Effects of Fire Regime on Nitrogen Distribution in Marshlands of the Sanjiang Plain (NE China)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Sampling and Chemical Analysis
2.4. Statistical Analysis
3. Results
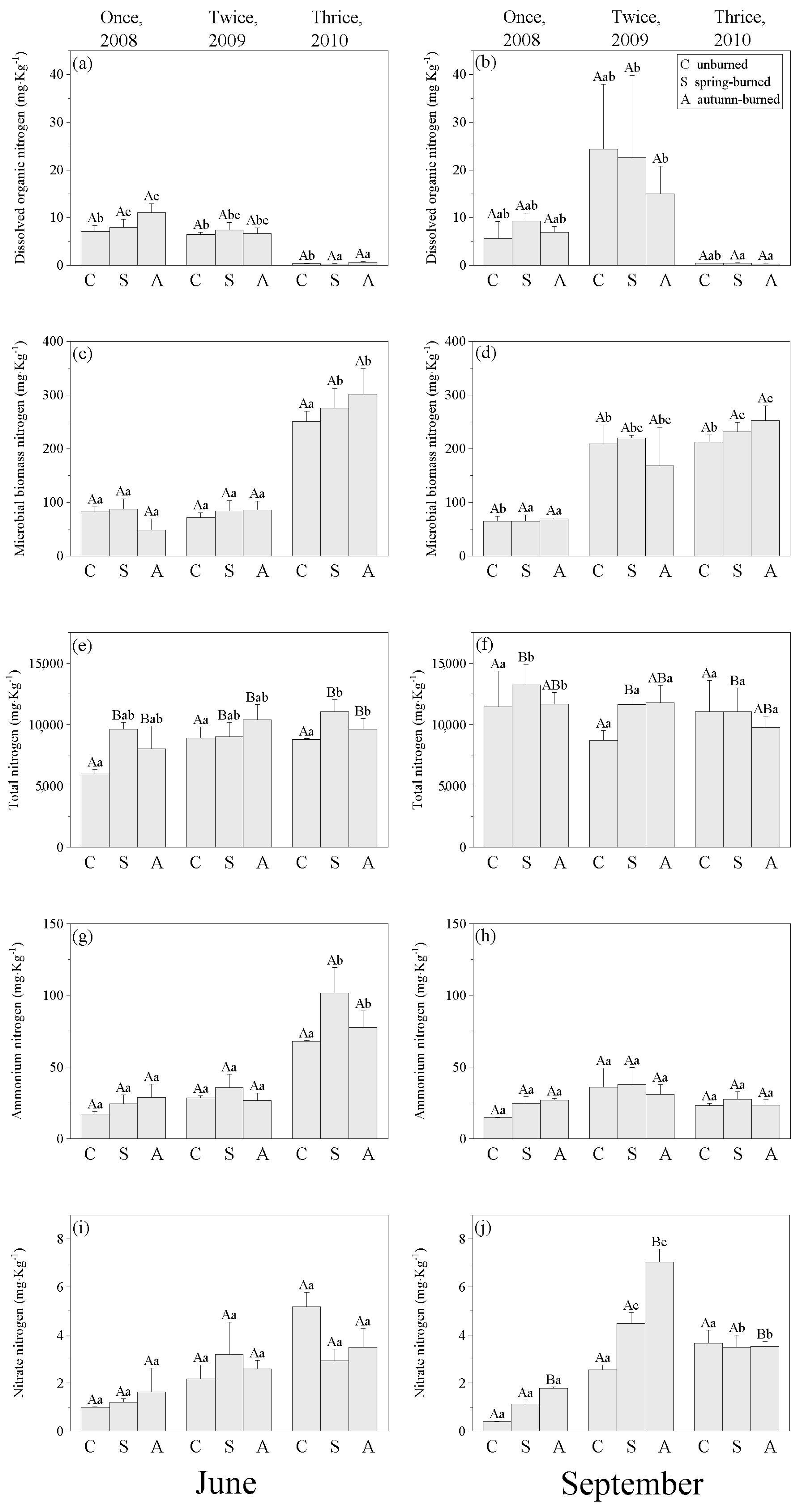
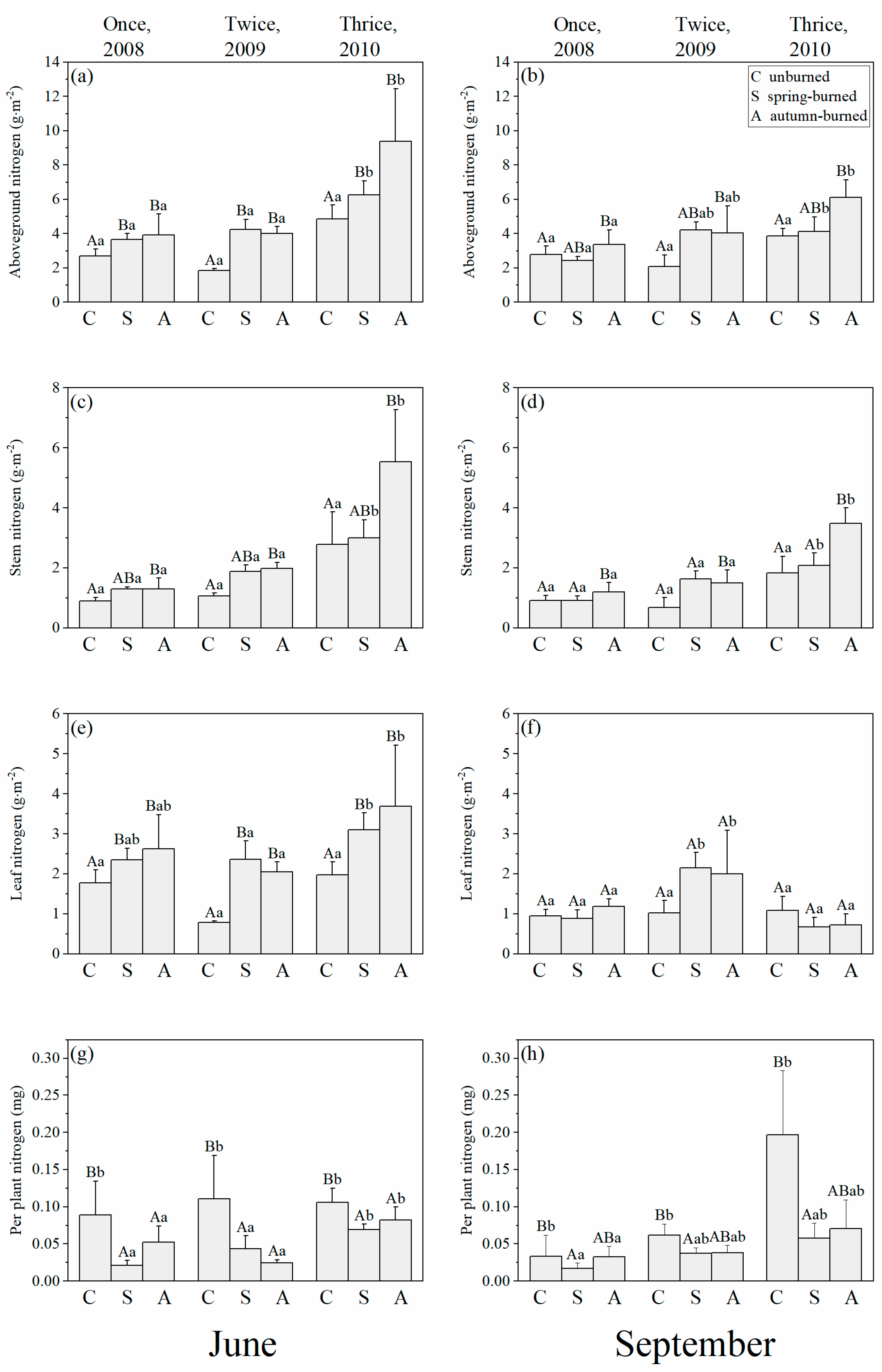
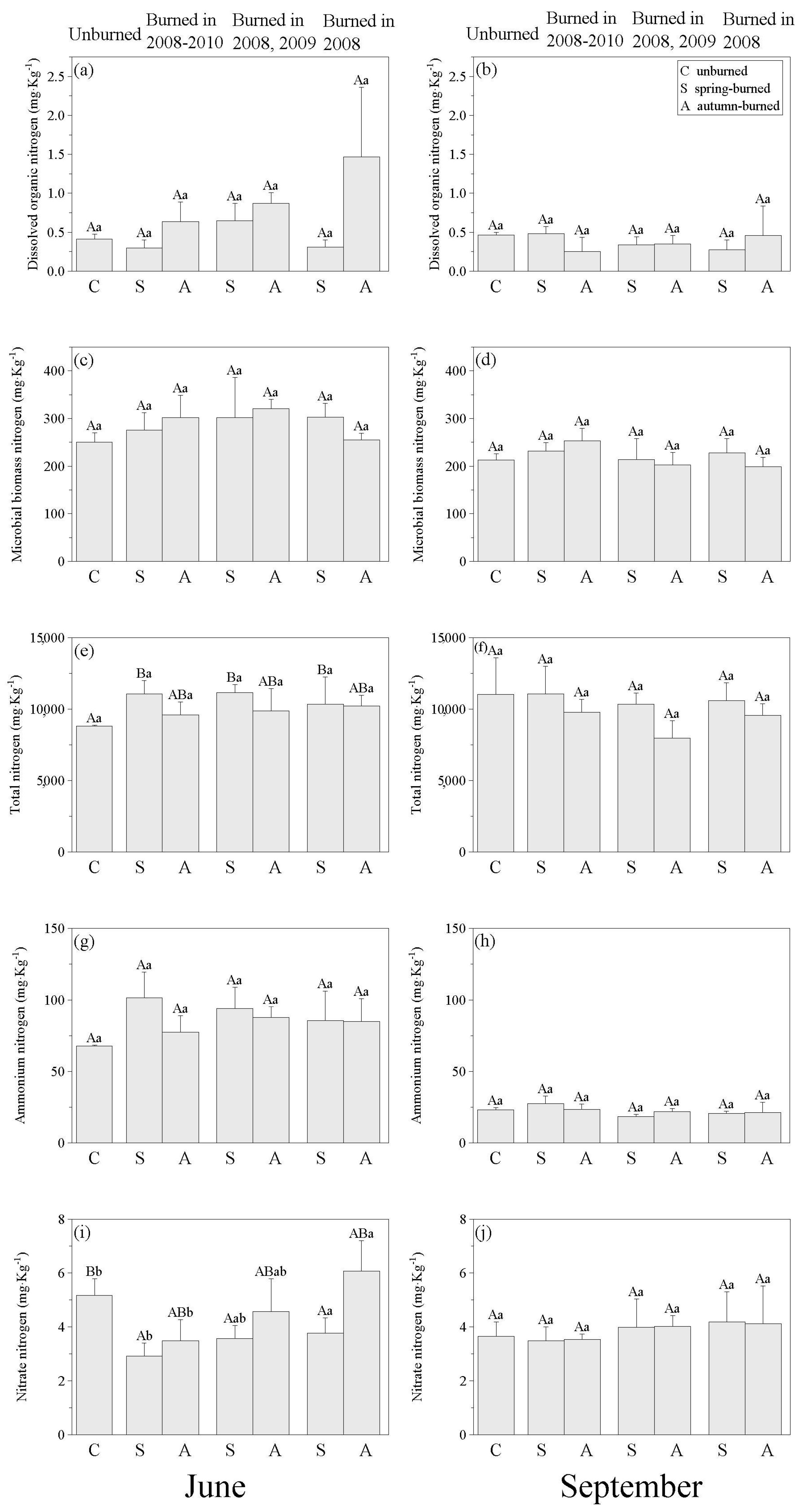
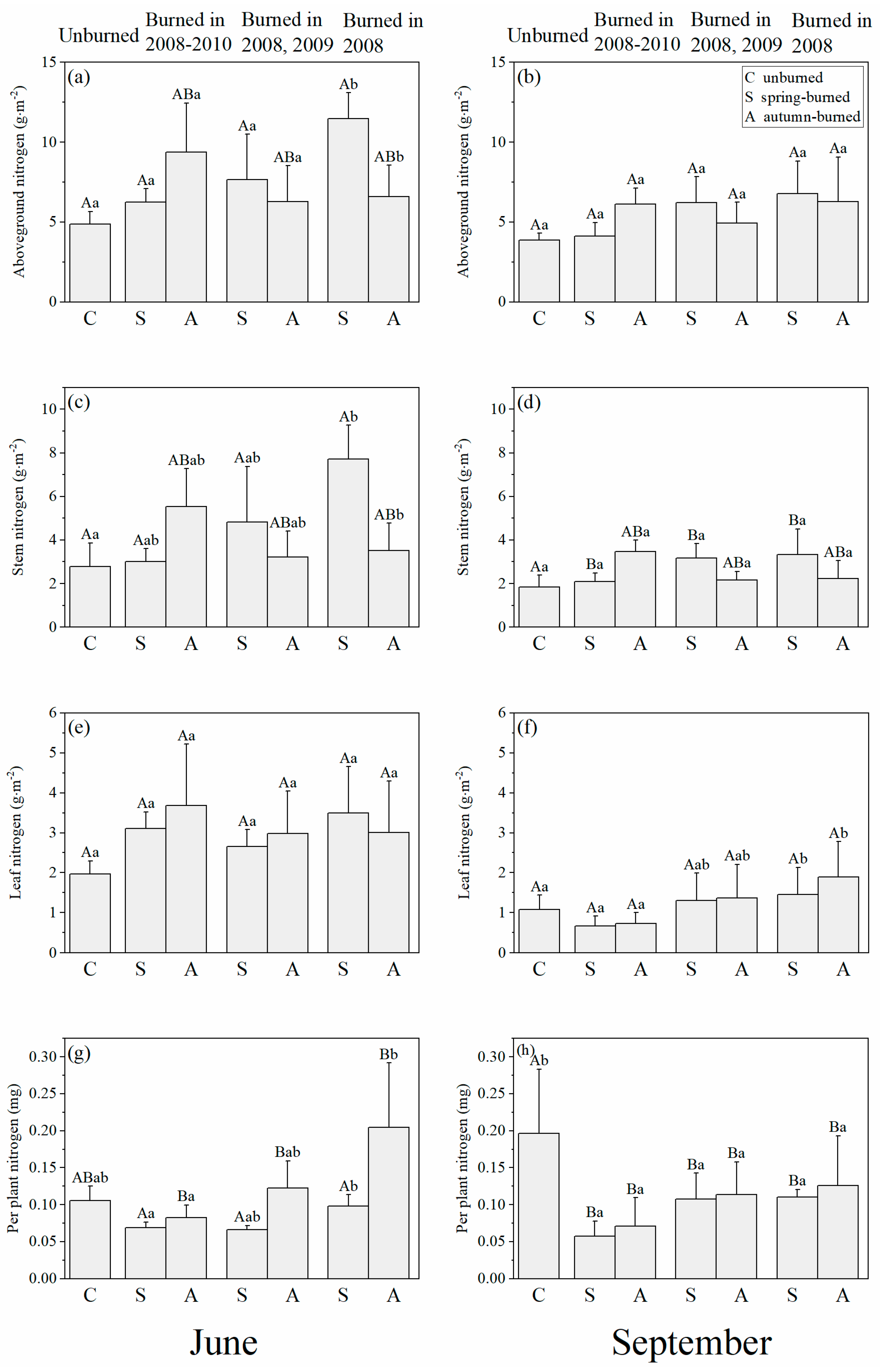
3.1. Changes in Nitrogen Distribution in Soils and Plants by Different Burn Frequency
3.2. Changes in Nitrogen Distribution in Soils and Plants in Time since Burning
4. Discussion
4.1. Effects of Burn Frequency on Marsh N Distribution
4.2. Effects of Time since Burning on Marsh N Distribution
4.3. Effects of Marsh Nitrogen Distribution under Different Burn Seasons
5. Conclusions and Management Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Penfound, W.T. Southern swamps and marshes. Bot. Rev. 1952, 18, 413–446. [Google Scholar] [CrossRef]
- Delle Grazie, F.M.; Gill, L.W. Review of the ecosystem services of temperate wetlands and their valuation tools. Water 2022, 14, 1345. [Google Scholar] [CrossRef]
- Kayranli, B.; Scholz, M.; Mustafa, A.; Hedmark, Å. Carbon storage and fluxes within freshwater wetlands: A critical review. Wetlands 2009, 30, 111–124. [Google Scholar] [CrossRef]
- Lolu, A.J.; Ahluwalia, A.S.; Sidhu, M.C.; Reshi, Z.A.; Shah, M.A.; Lolu, S.J. Wetlands: An imbroglio of interlinked carbon pools. Trop. Ecol. 2018, 59, 383–395. [Google Scholar]
- Wang, M.; Moore, T.R. Carbon, Nitrogen, Phosphorus, and Potassium Stoichiometry in an Ombrotrophic Peatland Reflects Plant Functional Type. Ecosystems 2014, 17, 673–684. [Google Scholar] [CrossRef]
- Wang, M.; Talbot, J.; Moore, T.R. Drainage and fertilization effects on nutrient availability in an ombrotrophic peatland. Sci. Total Environ. 2018, 621, 1255–1263. [Google Scholar] [CrossRef]
- Qian, Y.; Miao, S.L.; Gu, B.; Li, Y.C. Estimation of postfire nutrient loss in the Florida Everglades. J. Environ. Qual. 2009, 38, 1812–1820. [Google Scholar] [CrossRef]
- Hogue, B.A.; Inglett, P.W. Nutrient release from combustion residues of two contrasting herbaceous vegetation types. Sci. Total Environ. 2012, 431, 9–19. [Google Scholar] [CrossRef]
