Abstract
Coal-to-liquid technology is a key technology to ensuring national energy security, with the Fischer–Tropsch synthesis process at its core. However, in actual production, Fischer–Tropsch wax residue exhibits the characteristics of spontaneous combustion due to heat accumulation, posing a fire hazard when exposed to air for extended periods. This significantly threatens the safe production operations of coal-to-liquid chemical enterprises. This study primarily focuses on the experimental investigation of the oxidative spontaneous combustion process of three typical types of wax residues produced during Fischer–Tropsch synthesis. Differential Scanning Calorimetry (DSC) was used to test the thermal flow curves of the three wax residue samples. Kinetic analysis was performed using the Kissinger–Akahira–Sunose (KAS) and Flynn–Wall–Ozawa (FWO) methods to calculate their apparent activation energy. This study analyzed the thermal behavior characteristics, exothermic properties, and kinetic parameters of three typical wax residue samples, exploring the ease of reaction between wax residues and oxygen and their tendency for spontaneous combustion. The results indicate that Wax Residue 1 is rich in low-carbon chain alkanes and olefins, Wax Residue 2 contains relatively fewer low-carbon chain alkanes and olefins, while Wax Residue 3 primarily consists of high-carbon chain alkanes and olefins. This leads to different thermal behavior characteristics among the three typical wax residue samples, with Wax Residue 1 having the lowest heat release and average apparent activation energy and Wax Residue 3 having the highest heat release and average apparent activation energy. These findings suggest that Wax Residue 1 has a higher tendency for spontaneous combustion. This research provides a scientific basis for the safety management of the coal chemical industry, and further exploration into the storage and handling methods of wax residues could reduce fire risks in the future.
1. Introduction
Coal-to-liquid technology is one of the key technologies for ensuring national energy security [1]. The Fischer–Tropsch synthesis technology, the core of coal-to-liquid processes, converts coal into liquid fuels, facilitating the clean and efficient use of coal resources [2]. This process primarily uses an iron-based catalyst to induce polymerization reactions in synthesis gas (CO and H2), producing liquid hydrocarbons or hydrocarbon compounds [3]. In the Fischer–Tropsch slurry bed reactor, the main products—heavy oils and crude wax—are filtered using activated clay and diatomite to remove impurities, intercepting and eliminating some of the mixed catalysts and colored substances in the crude wax. The waste filter cake produced is referred to as “wax residue” [4,5]. The coal-based Fischer–Tropsch synthesis process has achieved industrial application on a megaton scale in China, with a total production capacity exceeding 8 million tons per year. For instance, the 4-million-ton-per-year coal indirect liquefaction unit at the National Energy Group Ningxia Coal Industry Company filters 16,600 tons of Fischer–Tropsch wax residue annually. Wax residues typically contain 40% to 60% paraffin and have the characteristics of spontaneous combustion due to heat accumulation [6]. The toxic gasses and thick smoke released during the combustion of paraffin pose a serious threat to the safety of on-site personnel. The fire can destroy surrounding equipment and buildings, causing huge economic losses. If there is an accumulation of combustible gasses or other flammable and explosive substances in the waste wax storage area, it may cause an explosion and further expand the scope of the accident. Meanwhile, the waste gas and residue generated from the combustion of waste wax can cause pollution in the atmosphere, soil, and water bodies and damage the ecological environment.
The oxidative spontaneous combustion process of the Fischer–Tropsch wax residue is influenced by various factors, primarily depending on the reaction intensity between the wax residue and oxygen. By calculating the apparent activation energy of the reaction between wax residue and oxygen, the tendency of wax residue for spontaneous combustion can be assessed [7,8]. However, there has been limited research, both domestically and internationally, on the oxidative spontaneous combustion process of the Fischer–Tropsch wax residue, with almost no related reports. Waste wax may undergo physical and chemical changes during storage, such as clumping and deterioration, which can affect its subsequent processing and utilization. Due to the flammability and other characteristics of waste wax, there are safety risks, such as fires and explosions, during storage. At present, the treatment technology for waste wax is relatively limited, making it difficult to achieve efficient and environmentally friendly treatment goals. At the same time, some advanced treatment technologies often have high costs, making it difficult to afford coal chemical enterprises economically. Some treatment methods may generate secondary pollution, such as wastewater and exhaust gasses, which increases the difficulty of environmental governance. Given the rapid development of the coal chemical industry and the stringent requirements for the safe production management of hazardous chemicals, studying the oxidation and spontaneous combustion mechanism of wax residues during storage holds significant practical importance. Since wax residue is a byproduct of indirect coal liquefaction [9] and is chemically similar to coal as a hydrocarbon substance, it can be inferred that the oxidative spontaneous combustion properties of wax residue might share some similarities with those of coal. Therefore, methods for studying the oxidative spontaneous combustion of coal can be adapted to investigate the mechanism for wax residue. Table 1 summarizes relevant research reviews on the spontaneous combustion tendencies associated with the kinetic reactions of coal.

Table 1.
Review of oxidative spontaneous combustion.
In recent years, numerous domestic and international scholars have conducted extensive research on the spontaneous combustion tendencies of coal by examining its thermal effects, characteristic temperatures, and kinetic properties using Differential Scanning Calorimetry (DSC). Therefore, adopting this research method to explore the oxidative spontaneous combustion tendencies of three typical Fischer–Tropsch wax residues separated during the coal-to-liquid process holds significant scientific value. This study offers forward-looking guidance for the management practices of the modern coal chemical industry and can provide a reference for preventing and addressing the fire risks associated with wax residues.
2. Materials and Methods
2.1. Experimental Sample
In the indirect coal liquefaction process, based on the process flow of the Fischer–Tropsch synthesis, it is observed that the sources of wax residue are mainly divided into three parts: (1) the slurry bed Fischer–Tropsch reactor: periodic filtering of the slurry bed Fischer–Tropsch reactor generates a wax residue containing iron-based catalysts and heavy wax oil, known as heavy oil filter cake. (2) Separation unit: during the solid–liquid separation of liquid-phase products produced by the slurry bed Fischer–Tropsch reactor, wax residue containing iron-based catalysts and heavy wax oil is generated, known as heavy wax filter cake. (3) Filtration unit: After the solid–liquid separation process in the Fischer–Tropsch filtration unit, wax residue containing heavy wax, filtration aids, and trace amounts of iron-based catalysts are generated, known as catalyst replacement filter cake. Photos of the three typical wax residues are shown in Figure 1. The compositional analysis of the three types of slag wax can be found in Table 2.

Figure 1.
Three typical Fischer–Tropsch wax residues.

Table 2.
Industrial analysis and elemental analysis of experimental samples.
