Experimental Study on the Influence of Staged Oxygen Consumption on the Oxidation Characteristics of Coal Spontaneous Combustion
Abstract
1. Introduction
2. Experimental Test of Staged Oxygen-Consumption and Oxidation
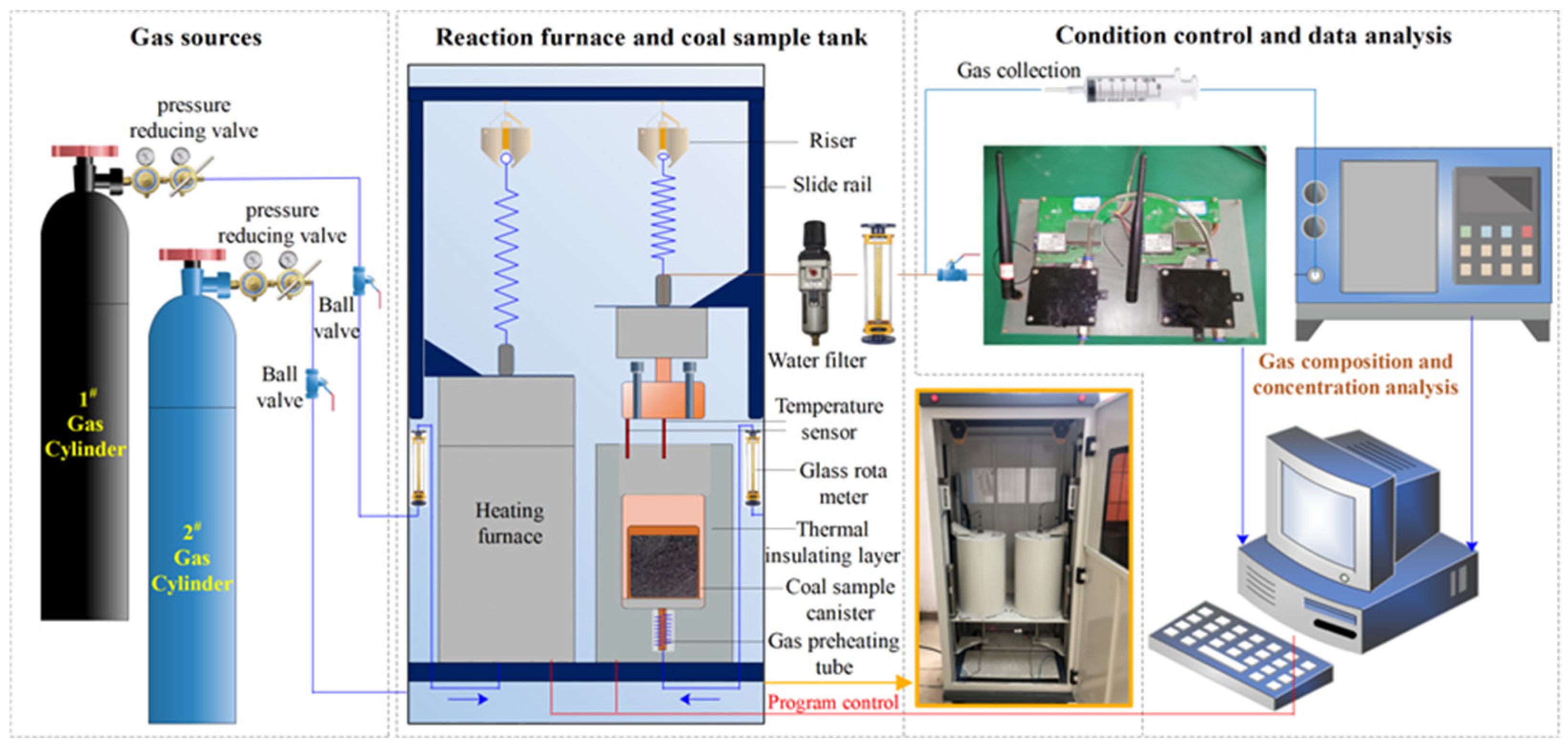
2.1. Experimental Apparatus
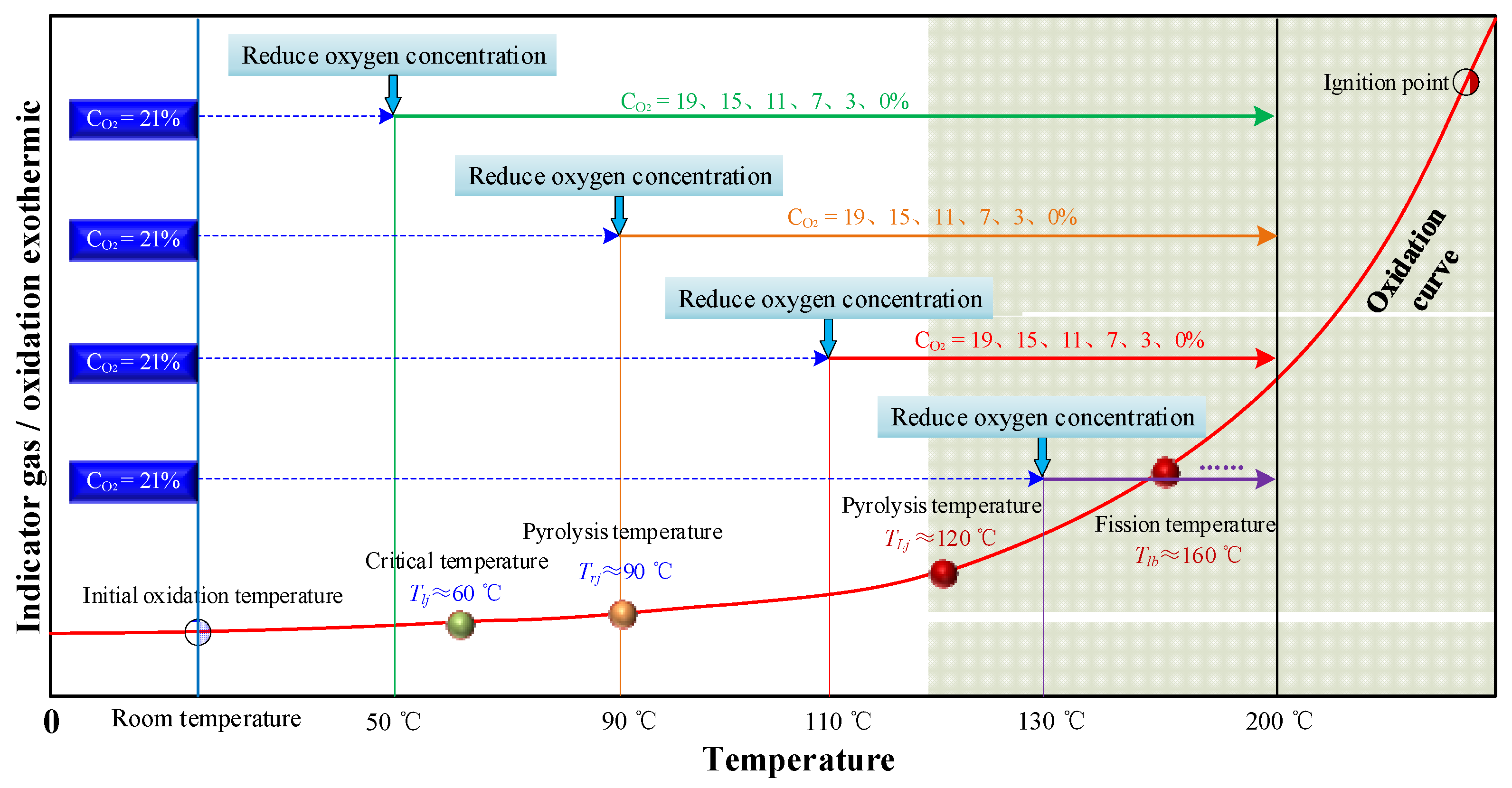
2.2. Experimental Process
3. Results and Analysis
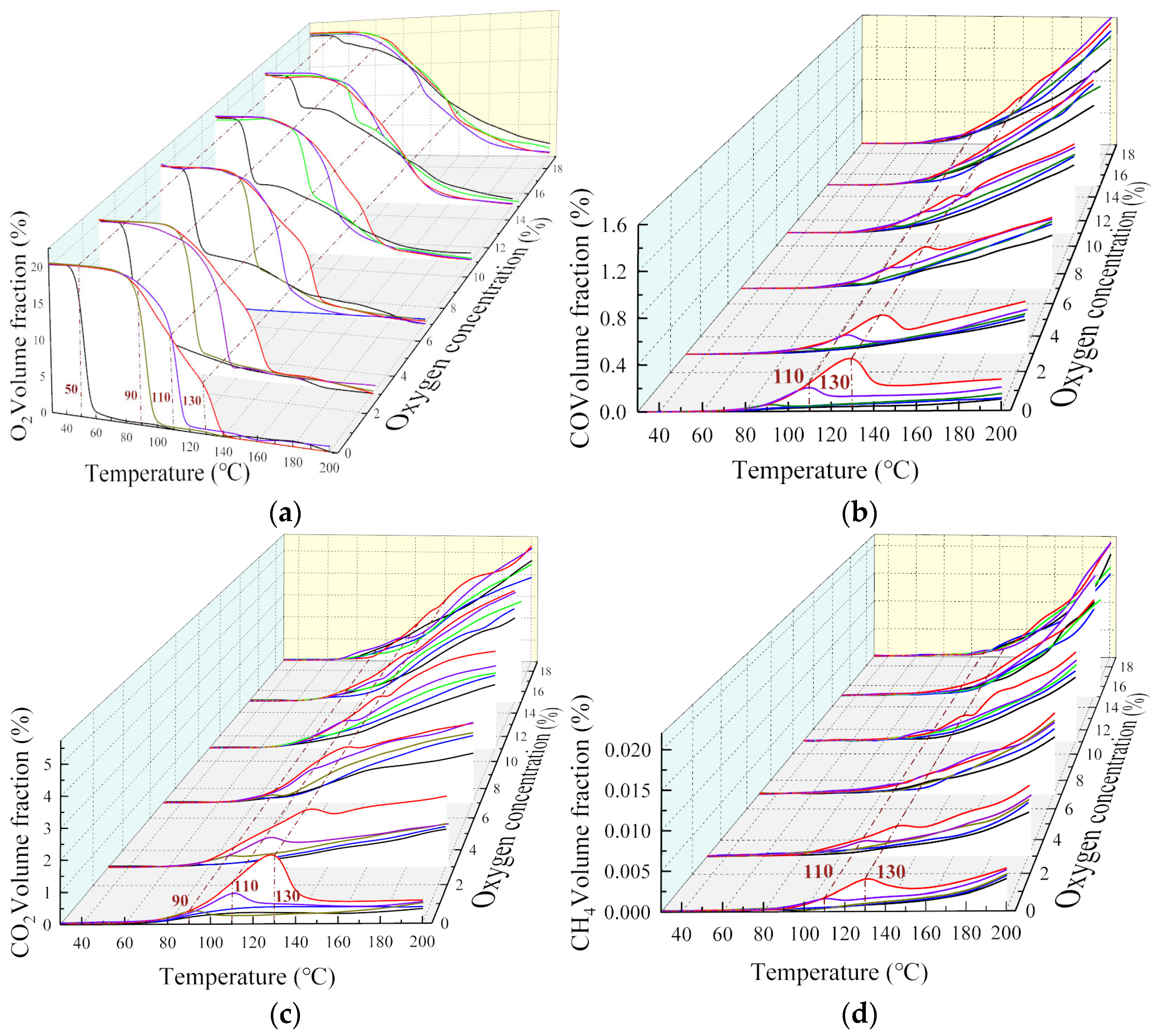
3.1. The Change Rule of Multi-Component Gas under the Condition of Staged Oxygen-Consumption
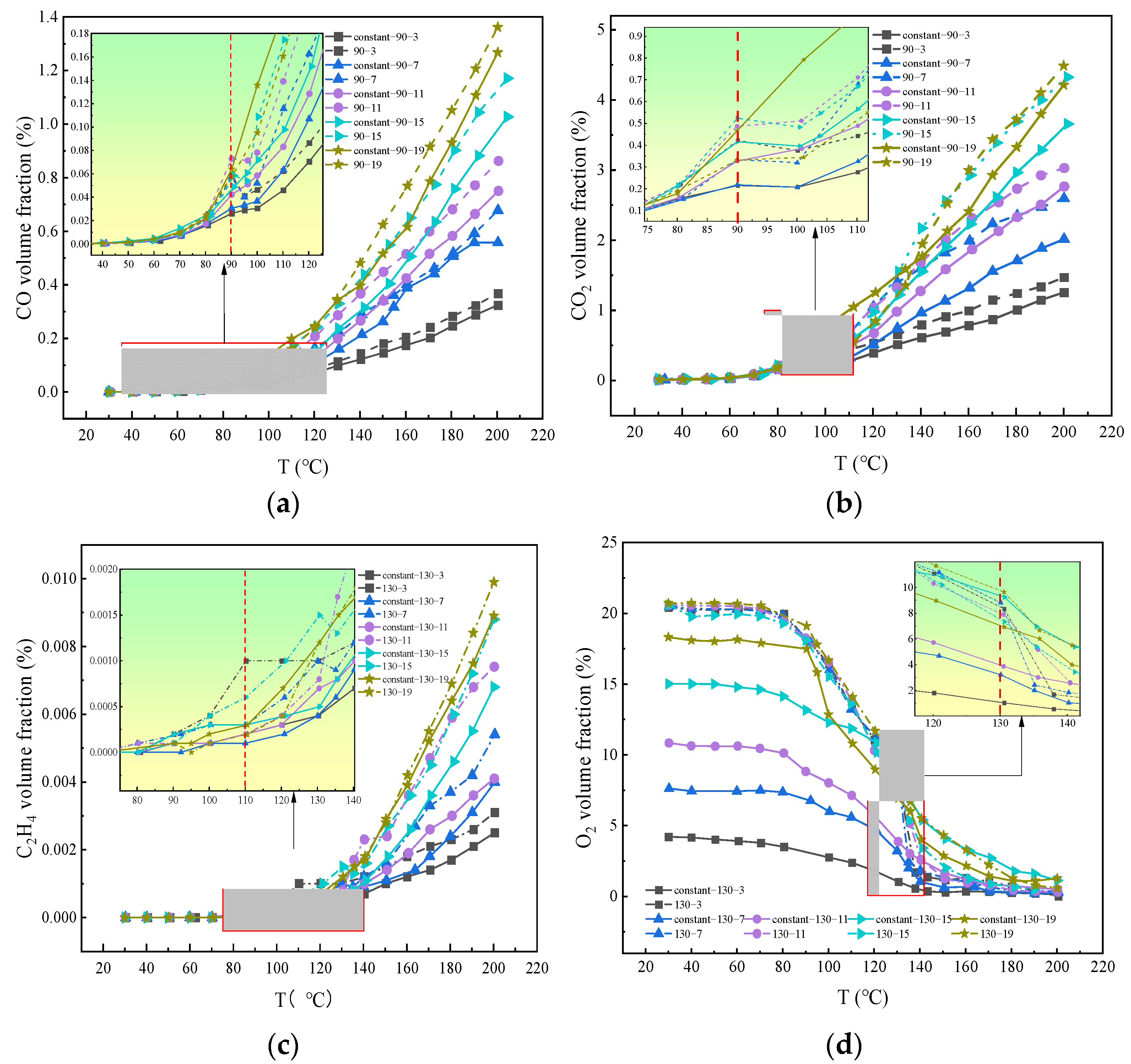
3.2. Comparison of Gas Change Rules under Staged Oxygen-Consumption and Constant Oxygen Conditions
3.3. The Variation Law of Coal Oxidation Characteristic Parameters under the Condition of Staged Oxygen-Consumption
3.3.1. Oxygen Consumption Rate
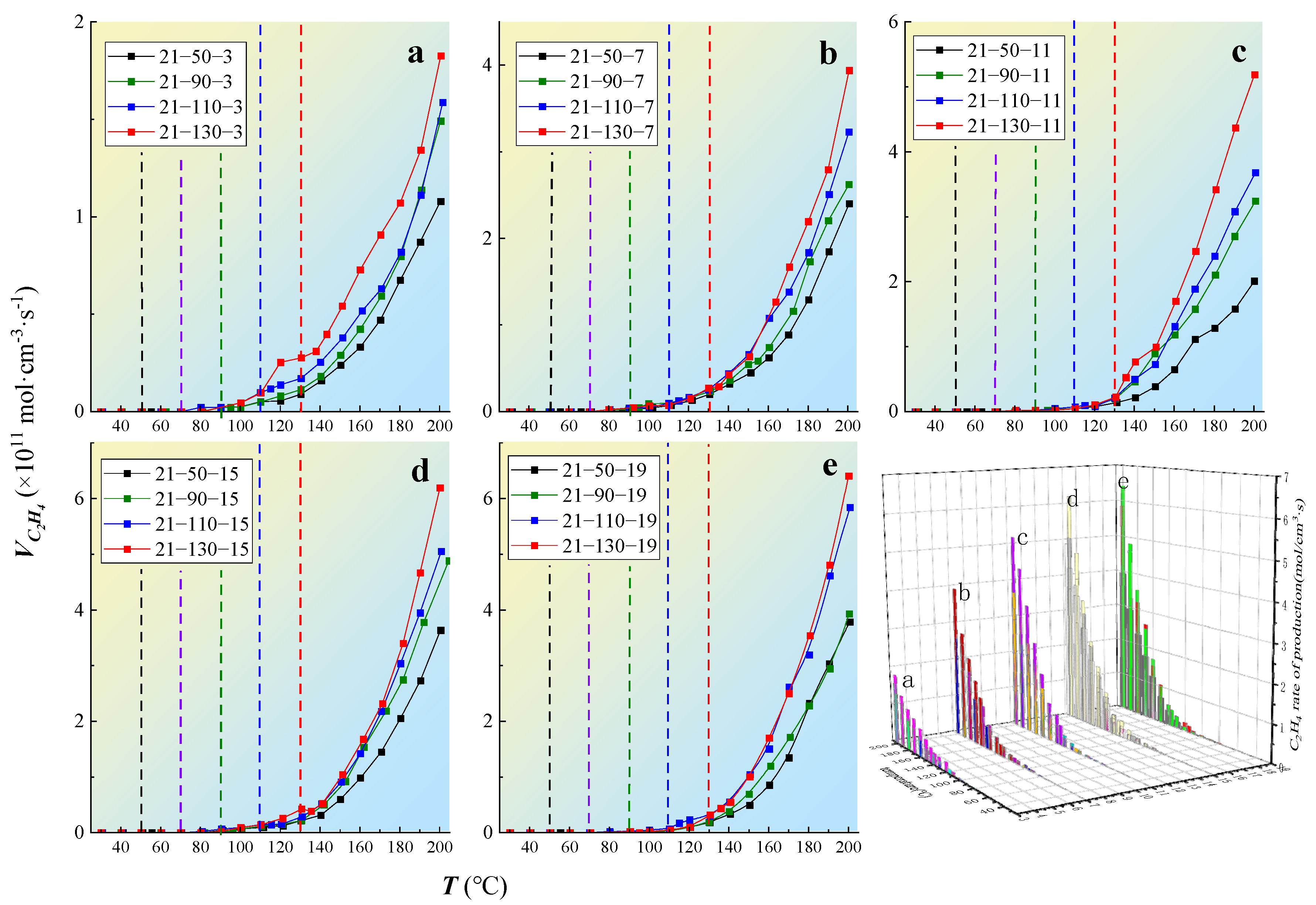
3.3.2. Gas Production Rate
- (1)
- CO production rates
- (2)
- C2H4 production rate
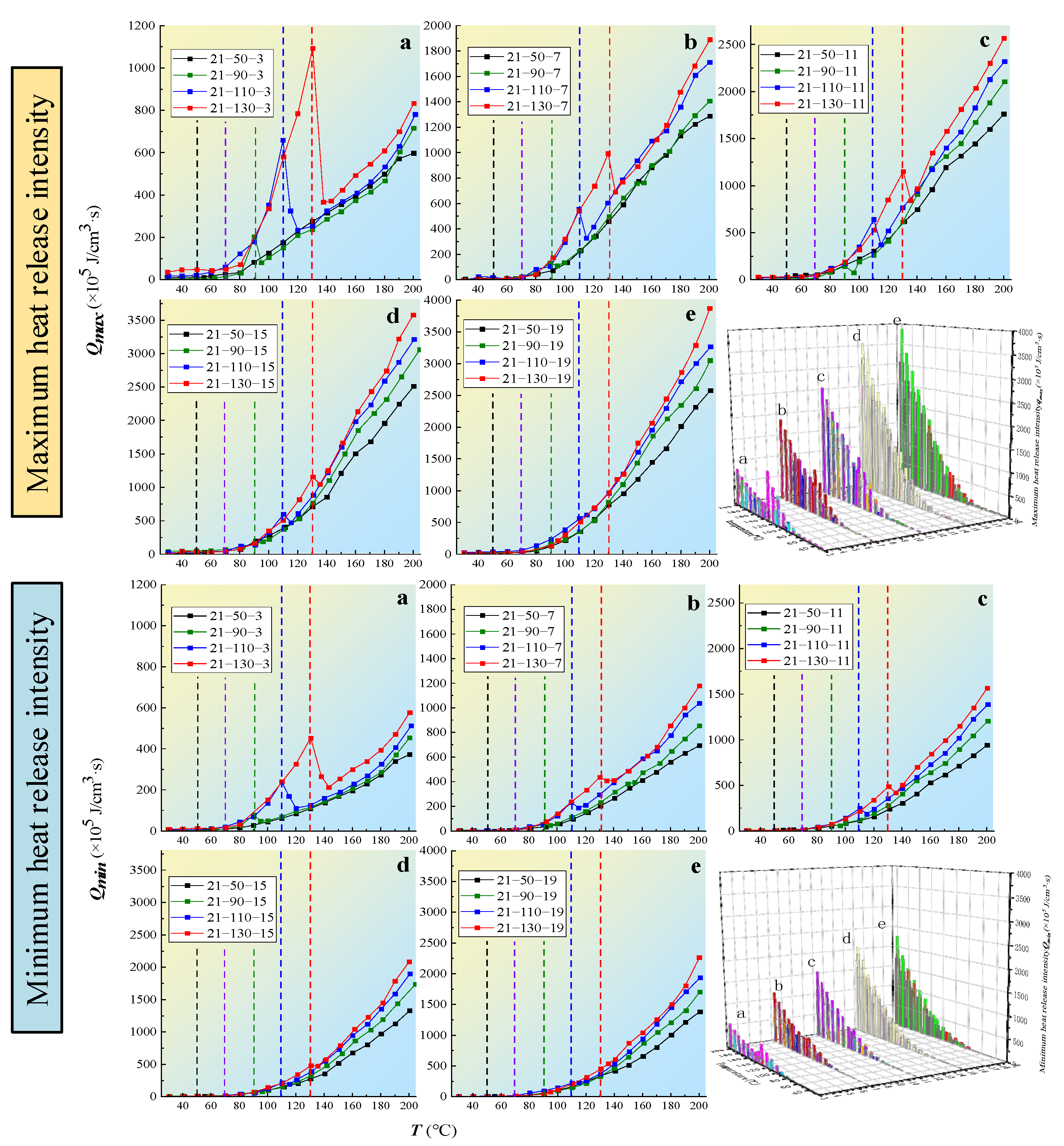
3.3.3. Exothermic Intensity
3.4. Correlation Analysis between Coal Oxidation Characteristic Parameters
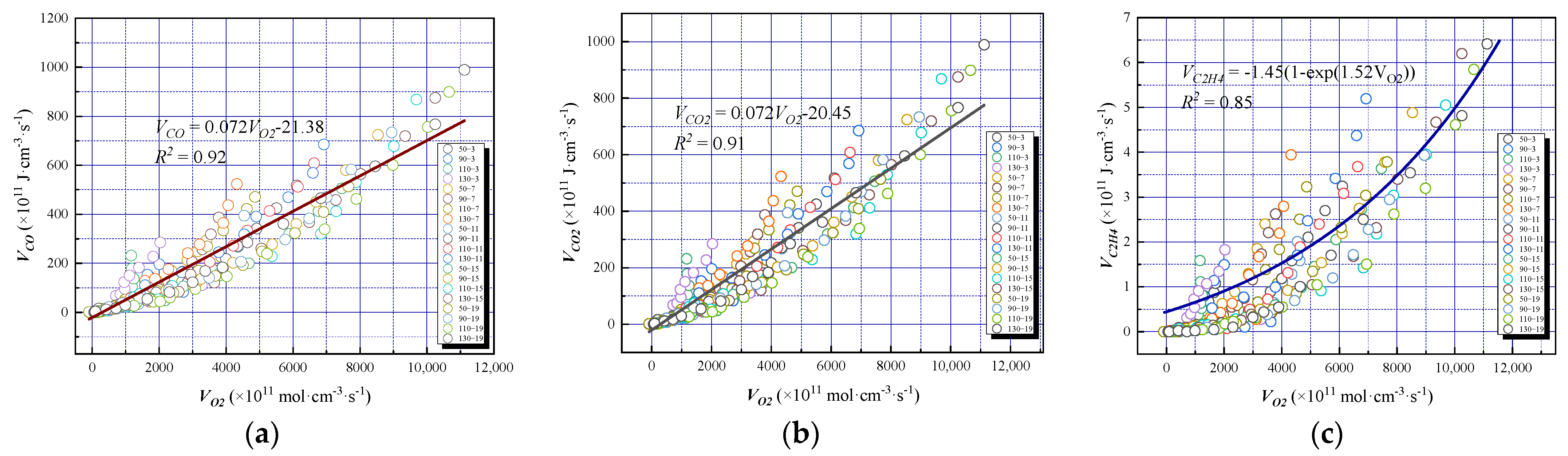
3.4.1. Relationship between Oxygen Consumption Rate and Gas Product Formation Rate
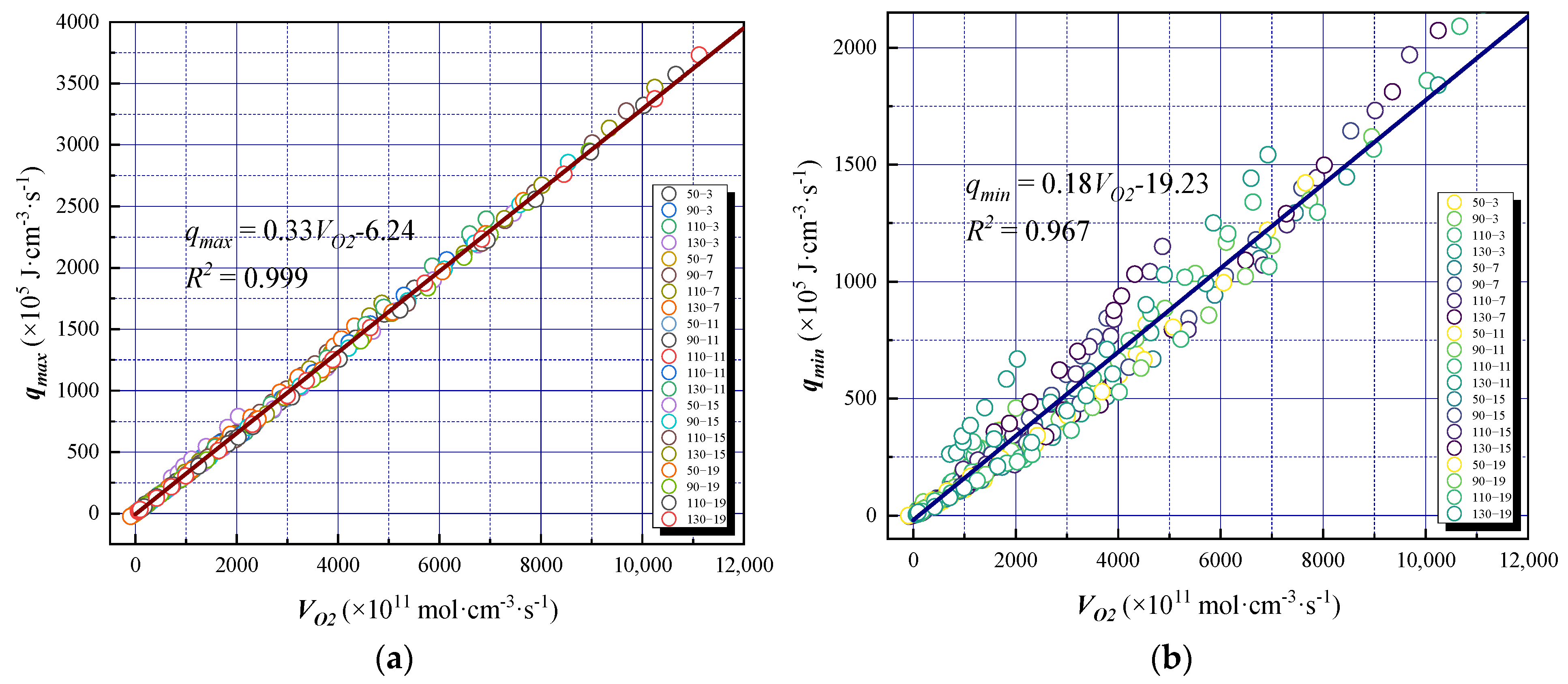
3.4.2. The Oxygen Consumption Rate and Exothermic Intensity
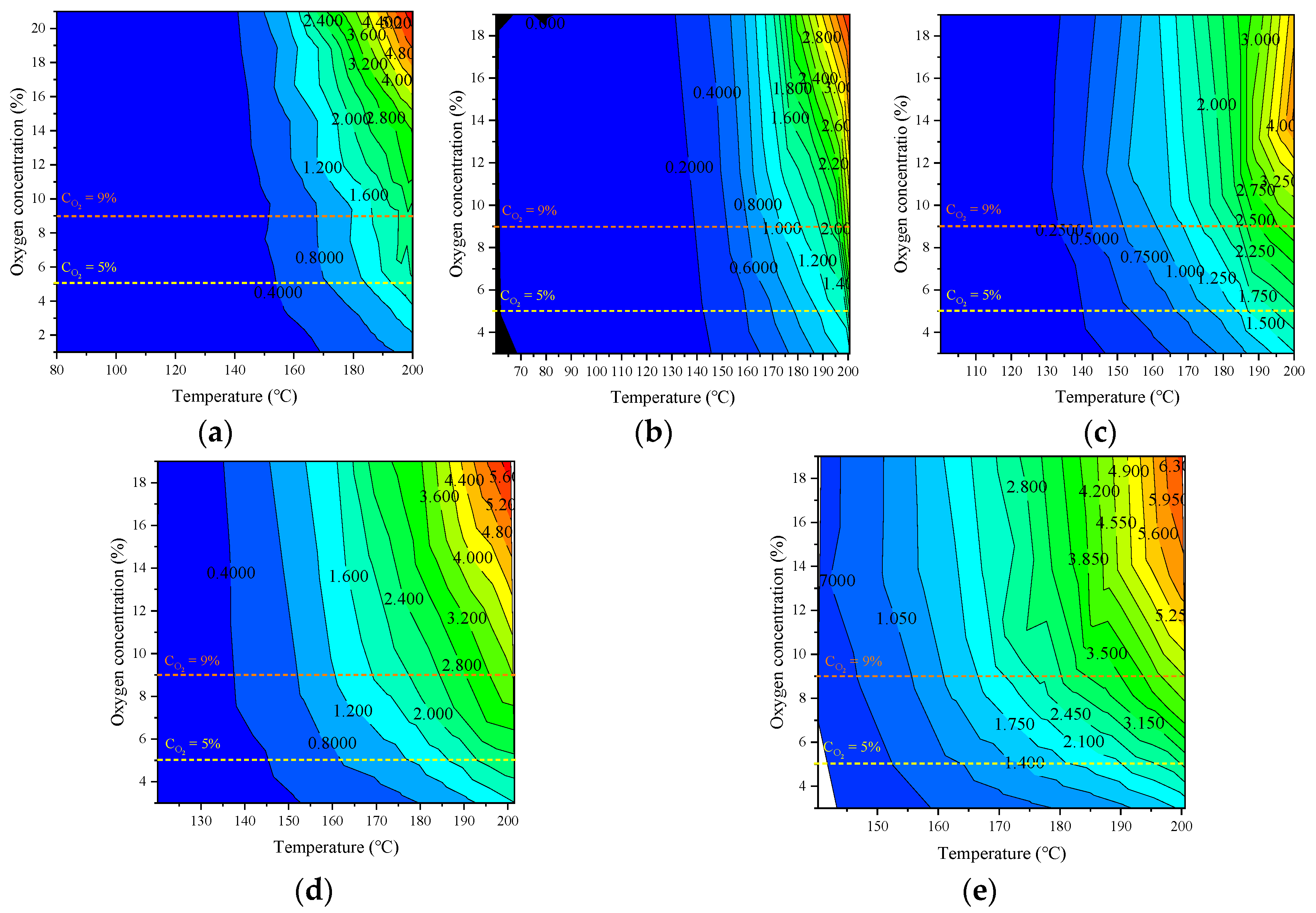
3.4.3. The Critical Oxygen Concentration Affecting the Oxidation Process of Coal under the Condition of Oxygen-Consumption
- (1)
- CO production rates
- (2)
- C2H4 production rate
4. Conclusions
- (1)
- After periodic oxygen reduction, the overall oxidation of coal was slightly stronger than that under constant low oxygen conditions. The decrease in oxygen concentration had a significant influence on the oxidation gas products and little effect on the pyrolysis gasses, such as CH4, C2H6 and C2H4. However, the relationships between the oxygen consumption rate, CO generation rate, and exothermic intensity did not change significantly. Therefore, under