Fire Retardance Methods and Materials for Phase Change Materials: Performance, Integration Methods, and Applications—A Literature Review
Abstract
1. Introduction
- I.
- Main class criteria:
- I.a.
- The temperature range over which the phase transition process occurs;
- I.b.
- Enthalpy variation during phase transition.
- II.
- Secondary class criteria:
- II.a.
- Chemical compatibility with the rest of the system components;
- II.b.
- Constraints related to mass/volume or storage density;
- II.c.
- Supercooling;
- II.d.
- Thermo-physical properties stability;
- II.e.
- Flammability;
- II.f.
- Cost;
- II.g.
- Lifecycle considerations and environmental impact.
2. Evaluation of PCMs Flammability
- The Critical Heat Flux (CHF) [43]—the lowest value of the thermal load per unit area at which a combustion reaction can be initiated, either spontaneous or piloted.
- The Heat Release Rate (HRR) is a quantitative metric of the rate at which the fire releases thermal energy [46]. This parameter is more important in assessing fire propagation dynamics. HRR is measured in flux per unit surface area and it is determined following ISO 5660-1.
2.1. UL 94 Horizontal Flammability Test [47]
- if the flame advances at a speed of less than 40 mm/min for sample of thickness ranging between 3 and 13 mm;
- if the rate flame advances at a speed of less than 75 mm/min for samples thinner than 3 mm;
- if the flame is extinguished before the first mark.
2.2. UL-94 Vertical Burning Test [48]
2.3. Limiting Oxygen Index (LOI) [49]
2.4. Cone calorimetry (ASTM E1354 [51], ISO 5660 [52])
3. Fire Retardants. Classification and Integration into PCM-Based Systems
- Creation of a heat sink that absorbs the heat generated by the combustion reaction. This can be achieved by using a substance that degrades in an endothermic process, releasing non-flammable volatile products, which isolate the flame and prevent the access of the oxygen. Aluminum or magnesium hydroxide are the typical compounds that achieve this mechanism.
- Increasing the loss rate of thermal energy and mass from the burning compound surface by melt dripping. Halogenated compounds with free radical initiators can be used for this purpose.
- Flame degradation by using chemical species (hydrogen halides or metal halides) that consume the main promoters of the thermo-oxidation in the flame (H and OH radicals).
- Suppressing the heat and mass transfer at the phase interface by the creation of a superficial charred layer on the surface of the burning compound with insulating properties. Intumescent char can be used for this purpose.
- Suppressing the thermal volatilization mechanism of the organic compound (or at least its rate) in order to reduce the flammability of the volatile products.
- Flame quencher, which is the most common FR, e.g., halogenated alkanes.
- FRs that act by local heat absorption (chemical compounds such as magnesium or aluminum hydroxide, which decompose endothermically preventing the heat from reaching the combustible material).
- Intumescent Flame Retardants, which operate by creating a high-volume char layer that prevents the exposure of the underlying substance to the source of ignition. APP, PER, and EG are the most commonly used IFRs (De Silva et al. [58]).
- Synergist FRs, of which two distinct types exist:
- ➢
- Chemical compounds that do not have flame retardancy properties if isolated but work with other FRs to improve the flame retardancy properties. For example, antimony oxide has a synergetic effect for halogenated alkanes.
- ➢
- Synergist systems, consisting of two or more FRs that work together to achieve a flame retardancy effect stronger than the effect of each FR alone. MMT clay is an FR that works together with heat absorbers and IFRs, Cai et al. [59]. APP used together with EG improves the fire retardancy effect, Cai et al. [60].
- ➢
- The acid source decomposes at high temperature generating an inorganic acid with low pH; the acid causes the dehydration of the carbonizing agent, the result being the apparition of a carbonaceous layer. The properties of the carbon layer depend on the number of carbon atoms; the reactive hydroxyl groups (OH) determine the intensity of the dehydration reaction.
- ➢
- The blowing agent undergoes decomposition and releases flammable gases; these can expand the carbonaceous layer converting it into an inflated multicellular layer. For this mechanism to be effective, the blowing agent must undergo decomposition during the carbonizing source dehydration reaction in order to trigger the expansion of the carbonaceous layer. Once this layer is formed, it insulates the underlying material from the thermal energy source and oxygen, achieving the flame retardancy effect.
- A.
- FR integration in the PCM mass:
- A.1.
- FRs integration for bulk PCMs;
- A.2.
- FRs for shape-stabilized PCMs;
- A.3.
- FRs for encapsulated PCMs.
- B.
- FR surface coatings.
- C.
- FR achieved through modification of the chemical bonds.
3.1. Flame Retardants for Bulk PCMs
3.2. Flame Retardants for Shape-Stabilized PCMs
- Horizontal burning test using samples with the dimensions 75 mm 10 mm 3 mm. Samples were clamped in a horizontal position at one end while the source of fire was applied at the other end for 10 s. The strip underwent combustion until the flame hit a mark at 5 cm distance from the clamps. The burning time was recorded and the sample mass before and after burning was also recorded.
- Thermal stability test, where 10 mg samples were heated from 40 °C to 600 °C at a constant heating rate of 20 °C/min under an air flow of 25 mL/min.
- Cone calorimetry, where samples with the dimensions 100 mm 100 mm 3 mm were subject to a heat flux of 35 kW/m2.
3.3. Flame Retardants in Microencapsulated PCMs
3.4. Flame Retardant Surface Coatings
3.5. Flame Retardancy Achieved through Modification of the Chemical Bonds
- (i)
- transesterification reaction of
- a.
- diethyl phosphite;
- b.
- 1-tetradecanol;
- c.
- 1-hexadecanol;
- d.
- 1-octadecanol.
- (ii)
- ring-opening reaction followed by hydrolytic condensation of 2, 3-epoxypropoxy propyl trimethoxysilane. The mixture consisting of the previously synthesized phosphite of higher alcohol (PHA) and KH-560 (0.11 mol) was stirred at a high speed for 6 h at the temperature 140 °C under a protective nitrogen atmosphere. Then, the liquid was transferred to an open dish, and distilled water was added to the mixture. In the last step of the process, a white solid powder was obtained after heating at 80 °C for 48 h. The synthesized PCMs were denoted as follows: sample based on 1-tetradecanol: PCM14; sample based on 1-hexadecanol: PCM16; sample based on 1-octadecanol: PCM18. The whole synthesis process is illustrated schematically in Figure 27.
4. Applications
5. Conclusions
- PCM containment method;
- PCM nature;
- PCM application;
- Flame retardancy performance required by the application;
- Economic, environmental, and lifecycle considerations.
- The FR stability and flame retardancy capability in the long-term. This issue deserves special attention since the degradation of the FR capability cannot be assessed once the PCM application is in service. Case studies investigated after long-term operation and many thermal cycles could provide key information in this regard. Two separate points must be included: long-term degradation/preservation of the fire-retardance capability; long-term degradation/preservation of the composite PCM thermo-physical properties.
- Degradation/alteration of the thermo-physical parameters relevant to the purpose of the PCM—heat storage. Any additive will definitely result in a decrease in the latent heat, since a PCM fraction is replaced by an equivalent amount of another material with a lower specific heat. Few studies (e.g., [113]) were identified discussing quantitatively the effect of FR additives on the MP, LH, and thermal conductivity. The studies discussing such issues converge in the conclusion that FR additives result in a degradation of the heat storage capacity, and a small alteration in the melting point.
- Scaling-up. Although many different laboratory-scale preparation methods were described in the literature, no discussion was identified on the upscaling of the processes. Either the integration into existing preparation processes or the development of new processes with FR integration phases would provide an interesting and useful discussion.
- The majority of the flammability assessments employ standard tests, such as LOI, vertical/horizontal burning tests, and most frequently the cone calorimetry test. However, it would be more interesting to evaluate quantitatively the flammability characteristics of the PCM integrated in the applications. It can be argued that the flammability test results could be more precise, reliable, and statistically significant if the tests were to be repeated under identical conditions, multiple times. No reports were identified mentioning multiple repetitions or statistical error margins for the results reported.
- Lifecycle analysis and circular economy integration are issues discussed scarcely in the analyzed references. The toxicity hazard is another matter of concern that is not addressed properly.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| APP | Ammonium polyphosphate | PA | Phosphoric Acid |
| BPO | Dibenzoyl peroxide | PC | Phosphorus Cellulose |
| BPOD | Phenyl phosphonic dichloride | PEG | Polyethylene Glycol |
| BTMS | Battery Thermal Management System | PEO | Polyethylene oxide |
| CA | Capric Acid | PEPA | Pentaerythritol phosphate |
| DSC | Differential Scanning Calorimetry | PER | Pentaerythritol |
| EDS | Energy Dispersive Spectroscope | PHRR | Peak Heat Release Rate (cone calorimetry parameter) |
| EG | Expanded Graphite | PM | Palmitic Acid |
| EGP | Expanded Graphite Plates | PMMA | Poly- Methyl Methacrylate |
| EP | Epoxy Resin | PN | Phosphorus-Nitrogen |
| FR | Flame Retardant | PP | Polypropylene |
| FP | Flash Point | PU | Polyurethane |
| FT-IR | Infrared Fourier-Transform Spectroscopy | RP | Red Phosphorus |
| LH | Latent Heat | SBS | Styrene-butadiene-styrene |
| HDPE | High Density Polyethylene | SS | Shape Stabilization |
| HNT | Halloysite Nanotubes | TBBP-A | Tetra bromo-bisphenol-A |
| HRR | Heat Release Rate | TC | Thermal Conductivity |
| IFR | Intumescent Flame Retardant | TCE | Thermal Conductivity Enhancer |
| IR | Infrared | TEM | Transmission Electron Microscope |
| MA | Myristic Acid | TGA | Thermo-gravimetric analysis |
| ML | Melamine | THR | Total Heat Release (cone calorimetry parameter) |
| MLR | Mass Loss Rate (cone calorimetry parameter) | TPH | Triphenyl phosphate |
| MF | Melamine Foam | TPP | Thermal Protective Performance |
| MMT | Montmorillonite | TR | Thermal runaway |
| MSDS | Material Safety Datasheet | TSP | Total Smoke Production (cone calorimetry parameter) |
| OMMT | Organo-montmorillonite | TTI | Time to Ignition (cone calorimetry parameter) |
References
- Wang, F.; Pang, D.; Liu, X.; Liu, M.; Du, W.; Zhang, Y.; Cheng, X. Progress in application of phase-change materials to cooling clothing. J. Energy Storage 2023, 60, 106606. [Google Scholar] [CrossRef]
- Mehrizi, A.A.; Karimi-Maleh, H.; Naddafi, M.; Karimi, F. Application of bio-based phase change materials for effective heat management. J. Energy Storage 2023, 61, 106859. [Google Scholar] [CrossRef]
- Meng, B.; Zhang, X.; Hua, W.; Liu, L.; Ma, K. Development and application of phase change material in fresh e-commerce cold chain logistics: A review. J. Energy Storage 2022, 55, 105373. [Google Scholar] [CrossRef]
- Sharshir, S.W.; Joseph, A.; Elsharkawy, M.; Hamada, M.A.; Kandeal, A.; Elkadeem, M.R.; Thakur, A.K.; Ma, Y.; Moustapha, M.E.; Rashad, M.; et al. Thermal energy storage using phase change materials in building applications: A review of the recent development. Energy Build. 2023, 285, 112908. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, J.; Pan, W. A review of battery thermal management systems about heat pipe and phase change materials. J. Energy Storage 2023, 62, 106827. [Google Scholar] [CrossRef]
- Gu, H.; Chen, Y.; Yao, X.; Huang, L.; Zou, D. Review on heat pump (HP) coupled with phase change material (PCM) for thermal energy storage. Chem. Eng. J. 2023, 455, 140701. [Google Scholar] [CrossRef]
- Sikiru, S.; Oladosu, T.L.; Amosa, T.I.; Kolawole, S.Y.; Soleimani, H. Recent advances and impact of phase change materials on solar energy: A comprehensive review. J. Energy Storage 2022, 53, 105200. [Google Scholar] [CrossRef]
- Chinnasamy, V.; Heo, J.; Jung, S.; Lee, H.; Cho, H. Shape stabilized phase change materials based on different support structures for thermal energy storage applications–A review. Energy 2023, 262, 125463. [Google Scholar] [CrossRef]
- Ghosh, D.; Ghose, J.; Datta, P.; Kumari, P.; Paul, S. Strategies for phase change material application in latent heat thermal energy storage enhancement: Status and prospect. J. Energy Storage 2022, 53, 105179. [Google Scholar] [CrossRef]
- Kumar, R.; Pandey, A.; Samykano, M.; Aljafari, B.; Ma, Z.; Bhattacharyya, S.; Goel, V.; Ali, I.; Kothari, R.; Tyagi, V. Phase change materials integrated solar desalination system: An innovative approach for sustainable and clean water production and storage. Renew. Sustain. Energy Rev. 2022, 165, 112611. [Google Scholar] [CrossRef]
- Panchal, J.M.; Modi, K.V.; Patel, V.J. Development in multiple-phase change materials cascaded low-grade thermal energy storage applications: A review. Clean. Eng. Technol. 2022, 8, 100465. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, P.; Wei, G.; Cui, L.; Xu, C.; Du, X. State-of-the-art review on performance enhancement of photovoltaic/thermal system integrated with phase change materials. J. Energy Storage 2022, 56, 106073. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Diaconu, B.M.; Cruceru, M.; Anghelescu, L. Phase Change Materials—Applications and Systems Designs: A Literature Review. Designs 2022, 6, 117. [Google Scholar] [CrossRef]
- Radouane, N. A Comprehensive Review of Composite Phase Change Materials (cPCMs) for Thermal Management Applications, Including Manufacturing Processes, Performance, and Applications. Energies 2022, 15, 8271. [Google Scholar] [CrossRef]
- Kotzé, J.P.; von Backström, T.; Erens, P.J. High Temperature Thermal Energy Storage Utilizing Metallic Phase Change Materials and Metallic Heat Transfer Fluids. J. Sol. Energy Eng. 2013, 135, 035001. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; De Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J.; Liu, J.; Sun, W.; Xu, H.; Liu, C. Self-healed inorganic phase change materials for thermal energy harvesting and management. Appl. Therm. Eng. 2023, 219, 119423. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Ge, R. A review of heat transfer performance enhancement and applications of inorganic salt based shape-stabilized composite phase change materials for medium and high temperature thermal energy storage. Energy Rep. 2022, 8, 12740–12764. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, R.; Ansu, A.; Goyal, R.; Sarı, A.; Tyagi, V. A comprehensive review on development of eutectic organic phase change materials and their composites for low and medium range thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2021, 223, 110955. [Google Scholar] [CrossRef]
- Huang, J.; Lu, S.; Kong, X.; Liu, S.; Li, Y. Form-Stable Phase Change Materials Based on Eutectic Mixture of Tetradecanol and Fatty Acids for Building Energy Storage: Preparation and Performance Analysis. Materials 2013, 6, 4758–4775. [Google Scholar] [CrossRef] [PubMed]
- Png, Z.M.; Soo, X.Y.D.; Chua, M.H.; Ong, P.J.; Suwardi, A.; Tan, C.K.I.; Xu, J.; Zhu, Q. Strategies to reduce the flammability of organic phase change Materials: A review. Sol. Energy 2021, 231, 115–128. [Google Scholar] [CrossRef]
- Bartkowiak, G.; Marszałek, A.; Dąbrowska, A. Thermal Load of Mine Rescuer in the Underwear and Protective Clothing with Phase Change Materials in Simulated Utility Conditions. Materials 2020, 13, 4320. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, X. A comprehensive review of integrating phase change materials in building bricks: Methods, performance and applications. J. Energy Storage 2023, 62, 106913. [Google Scholar] [CrossRef]
- Li, C.; Wen, X.; Cai, W.; Yu, H.; Liu, D. Phase change material for passive cooling in building envelopes: A comprehensive review. J. Build. Eng. 2023, 65, 105763. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Z.; Zhang, X.; Hu, M.; Zhang, L.; Fan, J. Adaptive dynamic building envelope integrated with phase change material to enhance the heat storage and release efficiency: A state-of-the-art review. Energy Build. 2023, 286, 112928. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Cui, H.; Bao, X.; Tang, W.; Sang, G.; Chen, X. Cementitious composites integrated phase change materials for passive buildings: An overview. Constr. Build. Mater. 2022, 361, 129635. [Google Scholar] [CrossRef]
- Liu, L.; Hammami, N.; Trovalet, L.; Bigot, D.; Habas, J.-P.; Malet-Damour, B. Description of phase change materials (PCMs) used in buildings under various climates: A review. J. Energy Storage 2022, 56, 105760. [Google Scholar] [CrossRef]
- Ismail, K.A.; Lino, F.A.; Machado, P.L.O.; Teggar, M.; Arıcı, M.; Alves, T.A.; Teles, M.P. New potential applications of phase change materials: A review. J. Energy Storage 2022, 53, 105202. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Organic phase change materials and their textile applications: An overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Wang, W.; Li, C.; Zeng, X.; Chen, J.; Sun, R. Application of polymer-based phase change materials in thermal safety management of power batteries. J. Energy Storage 2022, 55, 105646. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, B.; Zhang, T.; Jiang, Z.; Ding, J.; Ding, Y. A comprehensive review of composite phase change material based thermal management system for lithium-ion batteries. Renew. Sustain. Energy Rev. 2022, 167, 112667. [Google Scholar] [CrossRef]
- Sanker, S.B.; Baby, R. Phase change material based thermal management of lithium ion batteries: A review on thermal performance of various thermal conductivity enhancers. J. Energy Storage 2022, 50, 104606. [Google Scholar] [CrossRef]
- Zare, P.; Perera, N.; Lahr, J.; Hasan, R. Solid-liquid phase change materials for the battery thermal management systems in electric vehicles and hybrid electric vehicles—A systematic review. J. Energy Storage 2022, 52, 105026. [Google Scholar] [CrossRef]
- Luo, J.; Zou, D.; Wang, Y.; Wang, S.; Huang, L. Battery thermal management systems (BTMs) based on phase change material (PCM): A comprehensive review. Chem. Eng. J. 2021, 430, 132741. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, D.; Jiang, L.; Zhang, G.; Wu, H.; Day, R.; Jiang, W. Advanced thermal management system driven by phase change materials for power lithium-ion batteries: A review. Renew. Sustain. Energy Rev. 2022, 159, 112207. [Google Scholar] [CrossRef]
- Shen, Z.-G.; Chen, S.; Liu, X.; Chen, B. A review on thermal management performance enhancement of phase change materials for vehicle lithium-ion batteries. Renew. Sustain. Energy Rev. 2021, 148, 111301. [Google Scholar] [CrossRef]
- Chen, J.; Kang, S.; Jiaqiang, E.; Huang, Z.; Wei, K.; Zhang, B.; Zhu, H.; Deng, Y.; Zhang, F.; Liao, G. Effects of different phase change material thermal management strategies on the cooling performance of the power lithium ion batteries: A review. J. Power Sources 2019, 442, 227228. [Google Scholar] [CrossRef]
- Maqbool, Z.; Hanief, M.; Parveez, M. Review on performance enhancement of phase change material based heat sinks in conjugation with thermal conductivity enhancers for electronic cooling. J. Energy Storage 2023, 60, 106591. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, R.; Li, J. High latent heat phase change materials (PCMs) with low melting temperature for thermal management and storage of electronic devices and power batteries: Critical review. Renew. Sustain. Energy Rev. 2022, 168, 112783. [Google Scholar] [CrossRef]
- Yang, T.; King, W.P.; Miljkovic, N. Phase change material-based thermal energy storage. Cell Rep. Phys. Sci. 2021, 2, 100540. [Google Scholar] [CrossRef]
- Funt, J.M.; Magill, J.H. A method for the quantitative evaluation of flammability in polymers. J. Appl. Polym. Sci. 1974, 18, 1243–1245. [Google Scholar] [CrossRef]
- Stauffer, E.; Dolan, J.A.; Newman, R. Fire Debris Analysis; Academic Press: Cambridge, MA, USA, 2008; ISBN 978-0-12-663971-1. [Google Scholar] [CrossRef]
- Tewarson, A. Flammability Parameters of Materials: Ignition, Combustion, and Fire Propagation. J. Fire Sci. 1994, 12, 329–356. [Google Scholar] [CrossRef]
- Williams, F.A. Combustion Theory, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1994; ISBN 9780201407778. [Google Scholar]
- Garche, J.; Brandt, K. Electrochemical Power Sources: Fundamentals, Systems, and Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780444637772. [Google Scholar]
- Flammability UL 94 HB. 2021. Available online: https://www.impact-solutions.co.uk/plastic-testing/testing-capabilities-list/ul94-fire-testing/ul94-horizontal-burning-test/ (accessed on 11 March 2023).