- Liao, X.; Inglett, P.W.; Inglett, K.S. Fire effects on nitrogen cycling in Native and Restored Calcareous Wetlands. Fire Ecol. 2013, 9, 6–20. [Google Scholar] [CrossRef]
- Salvia, M.; Ceballos, D.; Grings, F.; Karszenbaum, H.; Kandus, P. Post-fire effects in wetland environments:landscape assessment of plant coverage and soil recovery in the Paraná River Delta Marshes, Argentina. Fire Ecol. 2012, 8, 17–37. [Google Scholar] [CrossRef]
- Heim, R.J.; Heim, W.; Darman, G.F.; Heinken, T.; Smirenski, S.M.; Holzel, N. Litter removal through fire—A key process for wetland vegetation and ecosystem dynamics. Sci. Total Environ. 2021, 755, 142659. [Google Scholar] [CrossRef]
- Weltzin, J.F.; Keller, J.K.; Bridgham, S.D.; Pastor, J.; Allen, P.B.; Chen, J. Litter controls plant community composition in a northern fen. Oikos 2005, 110, 537–546. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, X.; Li, Y.; Liu, Y.; Zhang, H.; Jia, Y.; Xia, S. Effects of burning on carbon utilization of soil microorganisms and plant growth of Carex brevicuspis communities at Lake Poyang Wetlands, China. Wetlands 2018, 39, 1–15. [Google Scholar] [CrossRef]
- Van Beest, C.; Petrone, R.; Nwaishi, F.; Waddington, J.M.; Macrae, M. Increased peatland nutrient availability following the fort McMurray Horse River wildfire. Diversity 2019, 11, 142. [Google Scholar] [CrossRef]
- DeBano, L.F. The role of fire and soil heating on water repellency in wildland environments: A review. J. Hydrol. 2000, 231, 195–206. [Google Scholar] [CrossRef]
- Mills, A.J.; Fey, M.V. Frequent fires intensify soil crusting: Physicochemical feedback in the pedoderm of long-term burn experiments in South Africa. Geoderma 2004, 121, 45–64. [Google Scholar] [CrossRef]
- Kotze, D.C. The effects of fire on wetland structure and functioning. Afr. J. Aquat. Sci. 2013, 38, 237–247. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Esque, T.C.; Kaye, J.P.; Eckert, S.E.; Defalco, L.A.; Tracy, C.R. Short-term soil inorganic N pulse after experimental fire alters invasive and native annual plant production in a Mojave Desert shrubland. Oecologia 2010, 164, 253–263. [Google Scholar] [CrossRef]
