Abstract
The oxidation characteristic parameters of residual coal in working face change with the advance of coal seam. To explore the influence of dynamic oxygen-consuming environments, we examined the influence of reducing the oxygen concentration on the formation characteristics and characteristic parameters of coal oxidation products, conducted with temperature-programmed experiments under staged oxygen consumption. The correlation between the characteristic oxidation parameters was determined, and the critical oxygen concentration that determined the gas yield was obtained. The results show that after staged oxygen-consumption, the oxidation of coal is stronger than that under constant low oxygen, the oxidation products are greatly affected, and the influence of pyrolysis gas is small. The oxidation characteristic parameters such as gas product volume fraction, production rate, and heat release intensity are positively correlated with the oxygen-consumed temperature. We found that the oxidation reaction heat of coal is 180~330 kJ·mol−1, and the maximum and minimum exothermic intensities are significantly linearly correlated with the oxygen consumption rate. Finally, the critical oxygen concentrations for gas production rate under oxygen-consumed conditions were 17, 9, and 5%. These results have practical significance for strengthening the prevention and control of spontaneous combustion disasters in goafs.
1. Introduction
Coal is the main source of nonrenewable energy in China. As of the end of 2023, national coal production reached 4.71 billion tons [1]. Yet the mining of coal is not without danger. Fire, in particular, is a major hazard, and 70% of coal fire accidents are caused by loose coal in goafs [2,3]. In these areas, the air flow is slow, and heat is not easily dissipated. The heat stored in the remaining coal undergoes spontaneous combustion mostly in an environment with poor oxygen (oxygen volume fraction < 21%) [4,5]. Consequently, it is of practical value to understand the variation law of the coal spontaneous combustion characteristic parameters under lean oxygen conditions.
Spontaneous coal combustion includes physical adsorption and chemical reactions of coal with oxygen. Many scholars have studied the characteristic parameters of coal oxidation, the spontaneous combustion of coal, and its influencing factors. Guo et al. [6] determined the index gas combined with the oxidation mechanism index curve point, and also determined the temperatures of six mutation points and the corresponding marker gas. Shao et al. [7] studied the formation of oxidation products under oxygen-poor conditions using temperature-programmed experiments and found that a low oxygen concentration causes a “hysteresis effect” in the oxidation products. Zhao et al. [8] carried out the oxidation of coals in an oxygen-poor environment with different metamorphic grades, finding that temperature had an effect on the oxidation products. Wen et al. [9] conducted temperature-programmed experiments under various oxygen concentration environments and determined that the oxygen concentration affects the index gas yield. Zhang et al. [10] experimentally tested and calculated the characteristic parameters under different oxygen concentrations. Wang et al. [11] reported that the oxygen consumption rate is easily affected by the oxygen concentration in a coal oxidation experiment. Liu et al. [12] conducted experiments on the effect of poor oxygen on the concentrations of CO, C2H4, and other indicator gasses, and obtained the micro principle of spontaneous combustion through coal heat storage. Chen et al. [13] studied the oxidizing parameters of lignite at various oxygen concentrations. The results showed that the CO volume fraction was positively correlated with coal temperature under the experimental conditions, and a higher oxygen concentration inhibited the activation energy. Jia et al. [14] performed oxidation experiments using six oxygen concentrations. The results showed that O-rich conditions promoted the complexation reaction, whereas O-poor conditions inhibited it. Finally, Qi et al. [15] conducted oxidation experiments on coal with different metamorphic grades and found that the fundamental factor affecting the change in the oxidation parameters was the coal oxidation stage.
In summary, domestic and foreign scholars have conducted extensive research on the oxidation characteristics and reaction process of spontaneous coal combustion as it relates to oxygen concentration, but these studies have been limited to the ideal condition of constant oxygen concentration. During the actual production of the mine, the environmental oxygen concentration of the residual coal in the working face gradually decreases with the advancement of the coal seam. The coal body continues to experience a transition from oxygen-rich to oxygen-poor oxidation, and the oxidation characteristic parameters under variable oxygen concentrations change accordingly. To understand the influence of staged oxygen consumption on the oxidation characteristics of coal, this study conducted a temperature-programmed experimental test on staged oxygen consumption, analyzed the formation characteristics of coal oxidation products under these conditions, and explored the influence of the reducing oxygen concentration on coal oxidation products and parameters in the oxidation process. These results provide new research ideas for the prevention and control of coal fires in goafs.
2. Experimental Test of Staged Oxygen-Consumption and Oxidation
2.1. Experimental Apparatus
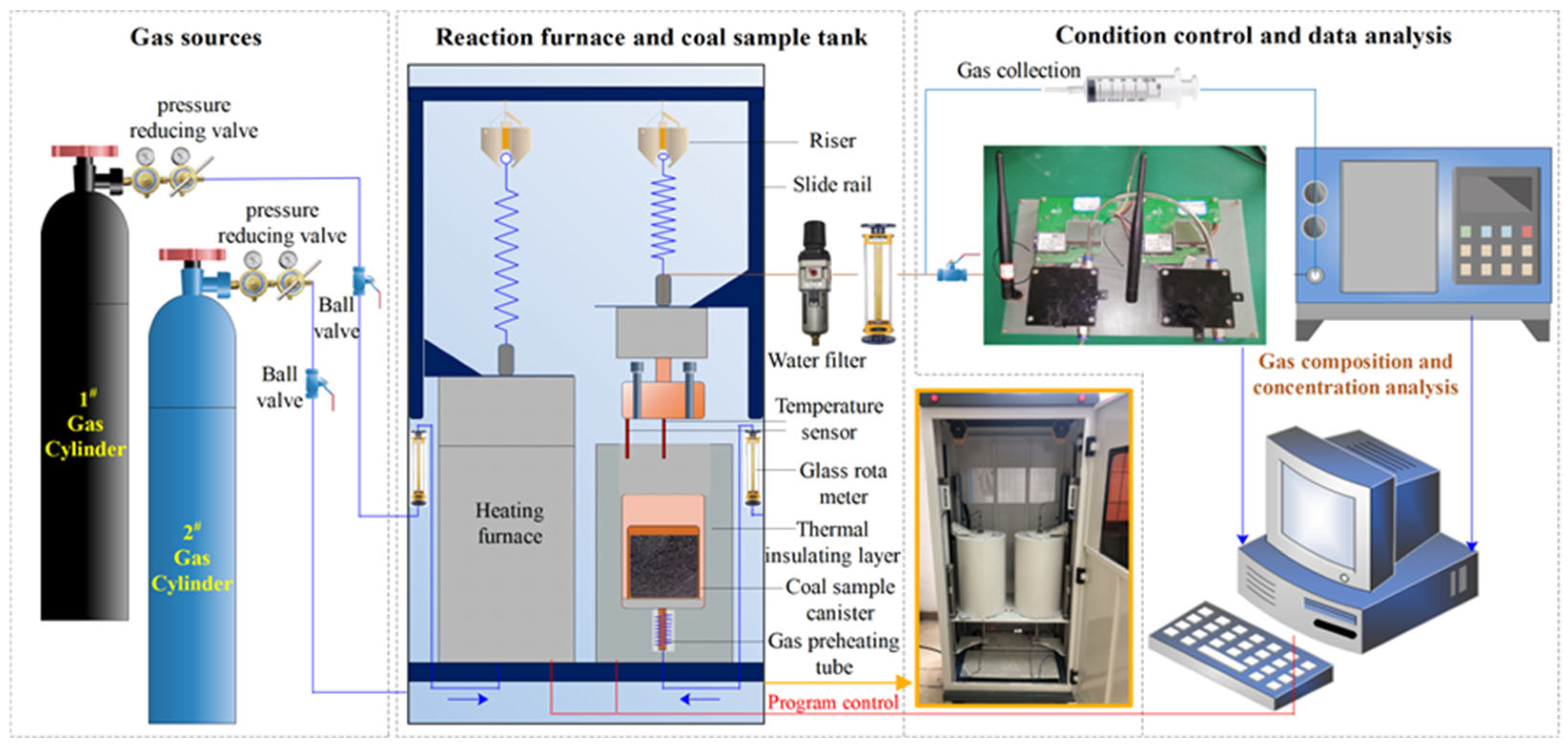
The step-by-step oxygen-consuming oxidation experiments adopted a programmed temperature increase system [16], as shown in Figure 1. The gas source consisted of two gas cylinders with different oxygen concentrations forming a dual-gas-source path. The reaction furnace was a fully automatic temperature control device, with a heating furnace wire on the inside, and a coal sample tank in the middle. The coal sample tank cover was arranged with an internal and external double thermocouple TM-902C temperature sensor, which was produced by Shenzhen Shiyan Electronic Factory (temperature measurement range: 50–1300 °C, accuracy ± 1 °C). The bottom of the tank was connected to a preheating winding pipe, and the top outlet was connected to a copper pipe, an external filter drying pipe, and a flowmeter. The gas collection bag (500 mL) was used to collect the index gas, and the gas content and composition collected at different coal temperatures were analyzed by a GC4000A mine gas chromatograph (sensitivity: 0.1 uv·s−1, accuracy ± 0.2%) from Shandong Lumei Heavy Industry Co., Ltd., Jining, China.

Figure 1.
Temperature programmed experimental setup.
In the experiment, long-flame coal with a low metamorphic degree was used to collect fresh coal samples from the working face, and the samples were crushed and screened to 100 g of 0–0.9 mm particle size for testing. The results of the industrial analysis of the coal samples are presented in Table 1.

Table 1.
Industrial analysis results of coal samples.
