Assessing the Response of Different Soil Arthropod Communities to Fire: A Case Study from Northwestern Africa
Abstract
1. Introduction
2. Materials and Methods
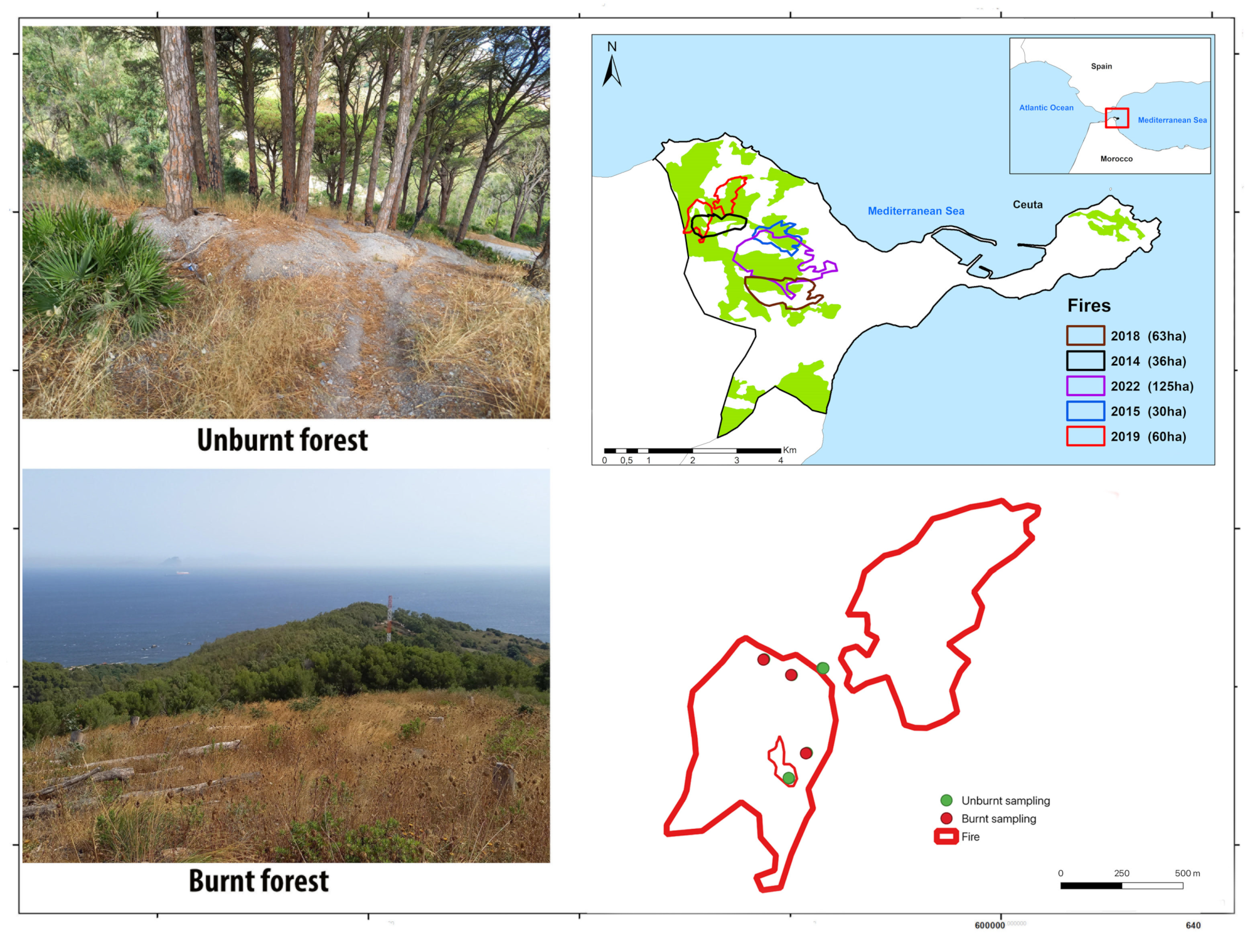
2.1. Study Area
2.2. Arthropod Sampling
3. Data Analysis
- (1)
- Abundance values of the top 10 most-abundant arthropod orders were compared between pitfalls in burnt and unburnt areas, using Generalized Linear Mixed Models (GLMMs). This analysis was carried out using a Poisson distribution due to the discrete nature of the dependent variables (counting the number of individuals captured). The fire condition (burnt or unburnt) of the sampling points was used as an independent variable, whereas the sampling point and the sampling month (July and October) were treated as random effects. Statistical analyses were performed using the lme4 package [42], and figures were performed using the ggplot 2 package [43].
- (2)
- The composition and species abundance of ant, beetle, and orthopteran communities found in the burnt vs. unburnt pitfall traps were compared by permutational analysis of variance (PERMANOVA). For each arthropod group, the pairwise similarity in species abundance and presence among pitfall traps were assessed using the Bray–Curtis similarity distance for the relative abundance data and using the adonis2 function from the vegan package [44]. Similar to the GLMM design, the fire condition was used as a fixed factor, whereas the sampling point and season were used as random factors in the PERMANOVAs. All analyses were performed using R software (Core Team 2021).