According to Table 2, the Vad content of the three types of slag wax exceeds 80%, with carbon (C) and hydrogen (H) as the main components, indicating that the Fischer–Tropsch synthesis slag wax is a hydrocarbon material. The low Mad content and high Vad content suggest that the slag wax has the characteristics of heat storage and spontaneous combustion. Comparing the three types of slag wax, it was found that the Vad content of slag wax 1 (82.60 wt.%) is lower than that of slag wax 2 (85.57 wt.%), and the Vad content of slag wax 2 is lower than that of slag wax 3 (90.32 wt.%). This corresponds with the elemental analysis results: the C content of slag wax 1 (81.92 wt.%) is lower than that of slag wax 2 (83.23 wt.%), and the C content of slag wax 2 is lower than that of slag wax 3 (83.95 wt.%). This indicates that slag wax 3 has a higher fire hazard than slag wax 2, and slag wax 2 has a higher fire hazard than slag wax 1.
2.2. DSC Apparatus
In this study, the thermal flow curves of three typical wax residue samples were tested using an STA 4439 F3 thermal analysis calorimeter. The working principle involves placing a small sample in an inert or oxidative gas environment at a certain heating rate. A computer is used to monitor and record the changes in the thermal flow curve of the experimental sample in real-time as the temperature changes. According to previous research in the literature [22], all samples need to be ground into powder with a particle size between 80 and 120 mesh. During the experiments, the gas flow rate was maintained at 100 mL/min, with the temperature range set between 40 and 600 °C, while the two slag wax samples were crushed using a crusher and then sieved with a plug to achieve a size of 5 ± 0.2 mg.
2.3. Thermokinetic Analysis Method
The Kissinger–Akahira–Sunose (KAS) method is a commonly used technique for determining the apparent activation energy in the thermal analysis of materials [23,24]. This method is applicable for data processing under non-isothermal conditions. In practical applications, by conducting experiments at different heating rates, different conversion ratios In(β/T2) corresponding to 1/T values can be obtained. By substituting these data into the following equation, the slope can be obtained through linear regression, thereby allowing for the calculation of the apparent activation energy of the material [25,26]. The calculation for this is as follows:
In Equation (1), α represents the conversion rate; β represents the heating rate (K/min); E is the activation energy (J/mol); T represents the temperature (K); A refers to the prefactor (min−1); G(α) represents the integral form of the conversion function; R represents the gas constant, 8.314 J/mol; and i is the index of different heating rates or temperature points.
Compared to KAS, the Flynn–Wall–Ozawa (FWO) method has the advantage of determining the apparent activation energy without the need to know the reaction mechanism beforehand. This model-free approach involves conducting experiments at different heating rates and measuring the temperature at the same conversion rate to plot a relationship between the logarithm of the heating rate, In(β), and the inverse conversion temperature (1/T) [27,28]. The specific steps are as follows:
The symbols and their meanings in the equation are consistent with those in Equation (1).
3. Experimental Results and Discussion
3.1. Analysis of Thermal Behavior Characteristics
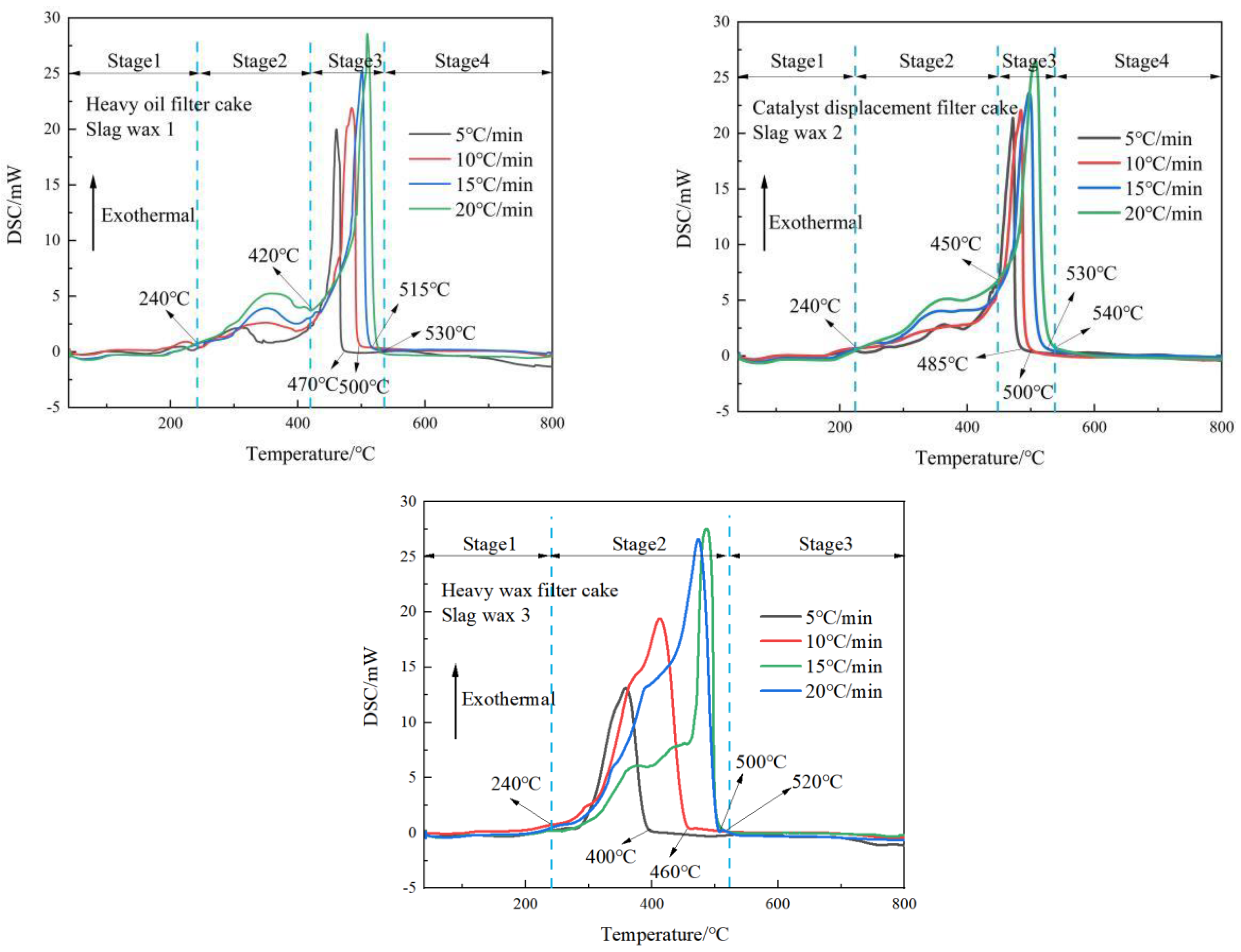
From the DSC curves of the three typical wax residues in Figure 2, it is observable that Wax Residue 1 and Wax Residue 2 exhibit similar trends, characterized by an initial gradual increase, followed by a sharp rise, then a rapid decline, and finally a gentle decrease. Based on these trends, the oxidative combustion processes of Wax Residue 1 and Wax Residue 2 can be divided into four stages: phase transition, pyrolysis, oxidative combustion, and burnout.