2.2. Experimental Process
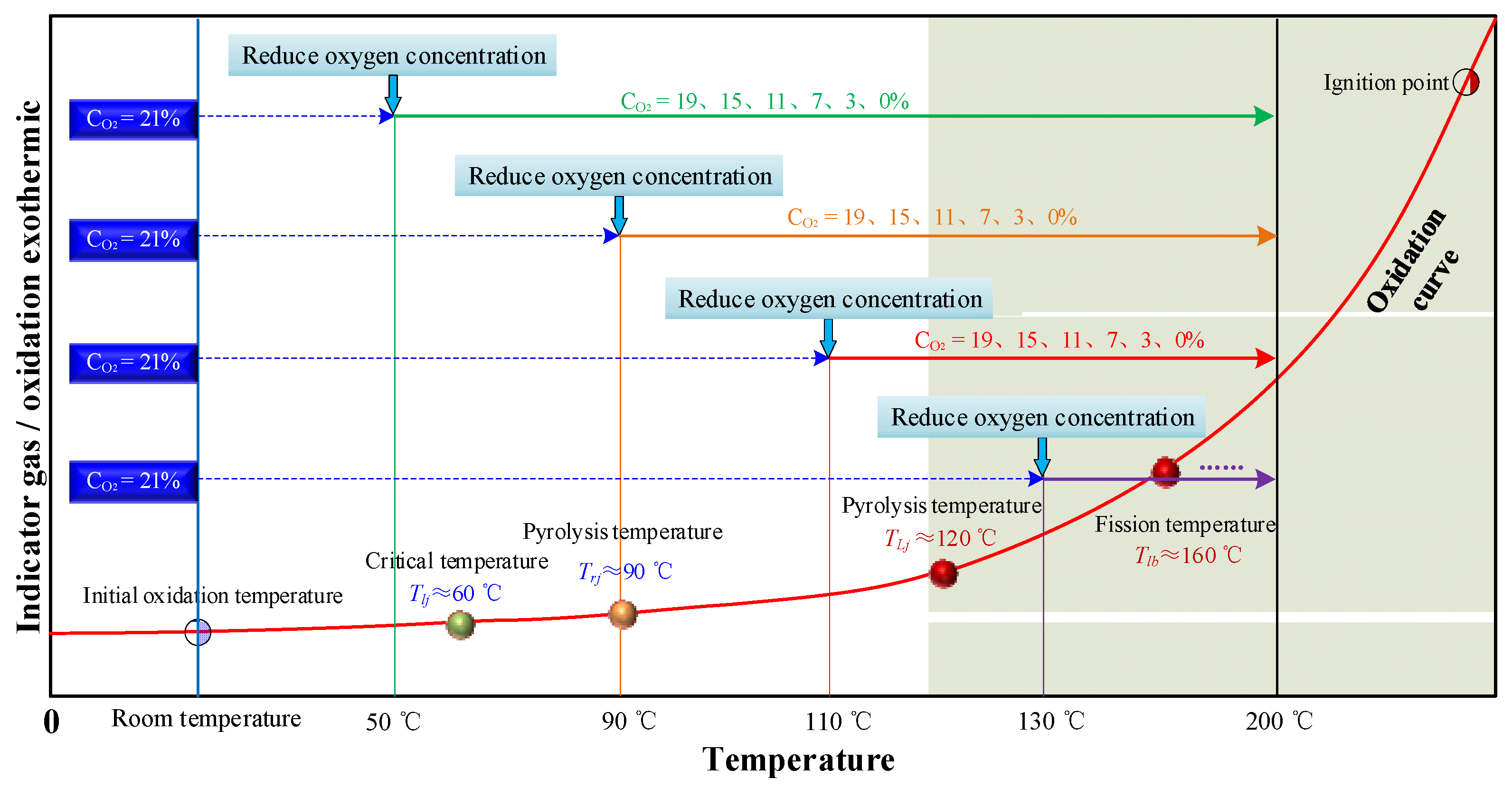
The experiment was divided into a spontaneous coal combustion constant oxygen heating experiment and a staged oxygen reduction experiment. In the constant oxygen heating experiment, the fresh coal sample was broken and sieved into 100 g of 0–0.9 mm particle size for testing. Temperature-programmed experiments on spontaneous coal combustion under five different constant oxygen concentration atmospheres were conducted. Considering the influence of coal pores in the goaf, gas was fed into the measured coal sample at a flow rate of 40 mL·min−1. Starting from room temperature, the coal body was heated to 200 °C at a heating rate of 0.3 °C·min−1. In the staged oxygen reduction experiment, based on the staged characteristics of coal oxidation, the coal spontaneous combustion process was segmented based on the critical and dry cracking temperature [17], and four temperature points of 50, 90, 110, and 130 °C were selected as the oxygen reduction points. The experimental scheme is shown in Figure 2. The temperature was increased to the oxygen reduction points under the air condition with the air supply volume of 40 mL·min−1. When the temperature reached the set values mentioned above, the experimental oxygen concentration was successively reduced to 19, 15, 11, 7, 3, and 0%, and the temperature was then continuously increased to 200 °C. During this period, the heating rate, air supply volume, and other experimental conditions remained constant. To ensure the reliability of the experimental data and to reduce accidental error, three experiments were repeated under each oxygen concentration condition, and the group data with the smallest error were used for analysis.

Figure 2.
Experimental scheme.
3. Results and Analysis
3.1. The Change Rule of Multi-Component Gas under the Condition of Staged Oxygen-Consumption
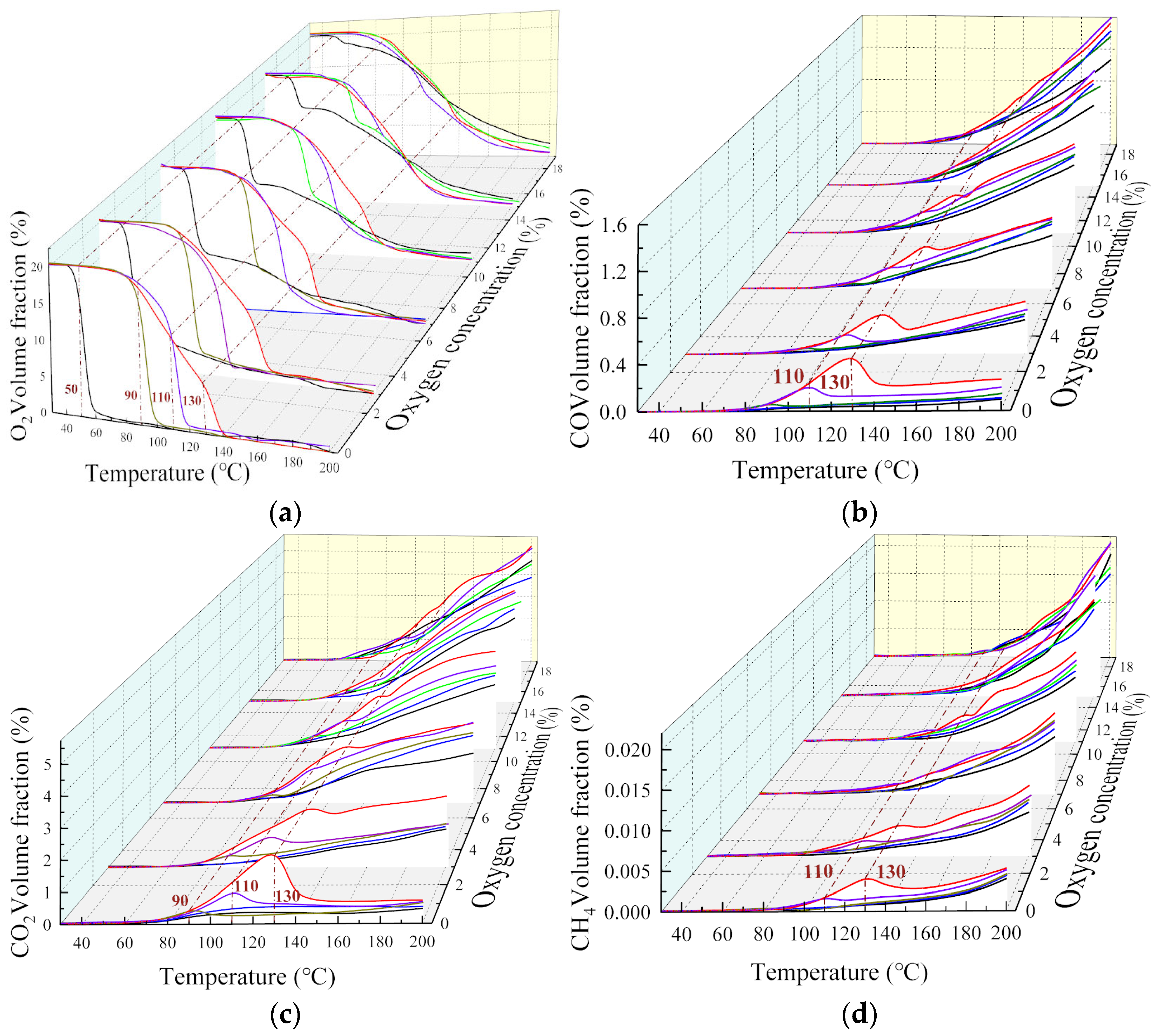
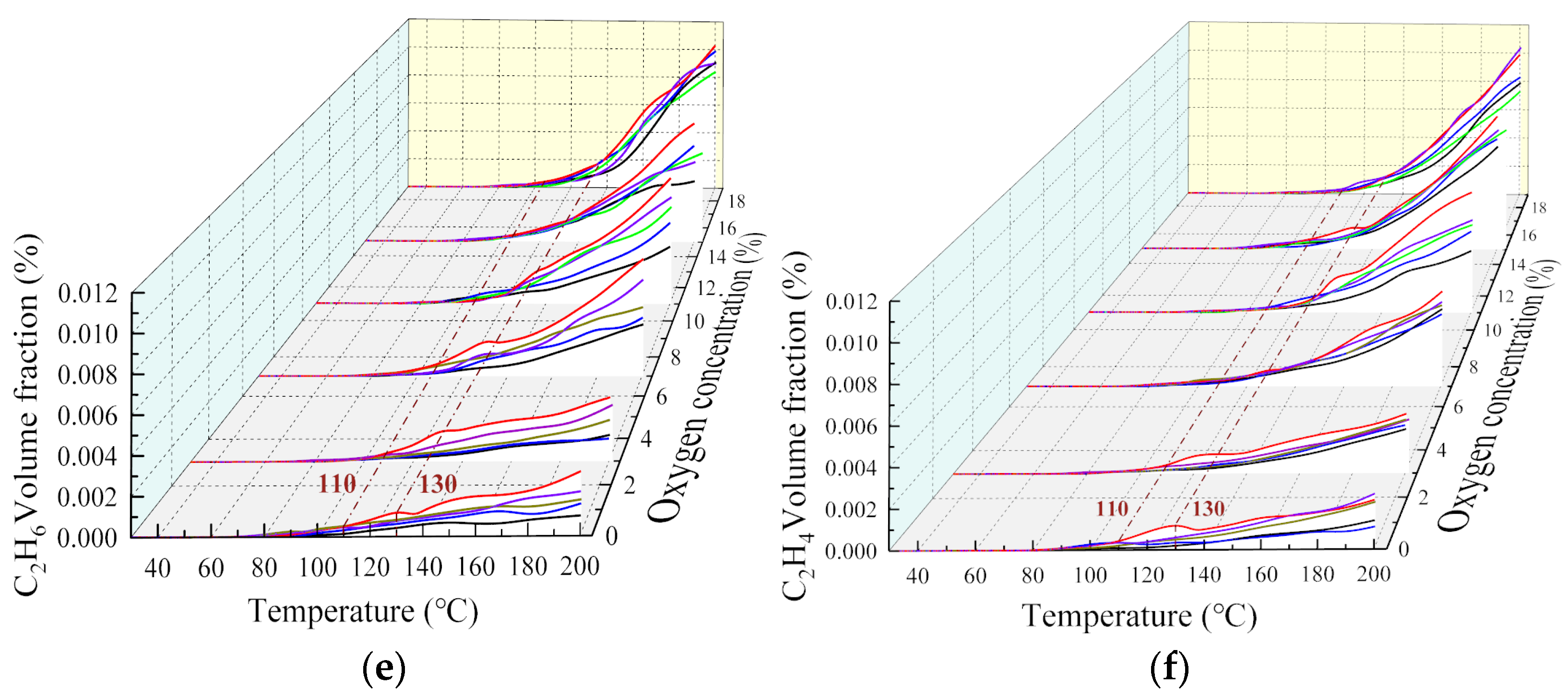
The change rule of the multicomponent gas under different oxygen consumption conditions is shown in Figure 3. When the oxygen-consuming temperature was not reached, the experiments and temperature rise tests under air conditions could be repeated.