the actual conditions of the site, the change in oxygen concentration should be comprehensively considered when using a CO concentration warning. It is feasible to determine the spontaneous coal combustion trend directly through the change in gas concentration when using C2H4 and other gasses for high-temperature warnings.
- (2)
- The coal oxidation characteristic parameters, such as the gas product volume fraction, oxygen consumption rate, gas production rate, and heat release intensity of the coal samples, are positively correlated with the oxygen-consumed temperature. The maximum and minimum exothermic intensities of coal oxidation were found to have a significant linear relationship with the oxygen consumption rate. The oxidation reaction heat of coal was obtained by numerical fitting to be 180–330 kJ·mol−1, and the degree of fitting was high, indicating that the calculated exothermic intensity was in line with the actual oxidation mode of coal.
- (3)
- The oxygen concentration had a significant effect on the formation rate of the oxygen-containing gaseous products. The critical oxygen concentrations that determined the formation rate of the oxidizing gas were 17, 9, and 5%. When the reaction rate was low, the effect of oxygen concentration on the production rate of hydrocarbon gas was weak; however, when the reaction was severe, the production rate of hydrocarbon gas increased exponentially with the oxygen consumption rate, indicating that there was also an indirect relationship between the formation of hydrocarbon gas and oxygen consumption. The critical oxygen concentrations leading to an abrupt change in the gas production rate were obtained, which were 9 and 5%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| reaction rate of gas component i (mol·cm−3·s−1) | |
| reactant O2 concentration (mol) | |
| ki | reaction constant |
| n | order of reaction |
| oxygen consumption rate (mol·cm−3·s−1) | |
| the experiment supplies oxygen content (%) | |
