Abstract
Wildfires cause profound challenges for animals to overcome due to their reliance on vegetation. This study addresses the impact of three levels of forest burn severity (unburned, low, and high burn severity) on the foraging behavior of small mammals in the Pinaleño Mountains (AZ, USA) using the giving up density (GUD) experiment approach. Overall, burn severity affected the foraging behavior of small mammals that spent less time foraging in high burn severity patches. Vegetation characteristics influenced GUD differently based on the level of burn severity. Higher canopy cover was perceived as areas with a higher predation risk (higher GUD) in unburned and low burn severity patches, while cover provided by logs and shrubs decreased the GUD (increased foraging). This suggests a complicated interaction between horizontal (logs, grass, shrub cover) and vertical vegetation cover in relation to burn severity. Fires affected the foraging behavior of the small mammals but did not impact all species in the same way. Generalists, such as Peromyscus sp. and Tamias dorsalis, seemed to forage across all burn severities, while specialist species, such as tree squirrels, tended to avoid the high burn severity patches. Clarifying the complex impacts of fires on small mammals’ foraging behaviors contributes to our understanding of the intricate interactions, at micro-habitat levels, between vegetation structure and the behavioral responses of animals and it can help managers to plan actions to reduce the negative impacts of wildfires.
1. Introduction
Climate change is increasing the magnitude of extreme environmental events [1,2], such as wildfire severity and frequency [3,4,5]. In the western United States, fires are estimated to increase at a rate of seven additional large events each year [6]. This phenomenon is quite alarming considering the profound changes caused by fire in the composition and abundance of plant and animal species [7,8,9]. The direct effects of wildfires, such as death due to injury, extreme temperature, or smoke inhalation [10,11], are not an exhaustive description of the consequences of wildfires on wildlife. Indirect effects, including habitat loss and environmental changes, arise later, but can extensively impact animals’ behavior and survival [10,11,12].
The largest obstacle animals have to overcome when their habitat is affected by fires is to suddenly deal with a new environment where the vegetation structure and composition have been modified and the quantity and distribution of resources have changed, while predation has potentially increased [11,13]. Changes in cover availability can influence the effective and perceived predation risk and subsequently alter the behavior, demographics, and growth rates of prey populations [14,15]. For example, periodically burned grassland fires influence herbivores’ forage availability and quality, consequently affecting bison’s (Bison bison) feeding behavior with an increase in bite mass and instantaneous intake rate per individual [16]. Woodpeckers (Picoides sp.) tend to select burned areas after a fire due to an increase in insects in dead logs [17]. The ability to adapt to those changes is reflected in fitness, and ultimately in the entire population affected by fire [10,18].
The effects of fire on wildlife populations depend upon the ecology of the species [19,20,21,22]. For example, the population of the insectivore rodent Akodon cursor increased after the fire, whereas frugivore/granivore Oecomys concolor decreased or disappeared in some areas [23]. Usually, fires affect greatly isolated populations or populations with small geographic ranges, threatening their potential for recovery [24]. Fires also have a larger effect on habitat specialists, such as small to medium forest-dwelling mammals, because of their narrow niche breadth and reliance on specific vegetation community types [25].
Small mammals are a crucial component of forest ecosystems; they play an important role in the dispersal of plant seeds and spores of mycorrhizal fungi and are prey for many avian and mammalian predators [26,27]. Therefore, any changes in small mammal abundance and behavior due to disturbances, such as fires, may affect how the forest ecosystem functions. Fire creates a mosaic landscape of different degrees of burn severity, which influences the foraging behavior of rodents [28]. The post-fire landscape offers heterogeneity in opportunities and risks, with patches characterized by a higher concentration of resources, but also patches with high visibility (high predator encounter risk) due to the reduction in canopy and shrub cover. Predators do not only influence their prey by killing them, but also by creating a landscape of fear that influences the prey’s activity time, foraging tactics, and microhabitat selection [29,30]. The experimental approach that has been long used to study spatial or temporal differences in animals’ perception of benefit versus cost is the giving up density (GUD) model [31,32,33,34]. The GUD corresponds to resource density in a patch at which an animal stops to forage because further time spent in the same patch will add more costs (energetic costs, risk of predation, missed opportunity cost of not engaging in alternative activities) than benefits [33,35].
The increased landscape fragmentation caused by fire can radically change habitat connectivity for long periods of time [36], especially for small mammals as they perceive barriers to movement at finer spatial scales [37]. Several studies have investigated the influence of fire and fuel management treatments on small mammal abundance [13,26,38,39,40]. However, research focused on how disturbance affects animal behavior is scarce and the response of animals to burned patches is not always clear [41].
In this study, we addressed the impact of different burned patches on the foraging behavior of small mammals in the forested areas of the Pinaleño Mountains of southeastern Arizona, USA. In the summer of 2017, the Frye Fire was ignited on Mount Graham by a lightning strike and in 3 months burned 20,000 ha of forest, creating a mosaic landscape of different burn severity patches. In particular, (1) we analyzed the foraging decisions made by small mammals in different burned severity patches as a trade-off between costs and benefits; (2) we studied the role of vegetation characteristics in the cost–benefit analysis of foraging decisions; (3) we determined if different species show variation in the use of various areas with different levels of burn severity. The results provide insights into the multivariate nature of the foraging process and the diversity of factors upon which foraging decisions are made. Through an integrated approach using of giving up density experiments and camera traps, it was possible to understand how the post-fire landscape affects foraging behavior in small mammals, including the differential use of burned patches.
2. Materials and Methods
2.1. Study Site
The study area is located above 2750 m a.s.l. in the Pinaleño Mountains, Graham County, AZ, USA where the federally endangered Mt. Graham red squirrel (Tamiasciurus fremonti grahamensis [42]) occurs. The vegetation is dominated by Engelmann spruce (Picea engelmannii) and Rocky Mountain fir (Abies lasiocarpa) at the highest elevations, with Douglas fir (Pseudotsuga menziesii) and Southwestern white pine (Pinus strobiformis) occurring more frequently as elevation decreases [43].
In 2017, the Frye Fire burned 20,000 ha and ranged in elevation from 1219 to above 3000 m a.s.l., impacting the spruce-fir forest as well as the mixed-conifer forest. The Frye Fire created a mosaic landscape of different burn severity patches [44]. Within this mosaic, we studied the effect of burn severity on small mammals from May to July during the two years following the fire (2018 and 2019), at elevations of 2750 m and above. We specifically selected this elevation range to include the habitat of the endangered Mt. Graham red squirrel. We selected 4 areas (Grant Hill, Soldier Trail, Bible Camp, and the vicinity of the Mt. Graham International Observatory) that were affected by the Frye Fire in the summer of 2017 and that were easily accessible.
2.2. Study Design
With the ArcGIS version 10.8.1 software we created a polygon of about 2 ha around each of the selected 4 study areas. Within each polygon, we generated 50 random points at least 50 m apart. We overlapped these points with the aerial image of the area post-fire (fire perimeter and burn severity were obtained from the US Forest Service Burned Area Emergency Response Program) and classified them into three categories of fire severity. We then randomly selected 5 points for each burn severity category within the area. Therefore, each area included 15 patches, 5 for each distinct burn severity. A patch was an area with a 10 m radius attributed to a burn severity category, following the classification by Parson et al. (2011) [45]. Class 1 “unburned” was categorized by almost all surface organics (soil, woody debris, surface roots, etc.) remaining intact with little to no charring, with vegetation remaining green, and tree canopies being unaltered. Classes 2 and 3 were identified under the term “low/moderate severity” and consisted of surface organics consumed leaving brown to black charring and gray ash, vegetation going from green to brown from scorching, and canopy foliage scorched but not completely consumed. Class 4 “high severity” was the most severe with almost all surface organics destroyed with heavy charring and more ash, with almost no vegetation remaining, and canopy foliage completely consumed. The distance between these patches was much smaller than the home range of a single small mammal and it allowed individuals to visit multiple patches during their daily movements [46,47,48].