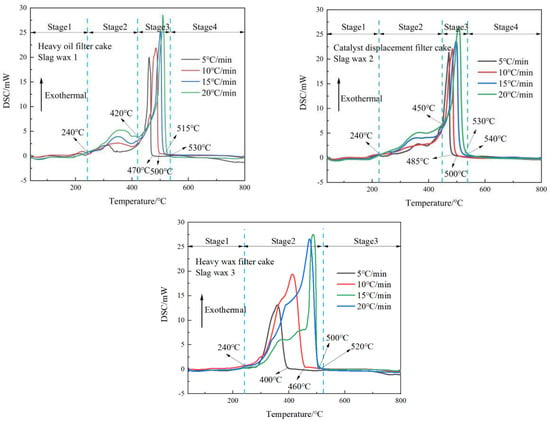
Figure 2.
DSC curves of three typical wax residues.
In contrast, the DSC curve of Wax Residue 3 indicates three stages in its oxidative combustion process, with the pyrolysis and oxidative combustion stages combined into a single combustion stage. During the phase transition stage, for Wax 1, Wax 2, and Wax 3, the temperature ranges from ambient up to 240 °C. During this phase, the heat flow curve is relatively smooth, primarily because the wax residues are decomposing from a solid to a liquid state under heat.
In the pyrolysis stage, both Wax 1 and Wax 2 experience the volatilization of low-carbon alkanes and low-carbon olefins present in the wax residues. For Wax Residue 1, the temperature ranges from 240 °C to 420 °C, while for Wax Residue 2, it ranges from 240 °C to 450 °C. This stage mainly involves the volatilization of low-carbon alkanes and olefins in Fischer–Tropsch wax residues. Since Wax Residue 2 undergoes further separation processes compared to Wax Residue 1, it contains fewer low-carbon chain substances and hence requires higher pyrolysis temperatures. Wax Residue 3, having undergone solid–liquid separation, almost lacks low-carbon chain substances; hence, it does not exhibit the significant volatilization of low-carbon alkanes and olefins. Therefore, the pyrolysis and oxidative combustion stages of Wax Residue 3 coalesce into a single combustion stage.
Upon comparison, it is evident that the peak heights of the characteristic peaks increase with the heating rate, indicating that the heating rate promotes the thermal decomposition of the wax residues. During the oxidative combustion stage, for Wax Residue 1 and Wax Residue 2, the temperature ranges from 420 °C to 530 °C. This stage mainly involves the oxidative combustion of organic matter and macromolecular compounds in the wax residues. The long-chain alkanes and olefins in the wax residues primarily undergo C-C bond and C-H bond cleavage reactions, generating small-molecule gaseous products and low-carbon alkanes and olefins, which release heat during combustion. For Wax Residue 3, this stage ends at around 500 °C, approximately 30 °C earlier than Wax Residue 1 and Wax Residue 2, likely because Wax Residue 3 releases more heat during this stage, accelerating its reaction progress.
Finally, after 550 °C, Wax 1, Wax 2, and Wax 3 enter the burnout stage, wherein the wax residues burn to completion.
3.2. Exothermic Characteristics Analysis
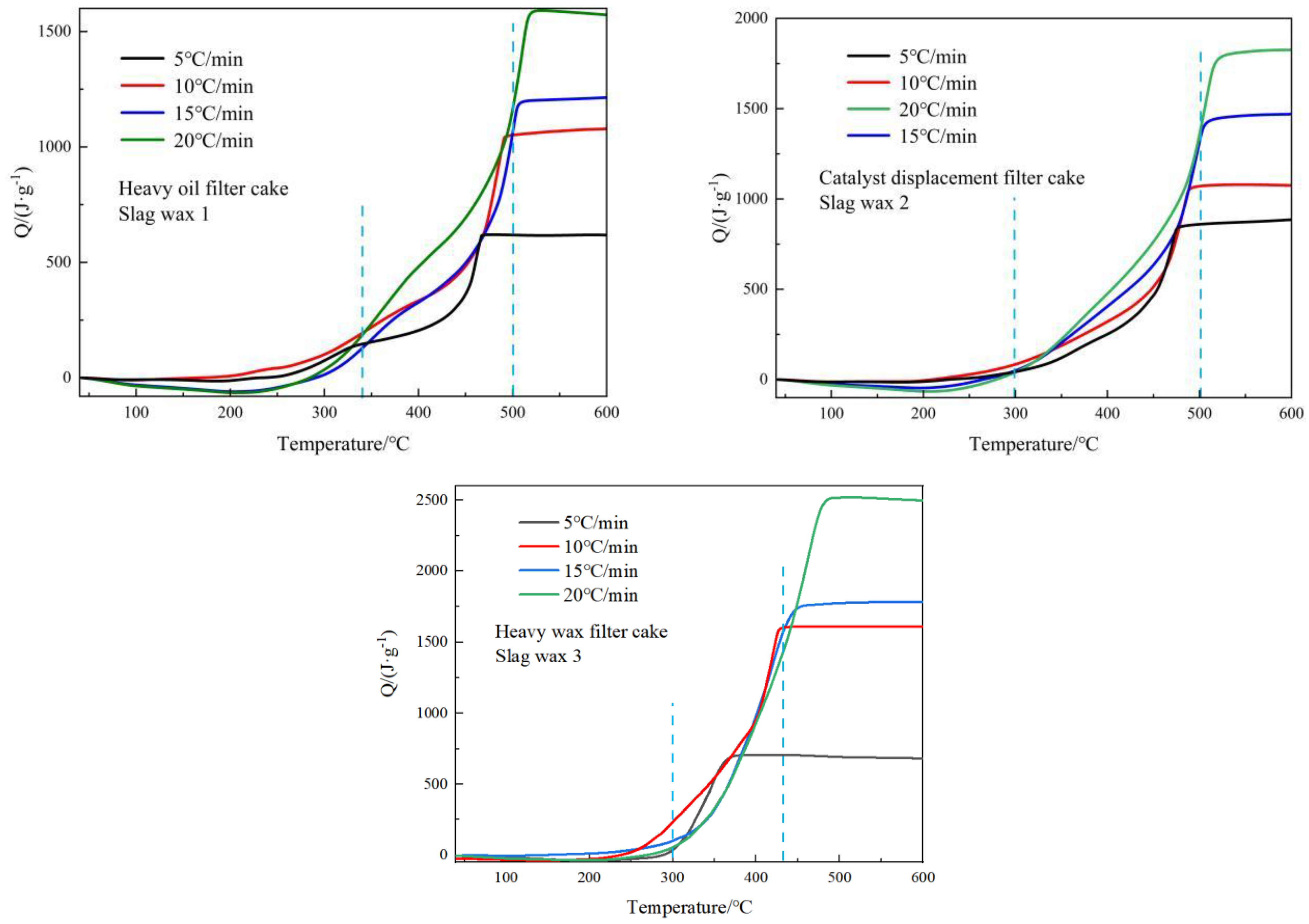
By integrating the thermal flow curves of the three typical wax residues, the heat released during the reaction process can be obtained. Figure 3 shows the heat release curves of the three Fischer–Tropsch wax residues as a function of temperature. In general, as the heating rate increases, the heat release of all three Fischer–Tropsch wax residues also increases correspondingly. Additionally, when comparing the three wax residues, it was found that the heat release was greatest for Wax 3 from the heavy wax filter cake, followed by Wax 2 from the catalyst replacement filter cake, and the least for Wax 1 from the heavy oil filter cake. The reason for this is that the content of the hydrocarbon compounds in Slag Wax 2 is between that of Slag Wax 1 and Slag Wax 3.