- (3)
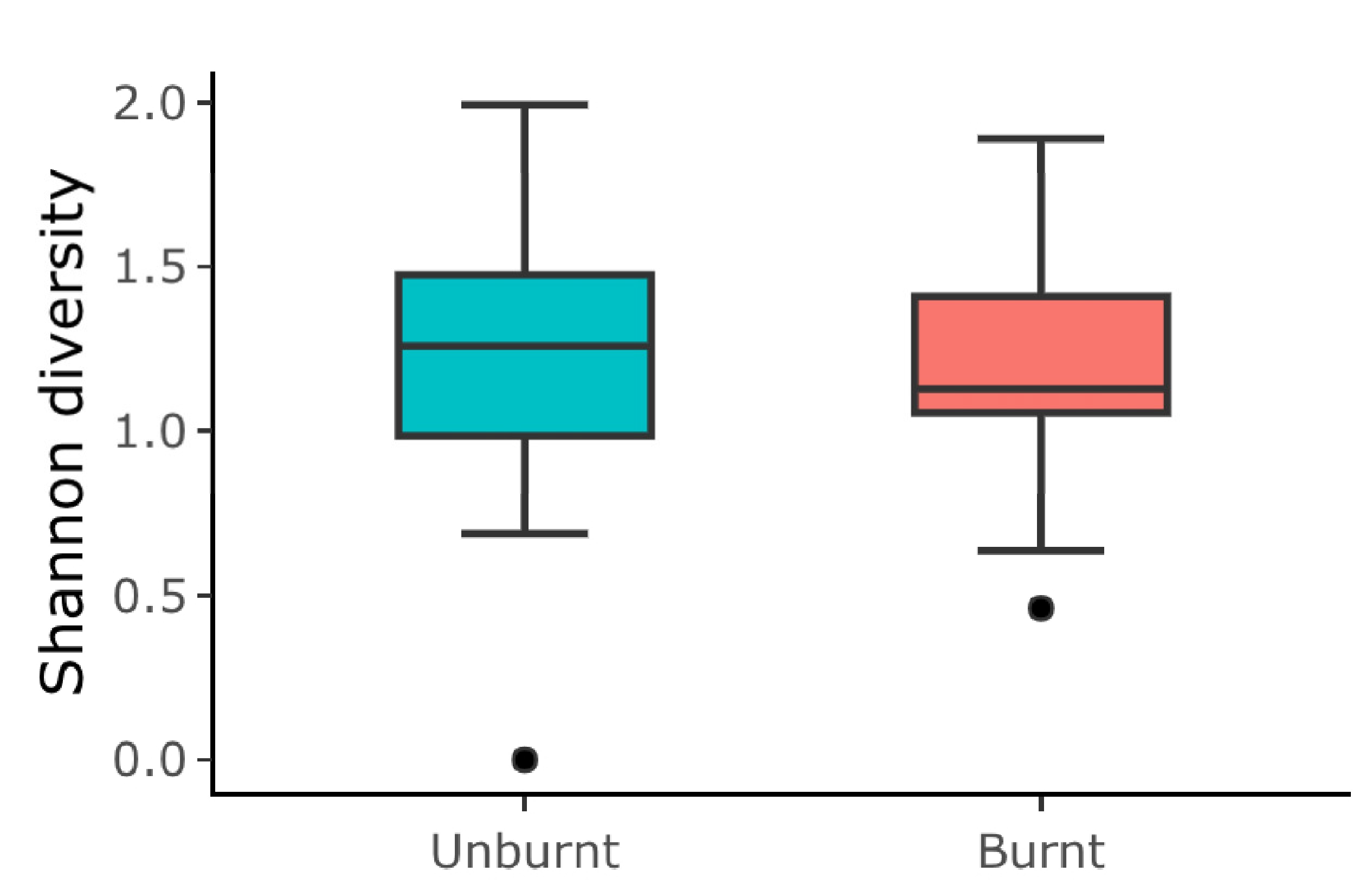
- Based on the abundance of beetle, ant, and grasshopper species per pitfall trap, we calculated the Shannon diversity index. Then, we used Generalized Linear Mixed Models (GLMMs) using the lme4 package [42], with a Gaussian distribution, to examine the effect of the fire condition (burnt and unburnt) on the Shannon diversity per trap. The sampling point and the sampling month (July and October) were treated as random effects.