| experimental tank volume (cm3) | |
| Q | supply flow (mL·min−1) |
| oxygen content of air inlet, outlet (%) | |
| CO content of air inlet, outlet (%) | |
| produces 1 mol of CO to release heat (J) | |
| produces 1 mol of CO2 to release heat (J) | |
| the heat released by the first and second reactions (J) | |
| the heat generated by chemical adsorption (J) | |
| x | axial length from tank bottom (cm) |
References
- Xie, H.P.; Wu, L.X.; Zheng, D.Z. Prediction on the energy consumption and coal demand of China in 2025. J. China Coal Soc. 2019, 44, 1949–1960. [Google Scholar]
- Chen, H.; Shao, H.; Jiang, S.G.; Huang, C.L.; Liu, G.Z. Study on the Cause of Hypoxia in the Corner of Return Air of Shallow Buried Flammable Coal Seam Group Mining Face and the Coordinated Prevention and Control of Coal Spontaneous Combustion. Appl. Sci. 2023, 13, 13. [Google Scholar] [CrossRef]
- Guo, J.; Chen, C.M.; Wen, H.; Cai, G.B.; Liu, Y. Prediction model of goaf coal temperature based on PSO-GRU deep neural network. Case Stud. Therm. Eng. 2024, 53, 103813. [Google Scholar] [CrossRef]
- Ren, L.F.; Li, Q.W.; Deng, J.; Ma, L.; Xiao, Y. Effect of Oxygen Concentration on the Oxidative Thermodynamics and Spontaneous Combustion of Pulverized Coal. ACS Omega 2021, 6, 26170–26179. [Google Scholar] [CrossRef]
- Zhao, J.R.; Xiao, Y.; Zhong, K.Q.; Li, Q.W.; Zhai, X.W. Effects of oxygen concentration and heating rate on coal spontaneous combustion characteristics. J. Therm. Anal. Calorim. 2023, 148, 4949–4958. [Google Scholar] [CrossRef]
- Guo, J.; Quan, Y.P.; Cai, G.B.; Jin, Y.F.; Zheng, X.Z. Meticulous Graded and Early Warning System of Coal Spontaneous Combustion Based on Index Gases and Characteristic Temperature. ACS Omega 2023, 8, 6801–6812. [Google Scholar] [CrossRef]
- Shao, H.; Zhou, F.B.; Chen, K.Y.; Cheng, J.W.; Palu, M. Study on the Hydrogen Generation Rules of Coal Oxidation at Low Temperature. J. Eng. Technol. 2014, 7, 90–95. [Google Scholar]