2.3. Vegetation Analysis
To investigate microhabitat features that could affect the foraging behavior of small mammals in addition to burn severity, we established a 10 m radius plot around the tray at each patch location, and we collected vegetation data both in 2018 and 2019 (Table A2). In each plot, we measured the diameter at breast height (DBH; cm) of all woody plants (shrub DBH < 10 cm, tree DBH > 10 cm; [49]), we recorded the species, and we classified the tree as alive and unburned, dead, or damaged by the fire. At 5 m and 10 m intervals from the center of the plot, we measured canopy density in each of the 4 cardinal directions, using a spherical densiometer and following the method proposed by Strickler (1959) [50]. For each piece of coarse woody debris (CWD) (defined as wood longer than 1 m with a diameter >20 cm), we recorded its length, smallest diameter, and largest diameter. The volume of CWD was determined using Smalian’s volume formula. To estimate the grass and shrub cover in each plot, we first recorded the percentage of each in the 4 quadrants and, subsequently, we averaged the values. Shrub cover was considered as any vegetation at least 40 cm tall (including ferns and small trees).
2.4. Giving up Density Experiment
We determined how the different burn severities affected the foraging behavior of small mammals using the giving-up density (GUD) experiment [31,32] in the 15 patches of each study area. We used one plastic tray (58 × 41 × 16 cm) placed in the center of the patch (2 L capacity each, 5 trays in each patch type, and 15 trays each study area) filled with 2 L of play sand mixed with 50 g of black oil sunflower seeds. We used sunflower seeds because have largely been utilized in similar experiments with many different rodents (Peromyscus sp. [51,52,53,54]; Tamiasciurus sp. [55,56]).
Trays also contained a lattice-like mesh to make the search for seeds more difficult. The seed trays were set out in the field for 3 consecutive days between May and July 2018 and 2019. In 2019 two repetitions were completed in each area. We considered the first day as a pre-baiting session, to let animals become familiar with the artificial food and tray, whereas we used the second and third days to measure the GUD. We sifted the trays once a day before sunset, and the sunflower seeds that remained in the tray were collected and replaced with another 50 g of seeds. Each time the sifted seeds were weighed with a precise digital milligram scale with a measurement range of less than 50 g and a precision sensor system of 0.001 g with an error within 0.005 g.
2.5. Burn Severity Patches Used by Different Species
We used a camera trap (Bushnell Trophy camera—model 119436) in video mode to enable the identification of the species visiting the trays [57]. From each video, we recorded the species, the day, and the time. Cameras were placed 40 cm above the ground facing the tray and set up to record 15 s videos, with 45 s intervals between consecutive videos. Cameras were active 24 h per day, and the batteries were checked every day.
2.6. Statistical Analysis
We performed all statistical analyses with R (Version 4.0.3; R Development Core Team, Auckland, New Zealand). Before proceeding with the data analysis, we averaged the grams of seeds retrieved in the tray (GUD) of the two consecutive nights of the experiment to avoid pseudo-replication. We applied a two-step analysis: we first assessed whether small mammals were more or less likely to visit the tray based on the burn severity of the patch where the tray occurred and, on the year, when the experiment occurred. Then, just for those trays that were visited, we assessed how the GUD was affected by the same two factors (burn severity and year). We investigated the presence/absence of any small mammals at the trays using a generalized linear model with binomial distribution and logit link function. We considered no seeds eaten as the absence of small mammals (value = 0), whereas when seeds were eaten from the tray (GUD < 50 g) we considered it as a presence (value = 1). The explanatory variables tested were year (2018 and 2019) and patch type (unburned, low burn severity, high burn severity), whereas the area was treated as a random factor. Next, we subset the data, considering only the trays visited by small mammals. We fitted a general linear model with Gaussian distribution and identity link, where the explanatory variable and random variables were the same as in the previous model. Model assumptions were verified by plotting residuals versus fitted values.
To determine which microhabitat characteristics influenced GUD in the 3 different burn severity patches, we fitted a linear model, grouping data by the burn severity of the patch. We verified possible correlation among the vegetation characteristics using the Pearson correlation test (r ≥ |0.7|). Area was included as a random factor, while the total volume of logs, percentage of grass cover, shrub cover, and canopy cover were included as fixed effects after being scaled.
We also assessed if the number of species present at each tray differed among patches with different burn severity using a generalized linear mixed model (family Poisson, log link), with area as a random effect and patch type as a fixed effect. For this analysis, we did not include large mammals detected by the camera (bear, fox, skunk) or birds (for a complete description of species detected by the camera see Table A1). We also qualitatively analyzed the absolute frequency of each species counting the number of trays they used in each patch type across the 2018 and 2019 field seasons.
3. Results
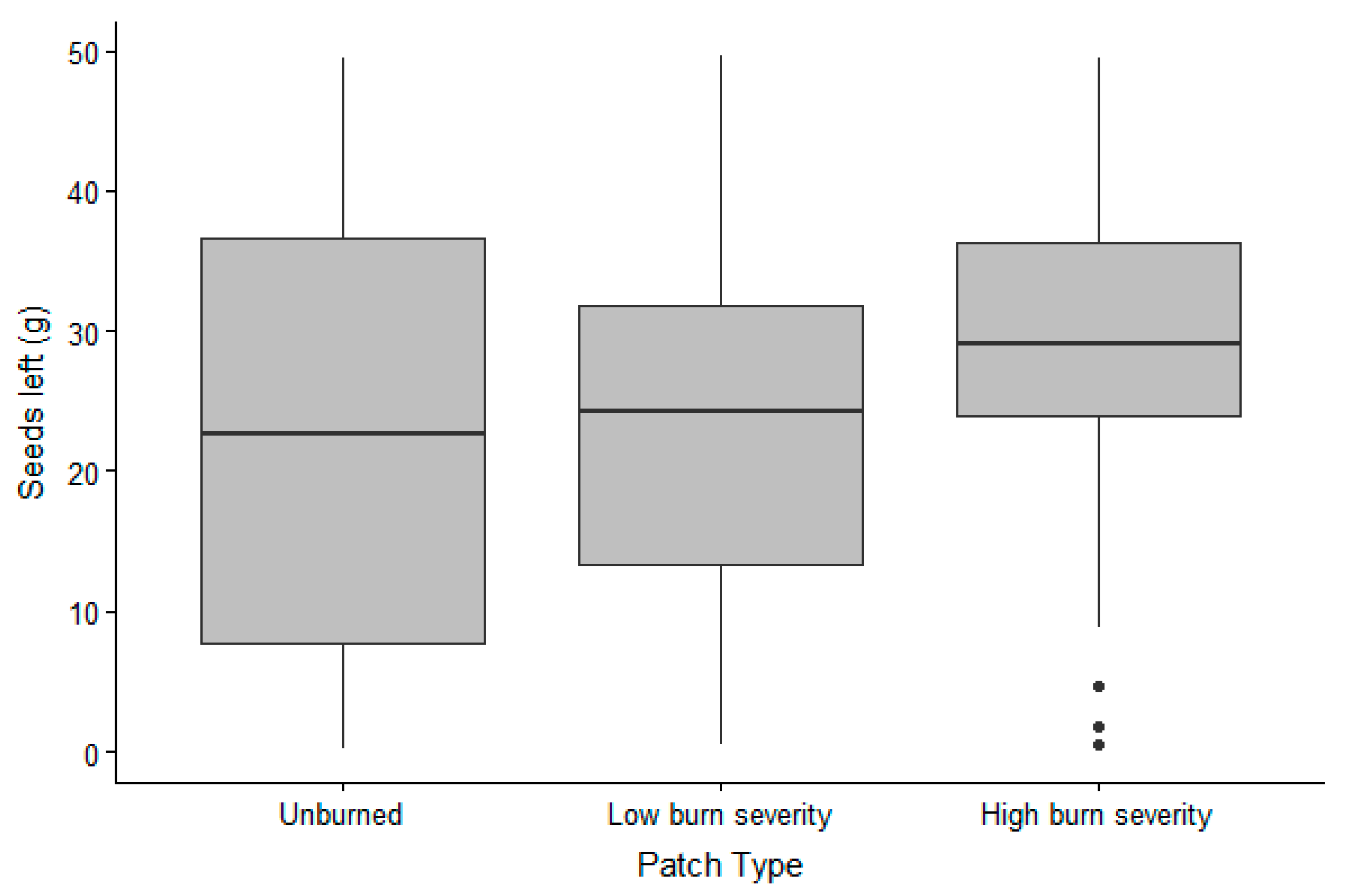
The presence of animals in the trays was lower in 2018 (65% of the trays visited) than in 2019 (all but one tray visited); however, the presence of small mammals was not affected by burn severity (Table 1). For the trays that were visited, GUD did not differ between years but was affected by the burn severity of the patch (Table 2). In fact, more grams of seeds were left in completely burned patches than in partially or unburned patches (Figure 1).

Table 1.
Results of the generalized linear mixed model (binomial distribution, logit link), examining the effect of burn severity and year on the GUD (grams of seeds left at the tray) in the Pinaleño Mountains, Graham County, AZ, USA, in 2018 and 2019.