4. Results
5. Discussion
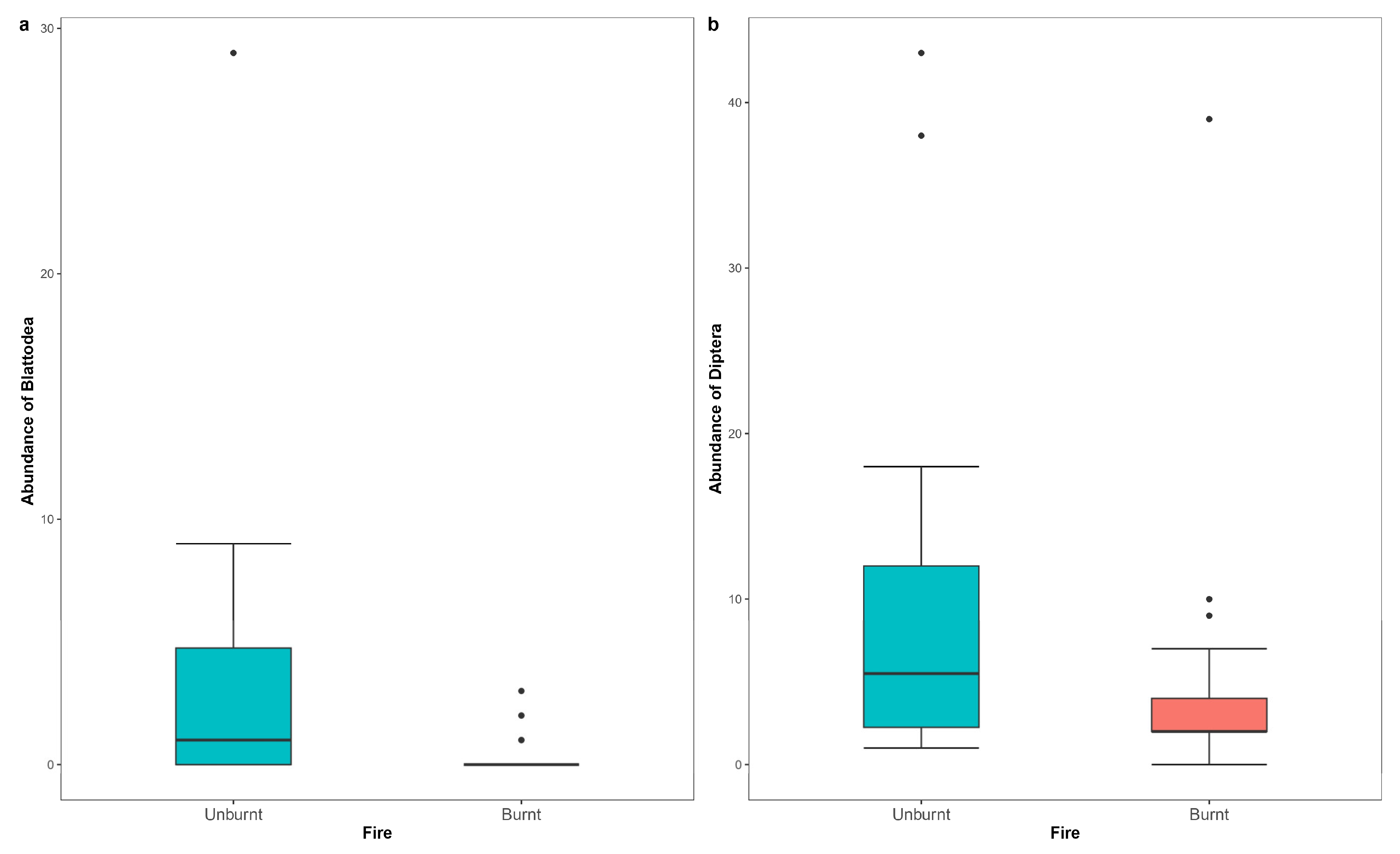
5.1. Effects of Fire on the Abundance of Arthropod Taxa
5.2. Effects of Fire on the Composition of Arthropod Communities
5.3. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keeley, J.E.; Bond, W.J.; Bradstock, R.A.; Pausas, J.G.; Rundel, P.W. Fire in Mediterranean Ecosystems: Ecology, Evolution and Management; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Kelly, L.T.; Giljohann, K.M.; Duane, A.; Aquilué, N.; Archibald, S.; Batllori, E.; Bennett, A.F.; Buckland, S.T.; Canelles, Q.; Clarke, M.F.; et al. Fire and biodiversity in the Anthropocene. Science 2020, 370, eabb0355. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G.; Fernaández-Munñoz, S. Fire regime changes in the Western Mediterranean Basin: From fuel-limited to drought-driven fire regime. Clim. Change 2012, 110, 215–226. [Google Scholar] [CrossRef]
- He, T.; Lamont, B.B.; Pausas, J.G. Fire as a key driver of Earth’s biodiversity. Biology 2019, 94, 1983–2010. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G.; Keeley, J.E. Wildfires as ecosystem services. Front. Ecol. Environ. 2019, 17, 289–295. [Google Scholar] [CrossRef]
- Trabaud, L. Man and fire: Impacts on Mediterranean vegetation. In Ecosystems of the World; Mediterranean-Type Shrublands; Di Castri, F., Goodall, D.W., Specht, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 1981; Volume 11. [Google Scholar]
- Trabaud, L.; Oustric, J. Heat requirements for seed germination of three Cistus species in the garrigue of southern France. Flora 1989, 183, 321–325. [Google Scholar] [CrossRef]
- Rundel, P.W.; Arroyo, M.T.K.; Cowling, R.M.; Keeley, J.E.; Lamont, B.B.; Vargas, P. Mediterranean biomes: Evolution of their vegetation, floras and climate. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 383–407. [Google Scholar] [CrossRef]
- Andersen, A.N.; Ribbons, R.R.; Pettit, M.; Parr, C.L. Burning for biodiversity: Highly resilient ant communities respond only to strongly contrasting fire regimes in Australia’s seasonal tropics. J. Appl. Ecol. 2014, 51, 1406–1413. [Google Scholar] [CrossRef]
- Vidal-Cordero, J.M.; Arnan, X.; Rodrigo, A.; Cerdá, X.; Boulay, R. Four-year study of arthropod taxonomic and functional responses to a forest wildfire: Epigeic ants and spiders are affected differently. For. Ecol. Manag. 2022, 520, 120–379. [Google Scholar] [CrossRef]
- Pausas, J.G.; Bradstock, R.A.; Keith, D.A.; Keeley, J.E.; The GCTE (Global Change of Terrestrial Ecosystems) Fire Network. Plant functional traits in relation to fire in crown-fire ecosystems. Ecology 2004, 85, 1085–1100. [Google Scholar] [CrossRef]
- Pryke, J.S.; Samways, M.J. Importance of Using Many Taxa and Having Adequate Controls for Monitoring Impacts of Fire for Arthropod Conservation. J. Insect Conserv. 2012, 16, 177–185. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M. Evolution of the Insects; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Gerlach, J.; Samways, M.; Pryke, J. Terrestrial invertebrates as bioindicators: An overview of available taxonomic groups. J. Insect Conserv. 2013, 17, 831–850. [Google Scholar] [CrossRef]
- Kremen, C.; Colwell, R.K.; Erwin, T.L.; Murphy, D.D.; Noss, R.F.; Sanjayan, M.A. Terrestrial arthropod assemblages: Their use in conservation planning. Conserv. Biol. 1993, 7, 796–808. [Google Scholar] [CrossRef]
- Samways, M.; McGeoch, M.; New, T. Insect Conservation a Handbook of Approaches and Methods; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Knicker, H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 2007, 85, 91–118. [Google Scholar] [CrossRef]
- Buckingham, S.; Murphy, N.; Gibb, H. Effects of fire severity on the composition and functional traits of litter-dwelling macroinvertebrates in a temperate forest. For. Ecol. Manag. 2019, 434, 279–288. [Google Scholar] [CrossRef]