- Zhao, X.G.; Dai, G.L.; Qin, R.X.; Zhou, L.; Li, J.L. Study on oxidation kinetics of low-rank coal during the spontaneous combustion latency. Fuel 2023, 339, 127441. [Google Scholar] [CrossRef]
- Wen, H.; Guo, J.; Jin, Y.F.; Wang, K.; Zhang, Y.T. Experimental study on the influence of different oxygen concentrations on coal spontaneous combustion characteristic parameters. Int. J. Oil Gas Coal Technol. 2017, 16, 187–202. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Shu, P.; Deng, J.; Duan, Z.X.; Li, L.L. Analysis of oxidation pathways for characteristic groups in coal spontaneous combustion. Energy 2022, 254, 124211. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, H.Q.; Huang, H.; Zhao, H.R.; Pan, R.L. Study on the thermal kinetics and microscopic characteristics of oxidized coal. Environ. Sci. Pollut. Res. 2023, 30, 85953–85967. [Google Scholar] [CrossRef]
- Liu, Z.J.; Xu, Y.L.; Wen, X.L.; Lv, Z.G.; Wen, J.D. Thermal Properties and Key Groups Evolution of Low-Temperature Oxidation for Bituminous Coal under Lean-Oxygen Environment. ACS Omega 2021, 6, 15115–15125. [Google Scholar] [CrossRef]
- Chen, J.; Jia, B.S.; Wen, Y.; Jing, Q.H.; Liu, L.J. Study on Spontaneous Combustion Characteristics and Oxidation Kinetic Parameters of Lignite at Different Oxygen Concentrations. ACS Omega 2022, 7, 38487–38495. [Google Scholar] [CrossRef]
- Jia, X.L.; Wu, J.K.; Lian, C.J.; Rao, J.L. Assessment of coal spontaneous combustion index gas under different oxygen concentration environment: An experimental study. Environ. Sci. Pollut. Res. 2022, 29, 87257–87267. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.Y.; Li, Q.Z.; Zhang, H.J.; Xin, H.H. Thermodynamic characteristics of coal reaction under low oxygen concentration conditions. J. Energy Inst. 2017, 90, 544–555. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, H.; Guo, J.; Jin, Y.F.; Fan, S.X. Correlation between oxygen concentration and reaction rate of low-temperature coal oxidation: A case study of long-flame coal. Energy 2023, 275, 127483. [Google Scholar] [CrossRef]