Figure 3.
Effect of oxygen-consumed at different temperature points on gas components: (a) O2, (b) CO, (c) CO2, (d) CH4, (e) C2H6, (f) C2H4.
The analysis showed that, when the coal body was heated to different temperatures, the index gas concentration tended to decrease as the oxygen concentration decreased, meaning the oxygen concentration and reaction process are inhibited. At the 40–200 °C stage, the higher the oxygen-consumed temperature point, the greater the decrease in the volume fraction of gas products. This is because the coal-oxygen composite reaction was weak at this stage, and the amount of gaseous products was small. As the temperature continues to increase, the coal oxidation process enters the pyrolysis stage, and the effect of temperature on the reaction dominates, and the product volume fraction shows an exponential growth trend again. In general, at the same oxygen concentration, the higher the temperature of oxygen reduction, the higher the concentration of gas products, indicating that the coal structure changes under high temperature conditions. In addition, the decrease in oxygen concentration had a significant inhibitory effect on the oxidation products but had little effect on pyrolysis gasses such as CH4, C2H6, and C2H4. The change rule for the multicomponent gas at each oxygen-consuming temperature point is listed in Table 2.

Table 2.
Variation law of multi-component gas.
3.2. Comparison of Gas Change Rules under Staged Oxygen-Consumption and Constant Oxygen Conditions
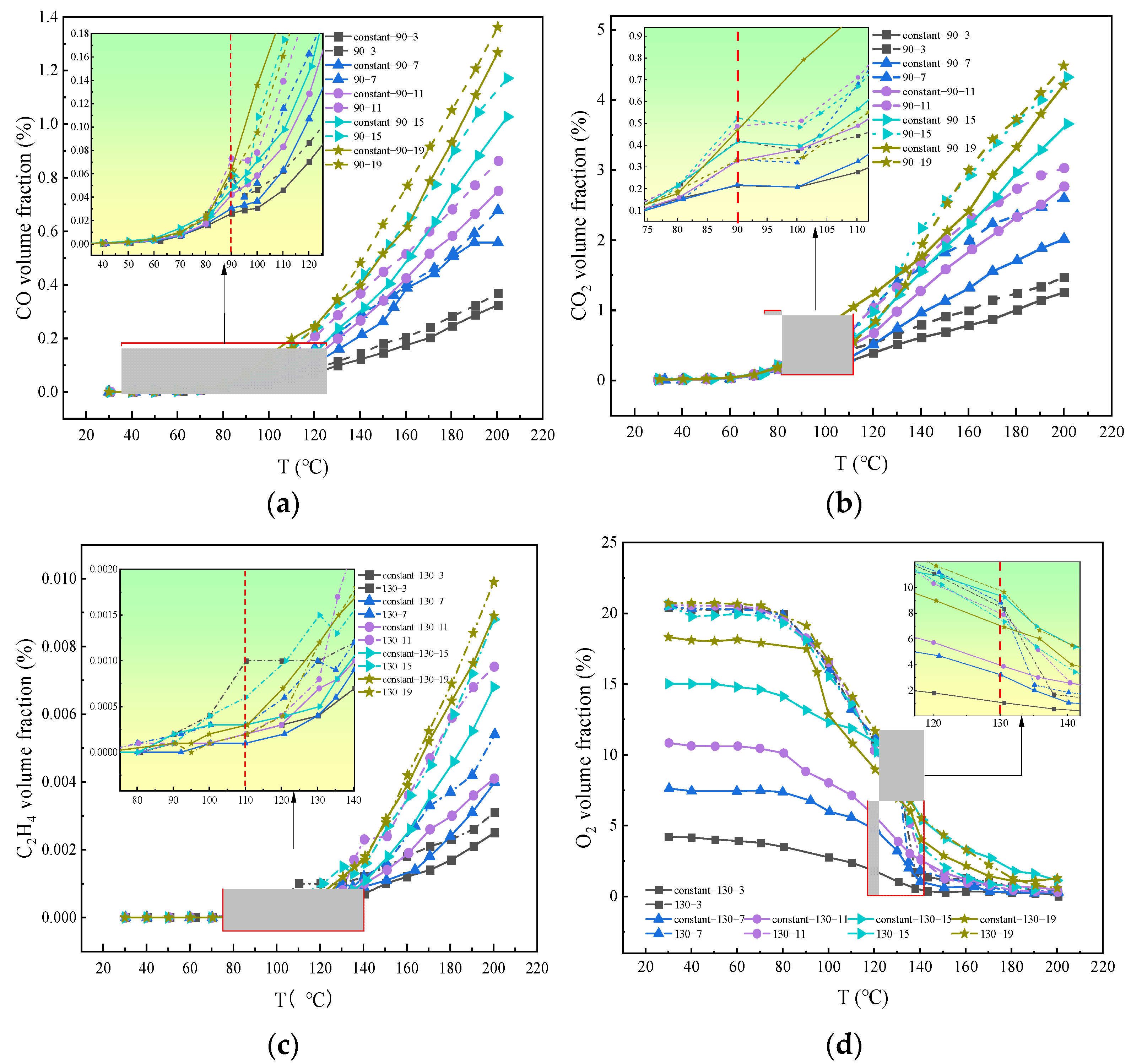
In contrast to the constant oxygen condition, the experimental coal sample exhibited a stage of sudden change from high to low oxygen concentrations, and the variation characteristics of the gas product concentration under the two conditions were compared. The hydrocarbon gas generation law exhibited characteristics similar to the experimental results. CO, CO2, and C2H4, as iconic gasses, are mainly generated by coal oxidation and pyrolysis [18]. The generation of these gasses was closely related to the characteristics of the spontaneous coal combustion stage. Therefore, only the volume fractions of CO and C2H4 in the different oxygen environments were analyzed, as shown in Figure 4.

Figure 4.
Variation of gas volume fraction under staged oxygen-consumption and constant lean oxygen conditions: (a) CO, (b) CO2, (c) C2H4, (d) O2.
The critical and fission stages were selected to compare the gas change rules under the conditions of staged and constant oxygen consumption. When the oxygen concentration was reduced at 90 °C, the highest CO concentration was 0.07%, which is far less than the highest CO2 concentration of 0.5%, because the low temperature stage was in oxygen-rich conditions for a long time. After oxygen reduction, both CO and CO2 showed a sudden short-term drop, with the overall trend being an ‘S’ type, decreasing first and then increasing. This was because with the increase in temperature, the pyrolysis reaction of coal occurred in the high-temperature section, causing the reaction activity to intensify, which promoted the production of CO and CO2. After 150 °C, the concentration of CO2 under 19% constant oxygen was greater than that under 19% oxygen reduction. The reason may be that in the self-heating and critical stage of oxidation at 30–100 °C, 19% constant oxygen condition was not fully oxidized. Compared with the 19% oxygen reduction condition, more CO accumulated, and CO2 was generated by the secondary reaction of pyrolysis [19]. Because C2H4 was the main pyrolysis product, the volume fractions at 19, 15, 11, and 7% oxygen concentration gradients were not affected by the reduction in oxygen concentration, and the overall oxygen reduction was still greater than that under constant oxygen conditions. The volume fraction, of which C2H4 is part after 130 °C, is greatly changed by the 11% oxygen concentration gradient, because the difference between 11% and initial oxygen concentration is greater than 15% and 19%, and the probability of oxygen molecules attacking the side chain of coal molecules increases, so the change in C2H4 volume fraction is greater [20]. The consumption of the O2 volume fraction was consistent with the experimental conditions and was affected by changes in the experimental oxygen environment. The experimental results of the staged oxygen consumption were more in line with the oxidation law of residual coal mined with the working face under variable oxygen conditions.