- Gongalsky, K.B.; Persson, T. Recovery of soil macrofauna after wildfires in boreal forests. Soil Biol. Biochem. 2013, 57, 182–191. [Google Scholar] [CrossRef]
- Swengel, A.B. A literature review of insect responses to fire, compared to other conservation managements of open habitat. Biodiver. Conserv. 2001, 10, 1141–1169. [Google Scholar] [CrossRef]
- Kral, K.C.; Limb, R.F.; Harmon, J.P.; Hovick, T.J. Arthropods and Fire: Previous Research Shaping Future Conservation. Rangel. Ecol. Manag. 2017, 70, 589–598. [Google Scholar] [CrossRef]
- Santos, X.; Mateos, E.; Bros, V.; Brotons, L.; De Mas, E.; Herraiz, J.A.; Herrando, S.; Miño, A.; Olmo-Vidal, J.M.; Quesada, J.; et al. Is response to fire influenced by dietary specialization and mobility? A comparative study with multiple animal assemblages. PLoS ONE 2014, 9, e88224. [Google Scholar] [CrossRef]
- Siemann, E.; Haarstad, J.; Tilman, D. Short-term and long-term effects of burning on oak savanna arthropods. Am. Mid. Nat. 1997, 137, 349–361. [Google Scholar] [CrossRef]
- Dietrich, C.H.; Harper, M.G.; Larimore, R.L.; Tessene, P.A. Insects and fire: Too much of a good thing? Ill. Nat. Hist. Survey Rep. 1998, 349, 4. [Google Scholar]
- Warren, S.D.; Scifres, C.J.; Teel, P.D. Response of grassland arthropods to burning: A review. Agric. Ecosyst. Environ. 1987, 19, 105–130. [Google Scholar] [CrossRef]
- Reed, C.C. Responses of prairie insects and other arthropods to prescription burns. Nat. Areas J. 1997, 17, 380–385. [Google Scholar]
- Rohde, A.T.; Pilliod, D.S.; Novak, S.J. Insect communities in big sagebrush habitats are altered by wildfire and post-fire restoration seeding. Insect Conserv. Divers. 2018, 12, 216–230. [Google Scholar] [CrossRef]
- Vasconcelos, H.L.; Pacheco, R.; Silva, R.C.; Vasconcelos, P.B.; Lopes, C.T.; Costa, A.N.; Bruna, E.M. Dynamics of the leaf-litter arthropod fauna following fire in a neotropical wood soil savanna. PLoS ONE 2009, 4, e7762. [Google Scholar] [CrossRef]
- Yekwayo, I.; Pryke, J.S.; Gaigher, R.; Samways, M.J. Only multi-taxon studies show the full range of arthropod responses to fire. PLoS ONE 2018, 13, e0195414. [Google Scholar] [CrossRef]
- Pressler, Y.; Moore, J.C.; Cotrufo, M.F. Belowground community responses to fire: Meta-analysis reveals contrasting responses of soil microorganisms and mesofauna. Oikos 2019, 128, 309–327. [Google Scholar] [CrossRef]
- Pausas, J.G.; Parr, C.L. Towards an understanding of the evolutionary role of fire in animals. Evol. Ecol. 2018, 32, 113–125. [Google Scholar] [CrossRef]
- Vasconcelos, H.L.; Maravalhas, J.B.; Cornelissen, T. Effects of fire disturbance on ant abundance and diversity: A global meta-analysis. Biodivers. Conserv. 2017, 26, 177–188. [Google Scholar] [CrossRef]
- Samways, M.J. Insects on the brink of a major discontinuity. Biodivers. Conserv. 1996, 5, 1047–1058. [Google Scholar] [CrossRef]
- Chamber, B.Q.; Samways, M.J. Grasshopper response to a 40-year experimental burning and mowing regime, with recommendations for invertebrate conservation management. Biodivers. Conserv. 1998, 7, 985–1012. [Google Scholar] [CrossRef]
- Yadav, S.; Stow, A.J.; Harris, R.M.B.; Dudaniec, R.Y. Morphological variation tracks environmental gradients in an agricultural pest, Phaulacridium vittatum (Orthoptera: Acrididae). J. Insect Sci. 2018, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.M.; Richards, L.A.; Wimp, G.M. Editorial: Arthropod interactions and responses to disturbance in a changing world. Front. Ecol. Evol. 2020, 8, 93. [Google Scholar] [CrossRef]
- Chamorro, S. El medio natural en Ceuta y su entorno: Concrecion y potencialidades para el desarrollo. In Monografía de los Cursos de Verano de la Universidad de Granada en Ceuta, 6th ed.; Instituto de Estudios Ceutíes: Ceuta, Spain, 1995; pp. 139–199. [Google Scholar]