- Vertical Burning Test for Classifying Materials. Available online: https://omnexus.specialchem.com/polymer-properties/properties/flammability-ul94 (accessed on 11 March 2023).
- Shrivastava, A. Introduction to Plastic Engineering; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 978-0-323-39500-7. [Google Scholar]
- ASTM D2863; Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-Like Combustion of Plastics (Oxygen Index). ASTM International: West Conshohocken, PA, USA, 2019. Available online: https://asrecomposite.com/wp-content/uploads/2021/09/ASTM-D2863_2013_427900547120.pdf (accessed on 11 March 2023).
- ASTM E1354-17; Standard Test Method For Heat And Visible Smoke Release Rates For Materials And Products Using An Oxygen Consumption Calorimeter. ASTM International: West Conshohocken, PA, USA, 1999.
- ISO 5660-1:2015; Reaction-to-Fire Tests—Heat Release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). ISO: Geneva, Switzerland, 2015.
- Dowbysz, A.M.; Samsonowicz, M. Smoke Generation Parameters from the Cone Calorimeter Method and Single-Chamber Test. EWaS5 2021, 9, 22. [Google Scholar] [CrossRef]
- De Silva, D.; Alam, N.; Nadjai, A.; Nigro, E.; Ali, F. Finite Element Modelling for Structural Performance of Slim Floors in Fire and Influence of Protection Materials. Appl. Sci. 2021, 11, 11291. [Google Scholar] [CrossRef]
- De Silva, D.; Bilotta, A.; Nigro, E. Approach for modelling thermal properties of intumescent coating applied on steel members. Fire Saf. J. 2020, 116, 103200. [Google Scholar] [CrossRef]
- Roshan, P. Functional Finishes for Textiles. Improving Comfort, Performance and Protection; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-0-85709-839-9. [Google Scholar]
- Sittisart, P.; Farid, M.M. Fire retardants for phase change materials. Appl. Energy 2011, 88, 3140–3145. [Google Scholar] [CrossRef]
- De Silva, D.; Nuzzo, I.; Nigro, E.; Occhiuzzi, A. Intumescent Coatings for Fire Resistance of Steel Structures: Current Approaches for Qualification and Design. Coatings 2022, 12, 696. [Google Scholar] [CrossRef]
- Cai, Y.; Hu, Y.; Song, L.; Kong, Q.; Yang, R.; Zhang, Y.; Chen, Z.; Fan, W. Preparation and flammability of high density poly-ethylene/paraffin/organophilic montmorillonite hybrids as a form stable phase change material. Energy Convers. Manag. 2007, 48, 462–469. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, Q.; Huang, F.; Gao, W. Preparation and properties studies of halogen-free flame retardant form-stable phase change materials based on paraffin/high density polyethylene composites. Appl. Energy 2008, 85, 765–775. [Google Scholar] [CrossRef]
- Fan, M.; Fu, F. Advanced High Strength Natural Fibre Composites in Construction; Woodhead Publishing: Sawston, UK, 2017; ISBN 978-0-08-100411-1. [Google Scholar] [CrossRef]
- Palacios, A.; De Gracia, A.; Haurie, L.; Cabeza, L.F.; Fernández, A.I.; Barreneche, C. Study of the Thermal Properties and the Fire Performance of Flame Retardant-Organic PCM in Bulk Form. Materials 2018, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- UNE-23727:1990; Ensayos de Reacción al Fuego de los Materiales de Construcción. Clasificación de los Materiales Utilizados en la Construcción. Asociacion Espanola de Normalizacion: Madrid, Spain, 1990.
- Palacios, A.; de Gracia, A.; Cabeza, L.F.; Julià, E.; Fernández, A.I.; Barreneche, C. New formulation and characterization of enhanced bulk-organic phase change materials. Energy Build. 2018, 167, 38–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Z.; Hai, A.M.; Zhang, S.; Tang, B. Shape-stabilization micromechanisms of form-stable phase change materials-A review. Compos. Part A: Appl. Sci. Manuf. 2022, 160, 107047. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Yang, R. Flame retardance property of shape-stabilized phase change materials. Sol. Energy Mater. Sol. Cells 2015, 140, 439–445. [Google Scholar] [CrossRef]
- He, H.; Liu, J.; Wang, Y.; Zhao, Y.; Qin, Y.; Zhu, Z.; Yu, Z.; Wang, J. An Ultralight Self-Powered Fire Alarm e-Textile Based on Conductive Aerogel Fiber with Repeatable Temperature Monitoring Performance Used in Firefighting Clothing. ACS Nano 2022, 16, 2953–2967. [Google Scholar] [CrossRef]
- Regulation (EU) 2016/425 of the European Parliament and of the Council of 9 March 2016 on Personal Protective Equipment and Repealing Council Directive 89/686/EEC; EU: Brussels, Belgium, 2016.
- EN ISO 11612:2015; Protective Clothing—Clothing to Protect against Heat and Flame—Minimum Performance Requirements. ISO: Geneva, Switzerland, 2015.
- Su, Y.; Fan, Y.; Ma, Y.; Wang, Y.; Liu, G. Flame-retardant phase change material (PCM) for thermal protective application in firefighting protective clothing. Int. J. Therm. Sci. 2023, 185, 108075. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Chen, R.; Su, Y. Thermal protective performance of firefighting protective clothing incorporated with phase change material in fire environments. Fire Mater. 2020, 45, 250–260. [Google Scholar] [CrossRef]
- Yin, G.-Z.; Yang, X.-M.; Hobson, J.; López, A.M.; Wang, D.-Y. Bio-based poly (glycerol-itaconic acid)/PEG/APP as form stable and flame-retardant phase change materials. Compos. Commun. 2022, 30, 101057. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, Y.; Song, L.; Ni, J.; Xing, W.; Wang, J. Effect of expanded graphite on properties of high-density polyethylene/paraffin composite with intumescent flame retardant as a shape-stabilized phase change material. Sol. Energy Mater. Sol. Cells 2010, 94, 360–365. [Google Scholar] [CrossRef]
- Huang, J.; Su, J.; Xu, W.; Lin, J.; Weng, M.; Liu, Y.; Min, Y. High enthalpy efficiency lignin-polyimide porous hybrid aerogel composite phase change material with flame retardancy for superior solar-to-thermal energy conversion and storage. Sol. Energy Mater. Sol. Cells 2022, 248, 112036. [Google Scholar] [CrossRef]
- Xue, F.; Huang, C.-H.; Qi, X.-D.; Yang, J.-H.; Zhao, C.-S.; Lei, Y.-Z.; Wang, Y. Largely improved thermal conductivity and flame resistance of phase change materials based on three-dimensional melamine foam/phosphorous cellulose/graphite nanoplatelets network with multiple energy transition abilities. Compos. Part A 2022, 156, 106898. [Google Scholar] [CrossRef]
- Zhou, R.; Ming, Z.; He, J.; Ding, Y.; Jiang, J. Effect of Magnesium Hydroxide and Aluminum Hydroxide on the Thermal Stability, Latent Heat and Flammability Properties of Paraffin/HDPE Phase Change Blends. Polymers 2020, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Qian, X.; Ma, L.; Song, L.; Hu, Y.; Liew, K.M. Preparation of a novel biobased flame retardant containing phosphorus and nitrogen and its performance on the flame retardancy and thermal stability of poly(vinyl alcohol). Polym. Degrad. Stab. 2014, 106, 47–53. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, Y.; Song, L.; Lu, H.; Wang, J.; Liu, Q. Synergistic effect of iron and intumescent flame retardant on shape-stabilized phase change material. Thermochim. Acta 2009, 487, 74–79. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Zhang, G.; Wu, H.; Rao, Z.; Guo, J.; Zhou, D. Experimental investigation of the flame retardant and form-stable composite phase change materials for a power battery thermal management system. J. Power Sources 2020, 480, 229116. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Li, X.; Zhang, G.; Chen, K.; Yang, W. Experimental investigation on thermal management system with flame retardant flexible phase change material for retired battery module. Appl. Energy 2022, 327, 120109. [Google Scholar] [CrossRef]
- Alkhazaleh, A.H.; Almanaseer, W.; Alkhazali, A. Experimental investigation on thermal properties and fire performance of lauric acid/diphenyl phosphate/expanded perlite as a flame retardant phase change material for latent heat storage applications. Sustain. Energy Technol. Assessments 2023, 56, 103059. [Google Scholar] [CrossRef]
- Tanwar, S.; Kaur, R. Fabrication and investigation on influence of metal oxide nanoparticles on thermal, flammability and UV characteristics of polyethylene glycol based phase change materials. J. Energy Storage 2022, 54, 105318. [Google Scholar] [CrossRef]
- Li, L.; Wang, G.; Guo, C. Influence of intumescent flame retardant on thermal and flame retardancy of eutectic mixed paraffin/polypropylene form-stable phase change materials. Appl. Energy 2016, 162, 428–434. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Zhang, G.; Weng, J.; Wang, Y.; Deng, J. Innovative thermal management and thermal runaway suppression for battery module with flame retardant flexible composite phase change material. J. Clean. Prod. 2021, 330, 129718. [Google Scholar] [CrossRef]
- Salgado-Pizarro, R.; Martín, M.; Svobodova-Sedlackova, A.; Calderón, A.; Haurie, L.; Fernández, A.I.; Barreneche, C. Manufacturing of nano-enhanced shape stabilized phase change materials with montmorillonite by Banbury oval rotor mixer for buildings applications. J. Energy Storage 2022, 55, 105289. [Google Scholar] [CrossRef]
- Yin, G.-Z.; Yang, X.-M.; Palencia, J.L.D.; Hobson, J.; López, A.M.; Wang, D.-Y. Phytic acid as a biomass flame retardant for polyrotaxane based phase change materials. J. Energy Storage 2022, 56, 105853. [Google Scholar] [CrossRef]
- Song, G.; Ma, S.; Tang, G.; Yin, Z.; Wang, X. Preparation and characterization of flame retardant form-stable phase change materials composed by EPDM, paraffin and nano magnesium hydroxide. Energy 2010, 35, 2179–2183. [Google Scholar] [CrossRef]
- Ma, T.; Li, L.; Wang, Q.; Guo, C. High-performance flame retarded paraffin/epoxy resin form-stable phase change material. J. Mater. Sci. 2018, 54, 875–885. [Google Scholar] [CrossRef]
- Huang, Y.; Stonehouse, A.; Abeykoon, C. Encapsulation methods for phase change materials – A critical review. Int. J. Heat Mass Transf. 2023, 200, 123458. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Du, W.; Liang, Z.; Li, F.; Yong, Y.; Li, Z. Construction of layered double hydroxide-modified silica integrated multilayer shell phase change capsule with flame retardancy and highly efficient thermoregulation performance. J. Colloid Interface Sci. 2023, 632, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Liu, Y.; Liang, C.; Lin, W.; Wang, C.; Li, C.; Zhang, F.; Cheng, J. Phase change material microcapsules with DOPO/Cu modified halloysite nanotubes for thermal controlling of buildings: Thermophysical properties, flame retardant performance and thermal comfort levels. Int. J. Heat Mass Transf. 2023, 207, 124045. [Google Scholar] [CrossRef]
- Liao, H.; Duan, W.; Liu, Y.; Wang, Q.; Wen, H. Flame retardant and leaking preventable phase change materials for thermal energy storage and thermal regulation. J. Energy Storage 2021, 35, 102248. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, L.; Chen, Z. Preparation and characterization of flame retardant phase change materials by microencapsulated paraffin and diethyl ethylphosphonate with poly(methacrylic acid-co -ethyl methacrylate) shell. J. Appl. Polym. Sci. 2015, 132, 41880. [Google Scholar] [CrossRef]
- Demirbağ, S.; Aksoy, S.A. Encapsulation of phase change materials by complex coacervation to improve thermal performances and flame retardant properties of the cotton fabrics. Fibers Polym. 2016, 17, 408–417. [Google Scholar] [CrossRef]
- Du, X.; Fang, Y.; Cheng, X.; Du, Z.; Zhou, M.; Wang, H. Fabrication and Characterization of Flame-Retardant Nanoencapsulated n-Octadecane with Melamine–Formaldehyde Shell for Thermal Energy Storage. ACS Sustain. Chem. Eng. 2018, 6, 15541–15549. [Google Scholar] [CrossRef]
- Kosny, J.; Kossecka, E.; Brzezinski, A.; Tleoubaev, A.; Yarbrough, D. Dynamic thermal performance analysis of fiber insulations containing bio-based phase change materials (PCMs). Energy Build. 2012, 52, 122–131. [Google Scholar] [CrossRef]
- Du, X.; Wang, S.; Du, Z.; Cheng, X.; Wang, H. Preparation and characterization of flame-retardant nanoencapsulated phase change materials with poly(methylmethacrylate) shells for thermal energy storage. J. Mater. Chem. A 2018, 6, 17519–17529. [Google Scholar] [CrossRef]
- Kazanci, B.; Cellat, K.; Paksoy, H. Preparation, characterization, and thermal properties of novel fire-resistant microencapsulated phase change materials based on paraffin and a polystyrene shell. RSC Adv. 2020, 10, 24134–24144. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.; Vicente, R.; Eisenblätter, J.; Marques, P. Thermal characterization of polyurethane foams with phase change material. Ciência Tecnol. Dos Mater. 2017, 29, 1–7. [Google Scholar] [CrossRef]
- Szczotok, A.M.; Carmona, M.; Serrano, A.; Kjøniksen, A.L.; Rodriguez, J.F. Development of thermoregulating microcapsules with cyclotriphosphazene as a flame retardant agent. IOP Conf. Series Mater. Sci. Eng. 2017, 251, 012120. [Google Scholar] [CrossRef]
- Kang, M.; Liu, Y.; Lin, W.; Liang, C.; Cheng, J. The thermal behavior and flame retardant performance of phase change material microcapsules with halloysite nanotube. J. Energy Storage 2023, 60, 106632. [Google Scholar] [CrossRef]
- Xu, L.; Liu, X.; An, Z.; Yang, R. EG-based coatings for flame retardance of shape stabilized phase change materials. Polym. Degrad. Stab. 2019, 161, 114–120. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Yang, R. A new flame retardance strategy for shape stabilized phase change materials by surface coating. Sol. Energy Mater. Sol. Cells 2017, 170, 87–94. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Cheng, Y.-X.; Zhao, R.; Cheng, W.-L. A high heat storage capacity form-stable composite phase change material with enhanced flame retardancy. Appl. Energy 2020, 262, 114536. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, P.; Wang, Y.; Zhou, C.; Lei, J. Form-stable phase change materials with enhanced thermal stability and fire resistance via the incorporation of phosphorus and silicon. Mater. Des. 2018, 160, 763–771. [Google Scholar] [CrossRef]
- Xu, L.; Liu, X.; Yang, R. Flame Retardant Paraffin-Based Shape-Stabilized Phase Change Material via Expandable Graphite-Based Flame-Retardant Coating. Molecules 2020, 25, 2408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, B.; Wang, L.; Lu, R.; Zhao, D.; Zhang, S. Novel hybrid form-stable polyether phase change materials with good fire resistance. Energy Storage Mater. 2017, 6, 46–52. [Google Scholar] [CrossRef]
- Chen, R.; Huang, X.; Zheng, R.; Xie, D.; Mei, Y.; Zou, R. Flame-retardancy and thermal properties of a novel phosphorus-modified PCM for thermal energy storage. Chem. Eng. J. 2020, 380, 122500. [Google Scholar] [CrossRef]
- Thakur, A.K.; Prabakaran, R.; Elkadeem, M.; Sharshir, S.W.; Arıcı, M.; Wang, C.; Zhao, W.; Hwang, J.-Y.; Saidur, R. A state of art review and future viewpoint on advance cooling techniques for Lithium–ion battery system of electric vehicles. J. Energy Storage 2020, 32, 101771. [Google Scholar] [CrossRef]
- Lamb, J.; Orendorff, C.J.; Steele, L.A.M.; Spangler, S.W. Failure propagation in multi-cell lithium ion batteries. J. Power Sources 2015, 283, 517–523. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, Y.; Zhao, L.; Zhu, F.; Li, L.; Kong, Q.; Chen, M. Investigation on the Properties of Flame-Retardant Phase Change Material and Its Application in Battery Thermal Management. Energies 2023, 16, 521. [Google Scholar] [CrossRef]
- Weng, J.; Xiao, C.; Ouyang, D.; Yang, X.; Chen, M.; Zhang, G.; Yuen, R.K.K.; Wang, J. Mitigation effects on thermal runaway propagation of structure-enhanced phase change material modules with flame retardant additives. Energy 2021, 239, 122087. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, W.; Wu, T.; Wang, C.; Liang, Z. Thermal management system study of flame retardant solid–solid phase change material battery. Surfaces Interfaces 2023, 36, 102558. [Google Scholar] [CrossRef]
- Haurie, L.; Mazo, J.; Delgado, M.; Zalba, B. Fire behaviour of a mortar with different mass fractions of phase change material for use in radiant floor systems. Energy Build. 2014, 84, 86–93. [Google Scholar] [CrossRef]
- UNE 23725; Reaction to Fire Tests of Building Materials. Dripping Test with Electrical Radiator Used for Melting Materials (Complementary Test). Spanish Association for Standardization and Certification (AENOR): Madrid, Spain, 1990.