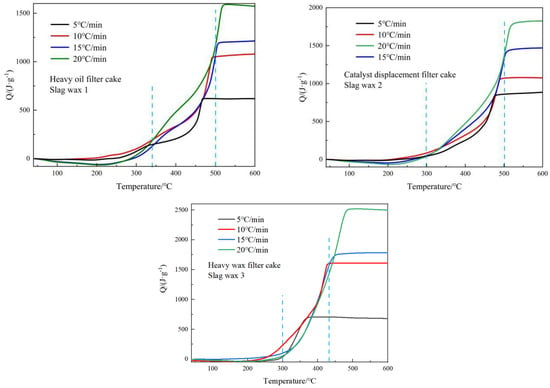
Figure 3.
Heat release curves of three typical wax residues.
The organic matter in Wax 1 primarily consists of heavy wax and a large amount of the Fischer–Tropsch iron catalyst. The high content of iron-based catalysts in this residue results in a lower organic content; hence, its heat release is relatively low. In Wax 2, the content of the iron-based catalysts is reduced, and the content of heavy oil is increased after solid–liquid separation in the separation unit, resulting in greater heat release compared to Wax 1. After solid–liquid separation through the filtering unit, Wax 3 contains heavy wax, filtration aids, and trace amounts of iron-based catalysts. At this stage, the content of heavy wax oil is the highest in Wax 3, leading to the highest heat release.
3.3. Kinetic Analysis
The magnitude of the activation energy reflects the ease or difficulty of the reaction between the wax residue samples and oxygen. A lower activation energy indicates the presence of a large number of molecules with sufficient activation energy, which reduces the energy required for effective collisions between the wax residue samples and oxygen, thereby