- Ruiz, J.L. Los Scarabaeoidea (Coleoptera) Coprófagos de la Región de Ceuta (Norte de África). Aproximación Faunística; Monografía 2; Estudios Sobre el Medio Natural de Ceuta y su Entorno; Transfretana: Ceuta, Spain, 1995; pp. 11–114. [Google Scholar]
- Taleb, M.S.; Fennane, M. Vascular Plant Communities of Morocco. Phytosociology, Ecology and Geography. In Geobotany Studies. Basics, Methods and Case Studies; Pedrotti, F., Ed.; Springer International Publishing: Cham, Switzerland, 2019; p. 161. [Google Scholar]
- Navarro Capel, M.C. El deterioro de los pinares ceutíes. Transfretana 1994, 6, 175–179. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Oksanen, J.F.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 January 2023).
- Kocher, L. Catalogue commenté des Coléoptères du Maroc. Fascicule I. Carabiques. Trav. l’Institut Sci. Chérifien Série Zool. 1963, 27, 1–171. [Google Scholar]
- Baraud, J. Coléoptères Scarabaeoidea. Faune du Nord de l’Afrique, du Maroc au Sinaï. Ed.; Lechevalier: Paris, France, 1985; p. 650. [Google Scholar]
- Viñolas, A.; Cartagena, M.C. Fauna de Tenebrionidae de la Península Ibérica y Baleares, Lagriinae y Pimeliinae; Argania: Barcelona, Spain, 2005; Volume 1, p. 428. [Google Scholar]
- Sánchez-Piñero, F.; Verdú, J.R.; Lobo, J.M.; Ruiz, J.L. Use of Quercus acorns and leaf litter by North African Thorectes species (Coleoptera: Scarabaeidae: Geotrupinae). Afr. Entomol. 2019, 27, 10–17. [Google Scholar] [CrossRef]
- Serrano, J. The genus Orthomus Chaudoir, 1838 in the Iberian Peninsula and Morocco. Russ. Entomol. J. 2021, 30, 430–447. [Google Scholar] [CrossRef]
- Gomy, Y.; Labrique, H.; Lackner, T. Les Histeridae du Maroc; Collection Systématique; Magellanes: Conflans-Sainte-Honorine, France, 2022; p. 292. [Google Scholar]
- Garcia-Villanueva, J.A.; Ena, V.; Tarrega, R.; Mediavilla, G. Recolonization of Two Burnt Quercus pyrenaica Ecosystems by Coleoptera. Int. J. Wildland Fire 1998, 8, 21. [Google Scholar] [CrossRef]
- Fernández Fernández, M.M.; Salgado Costas, J. Recolonization of a burnt pine forest (Pinus pinaster) by Carabidae (Coleoptera). Eur. J. Soil Biol. 2004, 40, 47–53. [Google Scholar] [CrossRef]
- Besuchet, C. Biologie, morphologie et systématique des Rhipidius (Col. Rhipiphoridae). Bul. Soc. Entomol. Suisse 1956, 29, 74–144. [Google Scholar]
- López-Colón, J.I. Los Rhipiphoridae Gemminger & Harold, 1870 de la fauna de la Península Ibérica e Islas Baleares (I) (Coleoptera). Lambillionea 1997, 97, 642–650. [Google Scholar]
- Pérez-Vera, F.; Ruiz, J.L.; Ávila, J.M. Descripción de una nueva especie de Asida Latreille, 1802 del subgénero Planasida Escalera, 1907, del norte de África (Coleoptera, Tenebrionidae). Bol. Asoc. Esp. Ent. 2012, 36, 381–400. [Google Scholar]
- Cagniant, H.; Espadaler, X. Les Leptothorax, Epimyrma et chalepoxenus du Maroc (Hyménoptera:Formicidae). Clé et catalogue des espèces. Ann. Soc. Entomol. Fr. 1997, 33, 259–284. [Google Scholar]
- Cagniant, H. Les Crematogaster du Maroc (Hym., Formicidae). Clé de détermination et commentaires. Orsis 2005, 20, 7–12. [Google Scholar]
- Borowiec, L.; Salata, S. Review of Mediterranean members of the Aphaenogaster cecconii group (Hymenoptera: Formicidae), with description of four new species. Zootaxa 2014, 3861, 40–60. [Google Scholar] [CrossRef]
- Taheri, A.; Reyes-López, J.L. Five new records of ants (Hymenoptera: Formicidae) from Morocco. J. Insect Sci. 2015, 15, 37. [Google Scholar] [CrossRef]
- Cagniant, H. Le genre Tetramorium au Maroc (Hymenoptera: Formicidae): Clé et catalogue des espèces. Ann. Soc. Entomol. Fr. 1997, 33, 89–100. [Google Scholar]
- Blatrix, R.; Galkowski, C.; Lebas, C.; Wegnez, P. Fourmis de France, de Belgique et du Luxembourg; Delachaux et Niestlé: Luçon, France, 2013; p. 288. [Google Scholar]
- Louveaux, A.; Amédégnato, C.; Poulain, S.; Desutter-Grandcolas, L. Orthoptères. Acridomorpha de l’Afrique du Nord-Ouest. Available online: http://acrinwafrica.mnhn.fr/ (accessed on 10 January 2023).