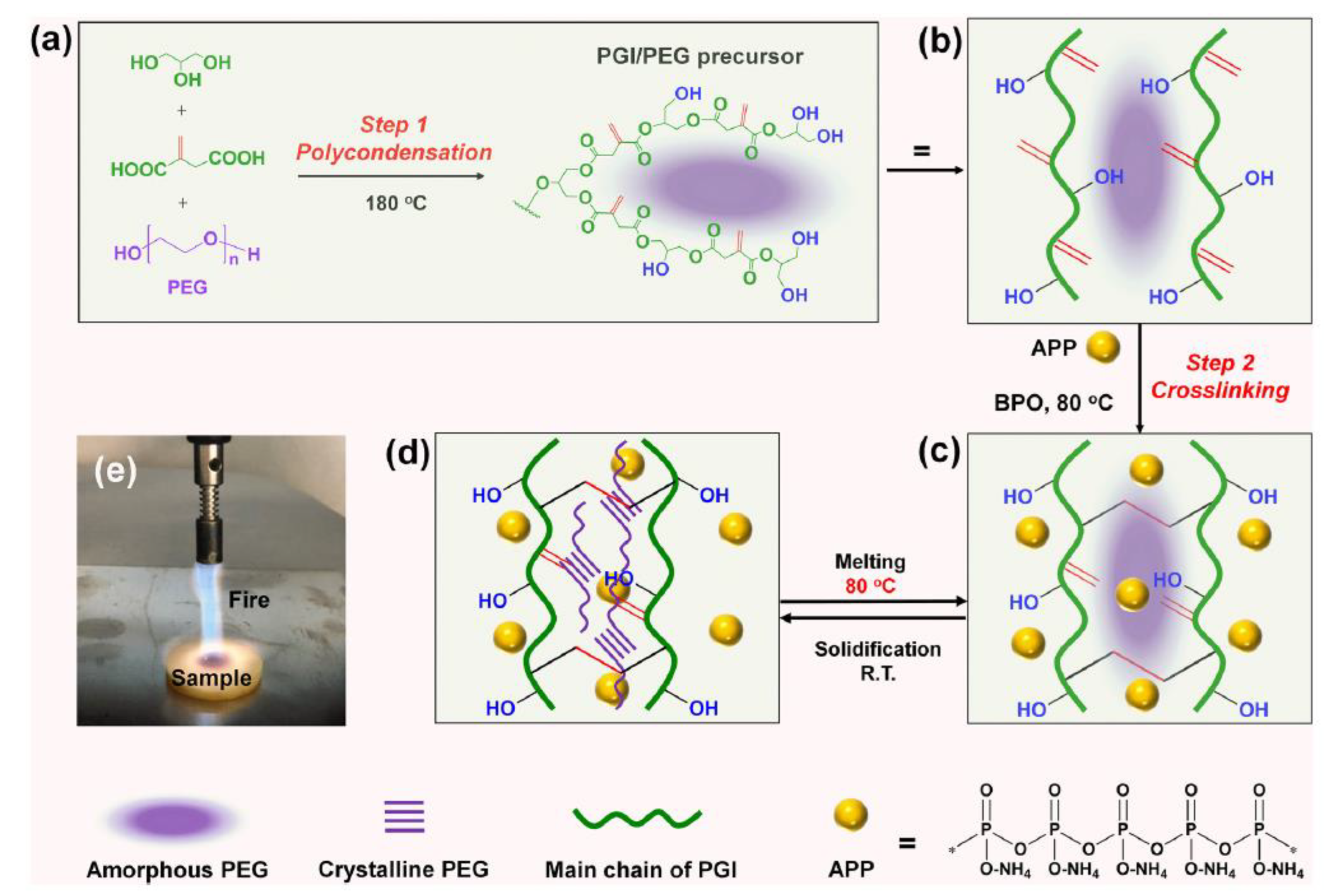
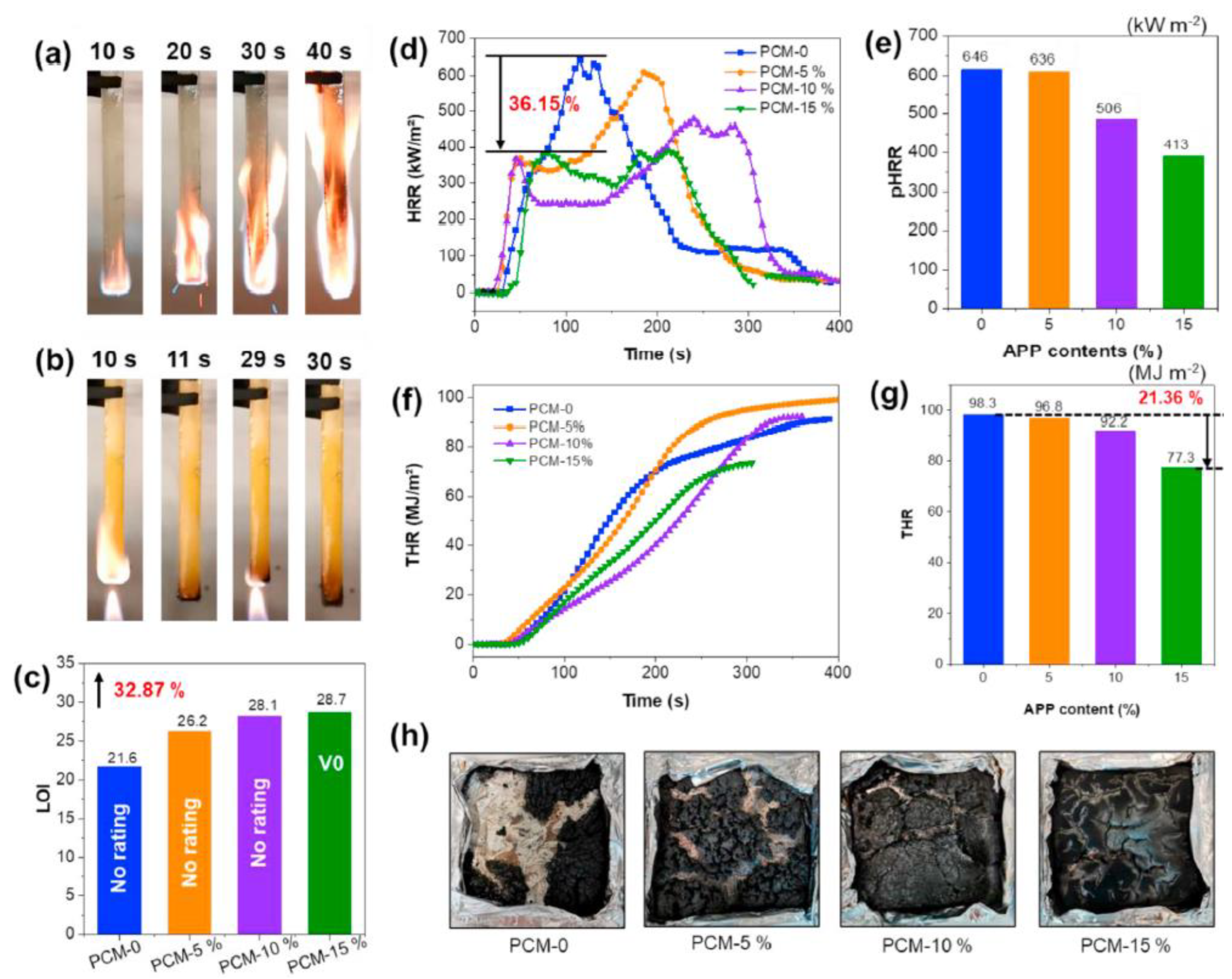
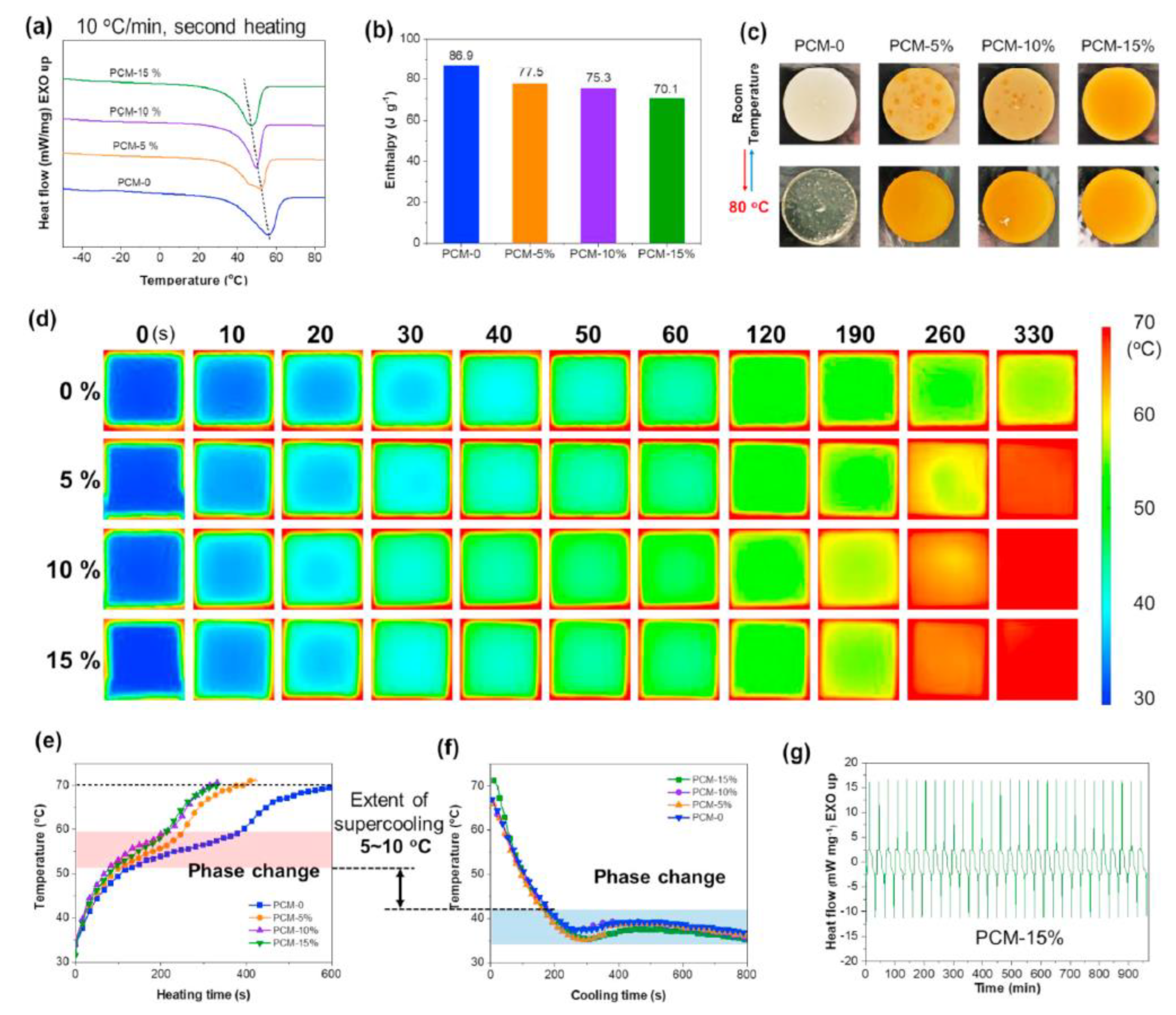

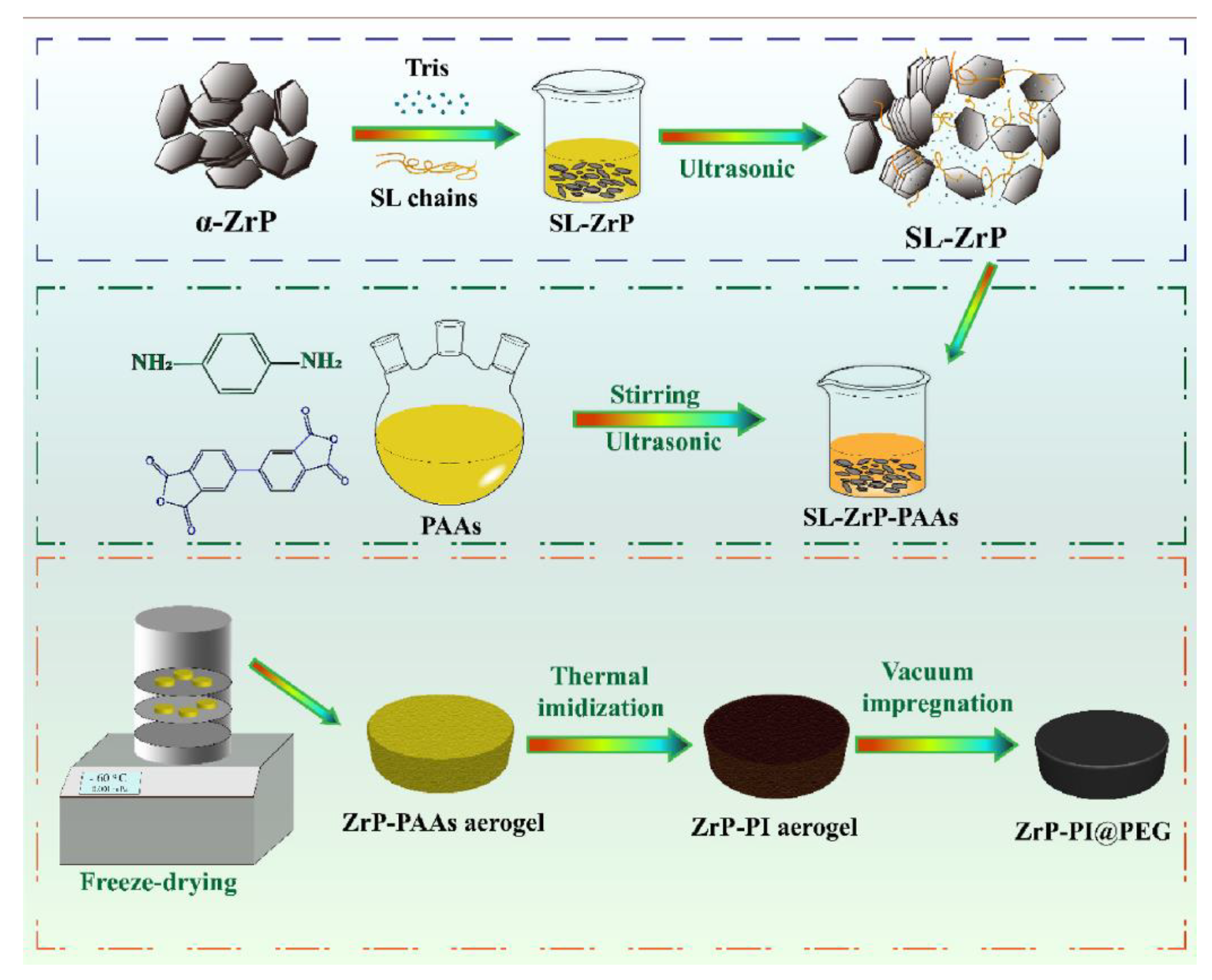
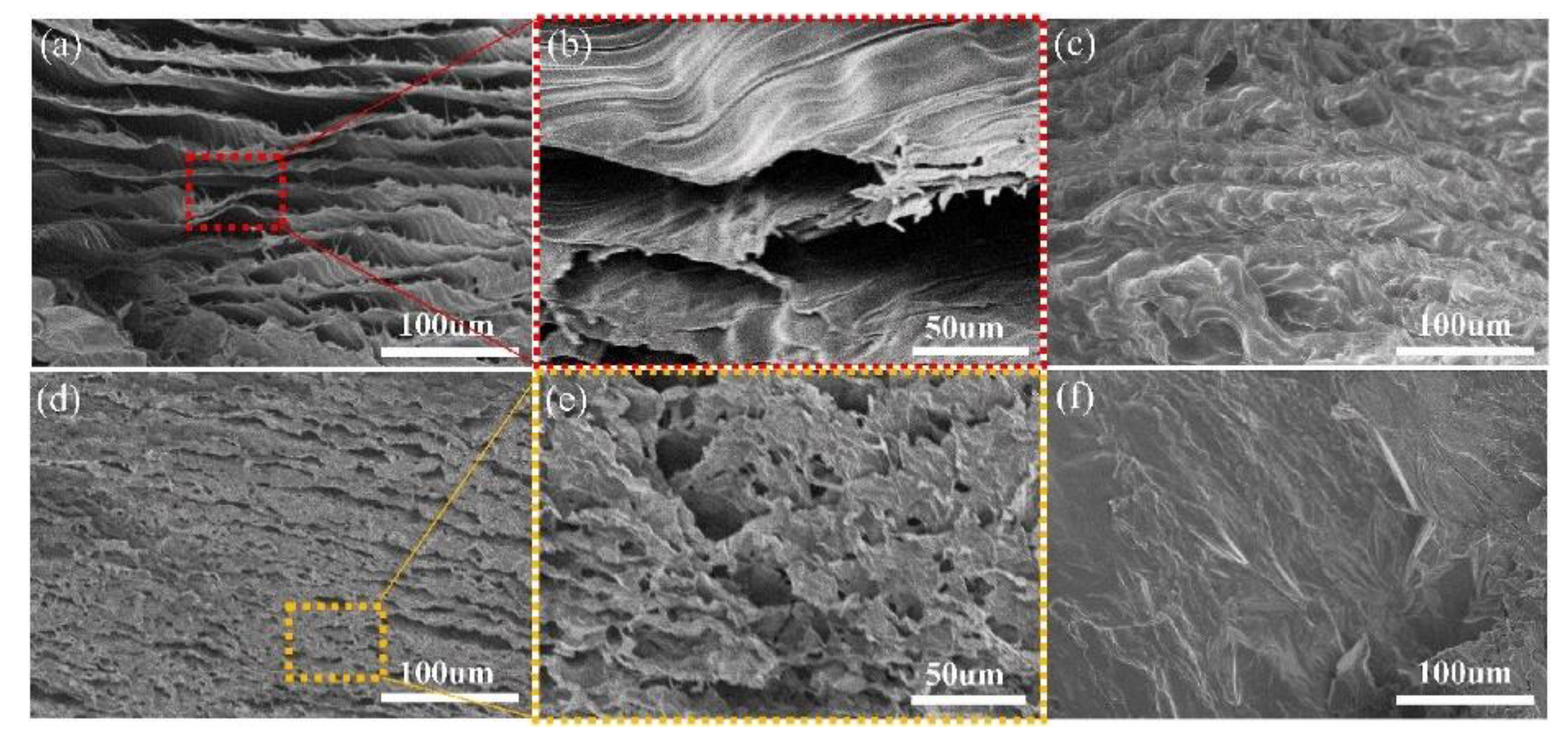
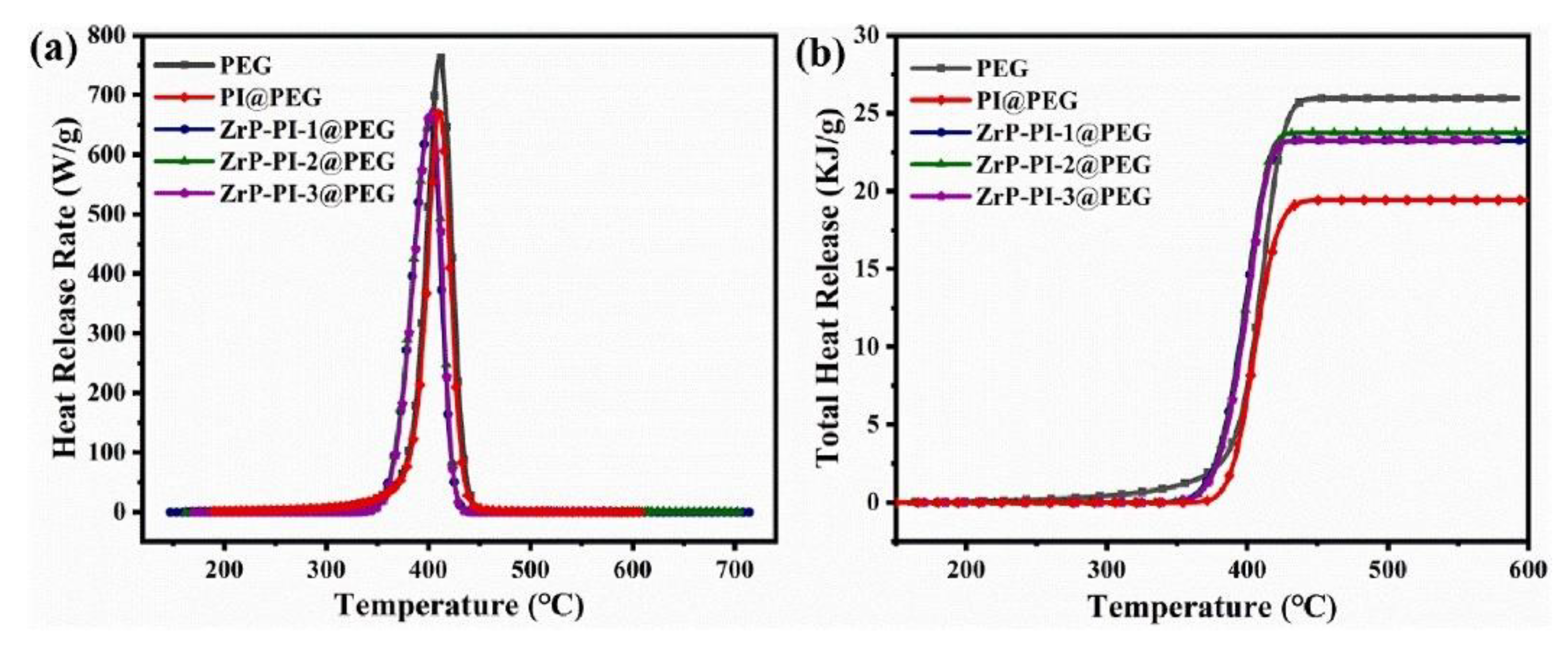
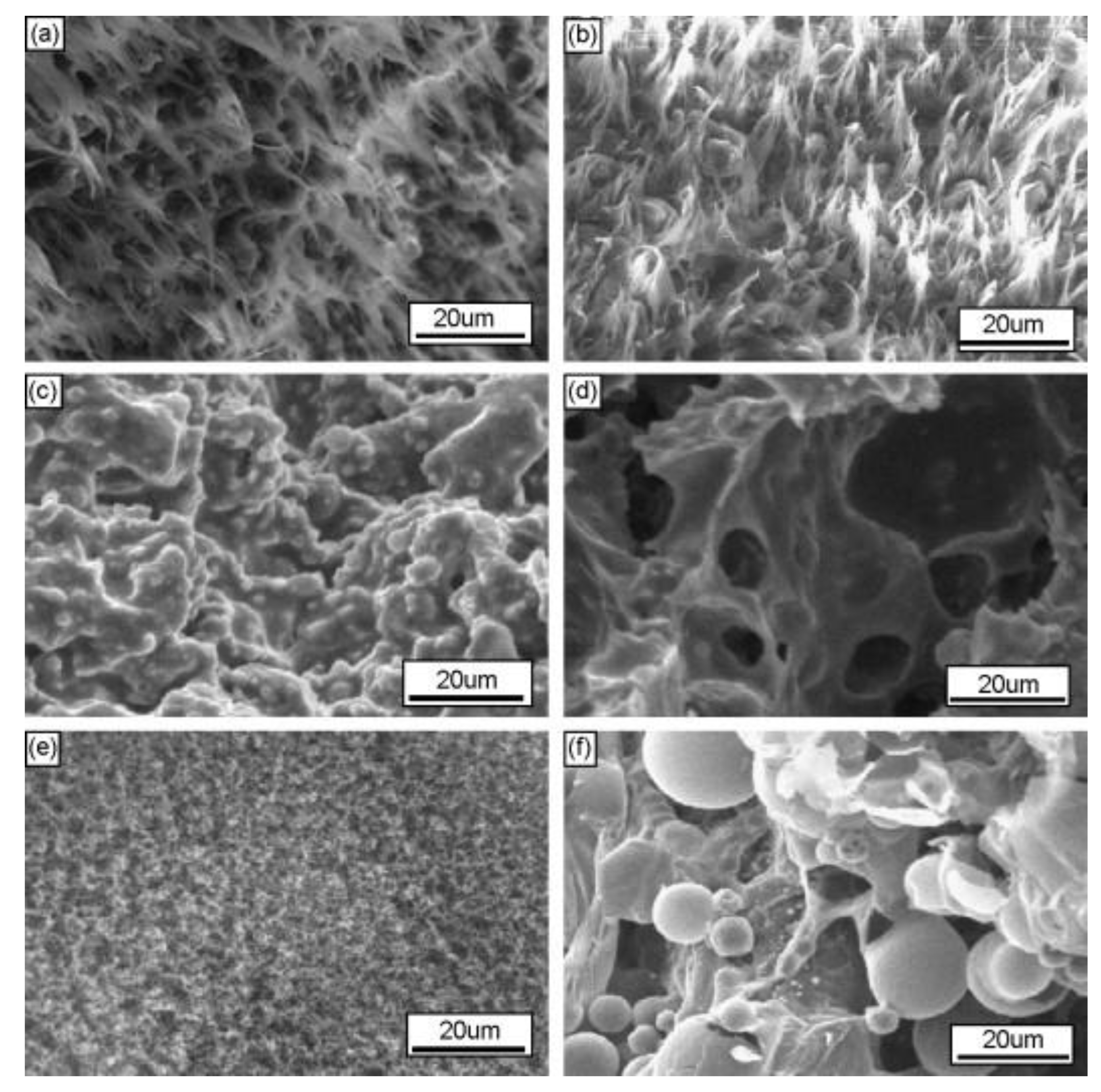
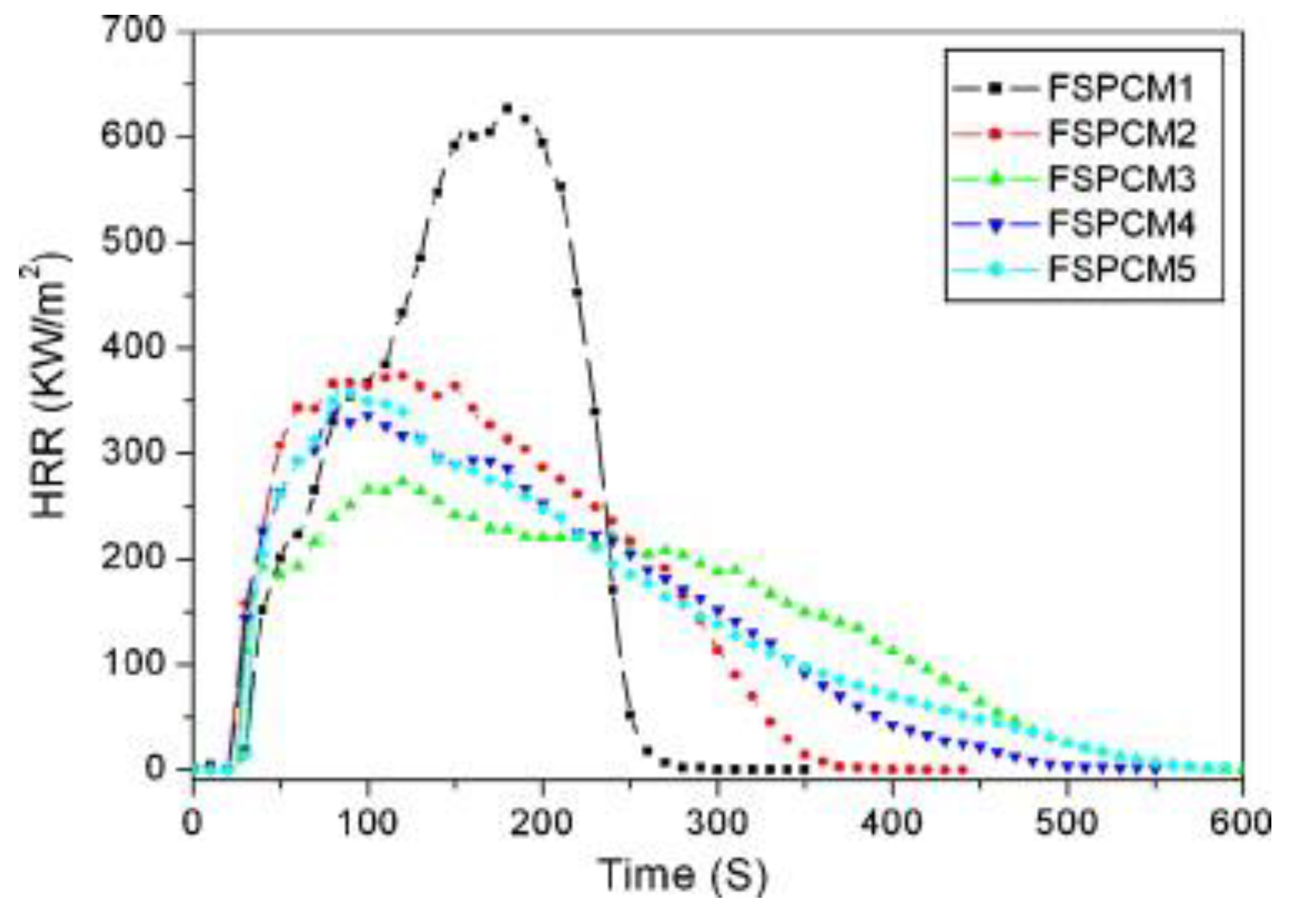

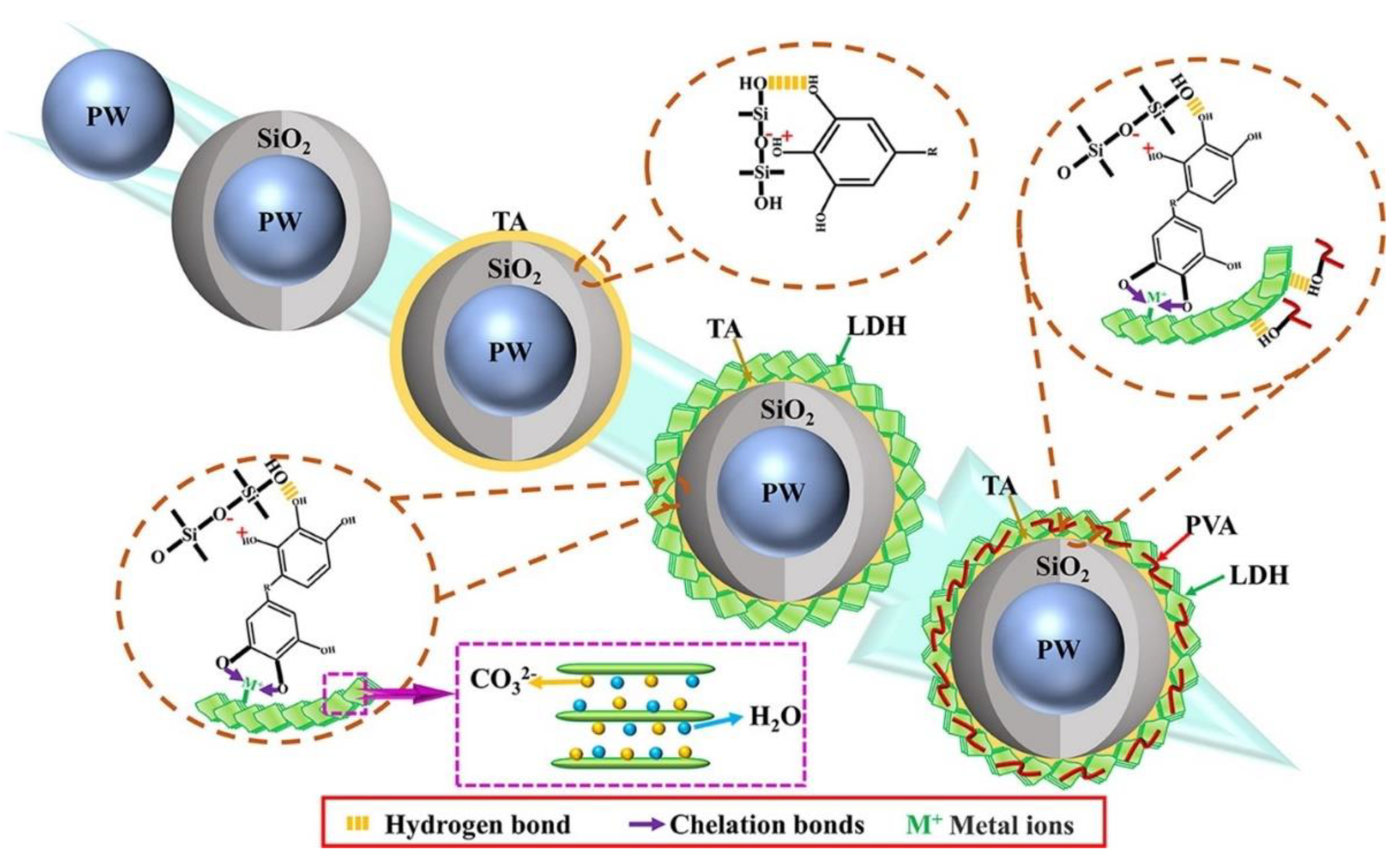
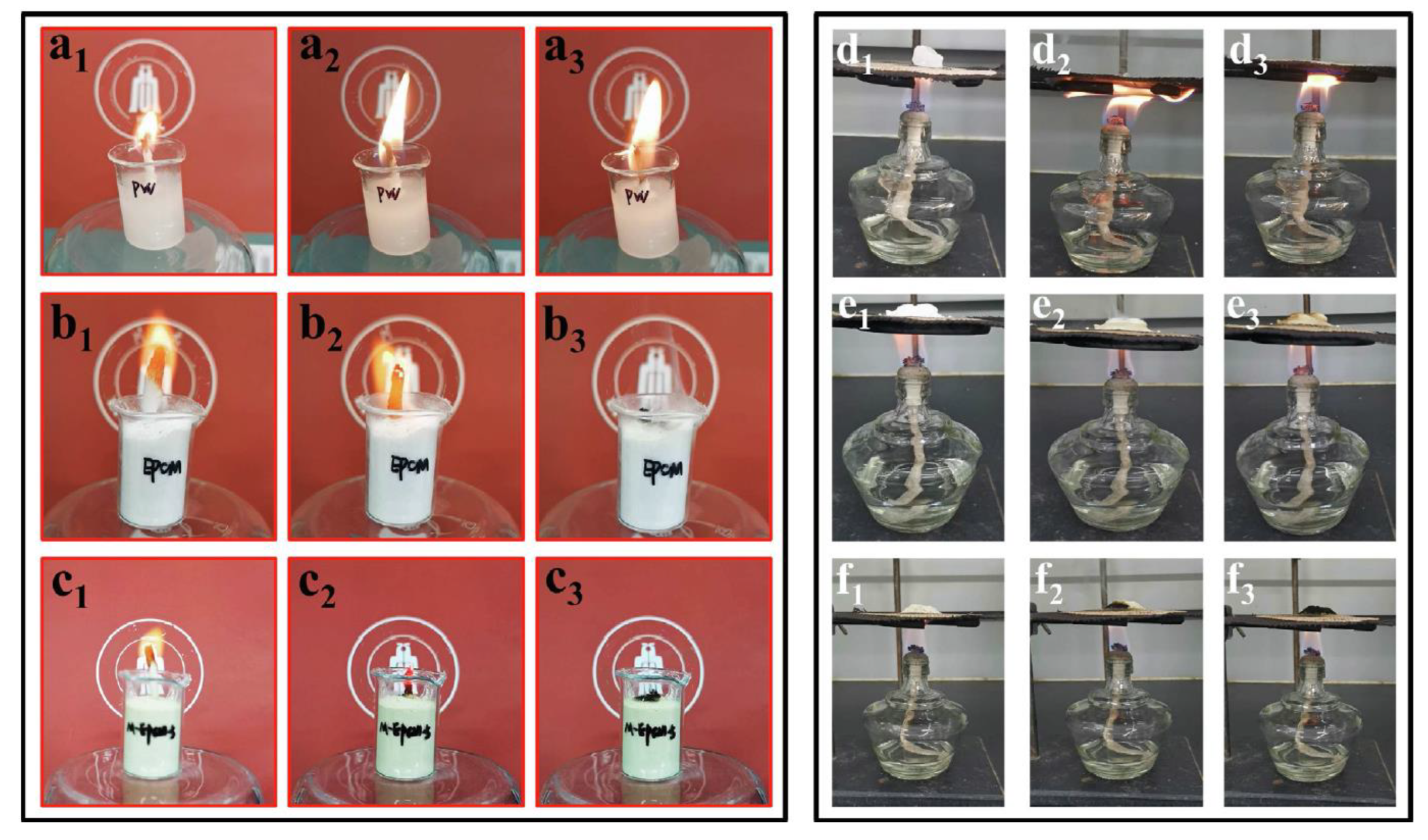

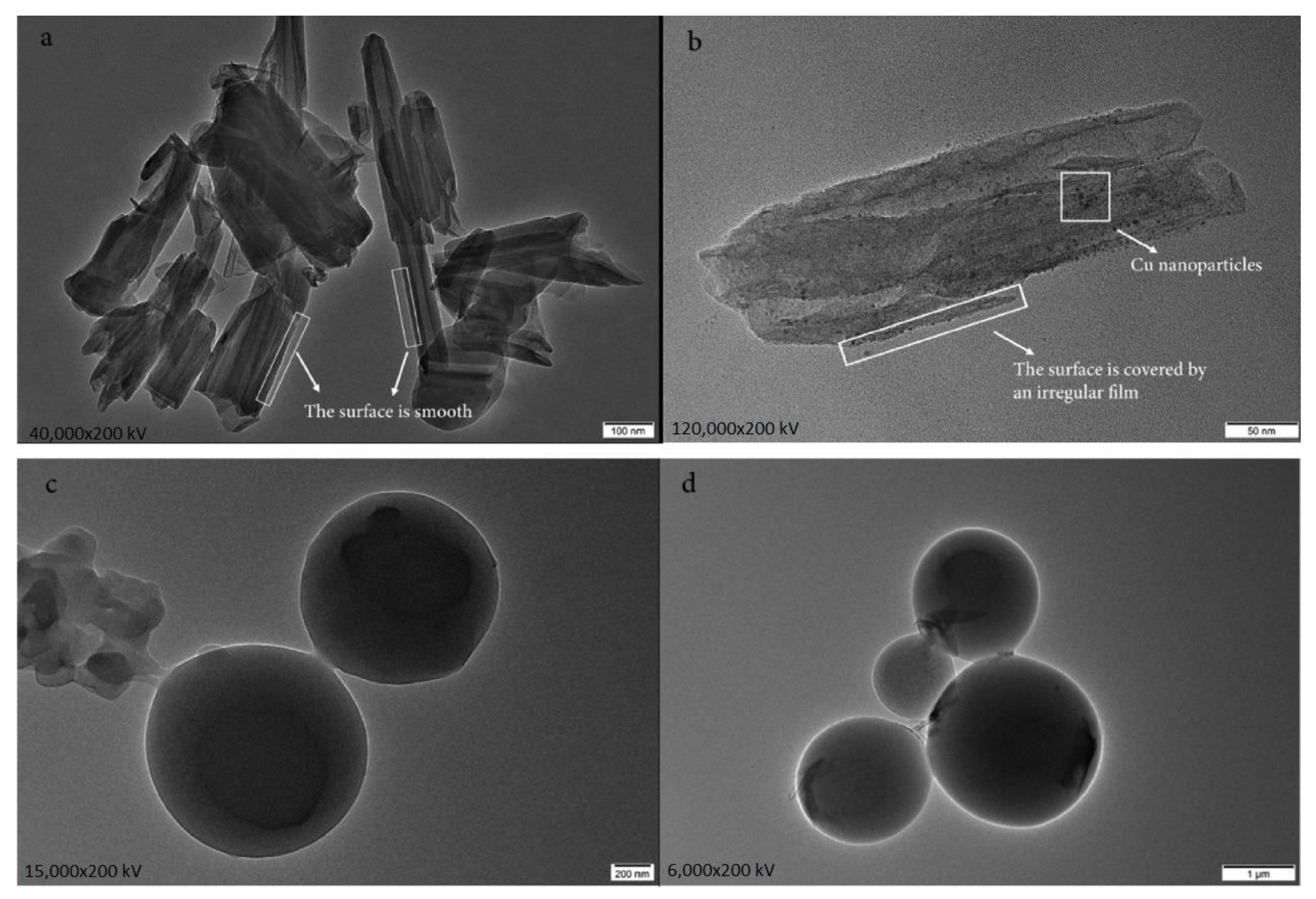
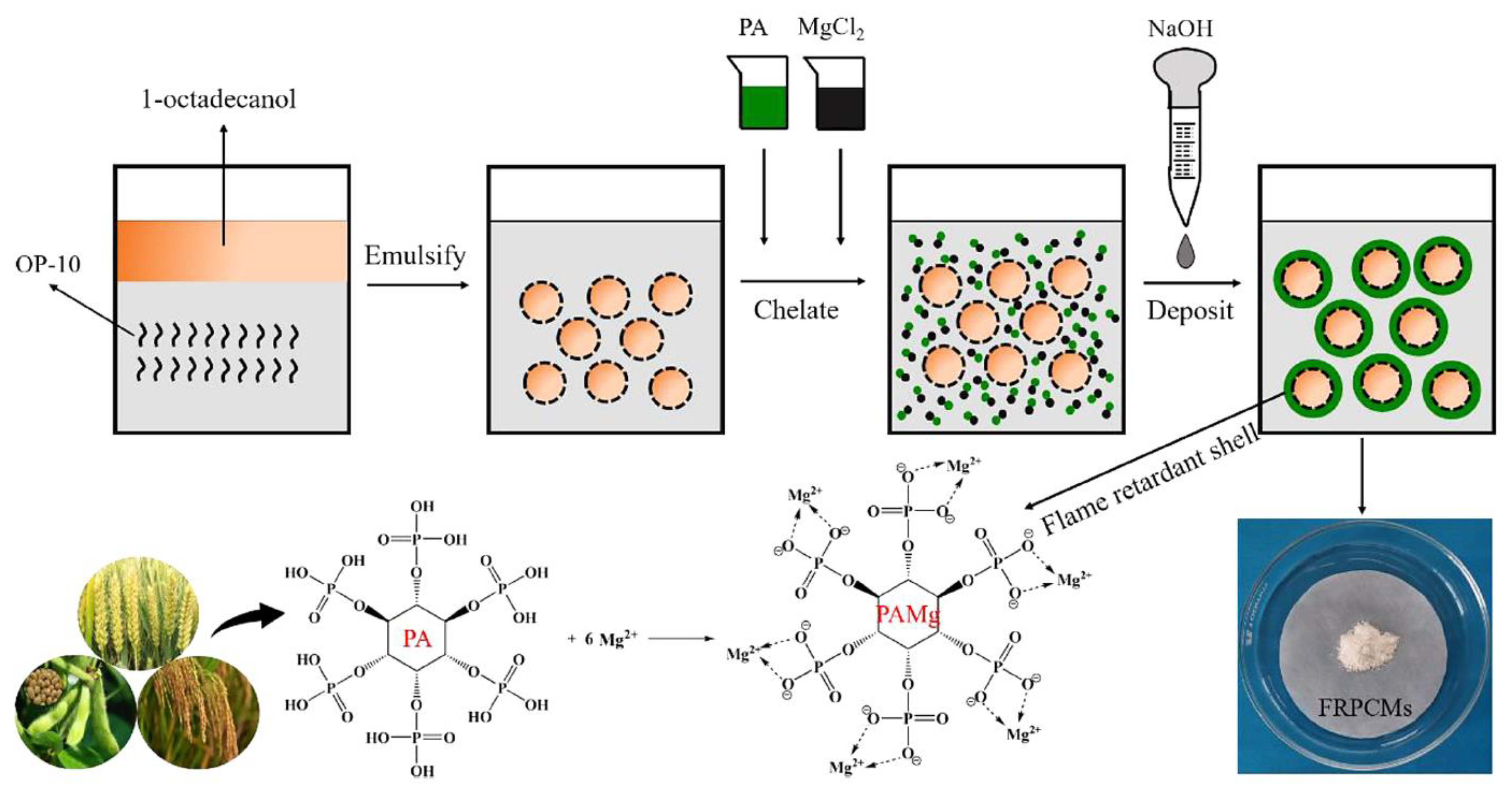

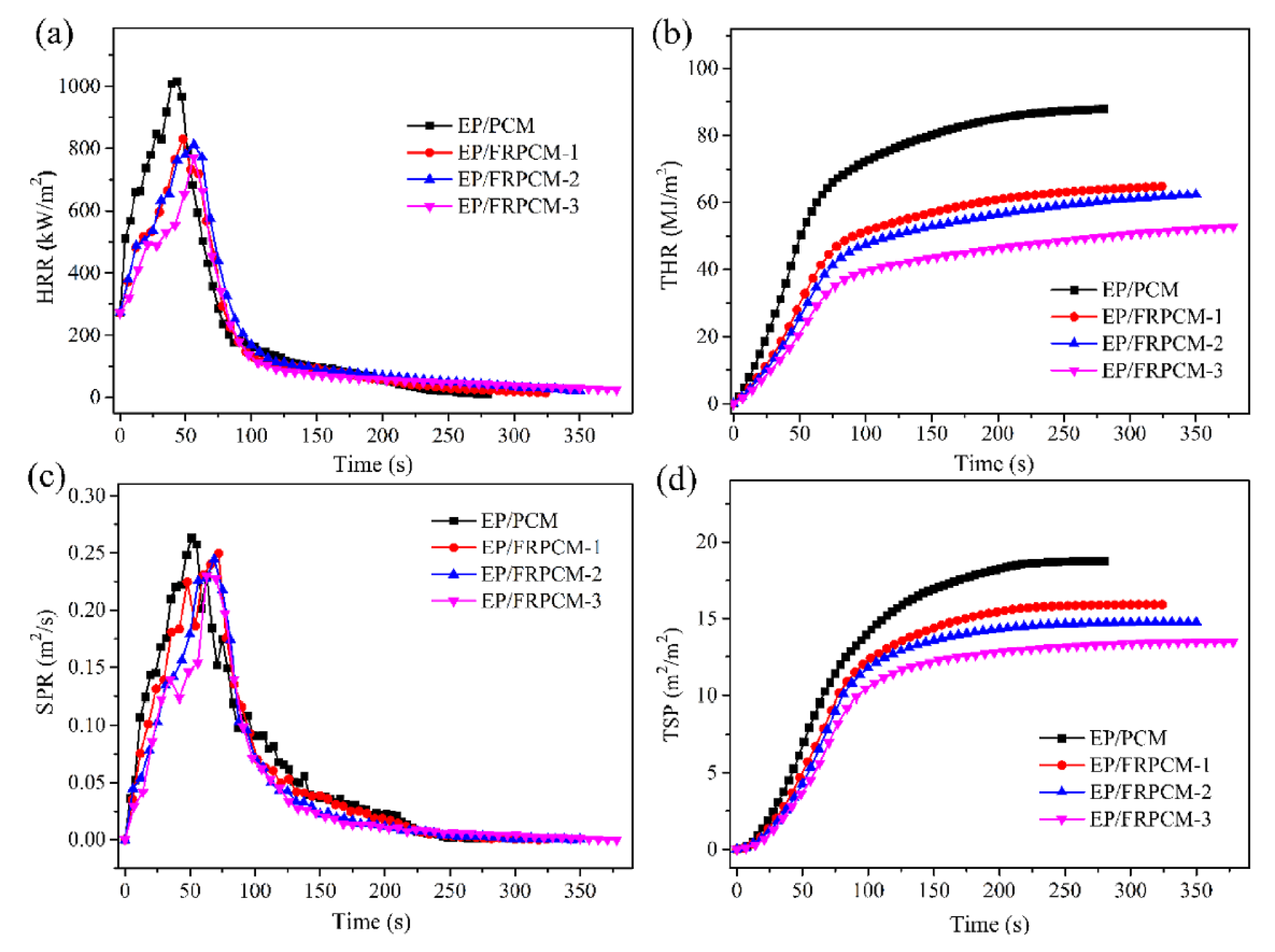
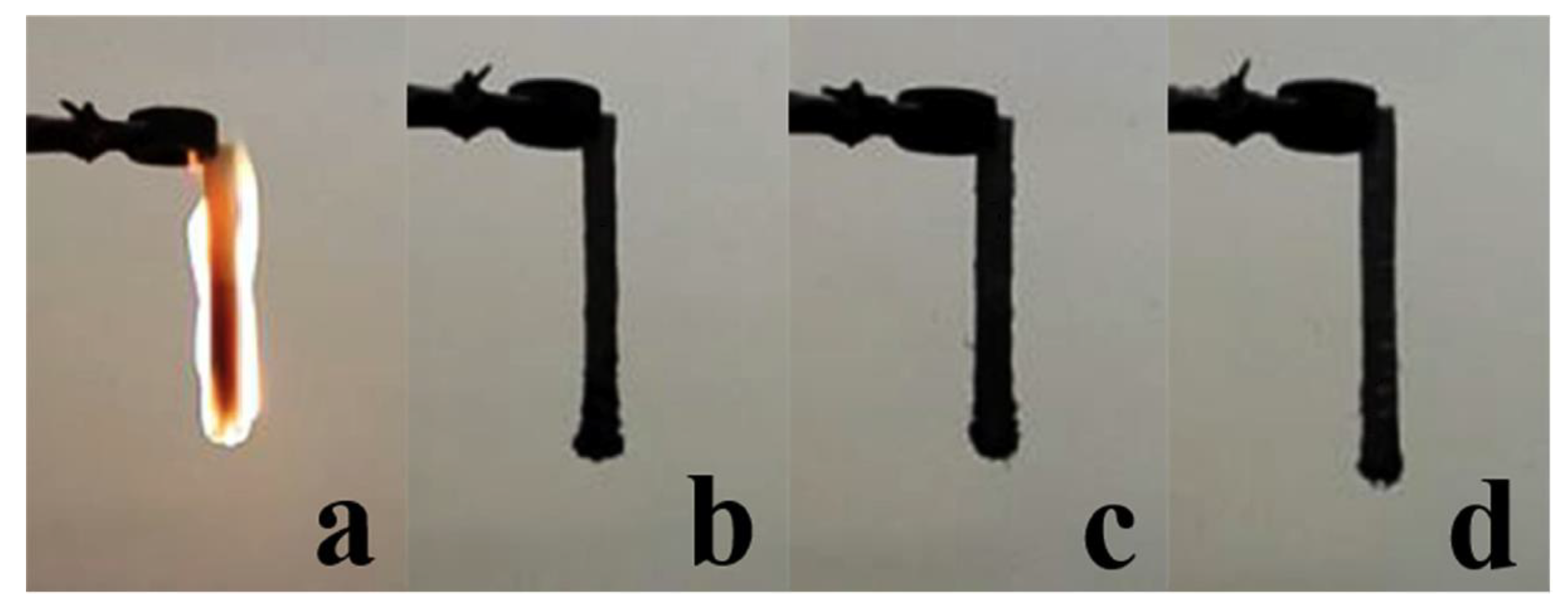
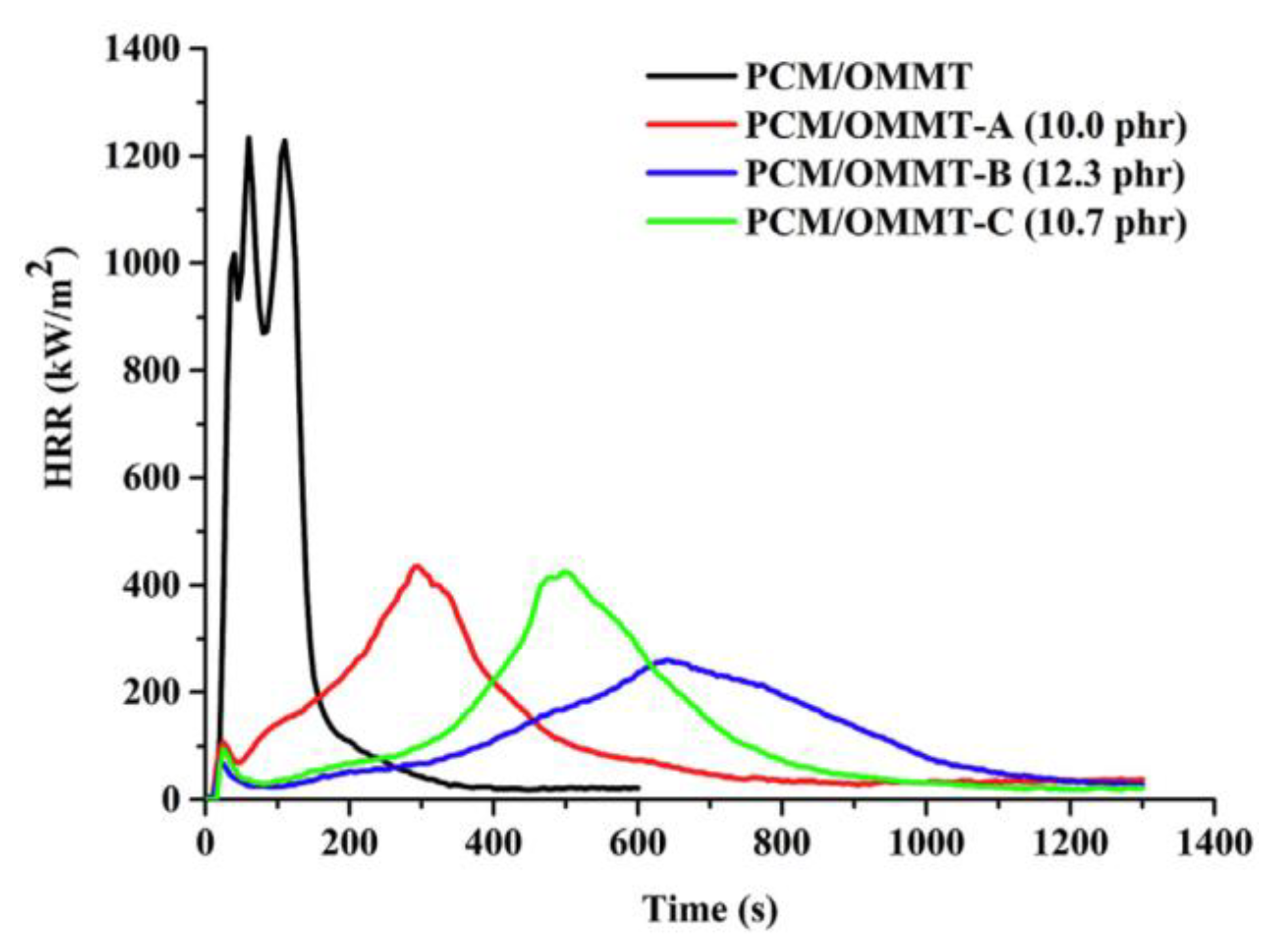
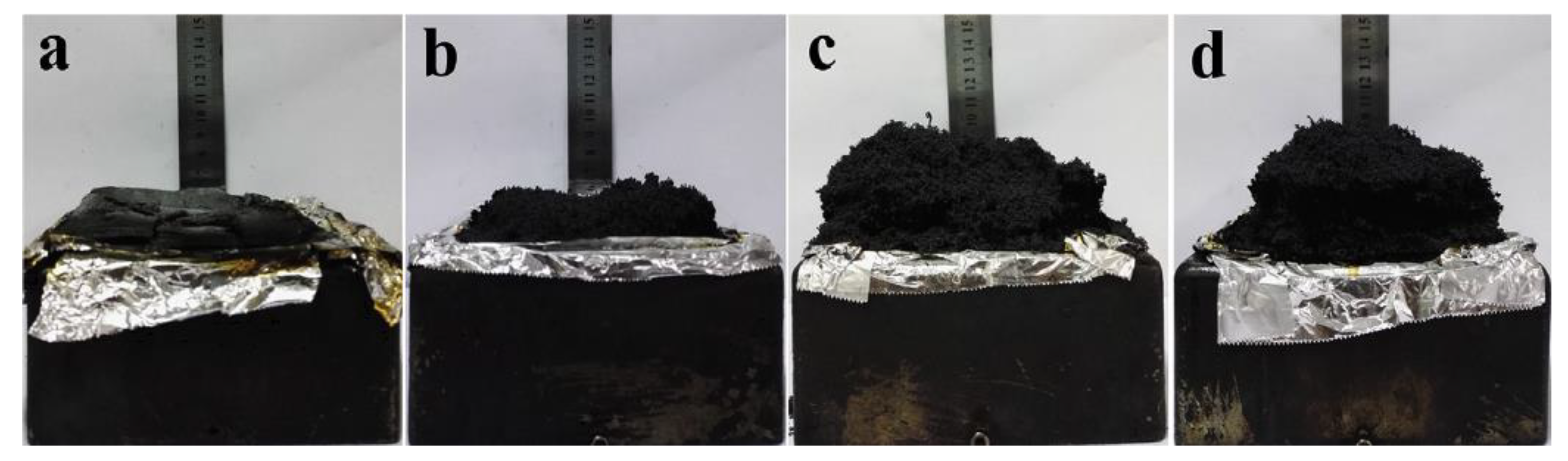
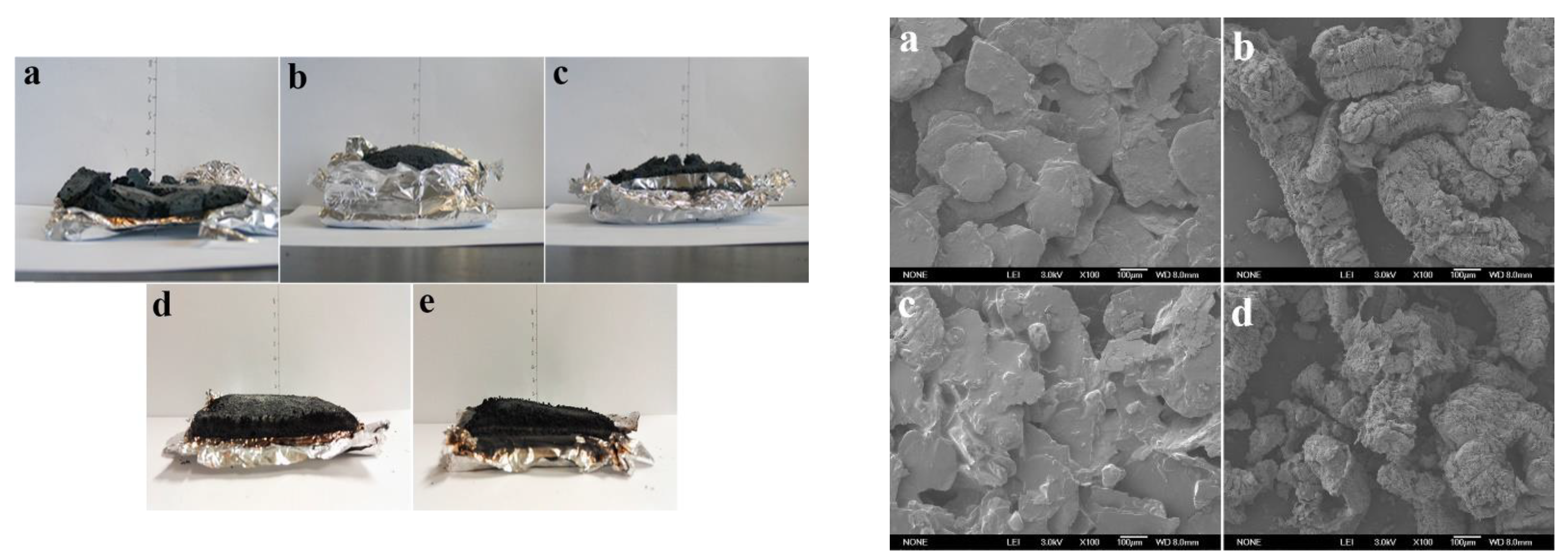
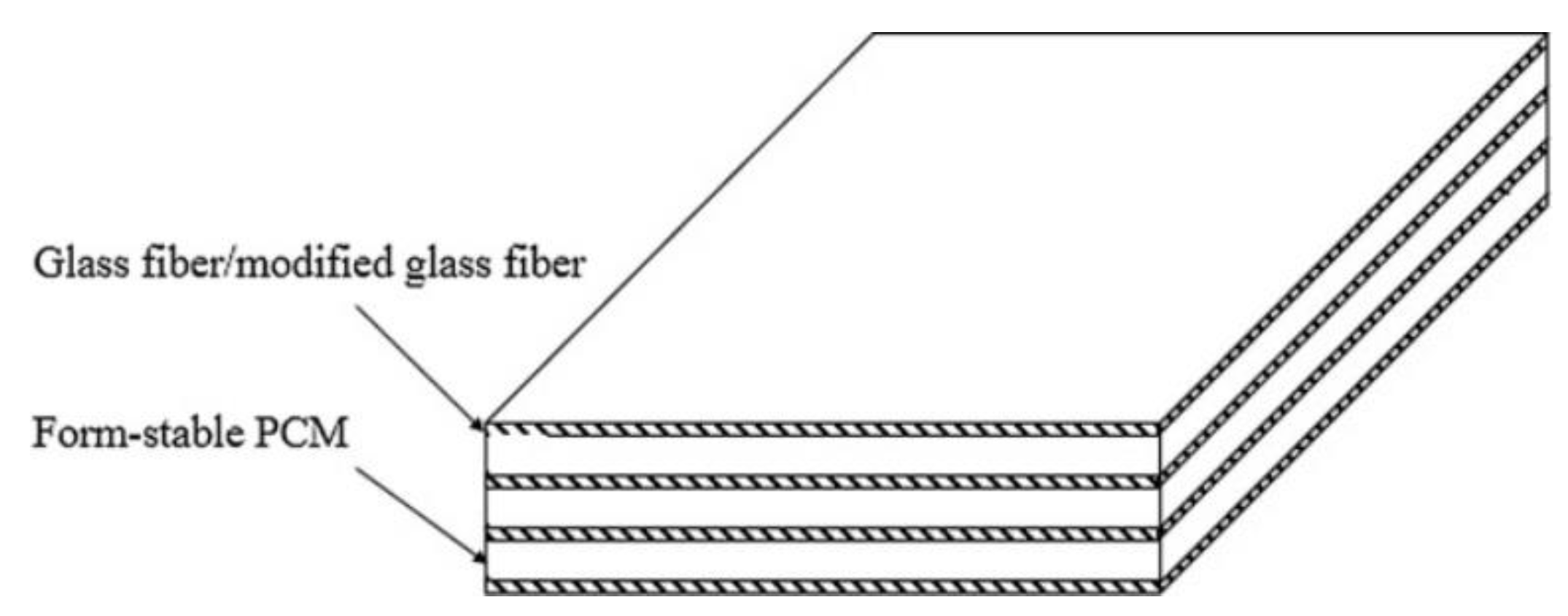

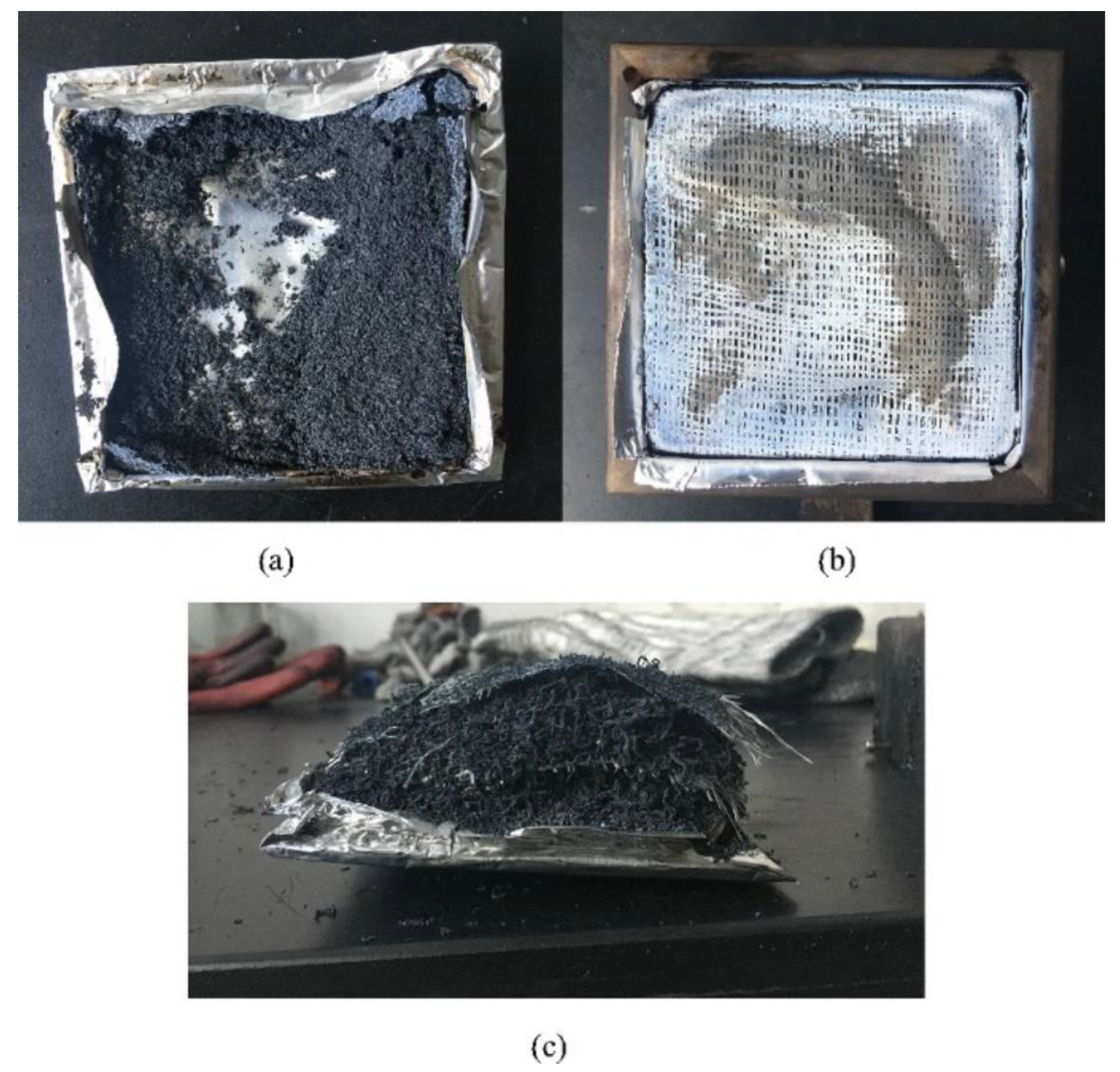

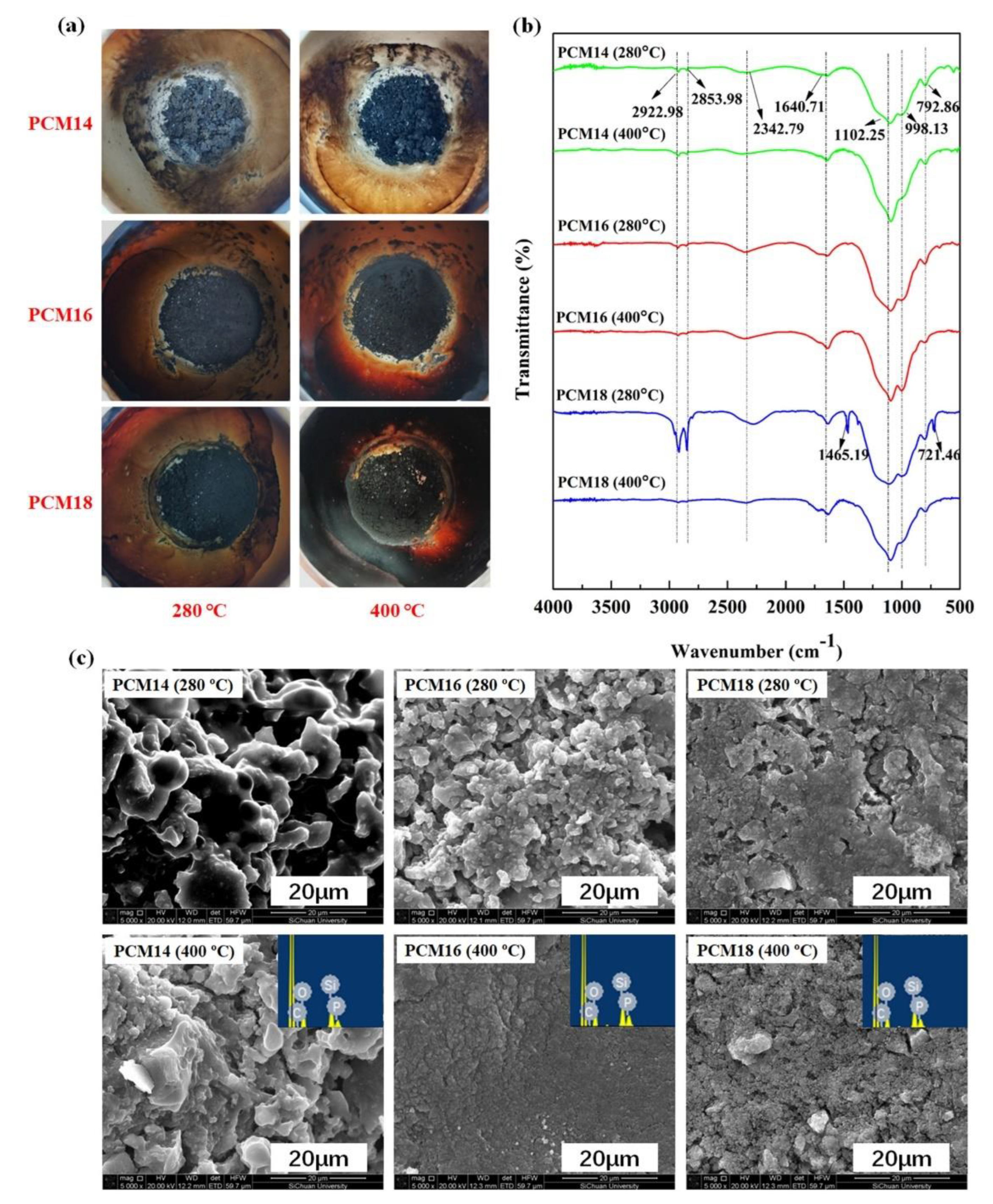


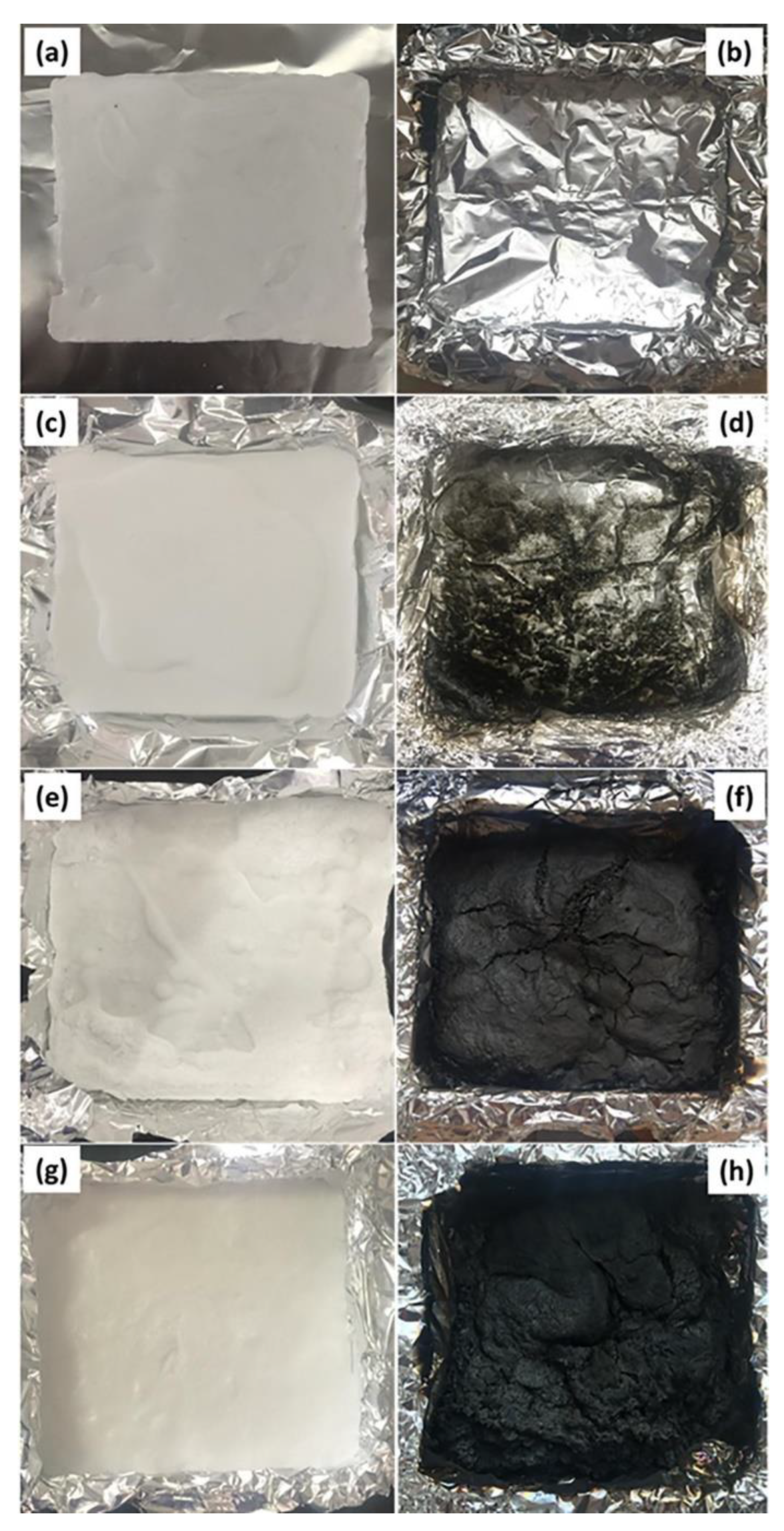


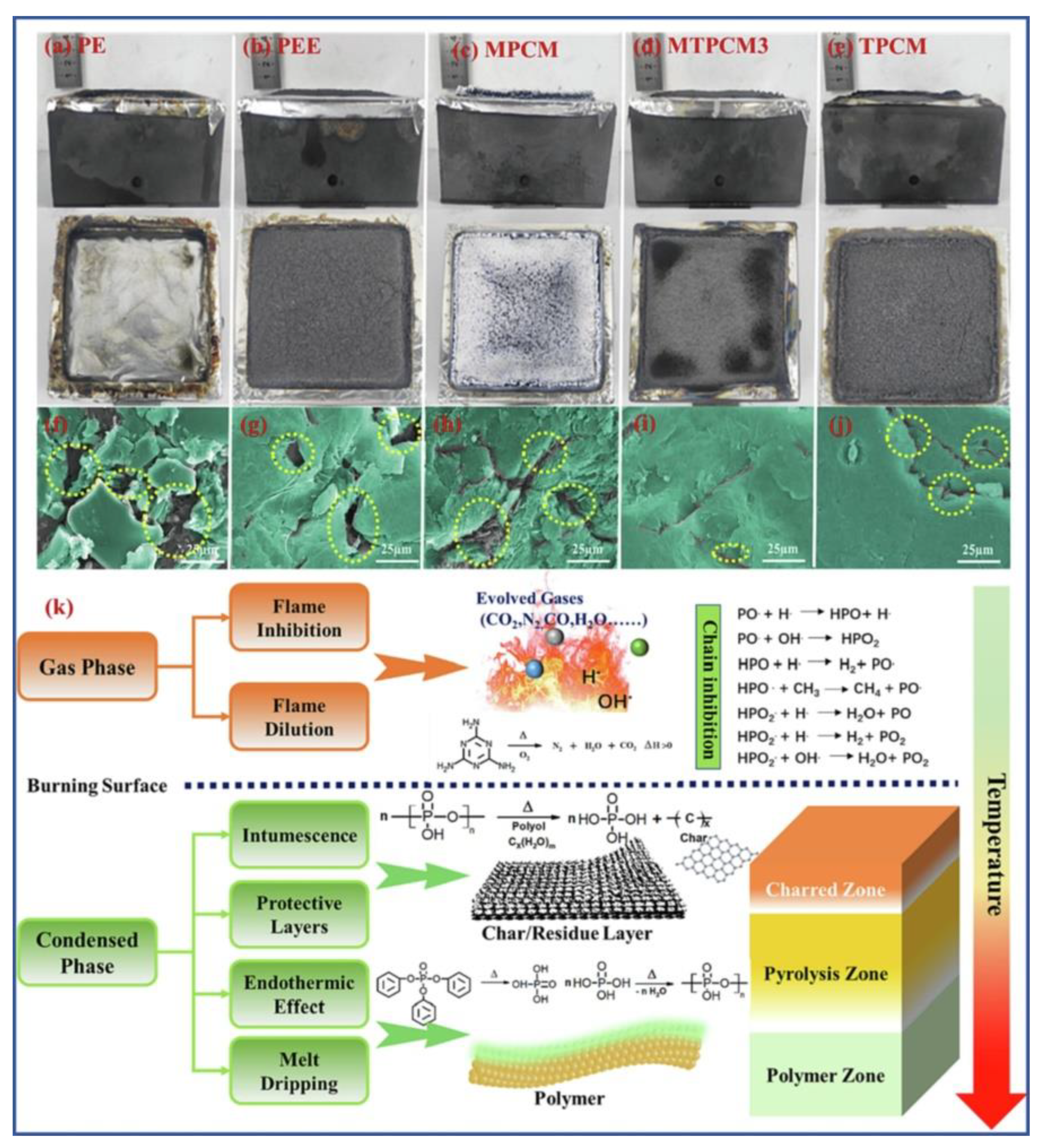



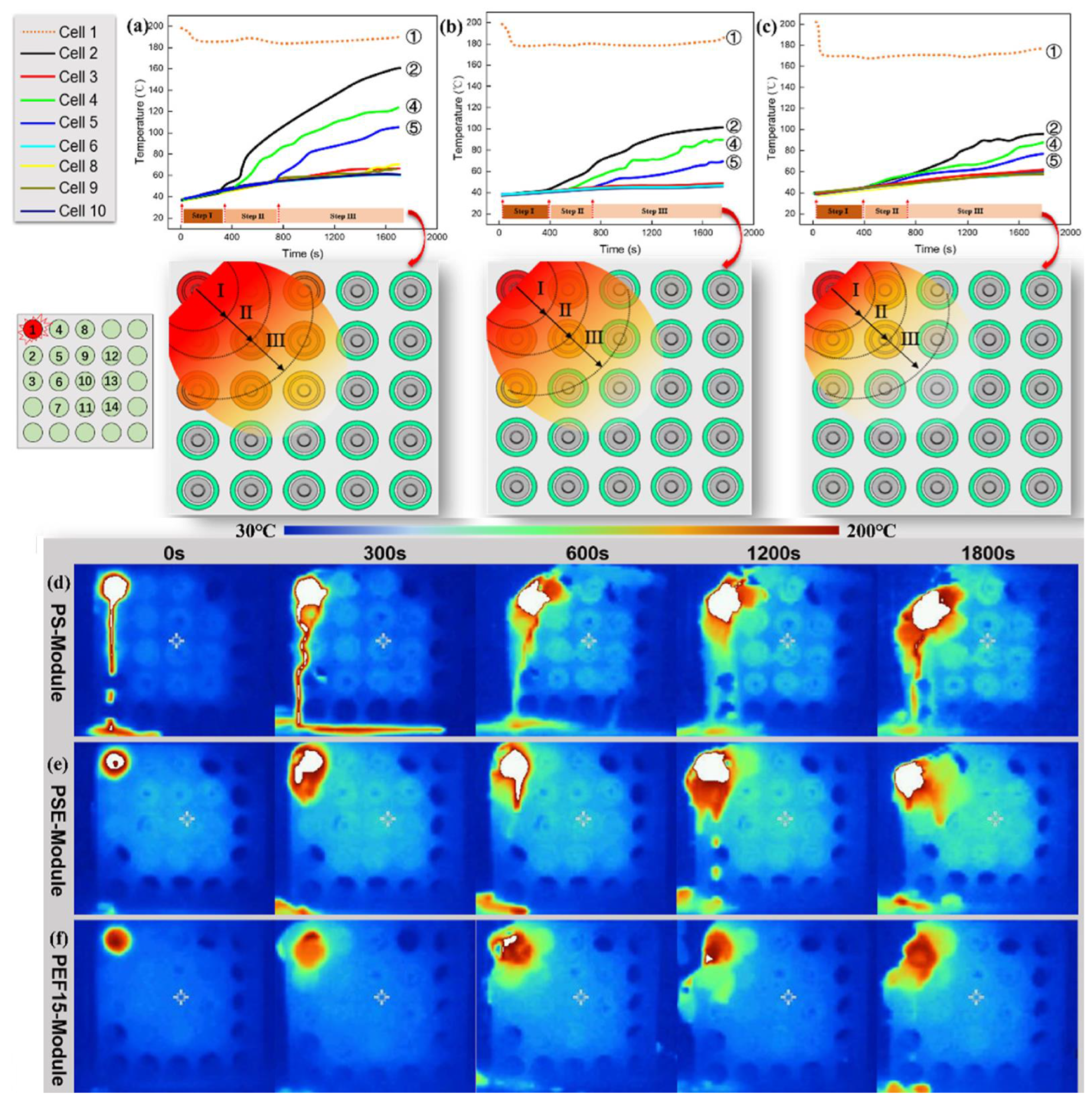

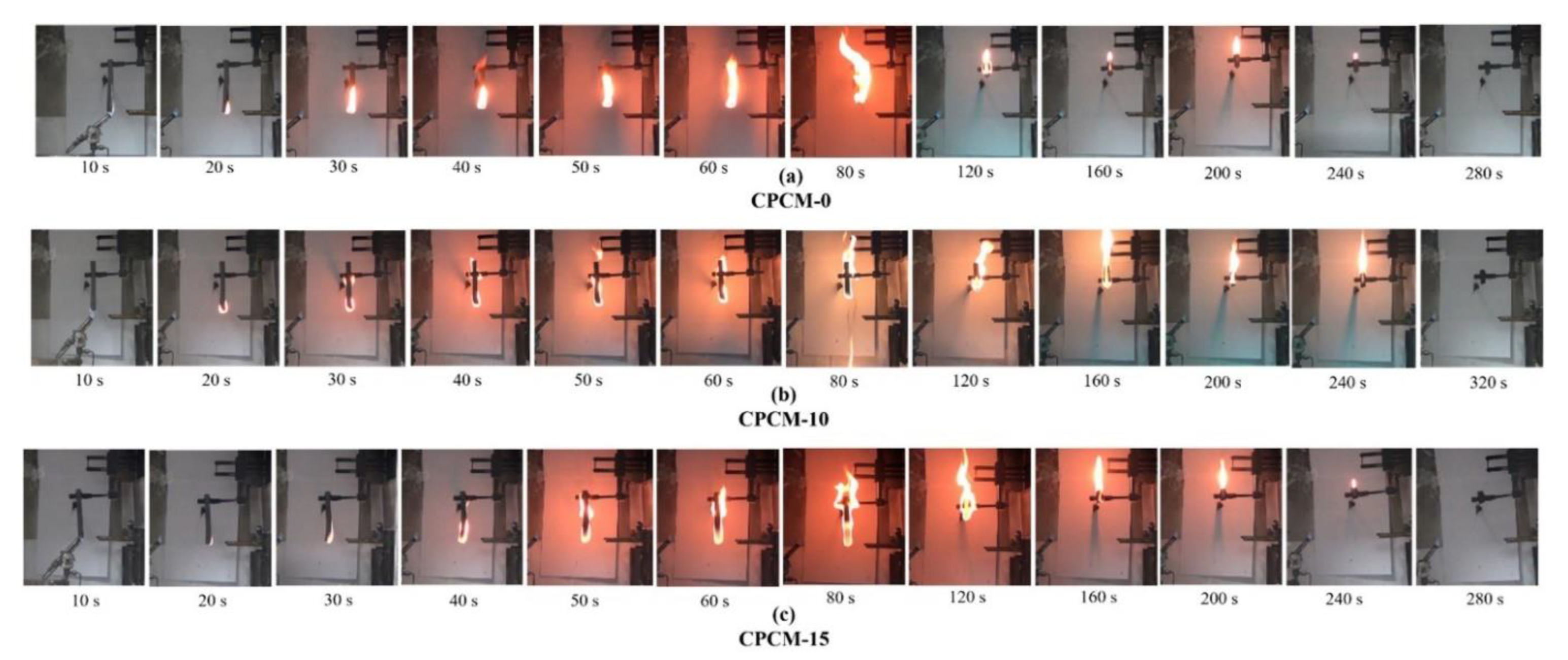
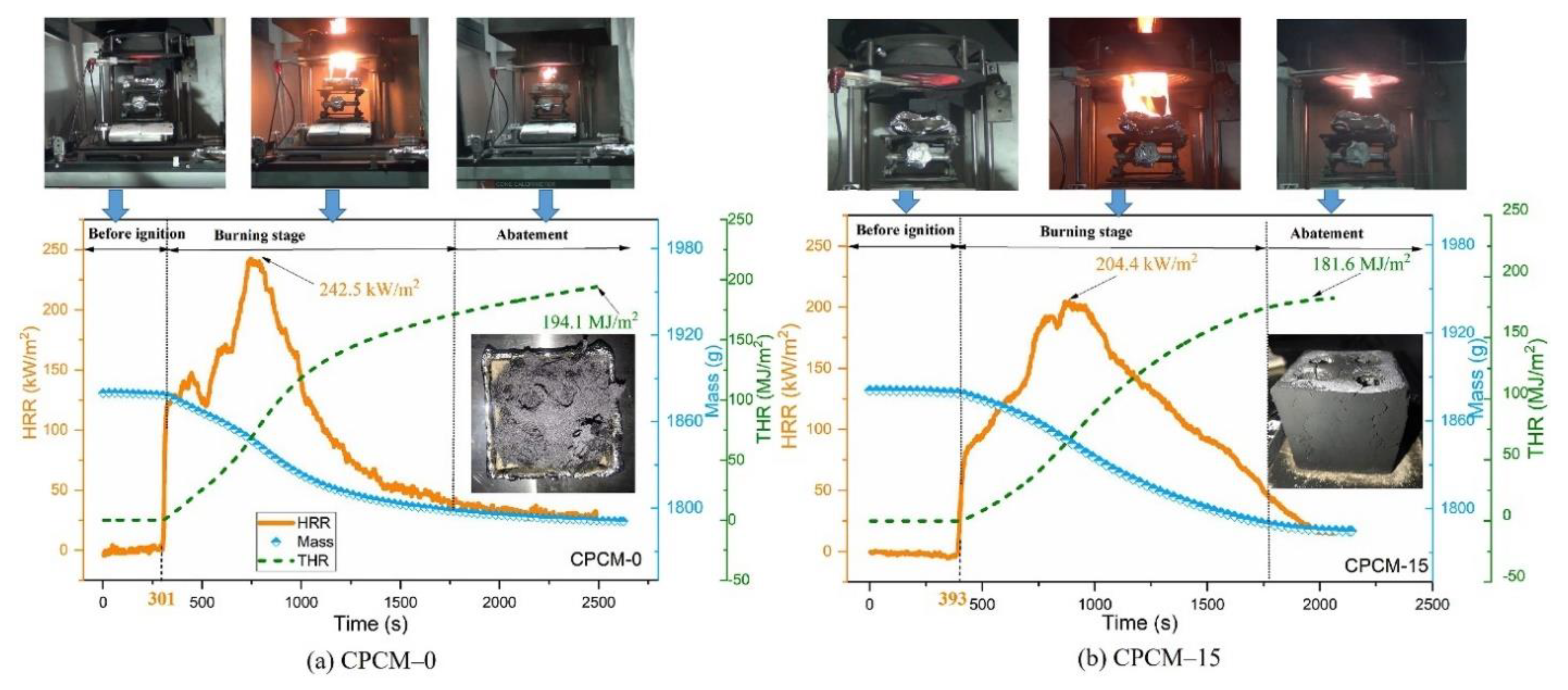
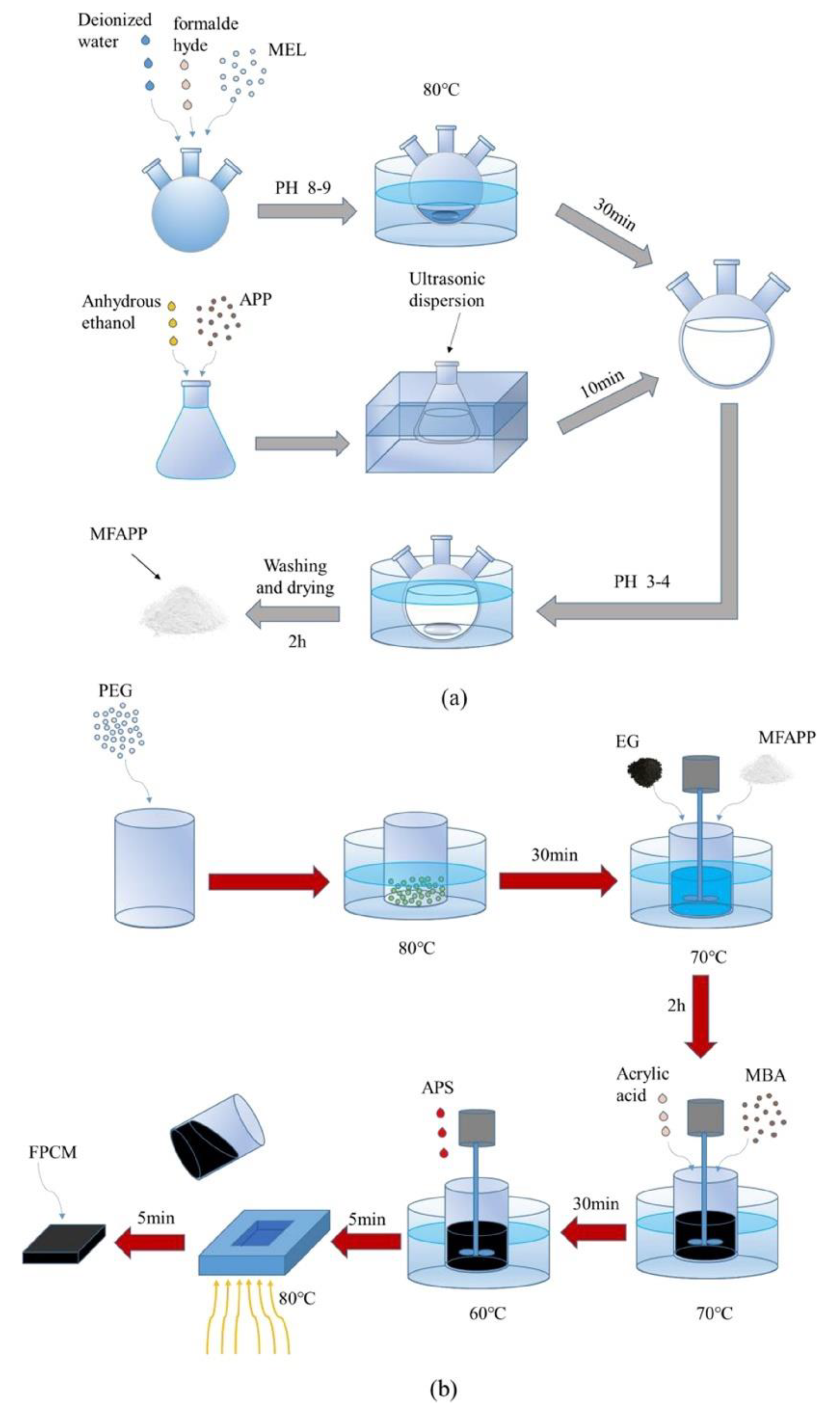

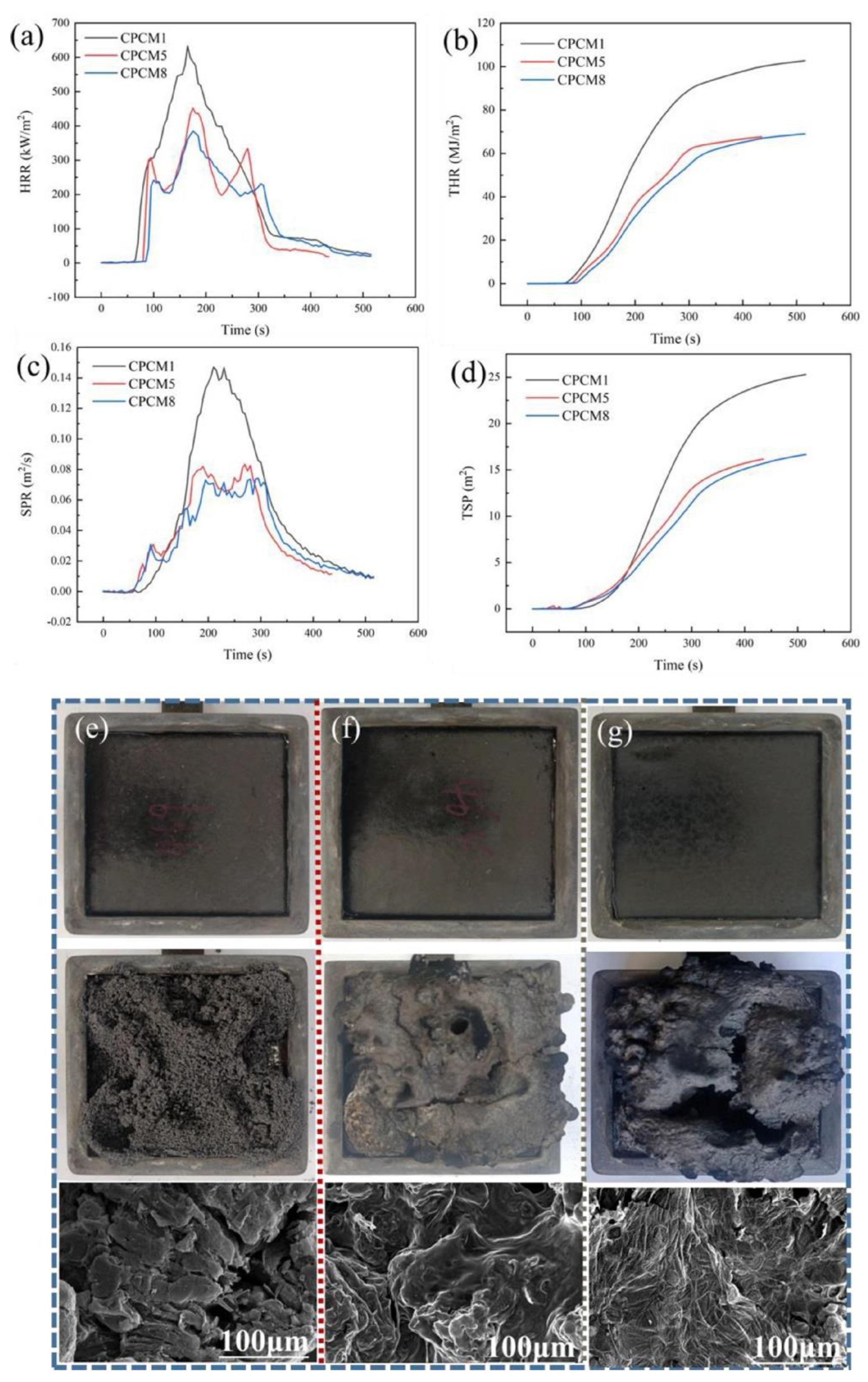
| PCM | Composition/Chemical Formula | Flammability Data |
|---|---|---|
| Paraffins | FP: 200–240 °C | |
| Fatty acids | Carboxylic acids with an aliphatic chain | FP: >200 °C May be ignited by heat, sparks, and flame |
| Esters | An acid in which the hydrogen atom of at least one acidic hydroxyl group of that acid is replaced by an organyl group | Highly flammable, low FP values |
| Polyethylene-Glycol | FP: 279 °C Explosive in presence of oxidizing materials | |
| Polybutadiene | FP: 113 °C | |
| Polyalcohol | Alcohol containing 2+ hydroxyl groups: D-mannitol D-sorbitol Xylitol Meso-erythrol | TTI: 108 s [23] TTI: 104 s [23] TTI: 77 s [23] TTI: 72 s [23] |
| Test Criteria | V-0 | V-1 | V-2 |
|---|---|---|---|
| Test specimen burning time (s) | ≤10 | ≤30 | ≤30 |
| Total burning time measured after the flame was applied 10 times (s) | ≤50 | ≤250 | ≤250 |
| Burning and afterglow time measured after second flame application (s) | ≤30 | ≤60 | ≤60 |
| Occurrence of dripping in burning specimens | No | No | Yes |
| Complete combustion to the holding clamp | No | No | No |