- Nelson, J.D.J.; Schoenau, J.J.; Malhi, S.S.; Gill, K.S. Burning and cultivation effects on greenhouse gas emissions and nutrients in wetland soils from Saskatchewan, Canada. Nutr. Cycl. Agroecosys. 2007, 78, 291–303. [Google Scholar] [CrossRef]
- Xu, T.; Weng, B.; Yan, D.; Wang, K.; Li, X.; Bi, W.; Li, M.; Cheng, X.; Liu, Y. Wetlands of international importance: Status, threats, and future protection. Int. J. Environ. Res. Public. Health 2019, 16, 1818. [Google Scholar] [CrossRef]
- Nichols, L.; Shinneman, D.J.; McIlroy, S.K.; de Graaff, M.-A. Fire frequency impacts soil properties and processes in sagebrush steppe ecosystems of the Columbia Basin. Appl. Soil Ecol. 2021, 165, 103967. [Google Scholar] [CrossRef]
- Hernandez, D.L.; Hobbie, S. Effects of fire frequency on oak litter decomposition and nitrogen dynamics. Oecologia 2008, 158, 535–543. [Google Scholar] [CrossRef]
- Carbone, L.M.; Aguilar, R. Contrasting effects of fire frequency on plant traits of three dominant perennial herbs from Chaco Serrano. Austral Ecol. 2016, 41, 778–790. [Google Scholar] [CrossRef]
- González-Pelayo, O.; Gimeno-García, E.; Ferreira, C.S.S.; Ferreira, A.J.D.; Keizer, J.J.; Andreu, V.; Rubio, J.L. Water repellency of air-dried and sieved samples from limestone soils in central Portugal collected before and after prescribed fire. Plant Soil 2015, 394, 199–214. [Google Scholar] [CrossRef]
- Cleavitt, N.L.; Fahey, T.J.; Groffman, P.M.; Hardy, J.P.; Henry, K.S.; Driscoll, C.T. Effects of soil freezing on fine roots in a northern hardwood forest. Can. J. Forest Res. 2008, 38, 82–91. [Google Scholar] [CrossRef]
- Tierney, G.L.; Fahey, T.J.; Groffman, P.M.; Hardy, J.P.; Fitzhugh, R.D.; Driscoll, C.T. Soil freezing alters fine root dynamics in a northern hardwood forest. Biogeochemistry 2001, 56, 175–190. [Google Scholar] [CrossRef]
- Guo, H.; Sun, Q.; Wu, Z.; Lu, C.; Qin, Z. Study on suitable rice planting scale based on balance of groundwater recharge and discharge in Sanjiang Plain. Water 2023, 15, 547. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, X.; Zhang, J.; Wang, Y.; Wu, L.; Ma, R.; Lu, X.; Jiang, M. Spatiotemporal variation in vegetation phenology and its response to climate change in marshes of Sanjiang Plain, China. Ecol. Evol. 2023, 13, e9755. [Google Scholar] [CrossRef]