- Liu, Y. Study on Nonlinear Evolution Law and Dynamic Early Warning Method of Residual Coal Spontaneous Combustion in Goaf. Ph.D. Thesis, Xi’an University of Science and Technology, Xi’an, China, 2022. [Google Scholar]
- Wang, B.F.; Lv, Y.H.; Liu, C.B. Research on fire early warning index system of coal mine goaf based on multi-parameter fusion. Sci. Rep. 2024, 14, 485. [Google Scholar] [CrossRef]
- Lu, B.; Zhang, X.; Qiao, L.; Ding, C.; Fan, N.; Huang, G. Experimental study on the effect of slow reaction process of the latent period on coal spontaneous combustion. Energy 2024, 302, 131927. [Google Scholar] [CrossRef]
- Zhou, B.Z.; Yang, S.Q.; Yang, W.M.; Jiang, X.Y.; Song, W.X.; Cai, J.W.; Xu, Q.; Tang, Z.Q. Variation characteristics of active groups and macroscopic gas products during low-temperature oxidation of coal under the action of inert gases N2 and CO2. Fuel 2022, 307, 121893. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, M.; Deng, J.; Xiao, Y.; Chen, W.L. Effects on environmental conditions and limiting parameters for spontaneous combustion of residual coal in underground goaf. Process Saf. Environ. Prot. 2024, 187, 1378–1389. [Google Scholar] [CrossRef]
- Zhao, X.G.; Dai, G.L.; Qin, R.X.; Zhou, L.; Li, J.H. Spontaneous combustion characteristics of coal based on the oxygen consumption rate integral. Energy 2024, 288, 129626. [Google Scholar] [CrossRef]
- Chao, W.Y.; Zhong, K.Q.; Xiao, Y.; Lai, X.P.; Li, Q.W. Determining the Spontaneous Combustion Period and Limit Parameters of Coal: A Large-Scale Furnace Experiment. Combust. Sci. Technol. 2023, 195, 494–507. [Google Scholar]
- Yang, Y.; Fei, J.B.; Luo, Z.M.; Wen, H.; Wang, H. Experimental study on characteristic temperature of coal spontaneous combustion. J. Therm. Anal. Calorim. 2023, 148, 10011–10019. [Google Scholar] [CrossRef]