| Sample | TTI [s] | Ignitions Count | Combustion Time [s] |
|---|---|---|---|
| Paraffin RT-21 | 26 | 1 | 300 |
| 60% paraffin + 1% EG + 40% APP | 25 | 9 | 21 |
| CA + MA | 19 | 1 | 200 |
| 50% (CA/MA) + 1% EG + 50% Mg(OH)2 | 22 | 21 | 4 |
| CA/PA | 12 | 1 | 224 |
| 50% (CA/PA) + 1% EG + 50% Mg(OH)2 | 25 | 27 | 5 |
| Sample | Components | Components Weight Ratio | Density [kg/m3] |
|---|---|---|---|
| PCM | SBS/HDPE/paraffin | 30/10/60 | 840 |
| PCM-E4 | PCM/EG4 | 100/20 | 930 |
| PCM-E5 | PCM/EG5 | 100/20 | 930 |
| PCM-E6 | PCM/EG6 | 100/20 | 930 |
| PCM-E7 | PCM/EG7 | 100/20 | 930 |
| PCM-E8 | PCM/EG8 | 100/20 | 930 |
| PCM-M | PCM/OMMT | 100/20 | 890 |
| PCM-M-T | PCM/OMMT/THBP | 100/20/1 | 930 |
| PCM-E4-M | PCM/OMMT/EG4 | 100/10/1 | 910 |
| PCM-E4-M-T | PCM/OMMT/EG4/THBP | 100/10/10/1 | 890 |
| Outside | ||
|---|---|---|
| OS | OS | OS |
| MB | MB | PCM-coated fabric |
| TL + Comfort layer | PCM-coated fabric | MB |
| PCM-coated fabric | TL + Comfort layer | TL + Comfort layer |
| Inside | ||
| Notation | Fiber Content | Fabric Type | Specific Mass [g/m2] | Thickness [mm] |
|---|---|---|---|---|
| F1 | 50% Nomex/50% flame-resistant viscose | Plain | 124.5 | 0.31 |
| F2 | Nomex IIIA | Twill | 228.5 | 0.49 |
| Sample | Recipe Components | Mass Weight [%] | TTI [s] | THR MJ/m2 | THR/Mass Lost [MJ/m2g] | Peak HRR [kW/m2] | Average HRR [kW/m2s] | TC [W/mK] |
|---|---|---|---|---|---|---|---|---|
| PCM1 | Paraffin HDPE | 60 40 | 58 | 101.1 | 4.47 | 1133.8 | 504.86 | 0.28 |
| PCM2 | Paraffin HDPE APP + PER + MMA | 40 20 20 | 27 | 90.4 | 4.09 | 627.25 | 368.42 | 0.29 |
| PCM3 | Paraffin HDPE APP + PER + MMA | 60 15 25 | 26 | 88.8 | 4.02 | 569.19 | 348.06 | 0.34 |
| PCM4 | Paraffin HDPE APP+PER+MMA EG | 60 20 15 5 | 34 | 80.4 | 3.79 | 501.48 | 314.90 | 0.51 |
| PCM5 | Paraffin HDPE APP+PER+MMA EG | 60 15 20 5 | 38 | 81.0 | 3.78 | 430.36 | 293.97 | 0.85 |
| Sample | Paraffin [g] | HDPE [g] | EG [g] | Al(OH)3 [g] | Mg(OH)2 [g] | Antioxidant [g] |
|---|---|---|---|---|---|---|
| SS-FR-PCM1 | 28 | 12.0 | 0 | 0 | 0 | 0.12 |
| SS-FR-PCM2 | 24 | 10.2 | 6 | 0 | 0 | 0.12 |
| SS-FR-PCM3 | 18 | 7.8 | 6 | 0 | 8 | 0.12 |
| SS-FR-PCM4 | 18 | 7.8 | 6 | 2 | 6 | 0.12 |