3.3. The Variation Law of Coal Oxidation Characteristic Parameters under the Condition of Staged Oxygen-Consumption
3.3.1. Oxygen Consumption Rate
Based on an analysis and calculation of the oxygen consumption rate, the physical and chemical reactions between coal and oxygen can be dynamically determined [21]. The relationship between the reaction rate of reactant component i and its initial concentration is as follows [22]:
when the temperature is constant, the initial oxygen content differs, and the oxygen consumption rate can be calculated as follows [23]:
According to Equation (2), the formula for calculating the oxygen consumption rate under experimental oxygen supply conditions is as follows [24]:
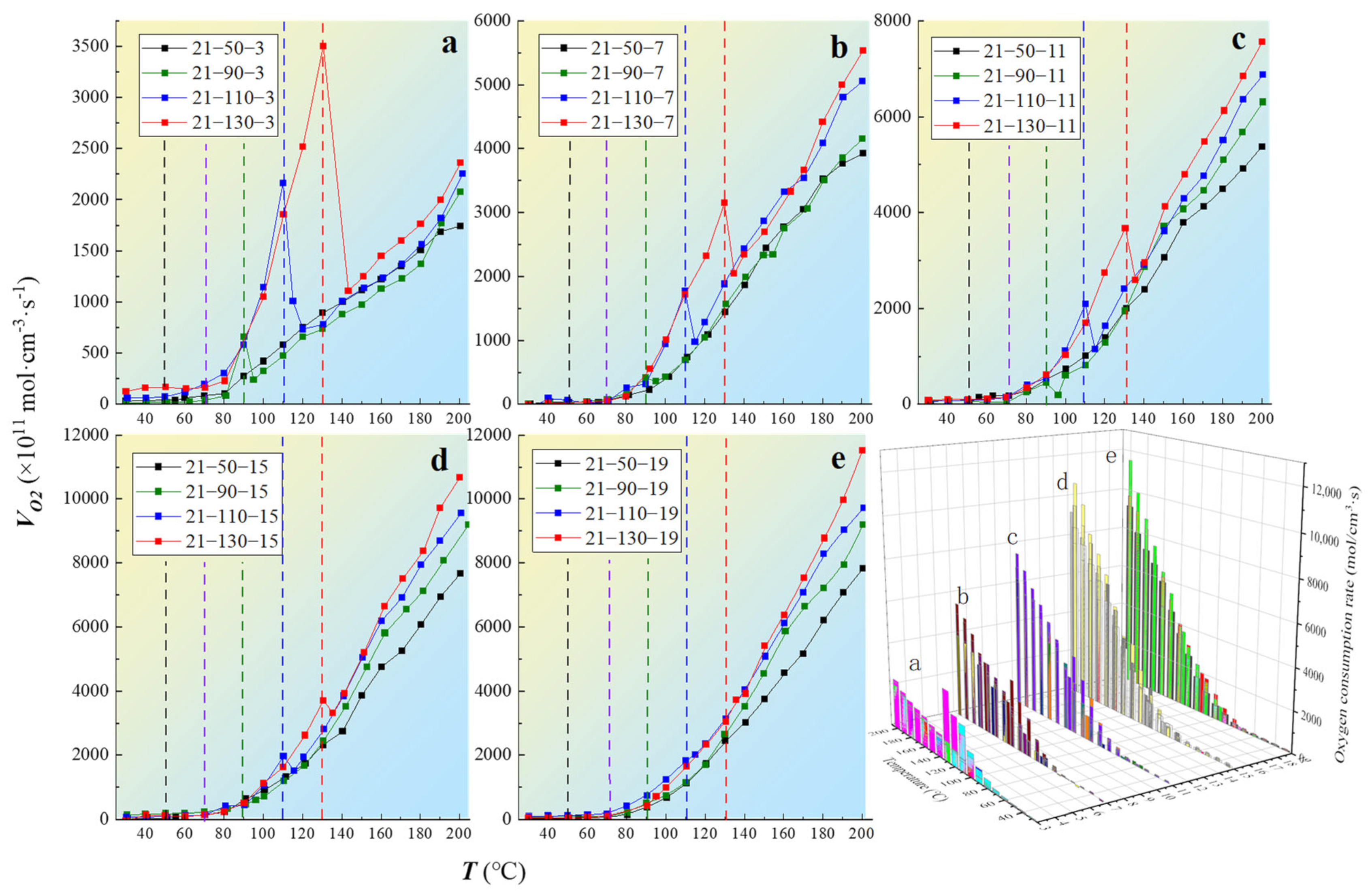
The changes in the coal oxygen consumption rate after oxygen consumption at different oxygen concentrations are shown in Figure 5.

Figure 5.
The variation law of coal oxygen consumption rate with different oxygen-consumed gradients: (a) 3% oxygen concentration, (b) 7% oxygen concentration, (c) 11% oxygen concentration, (d) 15% oxygen concentration, (e) 19% oxygen concentration.
Comprehensive analysis shows that is positively correlated with oxygen concentration and temperature and will increase with the latter. At the oxygen-consuming temperature point, when the oxygen concentration was 15% or less, decreased sharply with decreasing oxygen concentration. After oxygen consumption at different temperatures, the oxygen consumption rate of the continuous oxidation of the coal samples was related to the oxygen consumption temperature point. In the subsequent reactions, the oxygen consumed by at high temperatures was significantly stronger than that at low temperatures. This is because the coal samples started at room temperature, and a large number of intermediate products were produced during the heating and oxidation processes in a high-oxygen environment. When the coal temperature increased, the number of intermediate products also increased. When the oxygen concentration dropped suddenly, some oxygen easily reacted with the intermediate products, thus enhancing the .
3.3.2. Gas Production Rate
During the experimental oxidation process, the formation rates of CO and CO2 were different owing to the change in the oxygen concentration, but the overall curves showed similar trends. The gas generation law of hydrocarbons also has similar characteristics; therefore, only the gas generation rates of CO and C2H4 were analyzed.
- (1)
- CO production rates
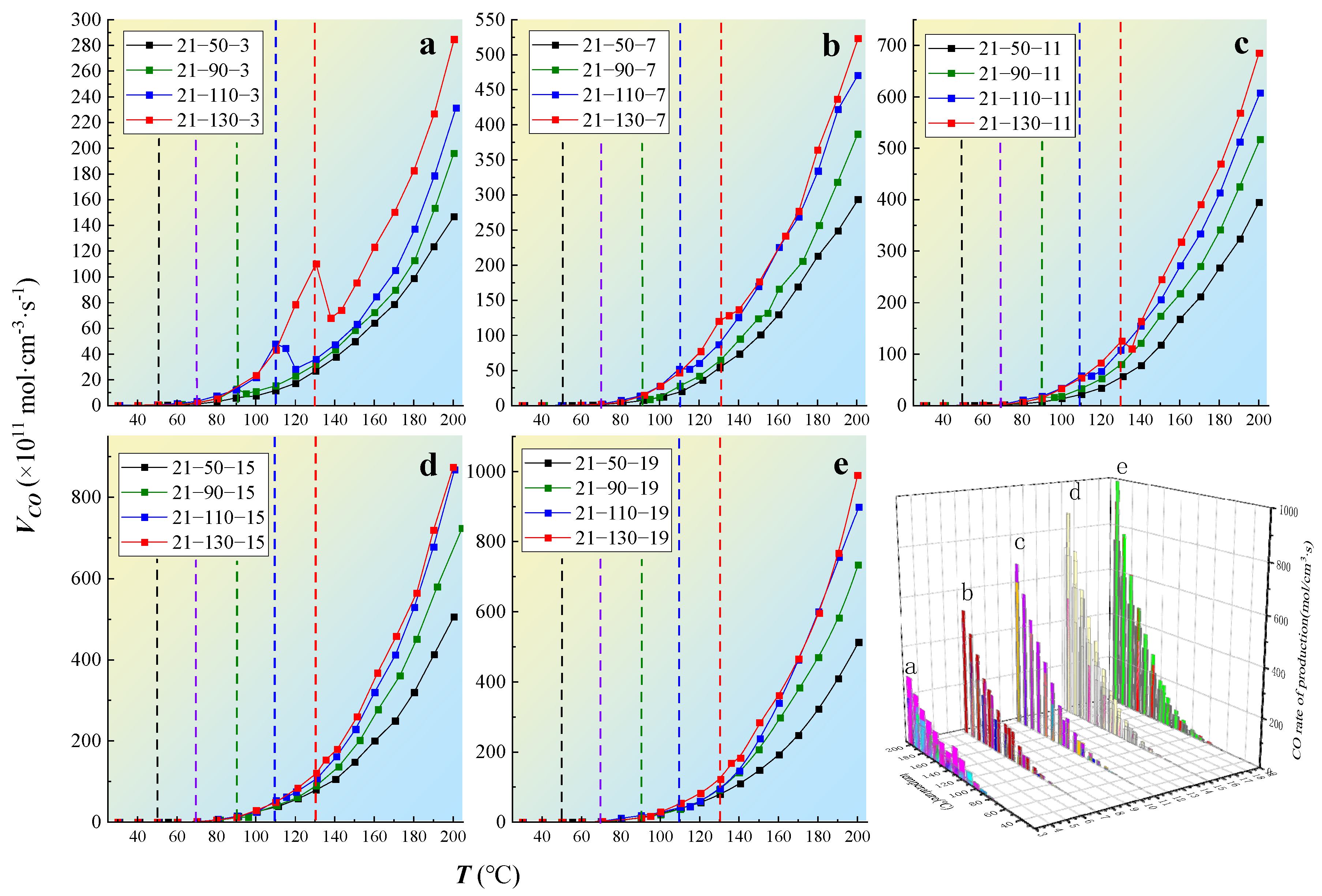
In a temperature-programmed experiment, the gas product formation rate along the wind flow direction is positively correlated with the oxygen consumption rate [25]. Consequently, [26]. is the production rate of CO when the coal temperature is T, in mol·cm−3·s−1). is the CO production rate at standard oxygen mass concentration. The CO production rate can be expressed as follows [27]:
After reduction to the same oxygen concentration at different temperatures, the CO gas production rate exhibited certain differences, as shown in Figure 6. The specific performance is that the higher the oxygen-consumed temperature, the greater the VCO. When the oxygen concentration is constant, the higher the oxidation temperature of the coal sample, there may be more intermediate products in the coal body. In the process of continuous increase in temperature, the intermediate products decompose to produce more oxygen-containing gasses [28,29].

Figure 6.
The variation law of coal CO production rate with different oxygen-consumed gradients: (a) 3% oxygen concentration, (b) 7% oxygen concentration, (c) 11% oxygen concentration, (d) 15% oxygen concentration, (e) 19% oxygen concentration.
When the oxygen-consumed temperature was 50 °C, the gas production rate changed slightly, which was due to the low coal temperature, and the CO was mainly derived from the oxidative decomposition of active functional groups [30]. At 90 °C, the gas production rate increased, indicating that reducing the oxygen content in the critical stage had little effect on the gas production rate. At 110 °C and 130 °C, the gas production rate increased significantly. After reducing the oxygen concentration, the production rate decreased briefly but then increased rapidly in a short time, indicating that reducing the oxygen concentration in the pyrolysis and fission stages had little effect on the gas yield. Temperature had a more significant effect on the product. When the coal sample exceeded the pyrolysis temperature, the decrease in oxygen content led to a decrease in the gas production rate, although it increased significantly compared to the steady-state oxygen concentration.