- Gao, C.; Wang, G.; Santin, C.; Doerr, S.H.; Cong, J.; Zhao, H. Response of Calamagrostis angustifolia to burn frequency and seasonality in the Sanjiang Plain wetlands (Northeast China). J. Environ. Manag. 2021, 300, 113759. [Google Scholar] [CrossRef]
- Zhao, H.; Tong, D.Q.; Lin, Q.; Lu, X.; Wang, G. Effect of fires on soil organic carbon pool and mineralization in a Northeastern China wetland. Geoderma 2012, 189–190, 532–539. [Google Scholar] [CrossRef]
- Wang, G.-P.; Liu, J.-S.; Wang, J.-D.; Yu, J.-B. Soil phosphorus forms and their variations in depressional and riparian freshwater wetlands (Sanjiang Plain, Northeast China). Geoderma 2006, 132, 59–74. [Google Scholar] [CrossRef]
- Gao, C.; Lin, Q.; Zhang, S.; He, J.; Lu, X.; Wang, G. Historical trends of atmospheric black carbon on Sanjiang Plain as reconstructed from a 150-year peat record. Sci. Rep. 2014, 4, 5723. [Google Scholar] [CrossRef]
- Recous, S.; Robin, D.; Darwis, D.; Mary, B. Soil inorganic N availability: Effect on maize residue decomposition. Soil Biol. Biochem. 1995, 27, 1529–1538. [Google Scholar] [CrossRef]
- Yin, Q.; Zhang, B.; Wang, R.; Zhao, Z. Biochar as an adsorbent for inorganic nitrogen and phosphorus removal from water: A review. Environ. Sci. Pollut. Res. Int. 2017, 24, 26297–26309. [Google Scholar] [CrossRef]
- Raison, R.J. Modification of the soil environment by vegetation fires, with particular reference to nitrogen transformations: A review. Plant Soil 1979, 51, 73–108. [Google Scholar] [CrossRef]
- Prober, S.M.; Lunt, I.D.; Thiele, K.R. Effects of fire frequency and mowing on a temperate, derived grassland soil in south-eastern Australia. Int. J. Wildland Fire 2008, 17, 586–594. [Google Scholar] [CrossRef]
- Montes-Morán, M.A.; Suárez, D.; Menéndez, J.A.; Fuente, E. On the nature of basic sites on carbon surfaces: An overview. Carbon 2004, 42, 1219–1225. [Google Scholar] [CrossRef]