Table 2.
Results of linear mixed model (Gaussian distribution, identity link), examining the effect of burn severity, year, and total volume of logs on the GUD (grams of seeds left at the tray) in the Pinaleño Mountains, Graham County, AZ, USA, in 2018 and 2019.
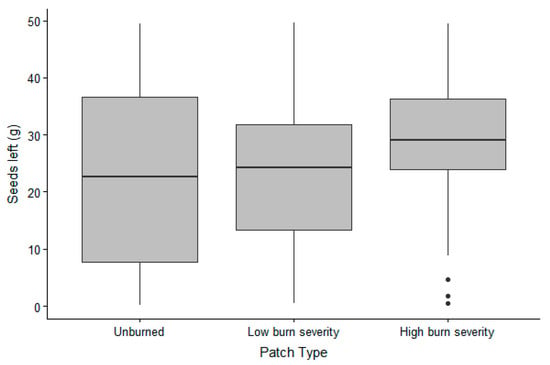
Figure 1.
Grams of sunflower seeds collected from the trays (therefore non-eaten by animals = high GUD) in the 3 burn severity patches in the Pinaleño Mountains, Graham County, AZ, USA, in 2018 and 2019.
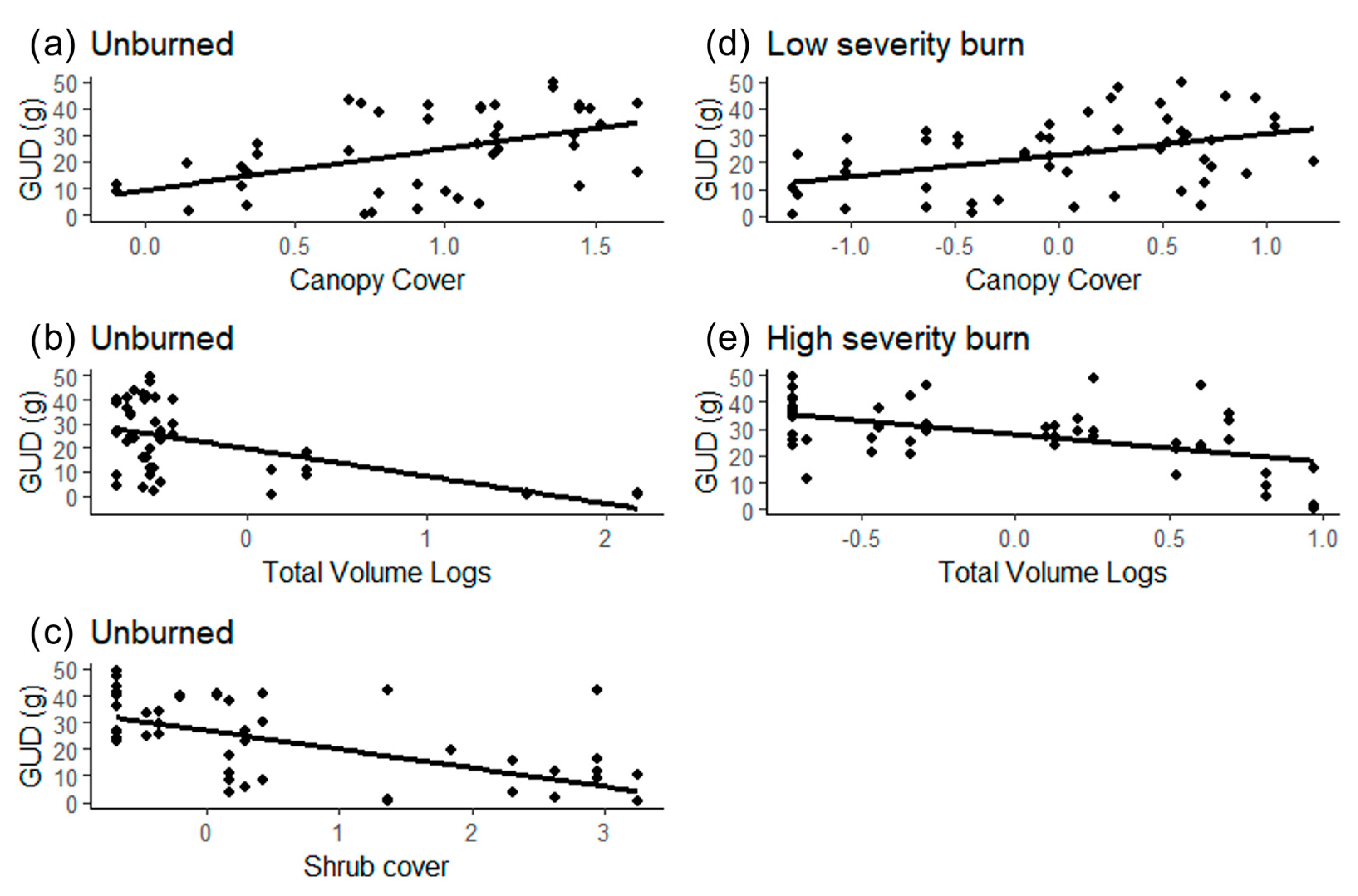
Vegetation characteristics affected GUD differently based on the patch type (Table 3). GUD was higher in unburned patches with little shrub cover but high canopy cover (Figure 2a–c). In the low burn severity patches, GUD was higher with high canopy and grass cover (Figure 2d). In the high burn severity patches, GUD was lower when there were more logs on the ground (Figure 2e).

Table 3.
Results of linear mixed models, examining the effect of vegetation characteristics on the GUD (grams of seeds left at the tray) in different burn severity patches in the Pinaleño Mountains, Graham County, AZ, USA, in 2018 and 2019.
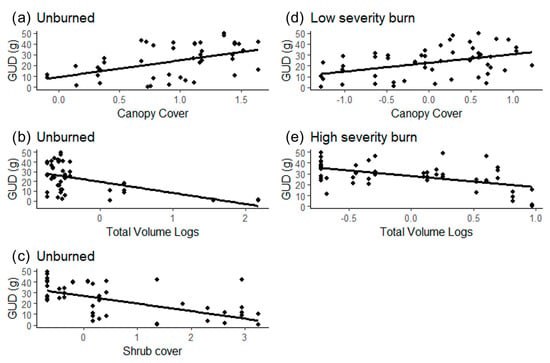
Figure 2.
Effect of statistically significant vegetation characteristics on the GUD (grams of seeds left at the tray) in different burn severity patches [(a–c) unburned, (d) low severity burn, (e) high severity burn] in the Pinaleño Mountains, Graham County, AZ, USA, in 2018 and 2019.
We detected the following species visiting the trays: Abert’s squirrel (Sciurus aberti), cliff chipmunk (Tamias dorsalis), Mt. Graham red squirrel (Tamiasciurus fremonti grahamensis), mouse (Peromyscus sp.), Mexican woodrat (Neotoma mexicana), rock squirrel (Otospermophilus variegatus), long-tailed vole (Microtus longicaudus), gray fox (Urocyon cinereoargenteus), black bear (Ursus americanus), bird (species unknown), and striped skunk (Mephitis mephitis) (Table A1).