- (2)
- C2H4 production rate
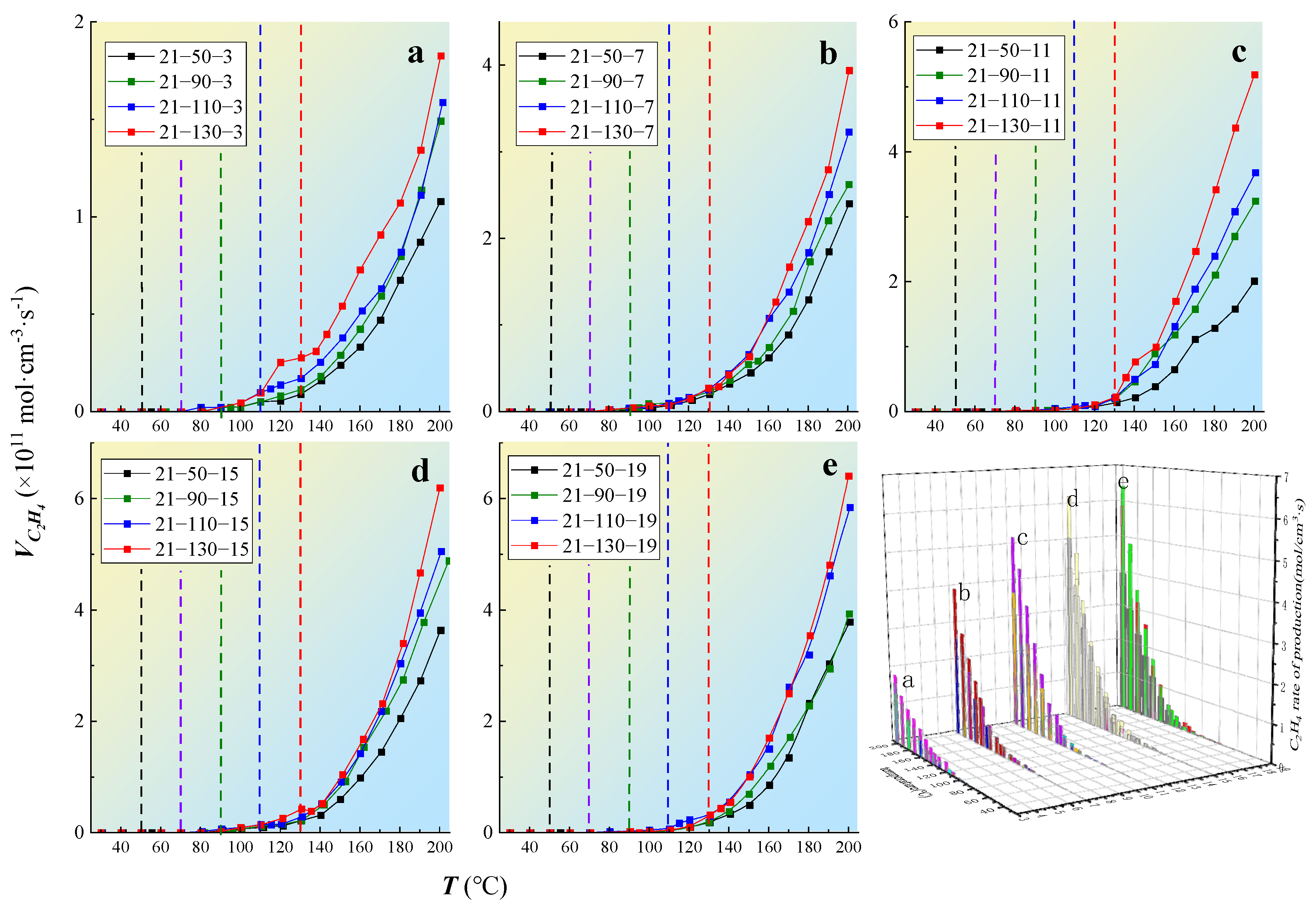
The variation law of coal C2H4 production rate with different oxygen consumption gradients is shown in Figure 7.

Figure 7.
The variation law of coal C2H4 production rate with different oxygen-consumed gradients: (a) 3% oxygen concentration, (b) 7% oxygen concentration, (c) 11% oxygen concentration, (d) 15% oxygen concentration, (e) 19% oxygen concentration.
C2H4 is mainly produced by the side-chain breakage of coal molecules caused by pyrolysis, and temperature has a significant effect on this process. However, in the process of coal oxidation, oxygen molecules attacking the benzene ring side chains of the coal molecules also produce hydrocarbon gasses [31]. When oxygen is reduced at different temperatures, the greater the difference in oxygen content, the more obvious the difference in the hydrocarbon gas production rate. This is because when the oxygen content and temperature are high, the attack probability of oxygen molecules on the side chains of the coal molecules increases, and the intermediate products are easily pyrolyzed to generate hydrocarbon gas after a sudden drop in the oxygen content. When the coal temperature is not high, the content of intermediate products and free radicals is low; therefore, the smaller the oxygen concentration difference before and after the oxygen consumption temperature, the smaller the production rate difference in hydrocarbon gasses.
3.3.3. Exothermic Intensity
The spontaneous combustion stage of coal releases heat; however, the physical adsorption stage releases less heat and is usually negligible [32]. When calculating the heat release intensity, the oxygen consumed by the coal was consumed by chemical adsorption, except for CO and CO2. The bond energy estimation method [33] can accurately calculate the maximum exothermic intensity qmax and minimum exothermic intensity qmin during the oxidation process [34]:
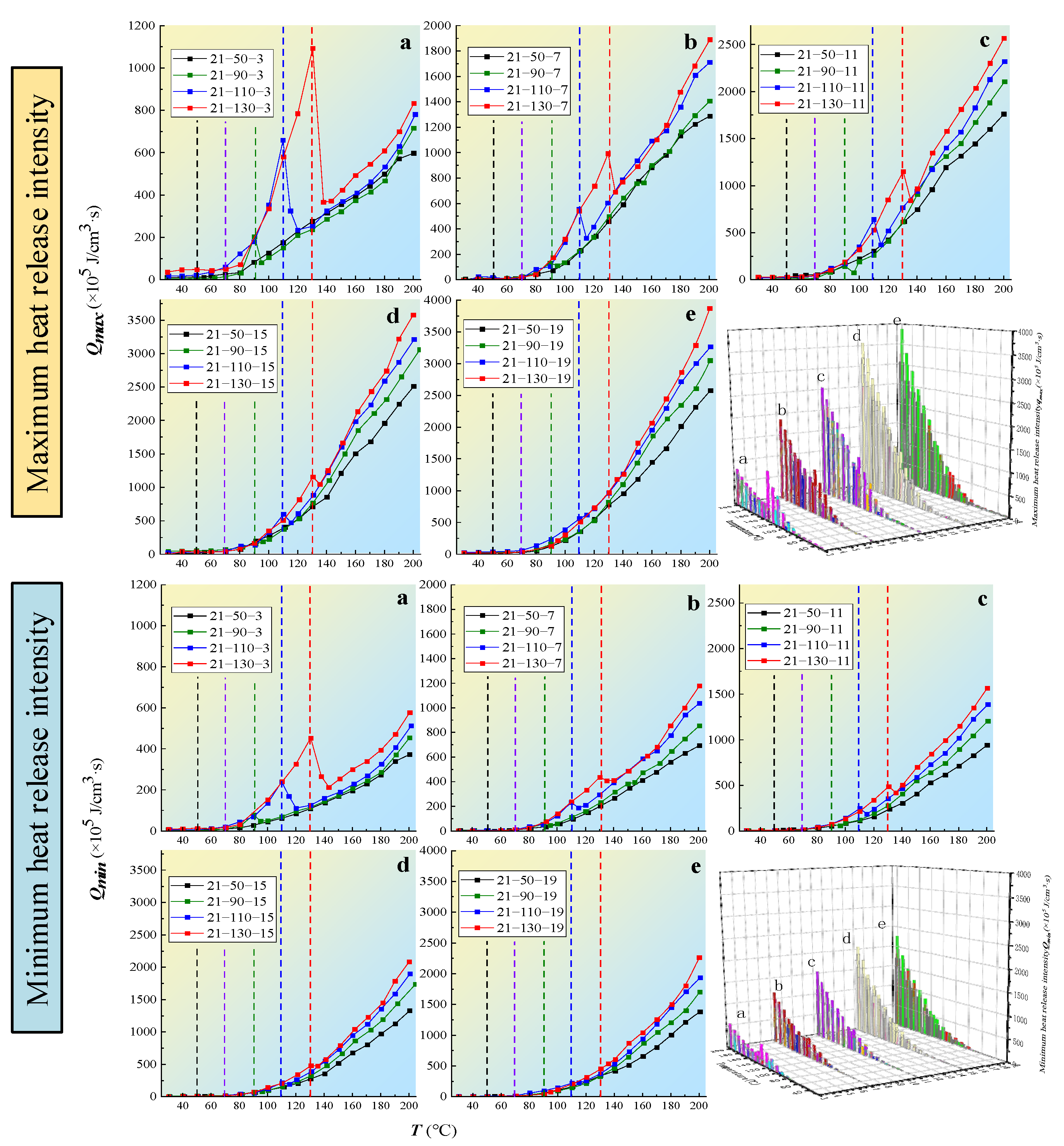
The variation law of the coal exothermic intensity with different oxygen consumption gradients is shown in Figure 8.

Figure 8.
The variation law of coal exothermic intensity with different oxygen-consumed gradients: (a) 3% oxygen concentration, (b) 7% oxygen concentration, (c) 11% oxygen concentration, (d) 15% oxygen concentration, (e) 19% oxygen concentration.
The heat release intensity of the coal was positively correlated with the oxygen concentration and oxygen reduction temperature. When the oxygen concentration was lower than 9%, the heat release intensity significantly decreased. When the oxygen concentration was lower than 5%, the heat release intensity sharply decreased. The heat release intensity also exhibited differences after the oxygen concentration was reduced at different temperature points. When the oxygen-consumed temperature reached above 110 °C, the oxygen content and exothermic intensity decreased, although the exothermic intensity increased significantly compared with the low temperature oxygen-consumption. This indicated that after the temperature exceeded the cracking temperature in a high-oxygen environment, even if the oxygen content decreased, the coal still released heat. This also indicated that it was difficult to suppress the heat storage during coal oxidation by reducing the oxygen content during the high-temperature stage.