- Li, Z.X.; Zhang, M.Q.; Yang, Z.B.; Liu, Y.; Yu, J.X. Exothermic characteristics of coal during low-temperature oxidation based on grey correlation method. Energy. Rep. 2022, 8, 86744–86752. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, C.; Lu, B.; Gao, F.; Shan, C. Study on the inhibitory mechanism of dehydrogenated antioxidants on coal spontaneous combustion. Sci Rep. 2022, 12, 21237. [Google Scholar] [CrossRef]
- Xu, X.F.; Zhang, F.J. Evaluation and Optimization of Multi-Parameter Prediction Index for Coal Spontaneous Combustion Combined with Temperature Programmed Experiment. Fire 2023, 6, 368. [Google Scholar] [CrossRef]
- Yan, H.W.; Nie, B.S.; Liu, P.J.; Chen, Z.Y.; Jin, F.-F. Experimental investigation and evaluation of influence of oxygen concentration on characteristic parameters of coal spontaneous combustion. Thermochim. Acta 2022, 717, 179345. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Yang, S.Q.; Zhou, B.Z.; Lan, L. The auto-oxidation characteristic of coal at different stages of the low-temperature oxidation process. Fuel 2023, 352, 129130. [Google Scholar] [CrossRef]
- Jin, Y.F.; Chai, Y.Y.; Liu, Y.; Guo, J.; Yan, H. Experimental study on the evolutionary characteristics of silicified coal functional groups during oxidation/pyrolysis. Combust. Sci. Technol. 2023, 195, 2491–2509. [Google Scholar] [CrossRef]
- Wang, H.Y.; Tian, Y.; Li, J.L.; Chen, X. Experimental study on thermal effect and gas release laws of coal-polyurethane cooperative spontaneous combustion. Sci. Rep. 2021, 11, 1994. [Google Scholar] [CrossRef]
- Ren, Z.J.; Wang, D.P.; Qin, Z.; Liu, Z.W. Effects of pore size, water content, and oxygen-containing functional groups on oxygen adsorption in bituminous coal. Sci. Rep. 2023, 13, 10373. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.C.; Wen, H.; Ge, L.M.; Zhang, X.H.; Deng, J. Determination and calculation of oxidation heat liberation intensity of loose coal at low temperature stage. J. China Coal Soc. 2000, 25, 387–390. [Google Scholar]