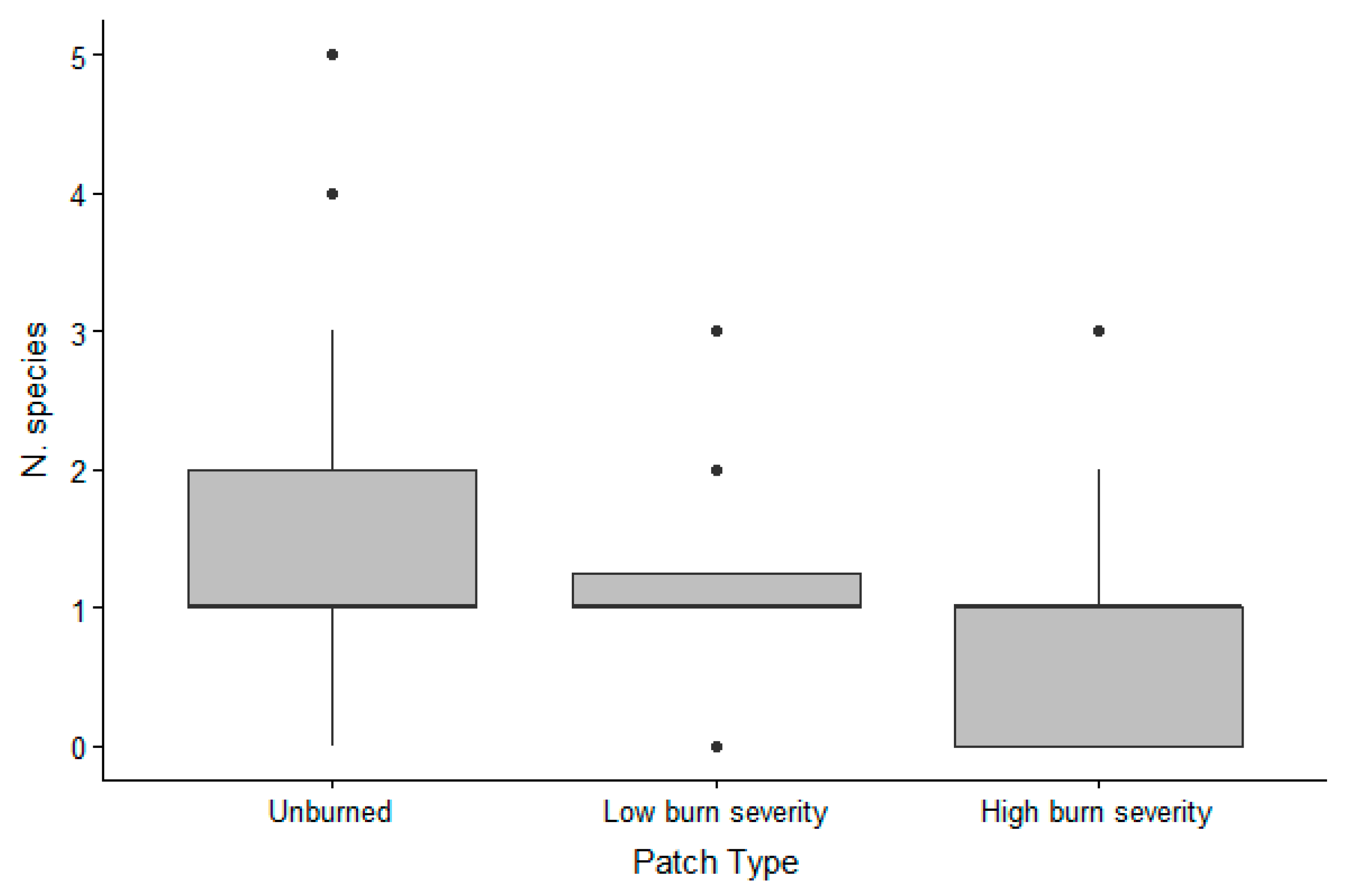
The different burn severity patches were used by a different number of species (Figure 3), with a mean of 1.72 (SD 1.03) species for unburned areas, 1.36 (SD 0.59) for partially burned areas, and 1.22 (SD 0.48) for completely burned areas. Species number was higher in unburned patches (beta = 0.55, 95% CI [0.17, 0.94], p = 0.005), compared to totally burned patches while there was no difference between the number of species detected in low burn severity patches versus totally burned patched (beta 0.23, 95% CI [−0.18, 0.64], p = 0.267), and between unburned and low burn severity (beta 0.32, 95% CI [0.05, 0.70], p = 0.09).
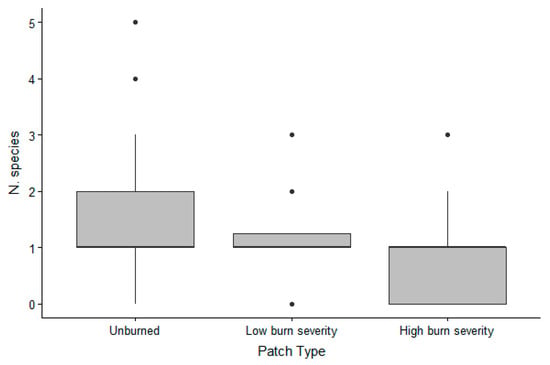
Figure 3.
Box plot of the number of species of vertebrates detected in each tray per burn severity in the Pinaleño Mountains, Graham County, AZ, USA, in 2018 and 2019.
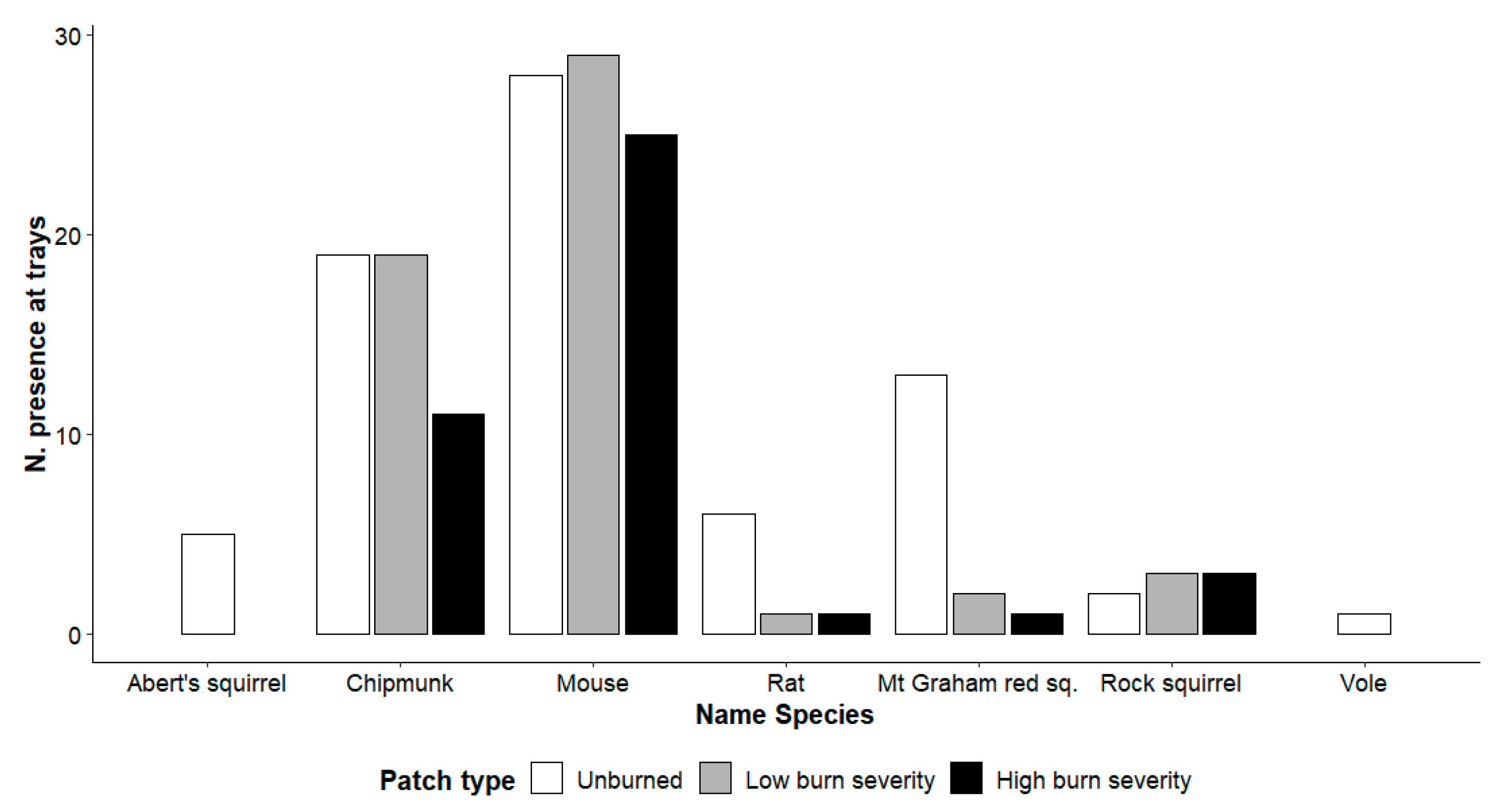
We observed a different species composition in the 3 categories of burned areas, although dominated by chipmunks and mice in all 3 patch types. Woodrat and Mt. Graham red squirrel used primarily unburned areas, whereas voles and Abert’s squirrels were only present in unburned areas (Figure 4).