| SS-FR-PCM5 | 18 | 7.8 | 6 | 4 | 4 | 0.12 |
| SS-FR-PCM6 | 18 | 7.8 | 6 | 6 | 2 | 0.12 |
| SS-FR-PCM7 | 18 | 7.8 | 6 | 8 | 0 | 0.12 |
| Sample | PHRR [kW/m2] | THR [MJ/m2] | TTI [s] | tPHRR [s] | MLR [g/s] |
|---|---|---|---|---|---|
| SS-FR-PCM1 | 1570 | 121 | 25 | 86 | 0.30 |
| SS-FR-PCM2 | 1098 | 109 | 28 | 108 | 0.20 |
| SS-FR-PCM3 | 860 | 108 | 30 | 98 | 0.17 |
| SS-FR-PCM4 | 828 | 112 | 31 | 94 | 0.15 |
| SS-FR-PCM5 | 763 | 110 | 34 | 96 | 0.14 |
| SS-FR-PCM6 | 656 | 103 | 38 | 90 | 0.13 |
| SS-FR-PCM7 | 703 | 106 | 29 | 86 | 0.16 |
| Ref | PCM | TCE/SS | Flame Retardant | Test Method | Preparation |
|---|---|---|---|---|---|
| Zhang [79] | Paraffin MP: 48 °C LH: 223 kJ/kg TC: 0.24 W/m·K | EG/- | APP Red phosphorus (RP) Epoxy resin (ER) | Cone UL-94 LOI | Mixing and dispersion. |
| Li [80] | Paraffin | EG/Maleic anhydride copolymers | EG/Melamine/Triphenyl phosphate | Cone UL 94 | Mixing, pouring into molds. |
| Alkhazaleh [81] | Dodecanoic acid (90%) MP: 40–45 °C | EG/Expanded perlite | Resorcinol bis(diphenyl phosphate) (10%) | Cone | Magnetic stirring. |
| Tanwar [82] | PEG | -/Polyvinyl Alcohol | Titanium Dioxide Nanoparticles | LOI | Stirring, casting, drying. |
| Li [83] | Solid paraffin wax/liquid paraffin (mineral oil)—Eutectic mixture | -/PP | Triazine char-forming agent APP | Cone TGA LOI UL 94 | Ultrasonic vibration (eutectic PCM), impregnation into matrix material, cut into sheets. |
| Huang [84] | Paraffin MP: 45 °C | EG/SBS | APP/PA/ZnO with the mass ratio 50/45/5%, respectively | UL 94 Cone LOI | Physical mixing, magnetic stirring, pouring into molds. |
| S.Pizzaro [85] | Palmitic acid MP: 60.45 °C LH: 221 kJ/kg | -/Ethylene propylene diene monomer, MMT | MMT | LOI Radiation test | Heating and mixing in a Banbury oval rotor mixer. |
| Yin [86] | Polyrotaxane | -/- | Phytic acid | Cone LOI UL 94 | PEO was dissolved in water then α-Cyclodextrin was added, followed by prolonged stirring. |
| Song [87] | Paraffin MP: 55–60 °C LH: 171.55 J/g | -/Nano structured magnesium hydroxide | RP | LOI | Nano-MH synthesized from MgCl2 6H2O and NaOH (precipitation). Raw materials were mixed and then shaped into a mold. |