increasing the likelihood of the reaction. Conversely, a higher activation energy implies that effective collisions between the wax residue and oxygen molecules require higher energy, thereby decreasing the likelihood of the reaction [29].
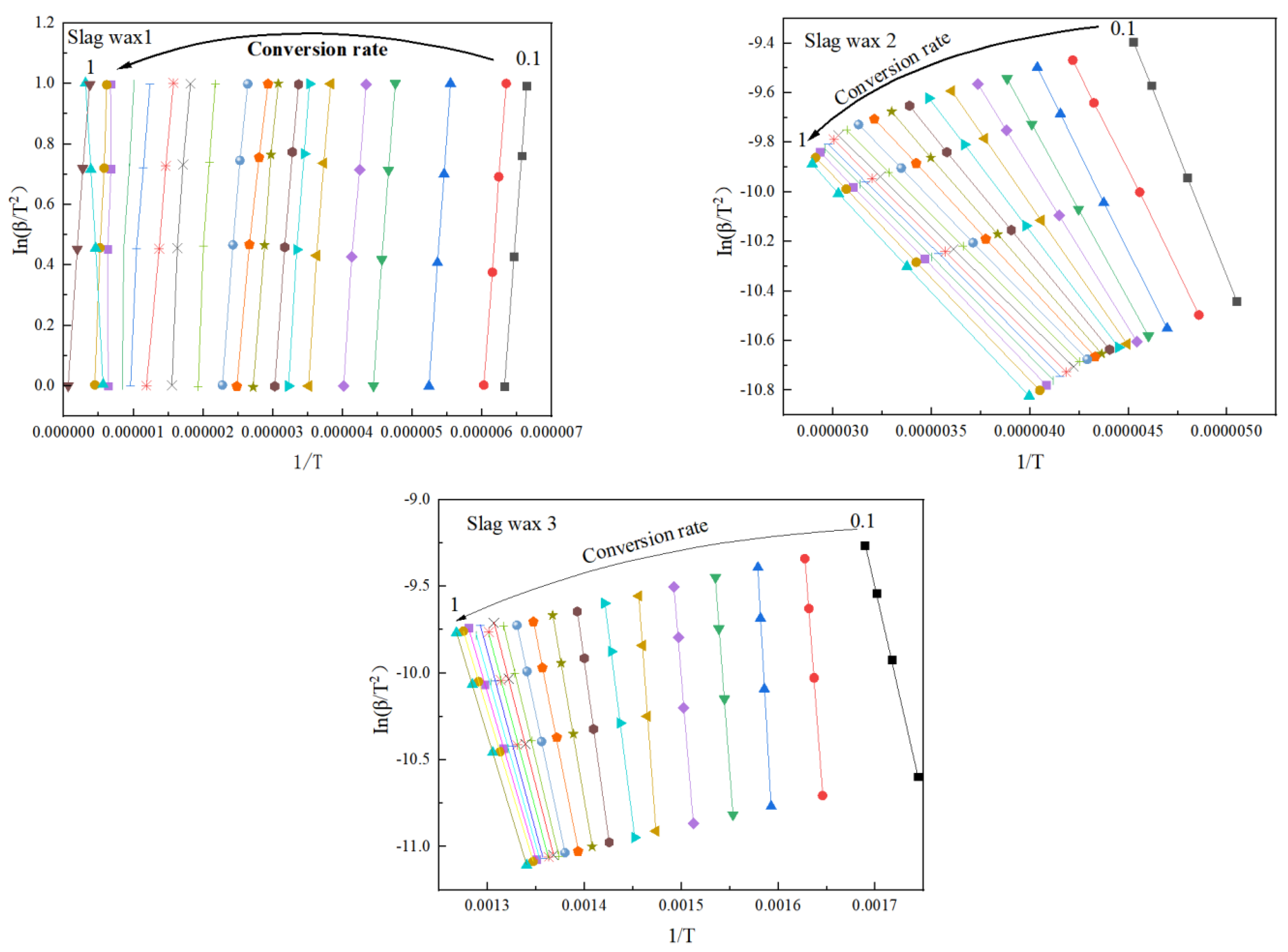
3.3.1. FWO
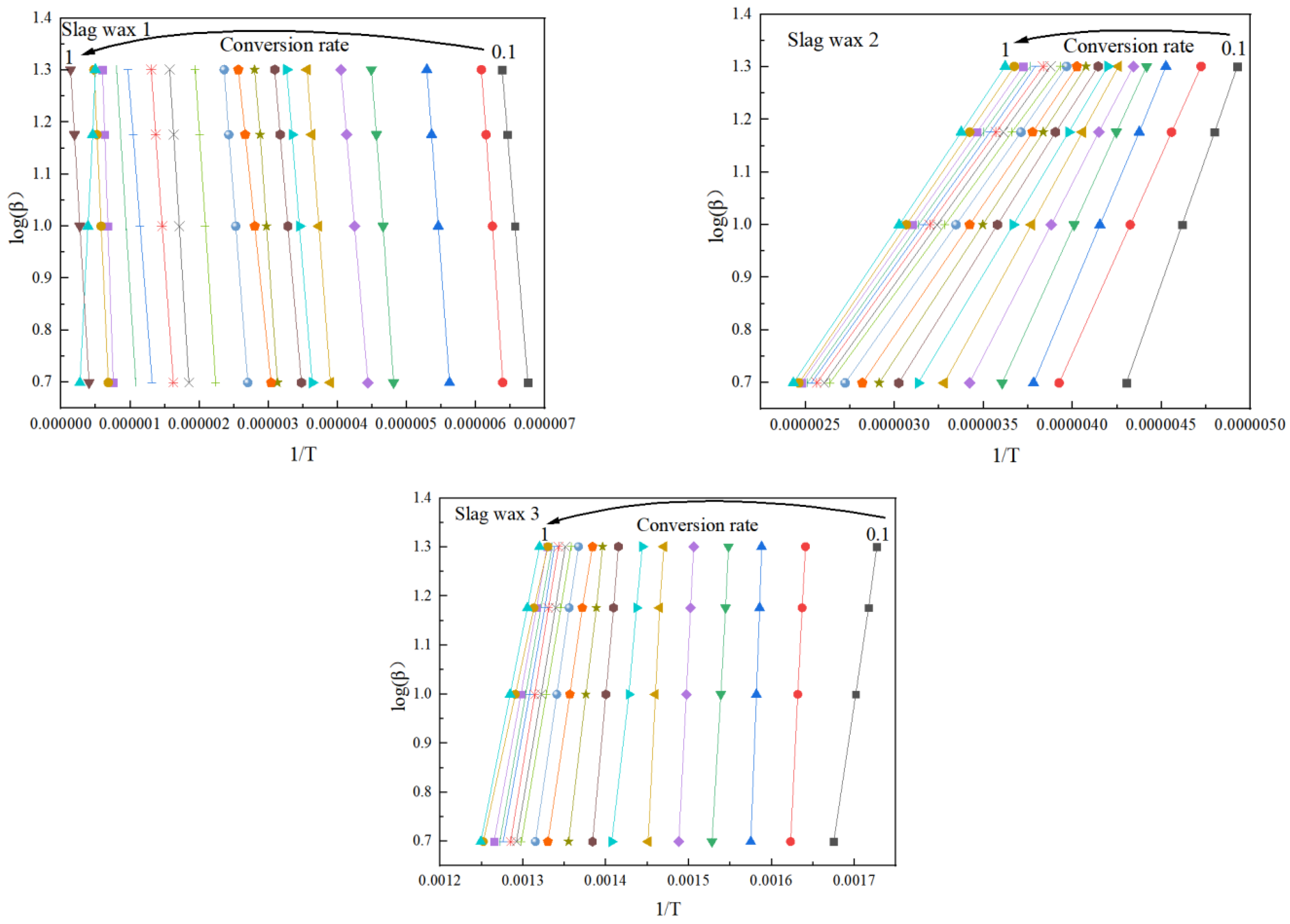
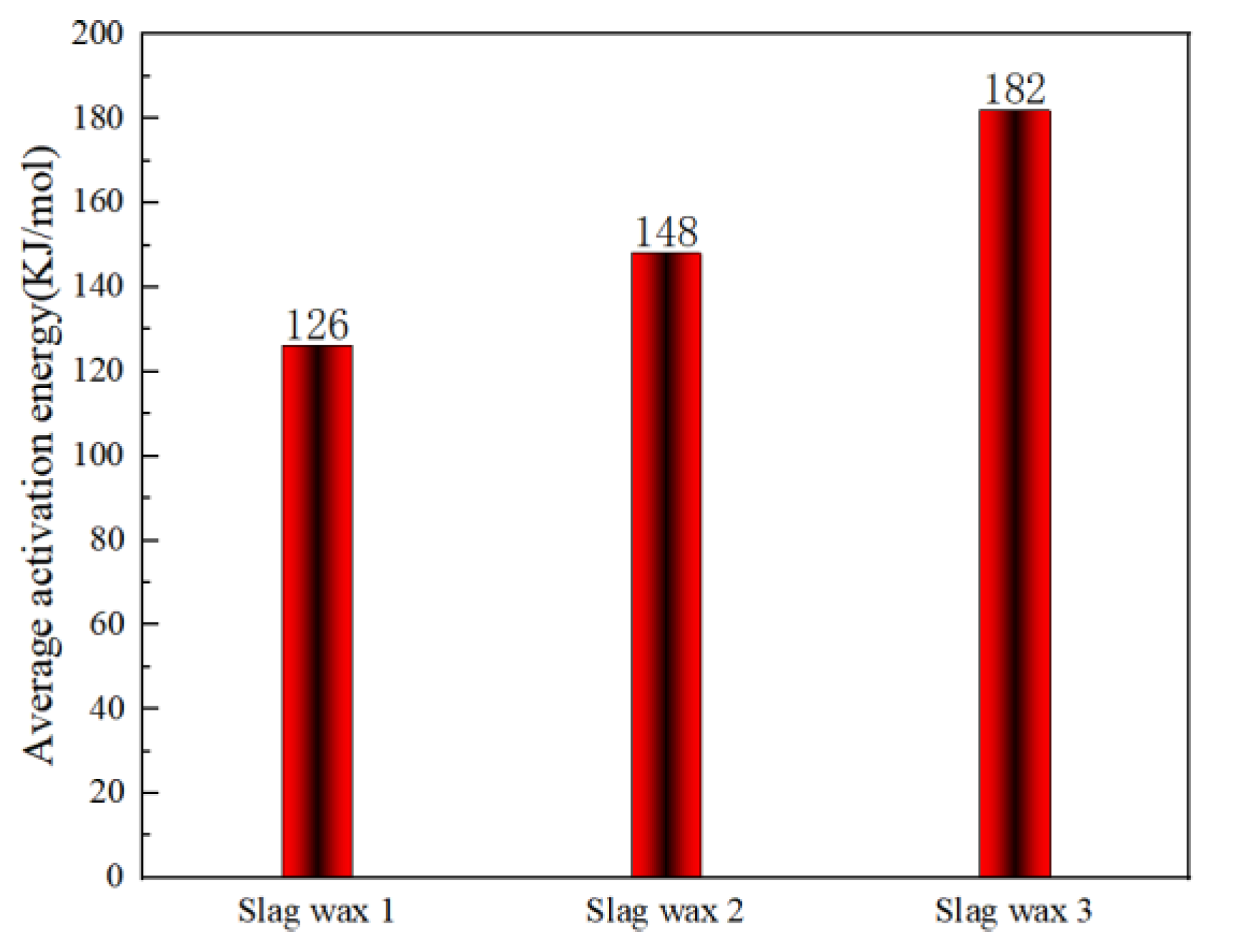
Figure 4 and Figure 5, respectively, show the kinetic parameters and average activation energies of the three wax residue samples calculated using the FWO method. The changes in controlling activation energy were calculated for conversion rates ranging from 0.1 to 1.0 with intervals of 0.1. Following a free radical reaction mechanism, it can be seen that the average activation energy of Wax 1 (126 KJ/mol) is lower than that of Wax 2 (148 KJ/mol), while Wax 3 has the highest average activation energy (182 KJ/mol). Despite the similarities in the raw materials of these wax samples, which are all hydrocarbon compounds produced from the Fischer–Tropsch synthesis process and follow a free radical reaction mechanism, their internal compositions differ, resulting in variations in their activation energies.