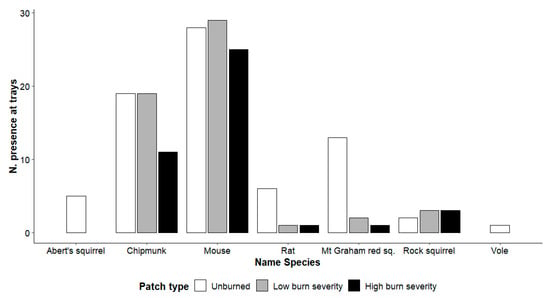
Figure 4.
Number of absolute frequencies of presence at the trays for each species in each patch type (unburned, low burn severity, high burn severity) in the Pinaleño Mountains, Graham County, AZ, USA, in 2018 and 2019. The total number of trays is 40 per patch type (20 each year).
4. Discussion
Varying levels of burn severity influence small mammal foraging behavior. In high burn severity patches, small mammals stopped foraging in trays earlier than in low burn severity and unburned patches. The larger amount of seeds left indicated greater costs than benefits in remaining to eat for longer times. The most known cost associated with foraging is predation risk. Vegetation characteristics in patches of different burn severities can influence the perception of predation risk [58] and consequently decisions regarding time spent foraging in a single location. In this study, we found that an increase in canopy cover corresponds to a lower number of seeds eaten, while an increase in horizontal cover (volume of logs and shrub cover) corresponds to a larger quantity of seeds eaten. We also found that fire affected the collective foraging behavior of small mammal communities but did not impact all species the same way, causing a smaller impact on generalist species.
The composition and abundance of small mammals varies as a function of time since the fire event [59] and depend upon the successional stages as well as the possibility of recolonization from nearby unburned habitats [60]. Immediately after a fire, populations can decline due to mortality [11,61], but the total small mammal biomass can increase in the two years after the fire event [26]. The higher presence of small mammals at the tray in 2019 suggests that the rodent populations in the study areas rebounded 2 years after the fire. In contrast with the presence/absence of mammals in patches, GUD is instead affected by differences in the level of burn severity, but not by year. Foraging behavior results in a complex response where benefits and costs are evaluated to maximize the energy intake. The main cost associated with high intensity burn areas is the risk of predation, generally perceived to be higher in the absence of vegetation cover [28,52,62]. Previous studies showed that rodent activity and occurrence are related to indirect (microhabitat) and not direct (olfactory and visual) cues of predation risk [63,64,65]. Thus, the presence of small mammals is more related to factors such as ground cover, refuges, and visibility to reduce the risk of predation than to the actual presence/activity of predators. As a result, GUD in high burn severity patches increased (foraging decreased) compared to unburned patches.
Predators create a landscape of fear that influences activity times, foraging tactics, and microhabitat selection of prey [29,66]. Foraging behavior may be influenced by different elements when considering microhabitats in different burn severity patches. In unburned areas and low burn severity patches, tree canopy cover has an important influence on GUD. However, we found that as canopy cover increased, GUD increased. Horizontal cover provides concealment from avian predators [67], but vegetation can also reduce the visual detectability of predators by prey [68,69]. Another possible explanation of this result is the presence in the Pinaleño Mountain of birds of prey that are adapted to hunt below the canopy of mature trees, such as Cooper’s hawk (Accipiter cooperii), sharp-shinned hawk (A. striatus), Mexican spotted owl (Strix occidentalis lucida), or great-horned owl (Bubo virginianus). With the canopy cover above the predators, prey species are no longer directly protected. Instead, logs or cover offered by lower vegetation can provide immediate refuge from predation [28] by birds of prey, as well as terrestrial predators such gray foxes (Urocyon cinereoargenteus) and bobcats (Lynx rufus) present in our study site. In fact, in unburned areas, an increase in shrub cover and volume of logs corresponded to a decrease in GUD, hence higher removal rates of seeds from the trays. A similar pattern was found in high burn severity patches where log volume significantly affected GUD, with more seeds removed from the trays when a larger volume of logs was present. The positive relationship between canopy cover and GUD, but the negative relationship between cover offered by horizontal cover (logs, shrub cover) and GUD, aligns with the explanation provided by Potash et al. [58,70], who demonstrated that prey could perceive different predation risks as a consequence of interactions between multiple environmental cues in heterogeneous landscapes [58,70]. This heterogeneity is created by a different distribution of predators in the landscape, creating spatial variation in a prey’s fear [71], and the interaction between vegetation cover along horizontal and vertical axes. In the absence of data collected in the same areas before the fire event, we cannot assess whether the small mammal response to vegetation changed after the fire.
The GUD experiment provides us with information on the assemblage of small mammals, but it does not provide information on the variability in gathering and/or feeding behavior by species. Thanks to the pairing of camera traps and trays we assessed that, while fire did affect the foraging behavior of small mammals, the effects varied among species. A lower number of species used the trays in high burn severity patches than in unburned patches. Fire did influence small mammal populations; however, the level of effect was not uniform and appeared to be associated with specific habitat requirements of individual species [72,73], and the home range size of the animal. In this context, we expected generalist species to be less impacted, whereas habitat specialists negatively affected [74,75]. We observed the use of all types of burn severity patches by the generalist T. dorsalis and Peromyscus spp., whereas voles and Abert’s squirrels were present only in unburned areas, and Mt. Graham red squirrel used mostly non burned areas, rarely visiting trays in other patch types. Previous studies showed the behavioral response of Mt. Graham red squirrel to fire, with an increased home range size and maximum distance traveled [25,46]. After the Frye Fire, surviving animals were forced to travel within and among isolated patches of live trees for cone harvesting and in search of new territories, leaving them more susceptible to predation [25]. In other studies, mice did not always modify their behavior as a consequence of disturbances or habitat types. For example, the white-footed mouse (Peromyscus leucopus) did not change its patterns of habitat use in response to fuel reduction treatments [76], and the deer mouse (Peromyscus maniculatus) did not change the quantity of foraging activity between habitats characterized by different shrub density [77].
5. Conclusions
Fire influences behavioral response in communities of small mammals, with high burn severity patches used by fewer species and perceived as riskier than unburned or lower burn severity patches. Vegetation variables play a different role in the foraging decision of small mammals showing a complicated interaction between horizontal (logs, grass, shrub cover) and vertical vegetation cover in relation to burn severity [58]. As wildfires threaten animals globally [78,79,80], and future increases in fire frequency and severity in the southwest USA will increase the loss of forest areas and potentially exacerbate the impact of predators on small mammals [81], understanding the relationship between animal foraging behavior, burn severity, and microhabitat can help managers plan actions to reduce the negative impacts of wildfires [28]. Fuel treatment is an important management technique in forests to reduce habitat loss in the long term; however, in the short term, it can exacerbate the impact on small mammals because of a lack of logs and shrubs that help mitigate the landscape of perceived fear, a factor that should be considered by forest managers when species of conservation concern are involved (e.g., the Mt. Graham red squirrel). Moreover, with the small mammals’ role in the food web, changes in small mammal populations can also affect predatory species of conservation concerns (e.g., Strix occidentalis [82], Martes caurina [83]). Therefore, while fuel treatment is a tool that can help forest-associated species over the long term by reducing wildfires and the consequent habitat loss and fragmentation, forest managers need to balance the long-term benefit with the risk of short-term impacts on small mammals and their predators [84].
Author Contributions
Conceptualization, methodology, data collection, data analysis and original draft preparation—M.M.; review and editing—M.V.M.; review, supervision, funding acquisition—J.L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Arizona Game and Fish Department, (grants no. I18005 and I16002), and T & E Inc. Grants for Conservation Biology.
Institutional Review Board Statement
All field work was conducted under the University of Arizona Institutional Animal Care and Use Committee protocol # 16-169, the Arizona Game and Fish Department scientific collecting permit # SP651773 for 2019, SP403044 for 2020, SP407072 for 2021, the U.S. Fish and Wildlife Service permit # TE041875-2, and adhered to the American Society of Mammologist’s guidelines for the use of wild mammals in research (Sikes and Gannon, 2011).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in FigShare at 10.6084/m9.figshare.20740765.
Acknowledgments
We would like to thank the Mt. Graham Red Squirrel Research Program grad-uate and undergraduate research assistants for valuable help in the field. This manuscript was improved by comments from R. W. Mannan, L. Wauters, and R. Steidl. Thanks to five anonymous reviewers who improved the initial version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Number of trays where each species was detected during the experiment (60 trays for three rounds) and percentage of each species detected over the total number of trays during the entire experiment (180 trays) on the Pinaleño Mountains, Graham County, AZ, USA, in 2018 and 2019.
Table A1.