3.4. Correlation Analysis between Coal Oxidation Characteristic Parameters
3.4.1. Relationship between Oxygen Consumption Rate and Gas Product Formation Rate
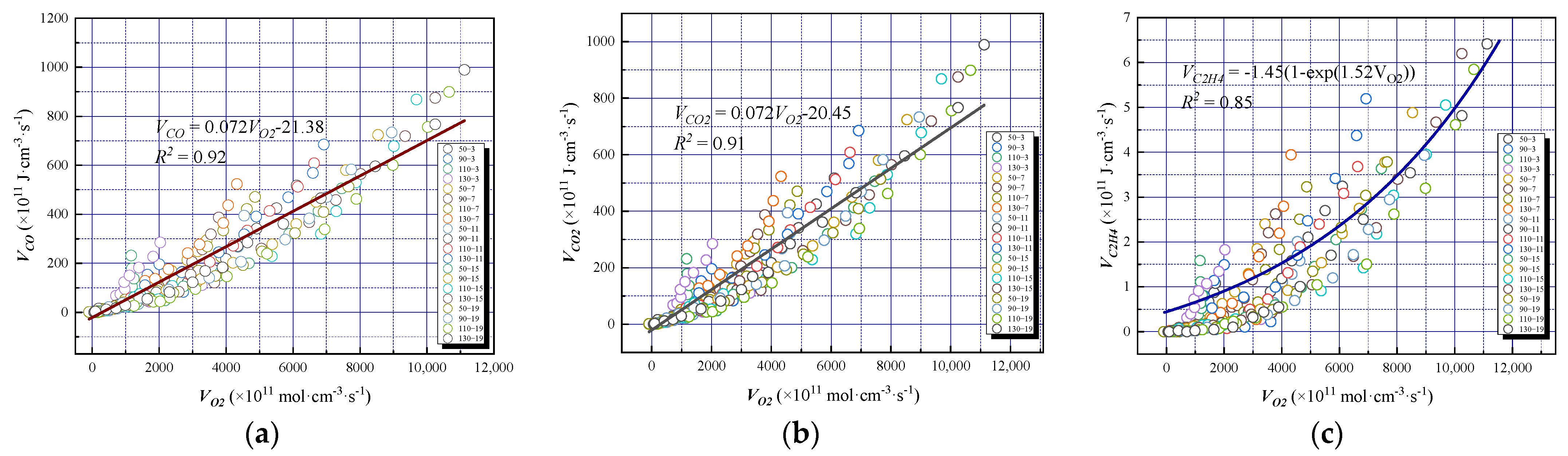
The relationship between the oxygen consumption rate , and the oxygen consumption rates VCO, , and is shown in Figure 9a–c, respectively.

Figure 9.
The relationship between the oxygen consumption rate and product content under different oxygen-consumed gradients: (a) Oxygen consumption rate and CO production rate, (b) Oxygen consumption rate and CO2 production rate, (c) Oxygen consumption rate and C2H4 production rate.
The oxygen consumption rate of the coal samples was linearly and positively correlated with VCO and . The formation rate of the oxidation products increased with increasing oxygen consumption rate, whereas tended to be exponentially related to the oxygen consumption rate. In addition, with VCO = 0.072 − 21.38, the correlation coefficient was 92%, since the formation of the CO path was single, and the carbon molecules could usually be directly oxidized. With = 0.072 − 20.45, the correlation coefficient was 91%. At the same oxygen consumption rate, was slightly larger than VCO because CO reacts with CO2 with an increasing degree of oxidation. With = 1.45(1 − exp(1.52)), the correlation coefficient was 85%, indicating that was low and had little effect on the production rate of ethylene; when increased, the production rate of ethylene increased rapidly. The lower may have been because coal was mainly oxidized by the surface-active structure, and the production of ethylene was small. A higher implied that the pyrolysis and fission processes were the main processes, and the generation of a large number of active structures consumed more oxygen. In addition, the accumulation of intermediate products during coal oxidation produced more hydrocarbon gasses, resulting in different degrees of response to the C2H4 production rate, and so a linear relationship with the oxygen consumption rate could not be established. Therefore, it was necessary to consider the ratio of CO concentration to oxygen concentration when monitoring the CO concentration to realize early coal fire prevention and control by analyzing the change in hydrocarbon gas concentration.
3.4.2. The Oxygen Consumption Rate and Exothermic Intensity
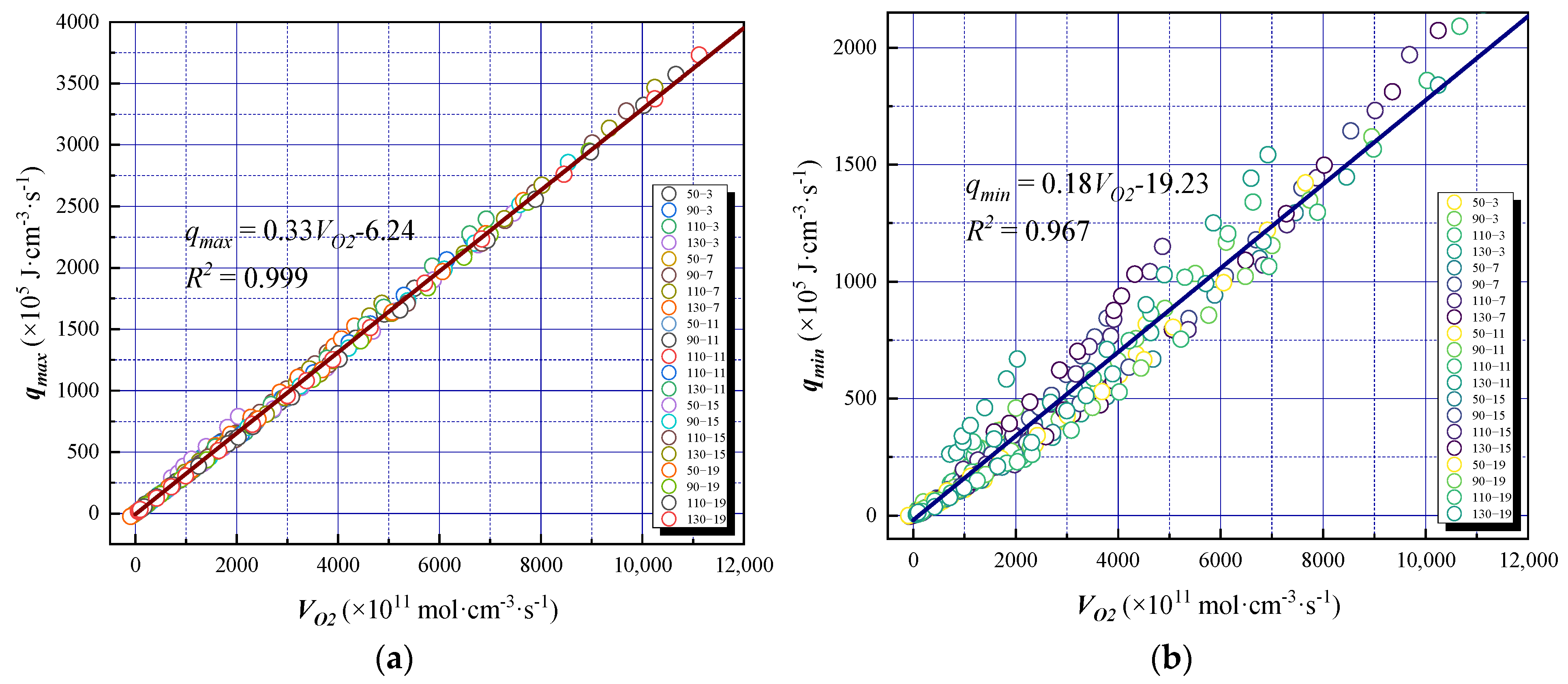
According to the analysis of the experimental results, the relationships between and both the maximum exothermic intensity qmax and the minimum exothermic intensity qmin are shown in Figure 10a,b, respectively.

Figure 10.
The relationship between oxygen consumption rate and exothermic intensity under different oxygen-consumed gradients: (a) and qmax, (b) and qmin.
Under different oxygen supply conditions, the is significantly linearly related to the exothermic intensity. As increases, the exothermic intensity increases linearly. For the same , the oxygen content had a weak effect on the exothermic intensity. The slope of the fitting line in this Figure represents the reaction heat of coal oxidation. It can be seen that the reaction heat of the experimental coal sample is 180–330 kJ·mol−1; that is, for every 1 mol of oxygen consumed, 180–330 kJ of heat was generated. In addition, qmax and qmin were strongly and linearly correlated with , and the correlation coefficient between qmin and was 96.7%, which was less than that between qmax and (99.9%). The discrete type was more significant and exhibits an exponential trend. This is because oxygen was consumed at this stage to generate CO and CO2 and release heat; qmax was in a completely oxidized state, and most chemical bonds in the coal body were completely broken; thus, the released heat was relatively stable and could be approximated as a continuous exothermic process. In the process of qmin formation, owing to the existence of other reactions, the distribution of chemical bonds in the coal samples was uneven, and the fracture rate and heat release were also different, which made qmin change significantly under the experimental conditions.