- Badih, A.; Pascual, F. Los Caelifera del Norte de Marruecos. Zool Baetica 1998, 9, 149–184. [Google Scholar]
- Gorochov, A.V.; Llorente, V. Estudio taxonómico preliminar de los Grylloidea de España (Insecta, Orthoptera). Graellsia 2001, 57, 95–139. [Google Scholar] [CrossRef]
- Gorochov, A.V. A study of the genus Gryllomorpha Fieber, 1853 (Orthoptera: Gryllidae: Gryllomorphinae). Zoosyst. Ross. 2009, 18, 25–47. [Google Scholar] [CrossRef]
- Shnoun, A.M.; Doumandi, S.E.; Desutter-Grandcolas, L. A check-list of Ensifera from Algeria (Insecta: Orthoptera). Zootaxa 2010, 2432, 1–44. [Google Scholar] [CrossRef]
- Silveira, J.M.; Barlow, J.; Louzada, J.; Moutinho, P. Factors affecting the abundance of leaf-litter arthropods in unburned and thrice-burned seasonally-dry Amazonian forests. PLoS ONE 2010, 5, e12877. [Google Scholar] [CrossRef] [PubMed]
- Arnan, X.; Cerdá, X.; Rodrigo, A.; Retana, J. Response of ant functional composition to fire. Ecography 2013, 36, 1182–1192. [Google Scholar] [CrossRef]
- Langlands, P.R.; Brennan, K.E.C.; Framenau, V.W.; Main, B.Y. Predicting the post-fire responses of animal assemblages: Testing a trait-based approach using spiders. J. Anim. Ecol. 2011, 80, 558–568. [Google Scholar] [CrossRef]
- Greenslade, P. Short term effects of a prescribed burn on invertebrates in grassy woodland in south-eastern Australia. Mem. Mus. Vic. 1997, 56, 305–312. [Google Scholar] [CrossRef]
- Neumann, F.G.; Tolhurst, K. Effects of fuel reduction burning on epigeal arthropods and earthworms in dry sclerophyll eucalypt forest of west-central Victoria. Austral. Ecol. 1991, 16, 315–330. [Google Scholar] [CrossRef]
- Frouz, J. Use of soil dwelling Diptera (Insecta, Diptera) as bioindicators: A review of ecological requirements and response to disturbance. Agric. Ecosyst. Environ. 1999, 74, 167–186. [Google Scholar] [CrossRef]
- Chergui, B.; Fahd, S.; Santos, X. Quercus suber forest and Pinus plantations show different post-fire resilience in Mediterranean north-western Africa. Ann. For. Sci. 2018, 75, 64. [Google Scholar] [CrossRef]
- Moretti, M.; Duelli, P.; Obrist, K.M. Biodiversity and resilience of arthropod communities after fire disturbance in temperate forests. Oecologia 2006, 149, 312–327. [Google Scholar] [CrossRef]
- Kaynas, B.Y. Long-term changes in surface-active beetle communities in a post-fire successional gradient in Pinus brutia forests. iFor. Biogeosci. For. 2017, 10, 376. [Google Scholar] [CrossRef]
- Pausas, J.G. Generalized fire response strategies in plants and animals. Oikos 2019, 128, 147–153. [Google Scholar] [CrossRef]
- Pausas, J.G.; Verdú, M. Fire reduces morphospace occupation in plant communities. Ecology 2008, 89, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Apigian, K.O.; Dahlsten, D.L.; Stephens, S.L. Fire and fire surrogate treatment effects on leaf litter arthropods in a western Sierra Nevada mixed-conifer forest. For. Ecol. Manag. 2006, 221, 110–122. [Google Scholar] [CrossRef]
- Moretti, M.; Obrist, M.K.; Duelli, P. Arthropod biodiversity after forest fires: Winners and losers in the winter fire regime of the southern Alps. Ecography 2004, 27, 173–186. [Google Scholar] [CrossRef]
- Pausas, J.G.; Lamont, B.B.; Paula, S.; Appezzato-da-Glória, B.; Fidelis, A. Unearthing belowground bud banks in fire-prone ecosystems. New Phytol. 2018, 21, 1435–1448. [Google Scholar] [CrossRef]
- Haydon, D.T.; Friar, J.K.; Pianka, E.R. Fire-driven dynamic mosaic in the Great Victoria Desert, Australia. I. Fire geometry. Landsc. Ecol. 2000, 15, 373–381. [Google Scholar] [CrossRef]
- Simila, M.; Kouki, J.; Martikainen, P.; Uotila, A. Conservation of beetles in boreal pine forests: The effects of forest age and naturalness on species assemblages. Biol. Conserv. 2002, 106, 19–27. [Google Scholar] [CrossRef]