| Ma [88] | Paraffin | -/Epoxy resin | Trimellitic anhydride reacted 2,6,7-trioxa-1-phosphabicyclo-[2.2.2]-octane-4-methanol Melamine cyanurate | UL94(V0 for IFR loading above 24%) Cone | Mixing, dispersion. |
| Sample | Nitrate (Total) [g] | Tannic Acid [g] | Poly Vinyl Alcohol [g] |
|---|---|---|---|
| Sample M-EPCM-1 | 0.8 | 1.6 | 1.6 |
| Sample M-EPCM-2 | 1.6 | 1.2 | 1.2 |
| Sample M-EPCM-3 | 2.0 | 1.0 | 1.0 |
| Sample M-EPCM-4 | 2.4 | 0.8 | 0.8 |
| Sample M-EPCM-5 | 3.2 | 0.4 | 0.4 |
| Sample | Peak HRR [kW/m2] | THR [MJ/m2] | TSP [m2] | TTI [s] |
|---|---|---|---|---|
| EP | 825 | 111 | 28.1 | 49.0 |
| EP/MPCM | 923 | 117 | 30.7 | 39.0 |
| EP/h-MPCM | 720 | 103 | 25.5 | 61.0 |
| EP/c-MPCM | 575 | 91 | 24.0 | 59.0 |
| Sample Reference | 1-Octadecanol [g] | Tween 80 [g] | PA (50% wt) | MgCl2 [g] |
|---|---|---|---|---|
| FRPCM-1 | 20 | 2 | 7.92 | 3.42 |
| FRPCM-2 | 20 | 2 | 13.2 | 5.7 |
| FRPCM-3 | 20 | 2 | 19.8 | 8.55 |
| Ref | PCM | Shell | Preparation/Size | Fire Retardant | Test Method/Results |
|---|---|---|---|---|---|
| Qiu [93] | Paraffin | Uncrosslinked and crosslinked poly (methacrylic acid-co-ethyl methacrylate | m | Diethyl ethylphosphonate | LOI/LOI value increased by 6–9% |
| Demirbağ [94] | n-eicosane | Gelatin/sodium alginate | Complex coacervation/1.37 1.6 m | Clay nanoparticles | Ignition time for the treated textiles with 25–50% longer |
| Du [95] | n-octadecane | PNDA-modified melamine-formaldehyde | m | PNDA | Cone, LOI PHRR dropped by 32.8% |
| Kosny [96] | Methyl ester | Not provided | Coating applied on the microcapsules/Not provided | Not provided | Not provided |
| Du [97] | n-octadecane | Poly (methyl methacrylate) | m | Crosslinking agent: Diethyl bis (2-hydroxyethyl acrylate) amino methyl phosphonate | Cone, LOI: % THR dropped % TSP dropped % LOI increased from 19.5% to 25.1% |
| Kazanci [98] | Paraffin 42–44 | Styrene monomer | Emulsion polymerization/Not provided | Ortho-Phosphoric acid PER | Gross heat of combustion (QPCS) Non-combustibility test ISO 1182: mass loss 6.2% ISO 11925-2: Single-flame source: “d0” class according to EN 13501-1 |
| Amaral [99] | Micronal®DS 5001X | PMMA | Not provided/Not provided | Melamine APP EG | Airbus test AITM 2002 F2 |