- Liu, H.; Li, Z.H.; Miao, G.D.; Yang, J.J.; Wu, X.Q. Insight into the chemical reaction process of coal during the spontaneous combustion latency. Energy 2023, 263, 125823. [Google Scholar] [CrossRef]




| Coal Samples | Mad% | Aad% | Ad% | Vad% | Vd% | Vdaf% | Fcad% | Fcd% |
|---|---|---|---|---|---|---|---|---|
| long-flame coal | 5.60 | 7.42 | 7.86 | 30.69 | 32.51 | 35.28 | 56.29 | 59.63 |
| Gas Component | Oxygen-Consumed Point Temperature/°C | |||
|---|---|---|---|---|
| 50 | 90 | 110 | 130 | |
| O2 | The variation of oxygen concentration groups at each level is similar, characterized by a ‘steady-accelerating-steady’ periodic downward trend. | The concentration of O2 content decreases rapidly and the oxidation rate increases. | The decrease is slow and more obvious at a high oxygen concentration, indicating that the effect of temperature on the coal oxidation reaction is greater than that of oxygen concentration. | |
| CO | The gas production rate is slow. At 50 °C, adsorption is the main effect. The oxygen concentration has little effect on the product concentration. | The CO concentration of coal samples with an oxygen concentration less than 11% decreases, indicating that the reaction rate of coal oxygen increases after exceeding the critical temperature, and that it is difficult to maintain the reaction at oxygen concentrations below 11%. | The CO content with oxygen concentrations less than 3% decreases sharply, while oxygen content greater than 15% can still maintain the coal-oxygen reaction. | The CO content with less than 15% oxygen concentration decreases sharply, while the CO growth trend in coal samples with 15 and 19% oxygen concentration is gentle. |
| CO2 | The gas volume fraction decreases at each oxygen concentration. | The CO2 content with an oxygen content higher than 7% increases, while that of an oxygen content lower than 7% decreases. Below 7%, it is approximately linear. When the temperature reaches the critical stage, the oxygen content decreases, indicating that the oxygen content is an important factor in inhibiting CO2 production. | ||