- Clough, T.; Condron, L.; Kammann, C.; Müller, C. A Review of Biochar and Soil Nitrogen Dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Noack, A.G. Black carbon in soils and sediments: Analysis, distribution, implications, and current challenges. Glob. Biogeochem. Cycles 2000, 14, 777–793. [Google Scholar] [CrossRef]
- De Marco, A.; Gentile, A.E.; Arena, C.; De Santo, A.V. Organic matter, nutrient content and biological activity in burned and unburned soils of a Mediterranean maquis area of southern Italy. Int. J. Wildland Fire 2005, 14, 365–377. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, Q.; Noll, L.; Hu, Y.; Wanek, W. Environmental effects on soil microbial nitrogen use efficiency are controlled by allocation of organic nitrogen to microbial growth and regulate gross N mineralization. Soil Biol. Biochem. 2019, 135, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Benson, E.J.; Hartnett, D.C. The role of seed and vegetative reproduction in plant recruitment and demography in tallgrass prairie. Plant Ecol. 2006, 187, 163–178. [Google Scholar] [CrossRef]
- Benson, E.J.; Hartnett, D.C.; Mann, K.H. Belowground bud banks and meristem limitation in tallgrass prairie plant populations. Am. J. Bot. 2004, 91, 416–421. [Google Scholar] [CrossRef]
- Dalgleish, H.J.; Hartnett, D.C. The effects of fire frequency and grazing on tallgrass prairie productivity and plant composition are mediated through bud bank demography. Plant Ecol. 2008, 201, 411–420. [Google Scholar] [CrossRef]
- Oliveras, I.; Meirelles, S.T.; Hirakuri, V.L.; Freitas, C.R.; Miranda, H.S.; Pivello, V.R. Effects of fire regimes on herbaceous biomass and nutrient dynamics in the Brazilian savanna. Int. J. Wildland Fire 2013, 22, 368–380. [Google Scholar] [CrossRef]
- Groves, R.H.; Hocking, P.J.; McMahon, A. Distribution of biomass, nitrogen, phosphorus and other nutrients in Banksia marginata and B. ornata Shoots of different ages after fire. Aust. J. Bot. 1986, 34, 709–725. [Google Scholar] [CrossRef]
- Rau, B.M.; Blank, R.R.; Chambers, J.C.; Johnson, D.W. Prescribed fire in a Great Basin sagebrush ecosystem: Dynamics of soil extractable nitrogen and phosphorus. J. Arid. Environ. 2007, 71, 362–375. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Siddiqi, M.Y.; Glass, A.D.M. Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 1997, 385, 59–61. [Google Scholar] [CrossRef]