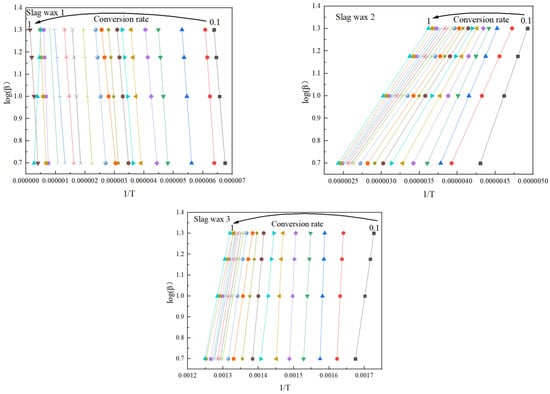
Figure 4.
Kinetic parameters calculated using the FWO method.
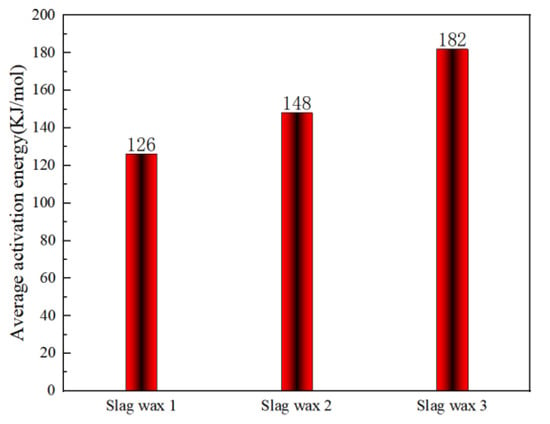
Figure 5.
Average apparent activation energy calculated using the FWO method.
Wax 3 is primarily composed of high-carbon chain alkanes and olefins, requiring higher temperatures to increase the collision probability between molecules, thereby necessitating a higher average activation energy for the oxidative combustion reaction. In contrast, Wax 1 contains a significant amount of low-carbon chain alkanes and olefins, which require lower temperatures for oxidation and combustion, resulting in a lower average apparent activation energy. Wax 2, generated during the solid–liquid separation process, contains iron-based catalysts and heavy wax oil. Therefore, its content of low-carbon chain alkanes and olefins is lower than that of Wax 1, but it has a higher content of iron-based catalysts compared to Wax 3, placing its average apparent activation energy between that of Wax 1 and Wax 3.
3.3.2. KAS
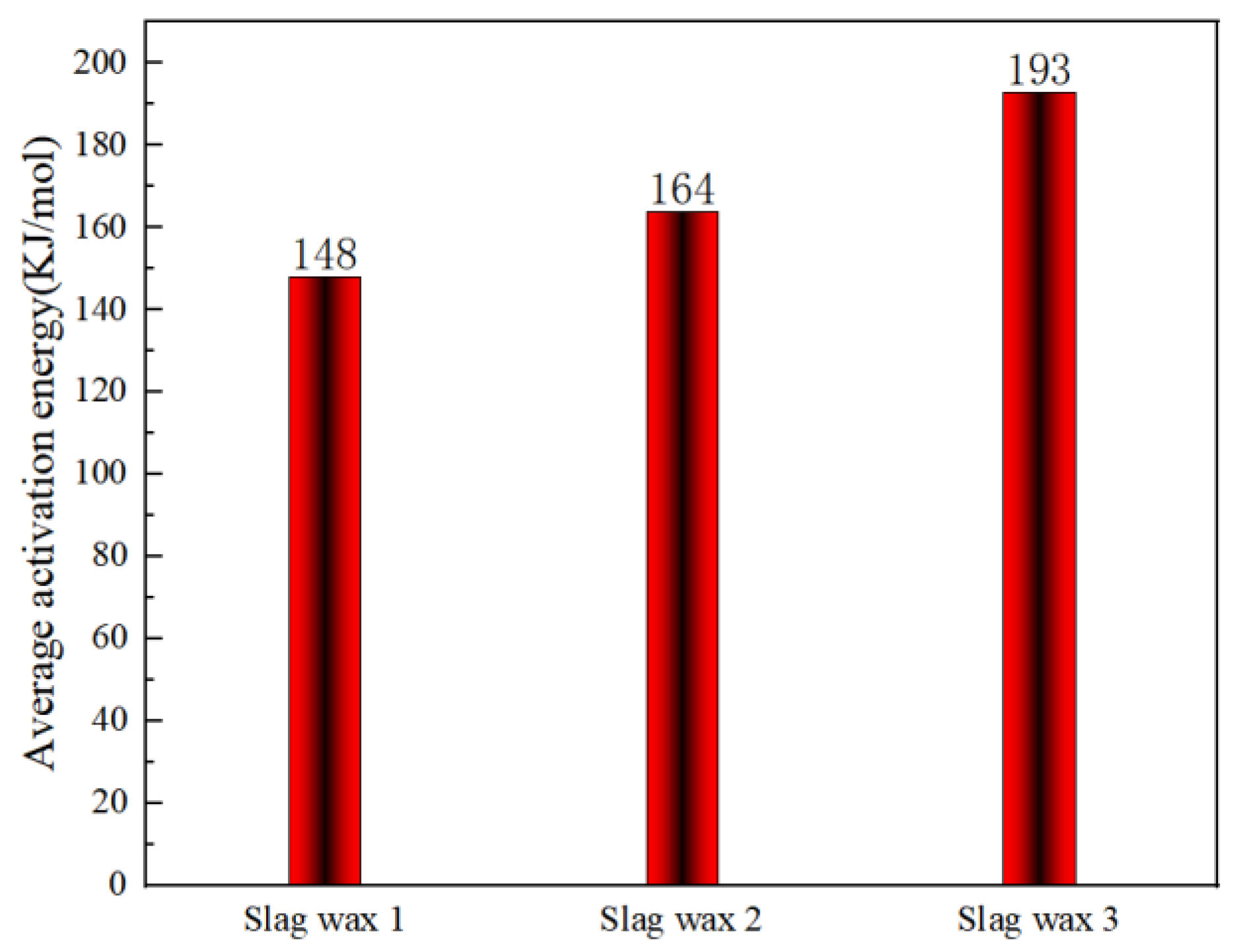
Figure 6 and Figure 7, respectively, show the kinetic parameters and average activation energies of the three wax residue samples calculated using the KAS method. The results from the KAS method indicate that the apparent activation energies calculated by this method are almost identical to those obtained using the FWO method. However, the average apparent activation energies of the three typical wax residue samples calculated by the KAS method are higher than those calculated by the FWO method. Specifically, the average activation energy of Wax 1 (KAS: 148 KJ/mol > FWO: 126 KJ/mol) is lower than that of Wax 2 (KAS: 164 KJ/mol > FWO: 148 KJ/mol), which, in turn, is lower than that of Wax 3 (KAS: 193 KJ/mol > FWO: 182 KJ/mol).
