023, 8, 11267–11280. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, M. Spectroscopic study and analysis of the content of residue elements in Marinol RG 1240 oil after working in various types of engines. Zesz. Nauk. Akad. Morskiej Gdyni 2017, 131–140. [Google Scholar]
- Usman, M.; Malik, M.A.I.; Ranjha, Q.A.; Arif, W.; Jamil, M.K.; Miran, S.; Siddiqui, S. Experimental assessment of performance, emission and lube oil deterioration using gasoline and LPG for a sustainable environment. Case Stud. Therm. Eng. 2023, 49, 103300. [Google Scholar] [CrossRef]






| Reference | Lubricant Oil Synthesis Composition | Lubricant Oil Testing | ||
|---|---|---|---|---|
| Physicochemical Testing | FTIR Testing | AAS Testing | ||
| Navada et al. [37] | CuO + pongamia oil | ✓ | ✓ | ✕ |
| Kumar et al. [38] | CuO + sunflower oil | ✕ | ✓ | ✕ |
| Gupta et al. [39] | CuO + 5W30 engine oil | ✓ | ✓ | ✕ |
| Subedi et al. [40] | CuO + 20W50 engine oil | ✓ | ✓ | ✕ |
| Ghaednia et al. [41] | CuO + sodium oleate | ✓ | ✕ | ✕ |
| Shah et al. [42] | CuO + hybrid paraffin oil | ✕ | ✕ | ✕ |
| Tabbakh et al. [36] | CuO + 60 stock (base oil) | ✓ | ✓ | ✕ |
| Current study | CuO + castor oil | ✓ | ✓ | ✓ |
| Properties | Units | Castor Oil |
|---|---|---|
| Cloud point | °C | 14.6 |
| Density | kg/m3 | 957.9 |
| Pour point | °C | −25 |
| Fire point | °C | 340 |
| Viscosity | mpa.s | 684.36 |
| Flash point | °C | 290 |
| Specific gravity Calorific value Cetane rating Oxidation stability | - MJ/kg - H | 0.9737 38.34 43.7 4.4 |
| Suppliers Information | CuO |
|---|---|
| Company | Sigma Aldrich (St. Louis, MO, USA) |
| Grade standard | Electron grade |
| Purity | 99% |
| Average particle size (nm) | 60 |
| Appearance | Black powder |
| Description | Specifications |
|---|---|
| Cylinders | 1 |
| Bore | 78 mm |
| Displacement | 315 cm3 |
| Stoke | 60 mm |
| Oil consumption | 0.0030 L |
| Oil sump capacity | 1.2 L |
| Dynamometer attached | Hydraulic dynamometer |
| Cooling System | Air-cooled |
| Mode of injection | Direct Injection |
| Compression ratio | 20.3:1 |
| Dry weight | 33 kg |
| Max. torque @ rpm | 15 Nm @ 2400 |
| Recommended battery | 12/44 (V/Ah) |
| Parameters | Description |
|---|---|
| Lubricant oil | Mineral oil and synthetic oil |
| Deterioration time | A total of 100 h of engine operations |
| Physicochemical testing | Kinematic viscosity at 100 and 40 °C, ash content, flash point, pour point, total base number, and specific gravity |
| FTIR testing | O-H, C-H, C=O, C-N and C-Br functional group |
| AAS testing | Iron, copper, chromium, zinc, calcium and aluminum |
| Mineral Oil (MO) | Measuring Units | Test Standards | Properties |
|---|---|---|---|
| TBN | mg KOH/g | D2896 [49] | 10.2 |
| (KV)40°C | mm2/s | D445 [50] | 115 |
| (KV)100°C | mm2/s | D445 [50] | 15.1 |
| Sulfated ash | % by mass | D784 [51] | 1.4 |
| Viscosity index | - | D2270 [52] | 137 |
| AAS of Distinct Elements | ||||
|---|---|---|---|---|
| Sr. | Element Types | Lubricant Types | Mean Absorbance | |
| Pre-Operation | Post-Operation | |||
| 1 | Chromium (Cr) | Mineral | 3.32 | 3.45 |
| Novel | 4.46 | 5.60 | ||
| 2 | Iron (Fe) | Mineral | 2.13 | 2.10 |
| Novel | 2.20 | 2.24 | ||
| 3 | Copper (Cu) | Mineral | 3.49 | 4.29 |
| Novel | 4.39 | 4.75 | ||
| 4 | Zinc (Zn) | Mineral | 5.61 | 3.81 |
| Novel | 3.1 | 2.77 | ||
| 5 | Aluminium (Al) | Mineral | 2.39 | 2.41 |
| Novel | 2.42 | 2.47 | ||
| 6 | Calcium (Ca) | Mineral | 2.48 | 2.37 |
| Novel | 6.51 | 5.07 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasir, A.S.; Usman, M.; Malik, M.A.I.; Shah, A.N.; Jafry, A.T.; Saleem, M.W.; Abbas, N.; Sajjad, U.; Karim, M.R.; Kalam, M.A. Investigation of Mineral Oil and CuO Mixed Synthetic Oil in Compression Ignition Engines: A Comparison of Physicochemical Attributes. Fire 2023, 6, 467. https://doi.org/10.3390/fire6120467
Nasir AS, Usman M, Malik MAI, Shah AN, Jafry AT, Saleem MW, Abbas N, Sajjad U, Karim MR, Kalam MA. Investigation of Mineral Oil and CuO Mixed Synthetic Oil in Compression Ignition Engines: A Comparison of Physicochemical Attributes. Fire. 2023; 6(12):467. https://doi.org/10.3390/fire6120467
Chicago/Turabian StyleNasir, Aamir Sajjad, Muhammad Usman, Muhammad Ali Ijaz Malik, Asad Naeem Shah, Ali Turab Jafry, Muhammad Wajid Saleem, Naseem Abbas, Uzair Sajjad, Mohammad Rezaul Karim, and Md Abul Kalam. 2023. "Investigation of Mineral Oil and CuO Mixed Synthetic Oil in Compression Ignition Engines: A Comparison of Physicochemical Attributes" Fire 6, no. 12: 467. https://doi.org/10.3390/fire6120467
APA StyleNasir, A. S., Usman, M., Malik, M. A. I., Shah, A. N., Jafry, A. T., Saleem, M. W., Abbas, N., Sajjad, U., Karim, M. R., & Kalam, M. A. (2023). Investigation of Mineral Oil and CuO Mixed Synthetic Oil in Compression Ignition Engines: A Comparison of Physicochemical Attributes. Fire, 6(12), 467. https://doi.org/10.3390/fire6120467