3.4.3. The Critical Oxygen Concentration Affecting the Oxidation Process of Coal under the Condition of Oxygen-Consumption
- (1)
- CO production rates
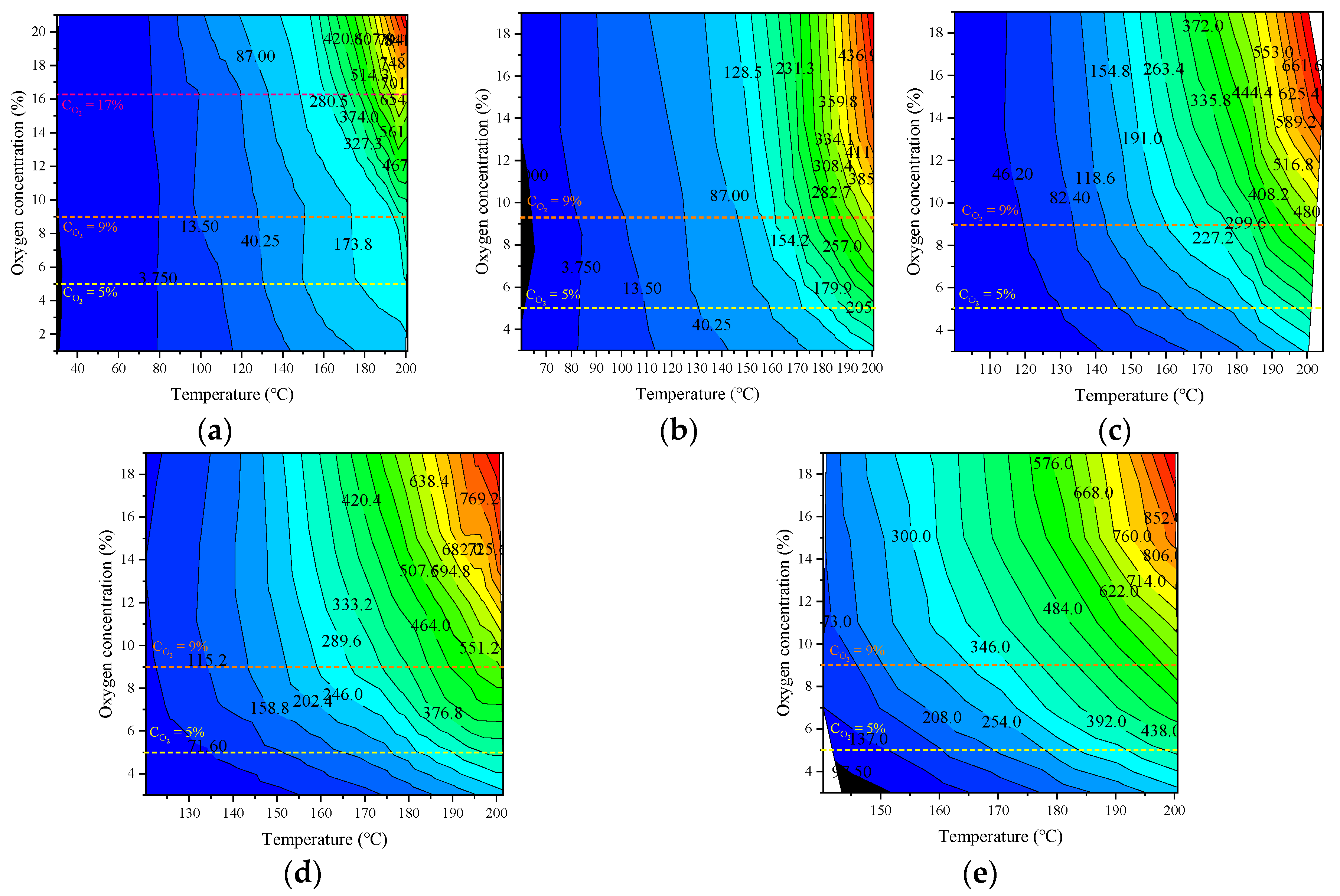
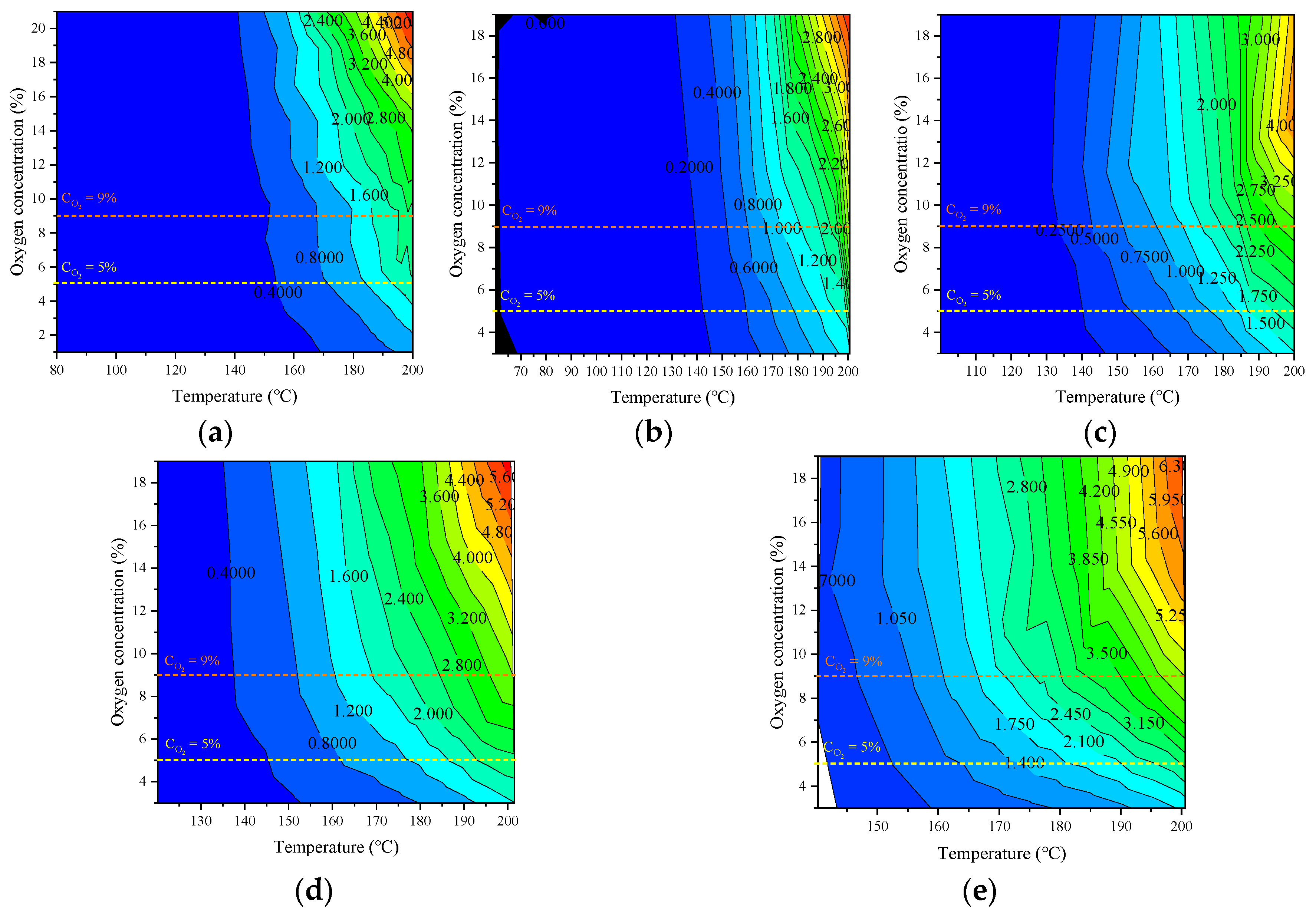
An isogram of the CO production rate of the coal samples with respect to temperature and oxygen content under various experimental conditions is shown in Figure 11.

Figure 11.
Contour diagram of CO production rate changing with coal temperature and oxygen concentration under different oxygen environments: (a) Raw coal, (b) Oxygen consumed at 50 °C, (c) Oxygen consumed at 90 °C, (d) Oxygen consumed at 110 °C, (e) Oxygen consumed at 130 °C.
The oxygen content in the low-temperature stage has little effect on the CO production rate. Under each oxygen content gradient, there are three critical oxygen concentrations that lead to changes in the CO production rate: 5, 9, and 17%. When the oxygen content decreased from 21 to 17%, the CO production rate decreased significantly; at 9–17%, the CO production rate decreased slowly; at 9–5%, the change in the CO production rate was weak; and when it was less than 5%, the CO production rate decreased rapidly. Because of the large oxygen concentration step (4%) in this experiment, no critical oxygen concentration of 17% was found, while the critical values of 5% and 9% also had a significant effect on the CO production rate, indicating a high-gradient oxygen content and high-temperature environment. Although the oxygen consumption rate increased, the critical oxygen content value did not change. In field applications, controlling the ambient oxygen concentration below the critical oxygen concentration according to demand is conducive to inhibiting CO production.
- (2)
- C2H4 production rate
An isogram of the C2H4 production rate of the coal samples with respect to temperature and oxygen content under various experimental conditions is shown in Figure 12.

Figure 12.
Contour of C2H4 production rate with temperature and oxygen concentration under different oxygen environments: (a) Raw coal, (b) Oxygen consumed at 50 °C, (c) Oxygen consumed at 90 °C, (d) Oxygen consumed at 110 °C, (e) Oxygen consumed at 130 °C.
When the oxygen content was above 9%, the C2H4 production rate of each coal sample changes slightly. At 5–9%, The gas production rate decreased slowly, and when it was less than 5%, the gas production rate decreased rapidly. This shows that, although the production rate of C2H4 was less affected by the oxygen content, there was also a sudden change in the oxygen concentration. Controlling the oxygen content below the critical temperature also inhibited the production rate of C2H4.
4. Conclusions
- (1)
- After periodic oxygen reduction, the overall oxidation of coal was slightly stronger than that under constant low oxygen conditions. The decrease in oxygen concentration had a significant influence on the oxidation gas products and little effect on the pyrolysis gasses, such as CH4, C2H6 and C2H4. However, the relationships between the oxygen consumption rate, CO generation rate, and exothermic intensity did not change significantly. Therefore, under the actual conditions of the site, the change in oxygen concentration should be comprehensively considered when using a CO concentration warning. It is feasible to determine the spontaneous coal combustion trend directly through the change in gas concentration when using C2H4 and other gasses for high-temperature warnings.
- (2)
- The coal oxidation characteristic parameters, such as the gas product volume fraction, oxygen consumption rate, gas production rate, and heat release intensity of the coal samples, are positively correlated with the oxygen-consumed temperature. The maximum and minimum exothermic intensities of coal oxidation were found to have a significant linear relationship with the oxygen consumption rate. The oxidation reaction heat of coal was obtained by numerical fitting to be 180–330 kJ·mol−1, and the degree of fitting was high, indicating that the calculated exothermic intensity was in line with the actual oxidation mode of coal.
- (3)
- The oxygen concentration had a significant effect on the formation rate of the oxygen-containing gaseous products. The critical oxygen concentrations that determined the formation rate of the oxidizing gas were 17, 9, and 5%. When the reaction rate was low, the effect of oxygen concentration on the production rate of hydrocarbon gas was weak; however, when the reaction was severe, the production rate of hydrocarbon gas increased exponentially with the oxygen consumption rate, indicating that there was also an indirect relationship between the formation of hydrocarbon gas and oxygen consumption. The critical oxygen concentrations leading to an abrupt change in the gas production rate were obtained, which were 9 and 5%.
In future research, the characteristics of oxygen consumption and the oxidation of coal should be considered. Combined with the correlation between parameters such as the critical oxygen concentration and oxygen consumption rate in this study, a dynamic coal fire perception, prevention, and control model can be established to provide theoretical support for safe coal mine production.