- Sippola, A.L.; Siitonen, J.; Punttila, P. Beetle diversity in timberline forests: A comparison between old-growth and regeneration areas in Finnish Lapland. Ann. Zool. Fenn. 2002, 39, 69–86. [Google Scholar]
- Muona, J.; Rutanen, I. The short-term impact of fire on the beetle fauna in boreal coniferous forest. Ann. Zool. Fenn. 1994, 31, 109–121. [Google Scholar]
- Campbell, E.M.; Alfaro, R.I.; Hawkes, B. Spatial distribution of mountain pine beetle outbreaks in relation to climate and stand characteristics: A dendroecological analysis. J. Integr. Plant Biol. 2007, 49, 168–178. [Google Scholar] [CrossRef]
- Castaño-Meneses, G.; Palacios-Vargas, J.G. Effects of fire and agricultural practices on neotropical ant communities. Biodivers. Conserv. 2003, 12, 1913–1919. [Google Scholar] [CrossRef]
- Parr, C.L.; Robertson, H.G.; Biggs, H.C.; Chown, S.L. Response of African savanna ants to long-term fire regimes. J. Appl. Ecol. 2004, 41, 630–642. [Google Scholar] [CrossRef]
- Arnan, X.; Rodrigo, A.; Retana, J. Post-fire recovery of Mediterranean ground ant communities follows vegetation and dryness gradients. J. Biogeogr. 2006, 33, 1246–1258. [Google Scholar] [CrossRef]
- Frizzo, T.L.; Campos, R.I.; Vasconcelos, H.L. Contrasting effects of fire on arboreal and ground-dwelling ant communities of a Neotropical savanna. Biotropica 2012, 44, 254–261. [Google Scholar] [CrossRef]
- Kwon, T.S.; Park, Y.K.; Lee, C.M.; Lim, J.H. Change of Ant Communities in the Burned Forests in Eastern Coastal Area; Korea Forest Research Institute: Seoul, Repubic of Korea, 2013; p. 150. [Google Scholar]
- Cagniant, H.; Espadaler, X. Le genre Messor au Maroc. Ann. Soc. Entomol. Fr. 1997, 33, 419–434. [Google Scholar]
- Arnan, X.; Molowny-Horas, R.; Bluüthgen, N. Food resource exploitation and functional resilience in ant communities found in common Mediterranean habitats. Sci. Total Environ. 2019, 684, 126–135. [Google Scholar] [CrossRef]
- Antunes, S.C.; Curado, N.; Castro, B.B.; Gonçalves, F. Short-term recovery of soil functional parameters and edaphic macro-arthropod community after a forest fire. J. Soils Sediments. 2009, 9, 267–278. [Google Scholar] [CrossRef]
- Taheri, A.; Reyes-Lopez, J.L.; Bennas, N. Contribution à l’étude de la faune myrmécologique du parc national de Talassemtane (nord du Maroc): Biodiversité, biogéographie et espèces indicatrices. Bol. Soc. Entomol. Aragonesa 2014, 54, 225–236. [Google Scholar]
- Bourke, A.F.G. Colony size, social complexity and reproductive conflict in social insects. J. Evolut. Biol. 1999, 12, 245–257. [Google Scholar] [CrossRef]
- Hood, W.G.; Tschinkel, W.R. Desiccation resistance in arboreal and terrestrial ants. Physiol. Entomol. 1990, 15, 23–35. [Google Scholar] [CrossRef]
- Kaspari, M. Body size and microclimate use in Neotropical granivorous ants. Oecologia 1993, 96, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, B. The Hot Blood Insects: Strategies and Mechanisms of Insect Thermoregulation; Harvard University Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Evans, E.W. Fire as a natural disturbance to grasshopper assemblages of tallgrass pairie. Oikos 1984, 43, 9–16. [Google Scholar] [CrossRef]
- Branson, D.H.; Vermiere, L.T. Grasshopper egg mortality mediated by oviposition tactics and fire intensity. Ecol. Entomol. 2007, 32, 128–134. [Google Scholar] [CrossRef]
- Barranco, P.; Pascual, F. Distribución de los ortópteros (Insecta, Orthoptera) en los campos de cultivo del valle del río Andarax (Almería, España). Boletín Sanid. Veg. Plagas. 1992, 18, 613–620. [Google Scholar]