Number of trays where each species was detected during the experiment (60 trays for three rounds) and percentage of each species detected over the total number of trays during the entire experiment (180 trays) on the Pinaleño Mountains, Graham County, AZ, USA, in 2018 and 2019.
| Species | N Detection | Percentage |
|---|---|---|
| Peromyscus sp.—Mouse | 82 | 45.55% |
| Tamias dorsalis—Cliff chipmunk | 49 | 27.22% |
| Tamiasciurus fremonti grahamensis—Mt. Graham red squirrel | 16 | 8.88% |
| Mephitis mephitis—Striped skunk | 10 | 5.55% |
| Otospermophilus variegatus—Rock squirrel | 8 | 4.44% |
| Neotoma mexicana—Mexican woodrat | 8 | 4.44% |
| Ursus americanus—Black bear | 6 | 3.33% |
| Sciurus aberti—Abert’s squirrel | 5 | 2.77% |
| Birds | 4 | 2.22% |
| Urocyon cinereoargenteus—Gray fox | 1 | 0.55% |
| Microtus longicaudus leucophaeus—Long-tailed vole | 1 | 0.55% |
| No species detected | 30 | 16.66% |
| NA (problems with camera, but seeds eaten) | 25 | 13.88% |

Table A2.
Mean and standard deviation (SD) for each vegetation characteristic in the three different burn severity patch type in the Pinalegno Mountain in 2018 and 2019.
Table A2.
Mean and standard deviation (SD) for each vegetation characteristic in the three different burn severity patch type in the Pinalegno Mountain in 2018 and 2019.
| Patch Type | Year | % Canopy Cover | % Shrub Cover | % Grass Cover | Volume of Logs m3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Unburned | 2018 | 83.72 | 10.81 | 8.92 | 9.15 | 19.99 | 22.32 | 133,374.45 | 217,664.65 |
| 2019 | 78.97 | 10.96 | 11.33 | 10.98 | 20.05 | 22.31 | 133,374.45 | 216,208.68 | |
| Low burn severity | 2018 | 69.63 | 11.03 | 0.86 | 1.72 | 12.56 | 13.01 | 208,708.30 | 225,619.18 |
| 2019 | 54.95 | 17.44 | 4.15 | 5.44 | 20.45 | 16.84 | 306,591.92 | 420,116.44 | |
| High burn severity | 2018 | 38.43 | 21.47 | 0.71 | 1.50 | 17.36 | 20.29 | 169,837.83 | 164,172.06 |
| 2019 | 38.43 | 21.33 | 2.25 | 2.73 | 17.23 | 17.77 | 169,837.83 | 163,129.69 | |
References
- Bailey, L.D.; van de Pol, M. Tackling Extremes: Challenges for Ecological and Evolutionary Research on Extreme Climatic Events. J. Anim. Ecol. 2016, 85, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Stott, P. How Climate Change Affects Extreme Weather Events. Science 2016, 352, 1517–1518. [Google Scholar] [CrossRef] [PubMed]
- Jolly, W.M.; Cochrane, M.A.; Freeborn, P.H.; Holden, Z.A.; Brown, T.J.; Williamson, G.J.; Bowman, D.M.J.S. Climate-Induced Variations in Global Wildfire Danger from 1979 to 2013. Nat. Commun. 2015, 6, 7537. [Google Scholar] [CrossRef] [PubMed]
- Littell, J.S.; Peterson, D.L.; Riley, K.L.; Liu, Y.; Luce, C.H. A Review of the Relationships between Drought and Forest Fire in the United States. Glob. Chang. Biol. 2016, 22, 2353–2369. [Google Scholar] [CrossRef]
- McKenzie, D.; Littell, J.S. Climate Change and the Eco-Hydrology of Fire: Will Area Burned Increase in a Warming Western USA? Ecol. Appl. 2017, 27, 26–36. [Google Scholar] [CrossRef]
- Dennison, P.E.; Brewer, S.C.; Arnold, J.D.; Moritz, M.A. Large Wildfire Trends in the Western United States, 1984–2011. Geophys. Res. Lett. 2014, 41, 2928–2933. [Google Scholar] [CrossRef]
- Smucker, K.M.; Hutto, R.L.; Steele, B.M. Changes in Bird Abundance after Wildfire: Importance of Fire Severity and Time since Fire. Ecol. Appl. 2005, 15, 1535–1549. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Blanchard, W.; MacGregor, C.; Barton, P.; Banks, S.C.; Crane, M.; Michael, D.; Okada, S.; Berry, L.; Florance, D.; et al. Temporal Trends in Mammal Responses to Fire Reveals the Complex Effects of Fire Regime Attributes. Ecol. Appl. 2016, 26, 557–573. [Google Scholar] [CrossRef]
- Camargo, A.C.L.; Barrio, R.O.L.; de Camargo, N.F.; Mendonça, A.F.; Ribeiro, J.F.; Rodrigues, C.M.F.; Vieira, E.M. Fire Affects the Occurrence of Small Mammals at Distinct Spatial Scales in a Neotropical Savanna. Eur. J. Wildl. Res. 2018, 64, 63. [Google Scholar] [CrossRef]
- Whelan, R.J.; Rodgerson, L.; Dickman, C.R.; Sutherland, E.F. Critical Life Processes of Plants and Animals: Developing a Process-Based Understanding of Population Changes in Fire-Prone Landscapes. In Flammable Australia: The Fire Regimes and Biodiversity of a Continent; Cambridge University Press: Cambridge, UK, 2002; pp. 94–124. [Google Scholar]
- Engstrom, R.T. First-Order Fire Effects on Animals: Review and Recommendations. Fire Ecol. 2010, 6, 115–130. [Google Scholar] [CrossRef]
- Koprowski, J.L.; Leonard, K.M.; Zugmeyer, C.A.; Jolley, J.L. Direct Effects of Fire on Endangered Mount Graham Red Squirrels. Southwest. Nat. 2006, 51, 59–63. [Google Scholar] [CrossRef]
- Lawes, M.J.; Murphy, B.P.; Fisher, A.; Woinarski, J.C.Z.; Edwards, A.C.; Russell-Smith, J. Small Mammals Decline with Increasing Fire Extent in Northern Australia: Evidence from Long-Term Monitoring in Kakadu National Park. Int. J. Wildland Fire 2015, 24, 712. [Google Scholar] [CrossRef]
- Arthur, A.D.; Pech, R.P.; Dickman, C.R. Habitat Structure Mediates the Non-Lethal Effects of Predation on Enclosed Populations of House Mice. J. Anim. Ecol. 2004, 73, 867–877. [Google Scholar] [CrossRef]
- Spencer, R.-J.; Thompson, M.B. Experimental Analysis of the Impact of Foxes on Freshwater Turtle Populations. Conserv. Biol. 2005, 19, 845–854. [Google Scholar] [CrossRef]
- Raynor, E.J.; Joern, A.; Briggs, J.M. Bison Foraging Responds to Fire Frequency in Nutritionally Heterogeneous Grassland. Ecology 2015, 96, 1586–1597. [Google Scholar] [CrossRef]
- Kreisel, K.J.; Stein, S.J. Bird Use of Burned and Unburned Coniferous Forests during Winter. Wilson Bull. 1999, 111, 243–250. [Google Scholar]
- Forsman, A. Rethinking Phenotypic Plasticity and Its Consequences for Individuals, Populations and Species. Heredity 2015, 115, 276–284. [Google Scholar] [CrossRef]
- Haim, A.; Izhaki, I.; Golan, A. Rodent Species Diversity in Pine Forests Recovering from Fire. Isr. J. Ecol. Evol. 1996, 42, 353–359. [Google Scholar]
- Sutherland, E.F.; Dickman, C.R. Mechanisms of Recovery after Fire by Rodents in the Australian Environment: A Review. Wildl. Res. 1999, 26, 405. [Google Scholar] [CrossRef]
- Horn, K.J.; McMillan, B.R.; St. Clair, S.B. Expansive Fire in Mojave Desert Shrubland Reduces Abundance and Species Diversity of Small Mammals. J. Arid Environ. 2012, 77, 54–58. [Google Scholar] [CrossRef]