Author Contributions
Conceptualization, supervision, formal analysis, J.G.; writing (original draft), L.W.; formal analysis, Y.L.; methodology, investigation, C.C.; methodology, investigation, G.C.; supervision, formal analysis, W.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (grant numbers 52004209, 52174198, and 52304251), the Shaanxi Science and Technology Association Young Talents Lifting Project (grant number 20240205), and the Shaanxi Postdoctoral Science Foundation (grant number 2023BSHEDZZ286).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We are grateful for the support of the laboratories and assistants who provided the experimental conditions for this study.
Conflicts of Interest
Wentao Du is employed at the Chenjiagou Coal Mine. Or. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Nomenclature
| reaction rate of gas component i (mol·cm−3·s−1) | |
| reactant O2 concentration (mol) | |
| ki | reaction constant |
| n | order of reaction |
| oxygen consumption rate (mol·cm−3·s−1) | |
| the experiment supplies oxygen content (%) | |
| experimental tank volume (cm3) | |
| Q | supply flow (mL·min−1) |
| oxygen content of air inlet, outlet (%) | |
| CO content of air inlet, outlet (%) | |
| produces 1 mol of CO to release heat (J) | |
| produces 1 mol of CO2 to release heat (J) | |
| the heat released by the first and second reactions (J) | |
| the heat generated by chemical adsorption (J) | |
| x | axial length from tank bottom (cm) |
References
- Xie, H.P.; Wu, L.X.; Zheng, D.Z. Prediction on the energy consumption and coal demand of China in 2025. J. China Coal Soc. 2019, 44, 1949–1960. [Google Scholar]
- Chen, H.; Shao, H.; Jiang, S.G.; Huang, C.L.; Liu, G.Z. Study on the Cause of Hypoxia in the Corner of Return Air of Shallow Buried Flammable Coal Seam Group Mining Face and the Coordinated Prevention and Control of Coal Spontaneous Combustion. Appl. Sci. 2023, 13, 13. [Google Scholar] [CrossRef]
- Guo, J.; Chen, C.M.; Wen, H.; Cai, G.B.; Liu, Y. Prediction model of goaf coal temperature based on PSO-GRU deep neural network. Case Stud. Therm. Eng. 2024, 53, 103813. [Google Scholar] [CrossRef]
- Ren, L.F.; Li, Q.W.; Deng, J.; Ma, L.; Xiao, Y. Effect of Oxygen Concentration on the Oxidative Thermodynamics and Spontaneous Combustion of Pulverized Coal. ACS Omega 2021, 6, 26170–26179. [Google Scholar] [CrossRef]
- Zhao, J.R.; Xiao, Y.; Zhong, K.Q.; Li, Q.W.; Zhai, X.W. Effects of oxygen concentration and heating rate on coal spontaneous combustion characteristics. J. Therm. Anal. Calorim. 2023, 148, 4949–4958. [Google Scholar] [CrossRef]
- Guo, J.; Quan, Y.P.; Cai, G.B.; Jin, Y.F.; Zheng, X.Z. Meticulous Graded and Early Warning System of Coal Spontaneous Combustion Based on Index Gases and Characteristic Temperature. ACS Omega 2023, 8, 6801–6812. [Google Scholar] [CrossRef]
- Shao, H.; Zhou, F.B.; Chen, K.Y.; Cheng, J.W.; Palu, M. Study on the Hydrogen Generation Rules of Coal Oxidation at Low Temperature. J. Eng. Technol. 2014, 7, 90–95. [Google Scholar]
- Zhao, X.G.; Dai, G.L.; Qin, R.X.; Zhou, L.; Li, J.L. Study on oxidation kinetics of low-rank coal during the spontaneous combustion latency. Fuel 2023, 339, 127441. [Google Scholar] [CrossRef]
- Wen, H.; Guo, J.; Jin, Y.F.; Wang, K.; Zhang, Y.T. Experimental study on the influence of different oxygen concentrations on coal spontaneous combustion characteristic parameters. Int. J. Oil Gas Coal Technol. 2017, 16, 187–202. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Shu, P.; Deng, J.; Duan, Z.X.; Li, L.L. Analysis of oxidation pathways for characteristic groups in coal spontaneous combustion. Energy 2022, 254, 124211. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, H.Q.; Huang, H.; Zhao, H.R.; Pan, R.L. Study on the thermal kinetics and microscopic characteristics of oxidized coal. Environ. Sci. Pollut. Res. 2023, 30, 85953–85967. [Google Scholar] [CrossRef]
- Liu, Z.J.; Xu, Y.L.; Wen, X.L.; Lv, Z.G.; Wen, J.D. Thermal Properties and Key Groups Evolution of Low-Temperature Oxidation for Bituminous Coal under Lean-Oxygen Environment. ACS Omega 2021, 6, 15115–15125. [Google Scholar] [CrossRef]
- Chen, J.; Jia, B.S.; Wen, Y.; Jing, Q.H.; Liu, L.J. Study on Spontaneous Combustion Characteristics and Oxidation Kinetic Parameters of Lignite at Different Oxygen Concentrations. ACS Omega 2022, 7, 38487–38495. [Google Scholar] [CrossRef]
- Jia, X.L.; Wu, J.K.; Lian, C.J.; Rao, J.L. Assessment of coal spontaneous combustion index gas under different oxygen concentration environment: An experimental study. Environ. Sci. Pollut. Res. 2022, 29, 87257–87267. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.Y.; Li, Q.Z.; Zhang, H.J.; Xin, H.H. Thermodynamic characteristics of coal reaction under low oxygen concentration conditions. J. Energy Inst. 2017, 90, 544–555. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, H.; Guo, J.; Jin, Y.F.; Fan, S.X. Correlation between oxygen concentration and reaction rate of low-temperature coal oxidation: A case study of long-flame coal. Energy 2023, 275, 127483. [Google Scholar] [CrossRef]
- Liu, Y. Study on Nonlinear Evolution Law and Dynamic Early Warning Method of Residual Coal Spontaneous Combustion in Goaf. Ph.D. Thesis, Xi’an University of Science and Technology, Xi’an, China, 2022. [Google Scholar]
- Wang, B.F.; Lv, Y.H.; Liu, C.B. Research on fire early warning index system of coal mine goaf based on multi-parameter fusion. Sci. Rep. 2024, 14, 485. [Google Scholar] [CrossRef]
- Lu, B.; Zhang, X.; Qiao, L.; Ding, C.; Fan, N.; Huang, G. Experimental study on the effect of slow reaction process of the latent period on coal spontaneous combustion. Energy 2024, 302, 131927. [Google Scholar] [CrossRef]
- Zhou, B.Z.; Yang, S.Q.; Yang, W.M.; Jiang, X.Y.; Song, W.X.; Cai, J.W.; Xu, Q.; Tang, Z.Q. Variation characteristics of active groups and macroscopic gas products during low-temperature oxidation of coal under the action of inert gases N2 and CO2. Fuel 2022, 307, 121893. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, M.; Deng, J.; Xiao, Y.; Chen, W.L. Effects on environmental conditions and limiting parameters for spontaneous combustion of residual coal in underground goaf. Process Saf. Environ. Prot. 2024, 187, 1378–1389. [Google Scholar] [CrossRef]
- Zhao, X.G.; Dai, G.L.; Qin, R.X.; Zhou, L.; Li, J.H. Spontaneous combustion characteristics of coal based on the oxygen consumption rate integral. Energy 2024, 288, 129626. [Google Scholar] [CrossRef]
- Chao, W.Y.; Zhong, K.Q.; Xiao, Y.; Lai, X.P.; Li, Q.W. Determining the Spontaneous Combustion Period and Limit Parameters of Coal: A Large-Scale Furnace Experiment. Combust. Sci. Technol. 2023, 195, 494–507. [Google Scholar]
- Yang, Y.; Fei, J.B.; Luo, Z.M.; Wen, H.; Wang, H. Experimental study on characteristic temperature of coal spontaneous combustion. J. Therm. Anal. Calorim. 2023, 148, 10011–10019. [Google Scholar] [CrossRef]
- Li, Z.X.; Zhang, M.Q.; Yang, Z.B.; Liu, Y.; Yu, J.X. Exothermic characteristics of coal during low-temperature oxidation based on grey correlation method. Energy. Rep. 2022, 8, 86744–86752. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, C.; Lu, B.; Gao, F.; Shan, C. Study on the inhibitory mechanism of dehydrogenated antioxidants on coal spontaneous combustion. Sci Rep. 2022, 12, 21237. [Google Scholar] [CrossRef]
- Xu, X.F.; Zhang, F.J. Evaluation and Optimization of Multi-Parameter Prediction Index for Coal Spontaneous Combustion Combined with Temperature Programmed Experiment. Fire 2023, 6, 368. [Google Scholar] [CrossRef]
- Yan, H.W.; Nie, B.S.; Liu, P.J.; Chen, Z.Y.; Jin, F.-F. Experimental investigation and evaluation of influence of oxygen concentration on characteristic parameters of coal spontaneous combustion. Thermochim. Acta 2022, 717, 179345. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Yang, S.Q.; Zhou, B.Z.; Lan, L. The auto-oxidation characteristic of coal at different stages of the low-temperature oxidation process. Fuel 2023, 352, 129130. [Google Scholar] [CrossRef]
- Jin, Y.F.; Chai, Y.Y.; Liu, Y.; Guo, J.; Yan, H. Experimental study on the evolutionary characteristics of silicified coal functional groups during oxidation/pyrolysis. Combust. Sci. Technol. 2023, 195, 2491–2509. [Google Scholar] [CrossRef]
- Wang, H.Y.; Tian, Y.; Li, J.L.; Chen, X. Experimental study on thermal effect and gas release laws of coal-polyurethane cooperative spontaneous combustion. Sci. Rep. 2021, 11, 1994. [Google Scholar] [CrossRef]
- Ren, Z.J.; Wang, D.P.; Qin, Z.; Liu, Z.W. Effects of pore size, water content, and oxygen-containing functional groups on oxygen adsorption in bituminous coal. Sci. Rep. 2023, 13, 10373. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.C.; Wen, H.; Ge, L.M.; Zhang, X.H.; Deng, J. Determination and calculation of oxidation heat liberation intensity of loose coal at low temperature stage. J. China Coal Soc. 2000, 25, 387–390. [Google Scholar]
- Liu, H.; Li, Z.H.; Miao, G.D.; Yang, J.J.; Wu, X.Q. Insight into the chemical reaction process of coal during the spontaneous combustion latency. Energy 2023, 263, 125823. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).