- Persson, J.; Hogberg, P.; Ekblad, A.; Hogberg, M.N.; Nordgren, A.; Nasholm, T. Nitrogen acquisition from inorganic and organic sources by boreal forest plants in the field. Oecologia 2003, 137, 252–257. [Google Scholar] [CrossRef]
- Mackenzie, M.; Deluca, T.; Sala, A. Fire exclusion and nitrogen mineralization in low elevation forests of western Montana. Soil Biol. Biochem. 2006, 38, 952–961. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Control of fossil-fuel particulate black carbon and organic matter, possibly the most effective method of slowing global warming. J. Geophys. Res. 2002, 107, ACH-16. [Google Scholar] [CrossRef]
- Hao, W.M.; Petkov, A.; Nordgren, B.L.; Corley, R.E.; Silverstein, R.P.; Urbanski, S.P.; Evangeliou, N.; Balkanski, Y.; Kinder, B.L. Daily black carbon emissions from fires in northern Eurasia for 2002-2015. Geosci. Model Dev. 2016, 9, 4461–4474. [Google Scholar] [CrossRef]
- Stohl, A.; Andrews, E.; Burkhart, J.F.; Forster, C.; Herber, A.; Hoch, S.W.; Kowal, D.; Lunder, C.; Mefford, T.; Ogren, J.A.; et al. Pan-Arctic enhancements of light absorbing aerosol concentrations due to North American boreal forest fires during summer 2004. J. Geophys. Res. 2006, 111, D22214. [Google Scholar] [CrossRef]
- Kuhlbusch, T.A.J. Method for determining black carbon in residues of vegetation fires. Environ. Sci. Technol. 1995, 29, 2695–2702. [Google Scholar] [CrossRef] [PubMed]
- Cleary, M.B.; Pendall, E.; Ewers, B.E. Aboveground and Belowground Carbon Pools After Fire in Mountain Big Sagebrush Steppe. Rangeland Ecol. Manag. 2010, 63, 187–196. [Google Scholar] [CrossRef]
- Li, X.; Song, K.; Liu, G. Wetland fire scar monitoring and its response to changes of the Pantanal Wetland. Sensors 2020, 20, 4268. [Google Scholar] [CrossRef]
- Postma, J.A.; Hecht, V.L.; Hikosaka, K.; Nord, E.A.; Pons, T.L.; Poorter, H. Dividing the pie: A quantitative review on plant density responses. Plant Cell Environ. 2021, 44, 1072–1094. [Google Scholar] [CrossRef]
- Reich, P.B.; Tjoelker, M.G.; Pregitzer, K.S.; Wright, I.J.; Oleksyn, J.; Machado, J.L. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol. Lett. 2008, 11, 793–801. [Google Scholar] [CrossRef]