- Mason, S.C.; Shirey, V.; Ponisio, L.C.; Gelhaus, J.K. Responses from bees, butterflies, and ground beetles to different fire and site characteristics: A global meta-analysis. Biol. Conserv. 2021, 261, 109265. [Google Scholar] [CrossRef]
- Brown, J.; York, A.; Christie, F.; McCarthy, M. Effects of fire on pollinators and pollination. J. Appl. Ecol. 2017, 54, 313–322. [Google Scholar] [CrossRef]
- Santos, X.; Belliure, J.; Gonçalves, J.; Pausas, J.G. Resilience of reptiles to megafires. Ecol. Appl. 2021, 32, e2518. [Google Scholar] [CrossRef]
- Rossetti, I.; Cogoni, D.; Calderisi, G.; Fenu, G. Short-Term Effects and Vegetation Response after a Megafire in a Mediterranean Area. Land 2022, 11, 23–28. [Google Scholar] [CrossRef]
| Class | Subclass/Order | Suborder/ Family | Fire Condition | Total | Proportion (%) | |
|---|---|---|---|---|---|---|
| Unburnt | Burnt | |||||
| Crustacea | Isopoda | 97 | 136 | 233 | 7.4 | |
| Arachnida | Acari | 442 | 390 | 832 | 26.6 | |
| Araneida | 47 | 64 | 111 | 3.5 | ||
| Pseudoescorpionida | 6 | 9 | 15 | 0.5 | ||
| Opiliones | 4 | 4 | 8 | 0.3 | ||
| Myriapoda | 0 | 3 | 3 | 0.1 | ||
| Insecta | Hymenoptera | Formicidae | 314 | 623 | 937 | 29.9 |
| Others | 18 | 74 | 92 | 2.9 | ||
| Diptera | 179 | 119 | 298 | 9.5 | ||
| Collembola | 118 | 123 | 241 | 7.7 | ||
| Coleoptera | 78 | 107 | 185 | 5.9 | ||
| Blattodea | 66 | 10 | 76 | 2.4 | ||
| Hemiptera | Heteroptera | 8 | 29 | 37 | 1.2 | |
| Others | 7 | 5 | 12 | 0.4 | ||
| Orthoptera | 3 | 8 | 11 | 0.4 | ||
| Archaeognatha | 6 | 0 | 6 | 0.2 | ||
| Thysanoptera | 2 | 1 | 3 | 0.1 | ||
| Neuroptera | 0 | 1 | 1 | 0.0 | ||
| Unknown nymph | 12 | 18 | 30 | 1.0 | ||
| Total | 1407 | 1724 | 3131 | 100 | ||
| Fire Condition (Burnt and Unburnt) | ||||
|---|---|---|---|---|
| Order | Estimate | Std. Error | Z | P |
| All arthropods | 0.222 | 0.174 | 1.272 | ns |
| Araneae | 0.168 | 0.192 | 0.876 | ns |
| Coleoptera | 0.131 | 0.219 | 0.595 | ns |
| Formicidae | −0.249 | 0.518 | −0.480 | ns |
| Isopoda | 0.479 | 0.841 | 0.570 | ns |
| Blattodea (unburnt) | 2.378 | 0.627 | 3.796 | 0.00015 |
| Diptera (unburnt) | 0.845 | 0.284 | 2.972 | 0.00296 |
| Collembola | 0.370 | 0.424 | 0.872 | ns |
| Orthoptera | −0.501 | 0.696 | −0.719 | ns |
| Acari | 0.253 | 0.633 | 0.400 | ns |
| Hymenoptera | −0.873 | 0.782 | −1.116 | ns |
| Order | Mean Abundance (SE) | PERMANOVA | |||
|---|---|---|---|---|---|
| Unburnt | Burnt | F | R2 | P | |
| Coleoptera | 5.2 (1.03) | 3.8 (0.43) | 2.6783 | 0.06132 | 0.005 |
| Formicidae (Hymenoptera) | 21 (6.5) | 23 (5.23) | 5.6046 | 0.1229 | 0.001 |
| Orthoptera | 0.4 (0.6) | 1.8 (0.37) | 1.1365 | 0.11212 | 0.429 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
EL Khayati, M.; Chergui, B.; Barranco, P.; Fahd, S.; Ruiz, J.L.; Taheri, A.; Santos, X. Assessing the Response of Different Soil Arthropod Communities to Fire: A Case Study from Northwestern Africa. Fire 2023, 6, 206. https://doi.org/10.3390/fire6050206
EL Khayati M, Chergui B, Barranco P, Fahd S, Ruiz JL, Taheri A, Santos X. Assessing the Response of Different Soil Arthropod Communities to Fire: A Case Study from Northwestern Africa. Fire. 2023; 6(5):206. https://doi.org/10.3390/fire6050206
Chicago/Turabian StyleEL Khayati, Mounia, Brahim Chergui, Pablo Barranco, Soumia Fahd, José L. Ruiz, Ahmed Taheri, and Xavier Santos. 2023. "Assessing the Response of Different Soil Arthropod Communities to Fire: A Case Study from Northwestern Africa" Fire 6, no. 5: 206. https://doi.org/10.3390/fire6050206
APA StyleEL Khayati, M., Chergui, B., Barranco, P., Fahd, S., Ruiz, J. L., Taheri, A., & Santos, X. (2023). Assessing the Response of Different Soil Arthropod Communities to Fire: A Case Study from Northwestern Africa. Fire, 6(5), 206. https://doi.org/10.3390/fire6050206