| CH4 | A small amount of CH4 appears, indicating that the coal sample itself has CH4, which is desorbed at low temperature. With the increase in temperature, the CH4 content of each group increases exponentially. | With the decrease of oxygen concentration, it decreases significantly, but the amplitude was significantly smaller than that of the oxidation products. The volume fraction of CH4 increases exponentially after oxygen consumption, indicating that the oxygen content had a certain effect on the CH4 production rate, but had little effect on its growth characteristics. | ||
| C2H6 C2H4 | None of them appear, indicating that the test coal samples do not contain adsorbed ethane and ethylene. | The influence of oxygen concentration is weak, because C2H6 and C2H4 are the pyrolysis products of coal, and temperature plays a decisive role. | Both fluctuate significantly, but soon grow exponentially. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Wang, L.; Liu, Y.; Chen, C.; Cai, G.; Du, W. Experimental Study on the Influence of Staged Oxygen Consumption on the Oxidation Characteristics of Coal Spontaneous Combustion. Fire 2024, 7, 359. https://doi.org/10.3390/fire7100359
Guo J, Wang L, Liu Y, Chen C, Cai G, Du W. Experimental Study on the Influence of Staged Oxygen Consumption on the Oxidation Characteristics of Coal Spontaneous Combustion. Fire. 2024; 7(10):359. https://doi.org/10.3390/fire7100359
Chicago/Turabian StyleGuo, Jun, Lei Wang, Yin Liu, Changming Chen, Guobin Cai, and Wentao Du. 2024. "Experimental Study on the Influence of Staged Oxygen Consumption on the Oxidation Characteristics of Coal Spontaneous Combustion" Fire 7, no. 10: 359. https://doi.org/10.3390/fire7100359
APA StyleGuo, J., Wang, L., Liu, Y., Chen, C., Cai, G., & Du, W. (2024). Experimental Study on the Influence of Staged Oxygen Consumption on the Oxidation Characteristics of Coal Spontaneous Combustion. Fire, 7(10), 359. https://doi.org/10.3390/fire7100359