- James, J.A.; Kern, C.C.; Miesel, J.R. Legacy effects of prescribed fire season and frequency on soil properties in a Pinus resinosa forest in northern Minnesota. Forest Ecol. Manag. 2018, 415–416, 47–57. [Google Scholar] [CrossRef]
- Dickson, T.L. Burning and mowing similarly increase prairie plant production in the spring, but not due to increased soil temperatures. Ecosphere 2019, 10, e02606. [Google Scholar] [CrossRef]
- Pellegrini, A.F.A.; Hedin, L.O.; Staver, A.C.; Govender, N. Fire alters ecosystem carbon and nutrients but not plant nutrient stoichiometry or composition in tropical savanna. Ecology 2015, 96, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Rehling, F.; Sandner, T.M.; Matthies, D.; Hector, A. Biomass partitioning in response to intraspecific competition depends on nutrients and species characteristics: A study of 43 plant species. J. Ecol. 2021, 109, 2219–2233. [Google Scholar] [CrossRef]

| Sampling Time | Burn Frequency df = 2 | Burn Season df = 1 | Time since Burning df = 2 | Burn Season df = 1 | Burn Frequency×Burn Season df = 2 | Time since Burning×Burn Season df = 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | ||
| Dissolved organic nitrogen | Jun. | 35.902 | 0.000 | 0.796 | 0.382 | 0.898 | 0.431 | 6.389 | 0.025 | 1.239 | 0.309 | 1.433 | 0.274 |
| Sep. | 6.470 | 0.007 | 0.805 | 0.380 | 0.062 | 0.940 | 0.533 | 0.478 | 0.347 | 0.711 | 4.269 | 0.038 | |
| Microbial biomass nitrogen | Jun. | 40.456 | 0.000 | 0.012 | 0.913 | 1.399 | 0.282 | 0.254 | 0.623 | 0.517 | 0.603 | 0.556 | 0.586 |
| Sep. | 28.130 | 0.000 | 0.006 | 0.939 | 1.574 | 0.244 | 2.976 | 0.108 | 0.103 | 0.903 | 1.391 | 0.284 | |
| Total nitrogen | Jun. | 2.116 | 0.144 | 0.972 | 0.335 | 0.007 | 0.993 | 2.285 | 0.155 | 3.220 | 0.059 | 0.502 | 0.617 |
| Sep. | 3.548 | 0.048 | 0.731 | 0.403 | 0.987 | 0.399 | 2.839 | 0.116 | 0.538 | 0.592 | 0.287 | 0.755 | |
| Nitrate nitrogen | Jun. | 2.962 | 0.073 | 0.153 | 0.699 | 14.131 | 0.001 | 26.703 | 0.000 | 0.607 | 0.554 | 5.855 | 0.015 |
| Sep. | 41.634 | 0.000 | 7.861 | 0.011 | 1.783 | 0.207 | 0.549 | 0.472 | 3.562 | 0.047 | 0.939 | 0.416 | |