- Mazzamuto, M.V.; Mazzella, M.N.; Merrick, M.J.; Koprowski, J.L. Fire Impacts on a Forest Obligate: Western Gray Squirrel Response to Burn Severity. Mamm. Biol. 2020, 100, 295–303. [Google Scholar] [CrossRef]
- De Souza Lima Figueiredo, M.; Fernandez, F.A.D.S. Contrasting Effects of Fire on Populations of Two Small Rodent Species in Fragments of Atlantic Forest in Brazil. J. Trop. Ecol. Camb. 2004, 20, 225–228. [Google Scholar] [CrossRef]
- Gerber, L.R.; Hilborn, R. Catastrophic Events and Recovery from Low Densities in Populations of Otariids: Implications for Risk of Extinction. Mammal Rev. 2001, 31, 131–150. [Google Scholar] [CrossRef]
- Merrick, M.J.; Morandini, M.; Greer, V.L.; Koprowski, J.L. Endemic Population Response to Increasingly Severe Fire: A Cascade of Endangerment for the Mt. Graham Red Squirrel. BioScience 2021, 71, 161–173. [Google Scholar] [CrossRef]
- Converse, S.J.; White, G.C.; Farris, K.L.; Zack, S. Small Mammals and Forest Fuel Reduction: National-Scale Responses to Fire and Fire Surrogates. Ecol. Appl. 2006, 16, 1717–1729. [Google Scholar] [CrossRef]
- Boone, S.R.; Brehm, A.M.; Mortelliti, A. Seed Predation and Dispersal by Small Mammals in a Landscape of Fear: Effects of Personality, Predation Risk and Land-Use Change. Oikos 2022, 2022, 1–15. [Google Scholar] [CrossRef]
- Doherty, T.S.; Davis, R.A.; van Etten, E.J.B. A Game of Cat-and-Mouse: Microhabitat Influences Rodent Foraging in Recently Burnt but Not Long Unburnt Shrublands. J. Mammal. 2015, 96, 324–331. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Beckerman, A.P.; O’Brien, K.M. Behaviorally Mediated Trophic Cascades: Effects of Predation Risk on Food Web Interactions. Ecology 1997, 78, 1388–1399. [Google Scholar] [CrossRef]
- Cowlishaw, G. Trade-Offs between Foraging and Predation Risk Determine Habitat Use in a Desert Baboon Population. Anim. Behav. 1997, 53, 667–686. [Google Scholar] [CrossRef]
- Newman, J.A.; Recer, G.M.; Zwicker, S.M.; Caraco, T. Effects of Predation Hazard on Foraging “Constraints”: Patch-Use Strategies in Grey Squirrels. Oikos 1988, 53, 93–97. [Google Scholar] [CrossRef]
- Bowers, M.A. Exploitation of Seed Aggregates by Merriam’s Kangaroo Rat: Harvesting Rates and Predatory Risk. Ecology 1990, 71, 2334–2344. [Google Scholar] [CrossRef]
- Brown, J.S. Patch Use as an Indicator of Habitat Preference, Predation Risk, and Competition. Behav. Ecol. Sociobiol. 1988, 22, 37–47. [Google Scholar] [CrossRef]
- Whitham, T.G. Coevolution of Foraging in Bombus and Nectar Dispensing in Chilopsis: A Last Dreg Theory. Science 1977, 197, 593–596. [Google Scholar] [CrossRef]
- Brown, J.S.; Morgan, R.A.; Dow, B.D. Patch Use under Predation Risk: II. A Test with Fox Squirrels, Sciurus Niger. Ann. Zool. Fenn. 1992, 29, 311–318. [Google Scholar]
- Parkins, K.; York, A.; Di Stefano, J. Edge Effects in Fire-Prone Landscapes: Ecological Importance and Implications for Fauna. Ecol. Evol. 2018, 8, 5937–5948. [Google Scholar] [CrossRef]
- Banks, S.C.; Lindenmayer, D.B.; Ward, S.J.; Taylor, A.C. The Effects of Habitat Fragmentation via Forestry Plantation Establishment on Spatial Genotypic Structure in the Small Marsupial Carnivore, Antechinus Agilis. Mol. Ecol. 2005, 14, 1667–1680. [Google Scholar] [CrossRef]
- Fisher, J.T.; Wilkinson, L. The Response of Mammals to Forest Fire and Timber Harvest in the North American Boreal Forest. Mammal Rev. 2005, 35, 51–81. [Google Scholar] [CrossRef]
- Amacher, A.J.; Barrett, R.H.; Moghaddas, J.J.; Stephens, S.L. Preliminary Effects of Fire and Mechanical Fuel Treatments on the Abundance of Small Mammals in the Mixed-Conifer Forest of the Sierra Nevada. For. Ecol. Manag. 2008, 255, 3193–3202. [Google Scholar] [CrossRef]
- Hutchen, J.; Volkmann, L.A.; Hodges, K.E.; Hutchen, J.; Volkmann, L.A.; Hodges, K.E. Experimental Designs for Studying Small-Mammal Responses to Fire in North American Conifer Forests. Int. J. Wildland Fire 2017, 26, 523–531. [Google Scholar] [CrossRef]
- Reed, T.M. Interspecific Territoriality in the Chaffinch and Great Tit on Islands and the Mainland of Scotland: Playback and Removal Experiments. Anim. Behav. 1982, 30, 171–181. [Google Scholar] [CrossRef]
- Hope, A.G.; Malaney, J.L.; Bell, K.C.; Salazar-Miralles, F.; Chavez, A.S.; Barber, B.R.; Cook, J.A. Revision of Widespread Red Squirrels (Genus: Tamiasciurus) Highlights the Complexity of Speciation within North American Forests. Mol. Phylogenet. Evol. 2016, 100, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Hutton, K.A.; Koprowski, J.L.; Greer, V.L.; Alanen, M.I.; Schauffert, C.A.; Young, P.J. Use of Mixed-Conifer and Spruce-Fir Forests by an Introduced Population of Abert’s Squirrels (Sciurus Aberti). Southwest. Nat. 2003, 48, 257–260. [Google Scholar] [CrossRef]
- McGuire, L.A.; Youberg, A.M. Impacts of Successive Wildfire on Soil Hydraulic Properties: Implications for Debris Flow Hazards and System Resilience. Earth Surf. Process. Landf. 2019, 44, 2236–2250. [Google Scholar] [CrossRef]
- Parsons, R.A.; Mell, W.E.; McCauley, P. Linking 3D Spatial Models of Fuels and Fire: Effects of Spatial Heterogeneity on Fire Behavior. Ecol. Model. 2011, 222, 679–691. [Google Scholar] [CrossRef]
- Koprowski, J.L.; King, S.; Merrick, M. Expanded Home Ranges in a Peripheral Population: Space Use by Endangered Mt. Graham Red Squirrels. Endanger. Species Res. 2008, 4, 227–232. [Google Scholar] [CrossRef]
- Abramson, G.; Giuggioli, L.; Kenkre, V.M.; Dragoo, J.W.; Parmenter, R.R.; Parmenter, C.A.; Yates, T.L. Diffusion and Home Range Parameters for Rodents: Peromyscus Maniculatus in New Mexico. Ecol. Complex. 2006, 3, 64–70. [Google Scholar] [CrossRef][Green Version]
- Edelman, A.J.; Koprowski, J.L. Selection of Drey Sites by Abert’s Squirrels in an Introduced Population. J. Mammal. 2005, 86, 1220–1226. [Google Scholar] [CrossRef]
- Doumas, S.L.; Koprowski, J.L. Return of Fire as a Restoration Tool: Long-Term Effects of Burn Severity on Habitat Use by Mexican Fox Squirrels. Restor. Ecol. 2013, 21, 133–139. [Google Scholar] [CrossRef]
- Strickler, G.S. Use of the Densiometer to Estimate Density of Forest Canopy on Permanent Sample Plots; U.S. Department of Agriculture: Washington, DC, USA, 1959; 5p.