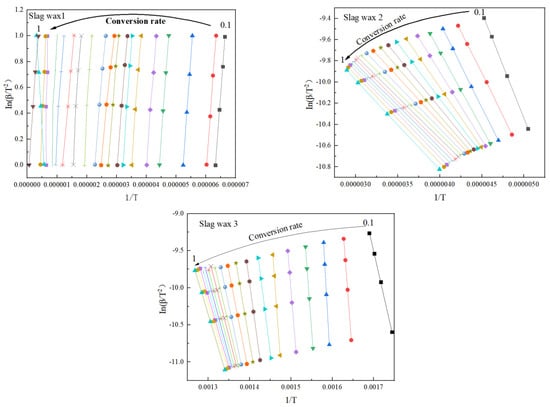
Figure 6.
Kinetic parameters calculated using the KAS method.
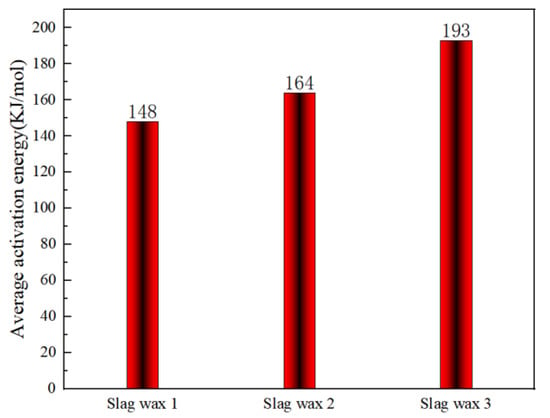
Figure 7.
Average apparent activation energy calculated using the KAS method.
The KAS method, which uses multiple heating rates and linear regression to process experimental data, can reduce the accumulation of experimental errors, better reflect the kinetic characteristics of the wax samples, and mitigate the effects of internal heterogeneity in the wax samples to some extent. On the other hand, the apparent activation energies calculated using the FWO method, a model-free approach, do not rely on specific reaction mechanisms and are applicable to various types of oxidative combustion reactions.
Since the results of the KAS and FWO methods are largely consistent, this validates the accuracy of the apparent activation energies calculated for the three typical wax residue samples.
4. Conclusions
In this paper, we employed Differential Scanning Calorimetry (DSC) to test the thermal flow curves of three typical Fischer–Tropsch wax residue samples analyzed their thermal behavior characteristics and exothermic properties and performed kinetic analysis using the Kissinger–Akahira–Sunose (KAS) and Flynn–Wall–Ozawa (FWO) methods to calculate their apparent activation energy. This revealed the mechanisms of their oxidative spontaneous combustion. The main findings of the study are as follows:
Thermal Behavior Characteristics: The oxidative combustion processes of the three typical Fischer–Tropsch wax residues (Wax 1, Wax 2, and Wax 3) exhibited distinct thermal behavior characteristics. The DSC thermal flow curves of Wax 1 and Wax 2 showed four stages: phase transition, pyrolysis, oxidative combustion, and burnout. However, the thermal flow curve of Wax 3 combined the pyrolysis and oxidative combustion stages into a single combustion stage, indicating that Wax 3 contains almost no low-carbon alkanes and olefins compared to Wax 1 and Wax 2.
Exothermic Properties: As the heating rate increased, the exothermic amounts of all three typical wax residue samples correspondingly increased. Wax 3 exhibited the highest exothermic amount, followed by Wax 2, with Wax 1 showing the least exothermic amount. This difference is mainly attributed to the variance in the content and composition of organic matter within the waxes. Wax 1 contained a significant number of iron-based catalysts, which resulted in a lower organic matter content and, subsequently, a lower exothermic amount.
Kinetic Analysis: The apparent activation energies calculated using the FWO and KAS methods indicated that the average activation energy of Wax 1 was less than that of Wax 2, which was, in turn, less than that of Wax 3. This suggests that although the raw materials of the three wax samples are similar, significant differences in their internal composition leads to considerable variations in their reactivity with oxygen. Wax 1 contains a substantial amount of low-carbon chain alkanes and olefins, and Wax 2 contains relatively fewer low-carbon chain alkanes and olefins, whereas Wax 3 is primarily composed of high-carbon chain alkanes and olefins. This results in the different average activation energies required for their oxidative combustion reactions, making Wax 1 more prone to oxidative spontaneous combustion.
Safety Management Significance: The research results provide scientific evidence for the safety management of the coal chemical industry, aiding in the prevention and handling of wax residue fire risks. Future research could conduct comprehensive tests on the physicochemical properties and compositional characteristics of the three typical Fischer–Tropsch synthesis wax residues, further revealing the oxidation spontaneous combustion behavior and kinetic characteristics of these three wax residues.
Author Contributions
Formal analysis, Writing—original draft preparation, T.L.; Supervision, investigation, J.D.; Validation, Supervision, M.Y.; Investigation, Resources, X.Y. (Xiaojing Yong); Conceptualization, Methodology, T.Z.; Methodology, Resources, X.Y. (Xin Yi); Formal analysis, Investigation, Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No. 52274225); the Key R&D Project in Ningxia Hui Autonomous Region (No. 2022BEE02001) and Special Key Research Project of Integration of Industry; the Innovation Chain of QINCHUANYUAN Industrial Cluster Programme (No. 2023QCY-LL-24); and the Collaborative innovation center program of Shaanxi Provincial Department of Education (22JY041).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.
Acknowledgments
This study’s relevant experimental tests were conducted at the Coal Chemical Industry Technology Research Institute of the Ningxia Coal Industry Company, a subsidiary of the National Energy Group. The research was supported by the institute’s scientific project “Development of Thermal Desorption Technology for the Utilization of Fischer–Tropsch Wax Residues”, as well as funding from the National Natural Science Foundation of China project “Study on the Mechanism of Inhibiting Coal Spontaneous Combustion by Aerobic Microorganisms” and the “Xi’an University of Science and Technology Outstanding Doctoral Dissertation Cultivation Project”.