| Szczotok [100] | Rubitherm®RT27 | Styrene divinylbenzene | m | Hexa(methacryloylethylenedioxy) cyclotriphosphazene | Thermal degradation |
| Kang [101] | CA | PMMA | PMMA polymerization | Halloysite nanotube | Cone PHRR decreased by 20% and 33% compared to EP and PCM-EP |
| Sample | Paraffin [wt%] | OBC [wt%] | EG [wt%] | CP, AT [wt%] | GF | Modified GF |
|---|---|---|---|---|---|---|
| CPCM-1 | 70 | 13 | 5 | 12 | ☐ | ☐ |
| CPCM-2 | 70 | 13 | 5 | 12 | ☒ | ☐ |
| CPCM-3 | 57 | 13 | 5 | 25 | ☒ | ☐ |
| CPCM-4 | 70 | 13 | 5 | 12 | ☐ | ☒ |
| Sample | PCM/TBBP-A Composite | PCM/TBBP-A/DBDPE Composite | |||
|---|---|---|---|---|---|
| TBBP-A [%] | LOI [%] | TBBP-A [%] | DBDPE [%] | LOI [%] | |
| 1 | 0 | 16.0 | 20 | 0 | 19.3 |
| 2 | 10 | 16.9 | 15 | 5 | 19.9 |
| 3 | 15 | 18.7 | 10 | 10 | 20.3 |
| 4 | 20 | 19.3 | 5 | 15 | 21.3 |
| 5 | 25 | 20.2 | 0 | 20 | 21.9 |
| Sample | PHRR [kW/m2] | TTI [s] | FPI [m2s/kW] | THR [MJ/m2] |
|---|---|---|---|---|
| Hexadecanol | 1551 | 116 | 0.075 | 354 |
| G | 1362 | 91 | 0.067 | 239 |
| G-20 | 679 | 142 | 0.209 | 125 |
| P-20 | 1088 | 116 | 0.107 | 239 |
| Sample | PCM [g] | PEPA [g] | Burning Time [s] | Residual Weight [g] |
|---|---|---|---|---|
| Hexadecanol | 10.0 | 0.0 | 110.0 | 0.05 |
| P20 | 8.0 | 2.0 | 90.0 | 2.33 |
| G | 10.0 | 0.0 | 3.5 | 9.76 |
| G20 | 8.0 | 2.0 | 2.5 | 9.92 |
| Sample | Paraffin [%] | EVA-g-MAH [%] | EG [%] | ML [%] | TPH [%] | Melting Point [°C] | Latent Heat [J/g] |
|---|---|---|---|---|---|---|---|
| PE | 60 | 40 | - | - | - | 47.3 | 122.5 |
| PEE | 60 | 36.5 | 3.5 | - | - | 47.6 | 109.9 |
| MPCM | 60 | 11.5 | 3.5 | 25 | - | 48.2 | 115.6 |
| MTPCM1 | 60 | 11.5 | 3.5 | 20 | 5 | 47.8 | 117.5 |
| MTPCM2 | 60 | 11.5 | 3.5 | 15 | 10 | 46.9 | 118.4 |
| MTPCM3 | 60 | 11.5 | 3.5 | 10 | 15 | 47.5 | 125.7 |
| MTPCM4 | 60 | 11.5 | 3.5 | 5 | 20 | 47.3 | 130.0 |
| TPCM | 60 | 11.5 | 3.5 | - | 25 | 47.3 | 130.6 |
| Paraffin | - | - | - | - | - | 48.8 | 225.7 |
| TPH | - | - | - | - | 50.7 | 82.4 |
| Sample | UL-94 Grading | PHRR [kW/m2] | THR [MJ/m2] | PSPR [m2/s] | TSP [m2] |
|---|---|---|---|---|---|
| PE | V2 | 2029.4 | 240.1 | 0.13 | 36.0 |
| PEE | V2 | 754.9 | 236.1 | 0.10 | 13.6 |
| MPCM | V2 | 696.5 | 226.9 | 0.06 | 11.7 |
| MTPCM3 | V0 | 499.4 | 193.8 | 0.03 | 7.8 |