- Jacob, S.A.; Matter, S.F.; Cameron, G.N. Interactive Effects of Vegetation and Illumination on Foraging Behavior of White-Footed Mice (Peromyscus Leucopus). J. Mammal. 2017, 98, 804–814. [Google Scholar] [CrossRef]
- Persons, W.E.; Eason, P. Human Activity and Habitat Type Affect Perceived Predation Risk in Urban White-Footed Mice (Peromyscus Leucopus). Ethology 2017, 123, 348–356. [Google Scholar] [CrossRef]
- Vander Wall, S.B.; Kuhn, K.M.; Gworek, J.R. Two-Phase Seed Dispersal: Linking the Effects of Frugivorous Birds and Seed-Caching Rodents. Oecologia 2005, 145, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Suazo, A.A.; Delong, A.T. Responses of Old-Field Mice (Peromyscus Polionotus) to Consecutive Days of Live Trapping. Am. Midl. Nat. 2007, 158, 395–402. [Google Scholar] [CrossRef]
- Wall, S.B.V.; Hager, E.C.H.; Kuhn, K.M. Pilfering of Stored Seeds and the Relative Costs of Scatter-Hoarding versus Larder-Hoarding in Yellow Pine Chipmunks. West. N. Am. Nat. 2005, 65, 248–257. [Google Scholar]
- Sullivan, T.P. Operational Application of Diversionary Food in Young Lodgepole Pine Forests to Reduce Feeding Damage by Red Squirrels. Proc. Vertebr. Pest Conf. 1992, 15, 340–343. [Google Scholar]
- Bedoya-Perez, M.A.; Carthey, A.J.R.; Mella, V.S.A.; McArthur, C.; Banks, P.B. A Practical Guide to Avoid Giving up on Giving-up Densities. Behav. Ecol. Sociobiol. 2013, 67, 1541–1553. [Google Scholar] [CrossRef]
- Potash, A.D.; Conner, L.M.; McCleery, R.A. Vertical and Horizontal Vegetation Cover Synergistically Shape Prey Behaviour. Anim. Behav. 2019, 152, 39–44. [Google Scholar] [CrossRef]
- Briani, D.C.; Palma, A.R.T.; Vieira, E.M.; Henriques, R.P.B. Post-Fire Succession of Small Mammals in the Cerrado of Central Brazil. Biodivers. Conserv. 2004, 13, 1023–1037. [Google Scholar] [CrossRef]
- Diffendorfer, J.; Fleming, G.M.; Tremor, S.; Spencer, W.; Beyers, J.L. The Role of Fire Severity, Distance from Fire Perimeter and Vegetation on Post-Fire Recovery of Small-Mammal Communities in Chaparral. Int. J. Wildland Fire 2012, 21, 436. [Google Scholar] [CrossRef]
- Whelan, R.J. Managing Fire Regimes for Conservation and Property Protection: An Australian Response. Conserv. Biol. 2002, 16, 1659–1661. [Google Scholar] [CrossRef]
- Bowers, M.A.; Jefferson, J.L.; Kuebler, M.G. Variation in Giving-up Densities of Foraging Chipmunks (Tamias Striatus) and Squirrels (Sciurus Carolinensis). Oikos 1993, 66, 229–236. [Google Scholar] [CrossRef]
- Orrock, J.L.; Danielson, B.J.; Brinkerhoff, R.J. Rodent Foraging Is Affected by Indirect, but Not by Direct, Cues of Predation Risk. Behav. Ecol. 2004, 15, 433–437. [Google Scholar] [CrossRef]
- Thorson, J.M.; Morgan, R.A.; Brown, J.S.; Norman, J.E. Direct and Indirect Cues of Predatory Risk and Patch Use by Fox Squirrels and Thirteen-Lined Ground Squirrels. Behav. Ecol. 1998, 9, 151–157. [Google Scholar] [CrossRef]
- Sivy, K.J.; Ostoja, S.M.; Schupp, E.W.; Durham, S. Effects of Rodent Species, Seed Species, and Predator Cues on Seed Fate. Acta Oecologica 2011, 37, 321–328. [Google Scholar] [CrossRef]
- Lima, S.L. Maximizing Feeding Efficiency and Minimizing Time Exposed to Predators: A Trade-off in the Black-Capped Chickadee. Oecologia 1985, 66, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Wywialowski, A.P. Habitat Structure and Predators: Choices and Consequences for Rodent Habitat Specialists and Generalists. Oecologia 1987, 72, 39–45. [Google Scholar] [CrossRef]
- Embar, K.; Kotler, B.P.; Mukherjee, S. Risk Management in Optimal Foragers: The Effect of Sightlines and Predator Type on Patch Use, Time Allocation, and Vigilance in Gerbils. Oikos 2011, 120, 1657–1666. [Google Scholar] [CrossRef]
- Camp, M.J.; Rachlow, J.L.; Woods, B.A.; Johnson, T.R.; Shipley, L.A. Examining Functional Components of Cover: The Relationship between Concealment and Visibility in Shrub-Steppe Habitat. Ecosphere 2013, 4, art19. [Google Scholar] [CrossRef]
- Laundré, J.W.; Hernández, L.; Altendorf, K.B. Wolves, Elk, and Bison: Reestablishing the “Landscape of Fear” in Yellowstone National Park, U.S.A. Can. J. Zool. 2001, 79, 1401–1409. [Google Scholar] [CrossRef]
- Brown, J.S.; Kotler, B.P. Hazardous Duty Pay and the Foraging Cost of Predation. Ecol. Lett. 2004, 7, 999–1014. [Google Scholar] [CrossRef]
- Griffiths, A.D.; Brook, B.W. Effect of Fire on Small Mammals: A Systematic Review. Int. J. Wildland Fire 2014, 23, 1034. [Google Scholar] [CrossRef]
- Torre, I.; Jaime-González, C.; Díaz, M. Habitat Suitability for Small Mammals in Mediterranean Landscapes: How and Why Shrubs Matter. Sustainability 2022, 14, 1562. [Google Scholar] [CrossRef]
- Waters, J.R.; Zabel, C.J. Northern Flying Squirrel Densities in Fir Forests of Northeastern California. J. Wildl. Manag. 1995, 59, 858. [Google Scholar] [CrossRef]
- Roberts, S.L.; Kelt, D.A.; van Wagtendonk, J.W.; Miles, A.K.; Meyer, M.D. Effects of Fire on Small Mammal Communities in Frequent-Fire Forests in California. J. Mammal. 2015, 96, 107–119. [Google Scholar] [CrossRef]
- Greenberg, C.H.; Otis, D.L.; Waldrop, T.A. Response of White-Footed Mice (Peromyscus Leucopus) to Fire and Fire Surrogate Fuel Reduction Treatments in a Southern Appalachian Hardwood Forest. For. Ecol. Manag. 2006, 234, 355–362. [Google Scholar] [CrossRef]
- Connolly, B.M.; Orrock, J.L. Habitat-Specific Capture Timing of Deer Mice (Peromyscus Maniculatus) Suggests That Predators Structure Temporal Activity of Prey. Ethology 2018, 124, 105–112. [Google Scholar] [CrossRef]
- Schoennagel, T.; Balch, J.K.; Brenkert-Smith, H.; Dennison, P.E.; Harvey, B.J.; Krawchuk, M.A.; Mietkiewicz, N.; Morgan, P.; Moritz, M.A.; Rasker, R.; et al. Adapt to More Wildfire in Western North American Forests as Climate Changes. Proc. Natl. Acad. Sci. USA 2017, 114, 4582–4590. [Google Scholar] [CrossRef]
- Ward, M.; Rhodes, J.R.; Watson, J.E.M.; Lefevre, J.; Atkinson, S.; Possingham, H.P. Use of Surrogate Species to Cost-Effectively Prioritize Conservation Actions. Conserv. Biol. 2020, 34, 600–610. [Google Scholar] [CrossRef]
- Ancillotto, L.; Fichera, G.; Pidinchedda, E.; Veith, M.; Kiefer, A.; Mucedda, M.; Russo, D. Wildfires, Heatwaves and Human Disturbance Threaten Insular Endemic Bats. Biodivers. Conserv. 2021, 30, 4401–4416. [Google Scholar] [CrossRef]
- Mueller, S.E.; Thode, A.E.; Margolis, E.Q.; Yocom, L.L.; Young, J.D.; Iniguez, J.M. Climate Relationships with Increasing Wildfire in the Southwestern US from 1984 to 2015. For. Ecol. Manag. 2020, 460, 117861. [Google Scholar] [CrossRef]
- Tempel, D.J.; Gutiérrez, R.J.; Whitmore, S.A.; Reetz, M.J.; Stoelting, R.E.; Berigan, W.J.; Seamans, M.E.; Peery, M.Z. Effects of Forest Management on California Spotted Owls: Implications for Reducing Wildfire Risk in Fire-Prone Forests. Ecol. Appl. 2014, 24, 2089–2106. [Google Scholar] [CrossRef]
- Moriarty, K.M.; Epps, C.W.; Zielinski, W.J. Forest Thinning Changes Movement Patterns and Habitat Use by Pacific Marten. J. Wildl. Manag. 2016, 80, 621–633. [Google Scholar] [CrossRef]
- Slauson, K.; Howard, B.; White, A.; Maxwell, C.; Holland, T. Evaluating the Effects of Alternative Landscape Management Scenarios on Three Old-Forest-Associated Predators over 100 Years in the Fire-Prone Forests of the Sierra Nevada, USA. Ecol. Soc. 2022, 27, 28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).