Conflicts of Interest
Author Tongshuang Liu was employed by the company Shaan Xi Anyitexin New Material Co., Ltd. Author Xiaojing Yong was employed by the company Ningxia Coal Industry Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Novikau, A. Energy security in security studies: A systematic review of twenty years of literature. Cent. Eur. J. Int. Secur. Stud. 2023, 17, 36–64. [Google Scholar] [CrossRef]
- Lin, T.; An, Y.; Yu, F.; Gong, K.; Yu, H.; Wang, C.; Sun, Y.; Zhong, L. Advances in selectivity control for Fischer–Tropsch synthesis to fuels and chemicals with high carbon efficiency. ACS Catal. 2022, 12, 12092–12112. [Google Scholar] [CrossRef]
- Okoye-Chine, C.; Mubenesha, S. The Use of Iron Ore as a Catalyst in Fischer–Tropsch Synthesis—A Review. Crystals 2022, 12, 1349. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, S.; Zhang, C.; Li, X.; Guo, Y. Adsorption decolorization and composition analysis of high melting point Fischer–Tropsch waxes. Asia-Pac. J. Chem. Eng. 2023, 18, e2857. [Google Scholar] [CrossRef]
- Chernyak, S.; Burtsev, A.; Maksimov, S.; Kupreenko, S.; Maslakov, K.; Savilov, S. Structural evolution, stability, deactivation and regeneration of Fischer-Tropsch cobalt-based catalysts supported on carbon nanotubes. Appl. Catal. A-Gen. 2020, 603, 117741. [Google Scholar] [CrossRef]
- Liang, C.; Yin, Z.; Sun, Y.; Xu, Y.; Yao, k.; Liu, Z.; Zhu, M. Pyrolysis of waste Fischer-Tropsch wax: An experimental study. J. Clean. Prod. 2022, 350, 131529. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, S.; Xie, Z.; Zhu, K.; Xu, X.; Ding, X.; Glowacz, A. Investigation of the pyrophoric tendency of the powder of corrosion products in an oil tank. Powder Technol. 2018, 339, 296–305. [Google Scholar] [CrossRef]
- Deng, J.; Liu, T.; Yao, M.; Yi, X.; Bai, G.; Huang, Q.; Li, Z. Comparative study of the combustion and kinetic characteristics of fresh and naturally aged pine wood. Fuel 2023, 343, 127962. [Google Scholar] [CrossRef]
- Kubicka, D.; Černý, R. Upgrading of Fischer-Tropsch waxes by fluid catalytic cracking. Ind. Eng. Chem. Res. 2012, 51, 8849–8857. [Google Scholar] [CrossRef]
- Zhao, J.; Li, R.; Song, J.; Lu, S.; Shu, C. Effect of oxygen concentration on the heat release behaviour of bituminous coal over the complete spontaneous combustion process. J. Therm. Anal. Calorim. 2024, 149, 10227–10240. [Google Scholar] [CrossRef]
- Deng, J.; Qu, G.; Ren, S.; Wang, C.; Su, H.; Yuan, Y.; Duan, X.; Yang, N.; Wang, J. Effect of water soaking and air drying on the thermal effect and heat transfer characteristics of coal oxidation at the low-temperature oxidation stage. Energy 2024, 288, 129705. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Z.; Zhai, X.; Wen, H. Thermodynamic behaviors of coal spontaneous combustion under different CO2 concentration. Int. J. Coal Prep. Util. 2023, 43, 1583–1596. [Google Scholar] [CrossRef]
- Zhu, J.; Gao, L.; Liu, H. Experimental research on the activation energy analysis of coal spontaneous combustion based on DSC. Disaster Adv. 2013, 6, 278–283. [Google Scholar]
- Yang, F.; Lai, Y.; Song, Y. Determination of the influence of pyrite on coal spontaneous combustion by thermodynamics analysis. Process Saf. Env. 2019, 129, 163–167. [Google Scholar] [CrossRef]
- Li, X.; Jin, Z.; Bai, G. Experimental study on the effect of acidity on coal spontaneous combustion at different oxygen concentrations. Energy Source Part A 2020, 1–10. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, T.; Jia, H.; Hu, D.; Wang, L. Study of the mutual coupling characteristics of the oxidation thermal effect and microstructural evolution of gas-containing coal. Sci. Total Env. 2024, 924, 171574. [Google Scholar] [CrossRef]
- Qin, B.; Dou, G.; Wang, D. Thermal analysis of vitamin C affecting low-temperature oxidation of coal. J. Wuhan Univ. Technol. 2016, 31, 519–522. [Google Scholar] [CrossRef]
- Dong, X.; Wang, F.; Guo, L.; Han, T. Study on the Influence of Coal Structure and Oxidation Performance by Endogenous Bacterium. Fire 2023, 6, 339. [Google Scholar] [CrossRef]
- Bekhouche, S.; Trache, D.; Akbi, H.; Abdelaziz, A.; Tarchoun, A.F.; Boudouh, H. Thermal decomposition behavior and ki-netic study of nitrocellulose in presence of ternary nanothermites with different oxi-dizers. FirePhysChem 2023, 3, 208–216. [Google Scholar] [CrossRef]
- Pal, Y.; Mahottamananda, S.N.; Subha, S.; Palateerdham, S.K.; Ingenito, A. Thermal decomposition kinetics and combustion performance of paraffin-based fuel in the presence of CeO2 catalyst. FirePhysChem 2023, 3, 217–226. [Google Scholar] [CrossRef]
- Chalghoum, F.; Jouini, M.; Abdelaziz, A.; Tarchoun, A.F.; Boukeciat, H.; Bekhouche, S.; Benziane, M.; Trache, D. Effect of the accelerated aging process on the thermal decomposition of LiAlH4-based composite solid propellants. FirePhysChem, 2024; in press. [Google Scholar] [CrossRef]
- Mohalik, N.; Mandal, S.; Ray, S.; Khan, A.; Mishra, D.; Pandey, J. TGA/DSC study to characterise and classify coal seams conforming to susceptibility towards spontaneous combustion. Int. J. Min. Sci. Technol. 2022, 32, 75–88. [Google Scholar] [CrossRef]
- Daoudi, M.; Triki, A.; Redjaimia, A. DSC study of the kinetic parameters of the metastable phases formation during non-isothermal annealing of an Al–Si–Mg alloy. J. Therm. Anal. Calorim. 2011, 104, 627–633. [Google Scholar] [CrossRef]
- Khedri, S.; Elyasi, S. Kinetic analysis for thermal cracking of HDPE: A new isoconversional approach. Polym. Degrad. Stabil. 2016, 129, 306–318. [Google Scholar] [CrossRef]
- Sopaci, S.; Nazir, H.; Emir, E.; Atakol, O.; Oz, S. Thermal kinetic analysis, theoretical thermodynamic calculations and antimicrobial activity of three new energetic materials. J. Therm. Anal. Calorim. 2018, 131, 3105–3120. [Google Scholar] [CrossRef]
- Wang, K.; Hu, L.; Deng, J.; Zhang, Y. Multiscale thermal behavioral characterization of spontaneous combustion of pre-oxidized coal with different air exposure time. Energy 2023, 262, 125397. [Google Scholar] [CrossRef]
- Hernowo, P.; Steven, S.; Restiawaty, E.; Bindar, Y. Nature of mathematical model in lignocellulosic biomass pyrolysis process kinetic using volatile state approach. J. Taiwan Inst. Chem. E 2022, 139, 104520. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Wang, G.; Xu, T.; Chai, Y.; Zheng, C.; Xu, R. Kinetics of petroleum coke/biomass blends during co-gasification. Int. J. Min. Met. Mater. 2016, 23, 1001–1010. [Google Scholar] [CrossRef]
- Aghili, A.; Shabani, A. A modification to the Friedman and Ortega isoconversional methods for evaluation of the activation energy as a function of conversion and temperature. Thermochim. Acta 2024, 736, 179748. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).