| TPCM | V0 | 688.1 | 193.8 | 0.04 | 11.2 |
| Sample | Component Ratio | Flammability Tests Results | |||||
|---|---|---|---|---|---|---|---|
| Paraffin [%] | SBS [%] | EG [%] | FR [%] | LOI [%] | UL-94 Grading | Total Duration of the Residual Flame [s] | |
| PS | 70 | 30 | 0 | 0 | 18.3 | V2 | 33.2 |
| PSE | 70 | 27 | 3 | 0 | 21.0 | V2 | 29.0 |
| PEF5 | 70 | 22 | 3 | 5 | 22.3 | V2 | 17.1 |
| PEF10 | 70 | 17 | 3 | 10 | 25.9 | V0 | 12.5 |
| PEF15 | 70 | 12 | 3 | 15 | 35.9 | V0 | 7.5 |
| PEF20 | 70 | 7 | 3 | 20 | 24.4 | V2 | 16.2 |
| Sample | OA [g] | HDDA [g] | BPO [g] | EG [g] | Al(OH)3 [g] | Total [g] |
|---|---|---|---|---|---|---|
| CPCM0 | 377 | 7 | 4 | 12 | 0 (0%) | 400 |
| CPCM10 | 338 | 6 | 4 | 12 | 40 (10%) | 400 |
| CPCM15 | 318 | 6 | 4 | 12 | 60 (15%) | 400 |
| Sample | EG [%] | MFAPP [%] | LOI [%] | UL-94 Grading |
|---|---|---|---|---|
| CPCM-0 | 0 | 0 | 17.8 | - |
| CPCM-1 | 3 | 0 | 19.2 | - |
| CPCM-2 | 3 | 13 | 27.3 | - |
| CPCM-3 | 3 | 15 | 29.0 | V2 |
| CPCM-4 | 3 | 17 | 30.8 | V1 |
| CPCM-5 | 3 | 19 | 32.6 | V0 |
| CPCM-6 | 3 | 21 | 35.1 | V0 |
| CPCM-7 | 3 | 23 | 38.6 | V0 |
| CPCM-8 | 3 | 25 | 43.2 | V0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaconu, B.; Cruceru, M.; Anghelescu, L. Fire Retardance Methods and Materials for Phase Change Materials: Performance, Integration Methods, and Applications—A Literature Review. Fire 2023, 6, 175. https://doi.org/10.3390/fire6050175
Diaconu B, Cruceru M, Anghelescu L. Fire Retardance Methods and Materials for Phase Change Materials: Performance, Integration Methods, and Applications—A Literature Review. Fire. 2023; 6(5):175. https://doi.org/10.3390/fire6050175
Chicago/Turabian StyleDiaconu, Bogdan, Mihai Cruceru, and Lucica Anghelescu. 2023. "Fire Retardance Methods and Materials for Phase Change Materials: Performance, Integration Methods, and Applications—A Literature Review" Fire 6, no. 5: 175. https://doi.org/10.3390/fire6050175
APA StyleDiaconu, B., Cruceru, M., & Anghelescu, L. (2023). Fire Retardance Methods and Materials for Phase Change Materials: Performance, Integration Methods, and Applications—A Literature Review. Fire, 6(5), 175. https://doi.org/10.3390/fire6050175