| Ammonium nitrogen | Jun. | 37.379 | 0.000 | 1.261 | 0.274 | 0.289 | 0.753 | 1.168 | 0.299 | 1.891 | 0.175 | 1.508 | 0.258 |
| Sep. | 6.865 | 0.005 | 2.769 | 0.112 | 1.645 | 0.231 | 0.100 | 0.757 | 2.059 | 0.154 | 1.963 | 0.180 | |
| Aboveground nitrogen | Jun. | 19.838 | 0.000 | 3.372 | 0.078 | 1.907 | 0.175 | 1.442 | 0.244 | 3.225 | 0.056 | 7.172 | 0.004 |
| Sep. | 11.367 | 0.000 | 5.594 | 0.026 | 1.536 | 0.240 | 0.009 | 0.926 | 2.642 | 0.090 | 2.118 | 0.146 | |
| Stem nitrogen | Jun. | 25.947 | 0.000 | 6.137 | 0.020 | 2.465 | 0.110 | 2.962 | 0.101 | 5.450 | 0.011 | 9.583 | 0.001 |
| Sep. | 29.143 | 0.000 | 7.207 | 0.012 | 0.061 | 0.941 | 0.739 | 0.400 | 5.830 | 0.008 | 8.270 | 0.002 | |
| Leaf nitrogen | Jun. | 5.917 | 0.008 | 0.381 | 0.542 | 0.703 | 0.507 | 0.109 | 0.744 | 0.790 | 0.465 | 0.610 | 0.553 |
| Sep. | 20.398 | 0.000 | 0.142 | 0.709 | 4.934 | 0.018 | 0.514 | 0.482 | 0.498 | 0.613 | 0.237 | 0.791 | |
| Per–plant nitrogen | Jun. | 6.934 | 0.004 | 0.718 | 0.404 | 8.438 | 0.002 | 14.038 | 0.001 | 2.062 | 0.148 | 2.946 | 0.076 |
| Sep. | 1.202 | 0.317 | 0.219 | 0.644 | 3.077 | 0.068 | 0.372 | 0.549 | 0.044 | 0.957 | 0.023 | 0.977 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, S.; Zhao, H.; Wang, G.; Cong, J.; Li, G.; Han, D.; Gao, C. Effects of Fire Regime on Nitrogen Distribution in Marshlands of the Sanjiang Plain (NE China). Fire 2024, 7, 339. https://doi.org/10.3390/fire7100339
Ji S, Zhao H, Wang G, Cong J, Li G, Han D, Gao C. Effects of Fire Regime on Nitrogen Distribution in Marshlands of the Sanjiang Plain (NE China). Fire. 2024; 7(10):339. https://doi.org/10.3390/fire7100339
Chicago/Turabian StyleJi, Shengzhen, Hongmei Zhao, Guoping Wang, Jinxin Cong, Guangxin Li, Dongxue Han, and Chuanyu Gao. 2024. "Effects of Fire Regime on Nitrogen Distribution in Marshlands of the Sanjiang Plain (NE China)" Fire 7, no. 10: 339. https://doi.org/10.3390/fire7100339
APA StyleJi, S., Zhao, H., Wang, G., Cong, J., Li, G., Han, D., & Gao, C. (2024). Effects of Fire Regime on Nitrogen Distribution in Marshlands of the Sanjiang Plain (NE China). Fire, 7(10), 339. https://doi.org/10.3390/fire7100339









