Short-Term Impacts of Fire and Post-Fire Restoration Methods on Soil Properties and Microbial Characteristics in Southern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sampling and Chemical Analyses
2.3. Soil Microbial Biomass, Enzyme Activity and Microbial Characteristics Analyses
2.4. Statistical Analyses
3. Results
3.1. Impacts of Fire, Soil Depth, and Post-Fire Restoration Methods on Soil Physiochemical Properties
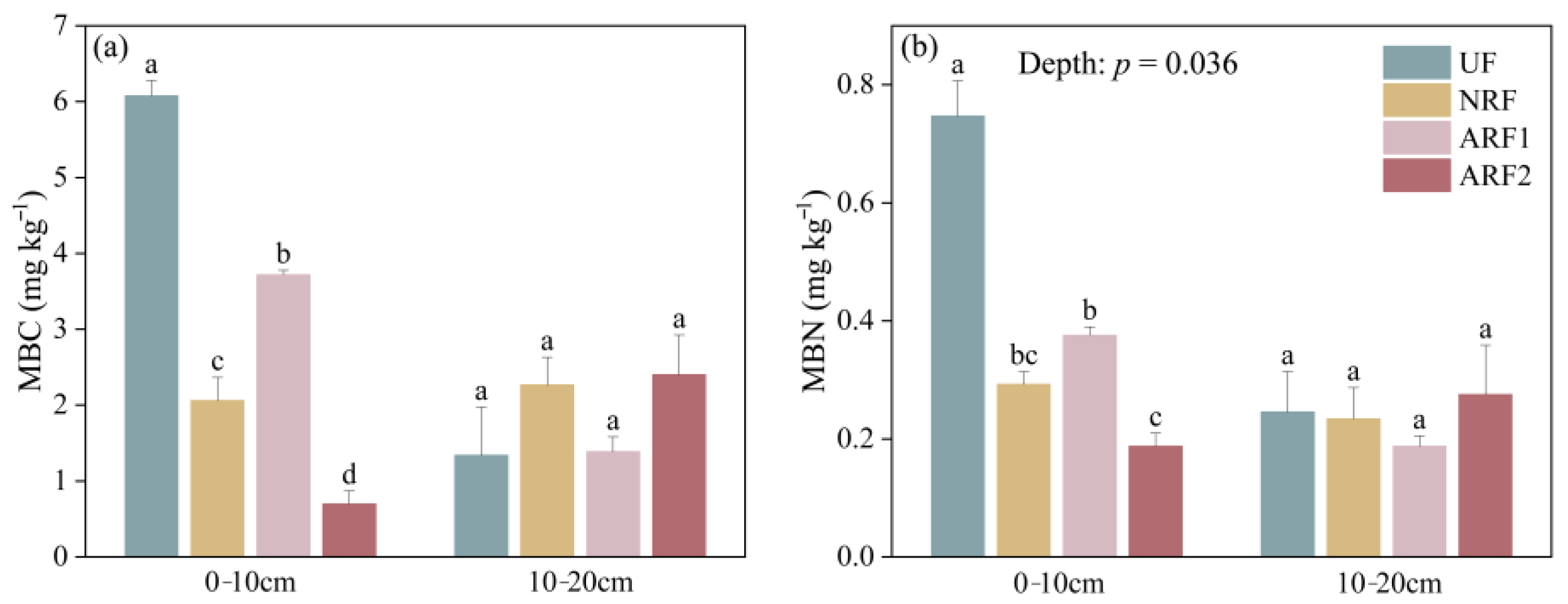
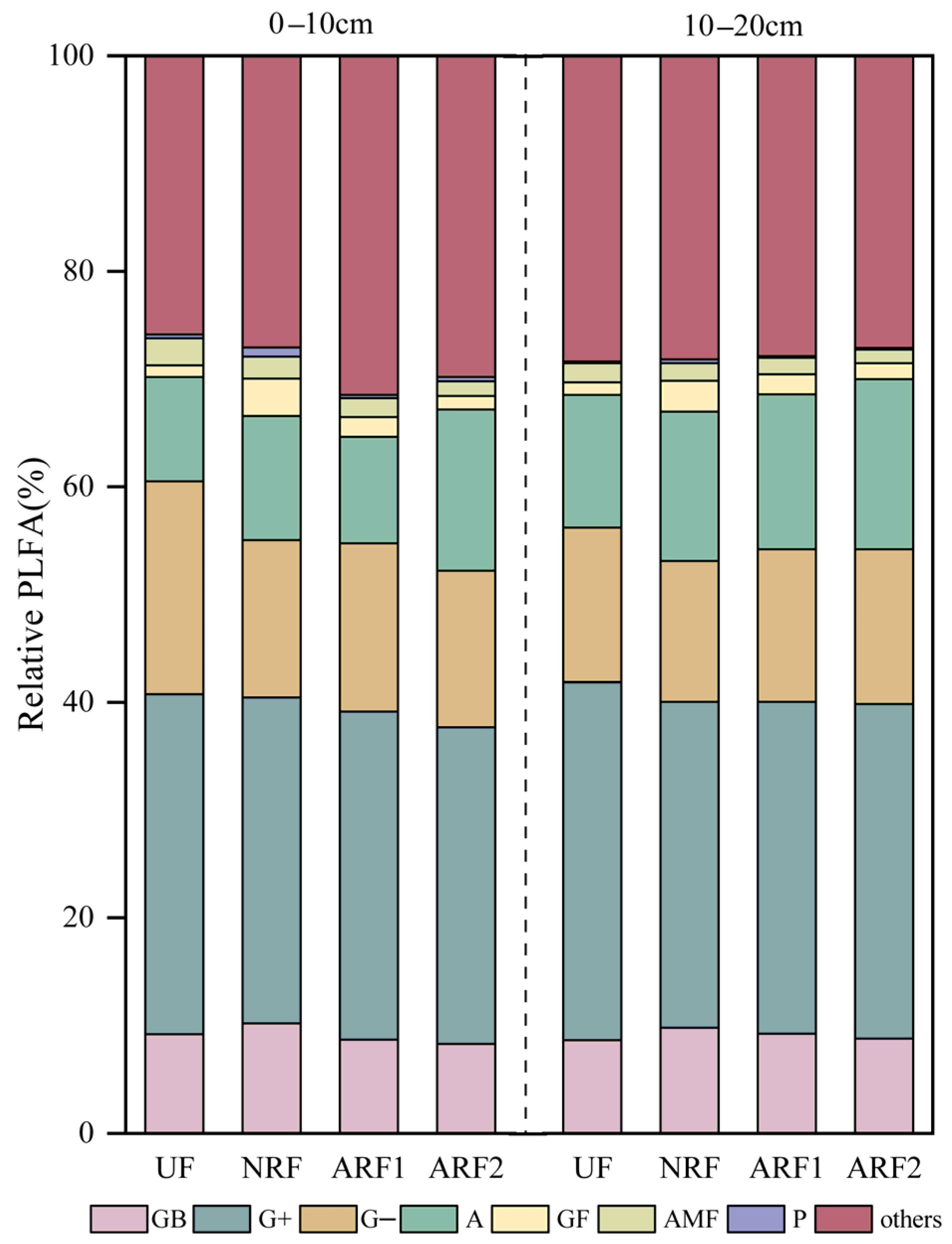
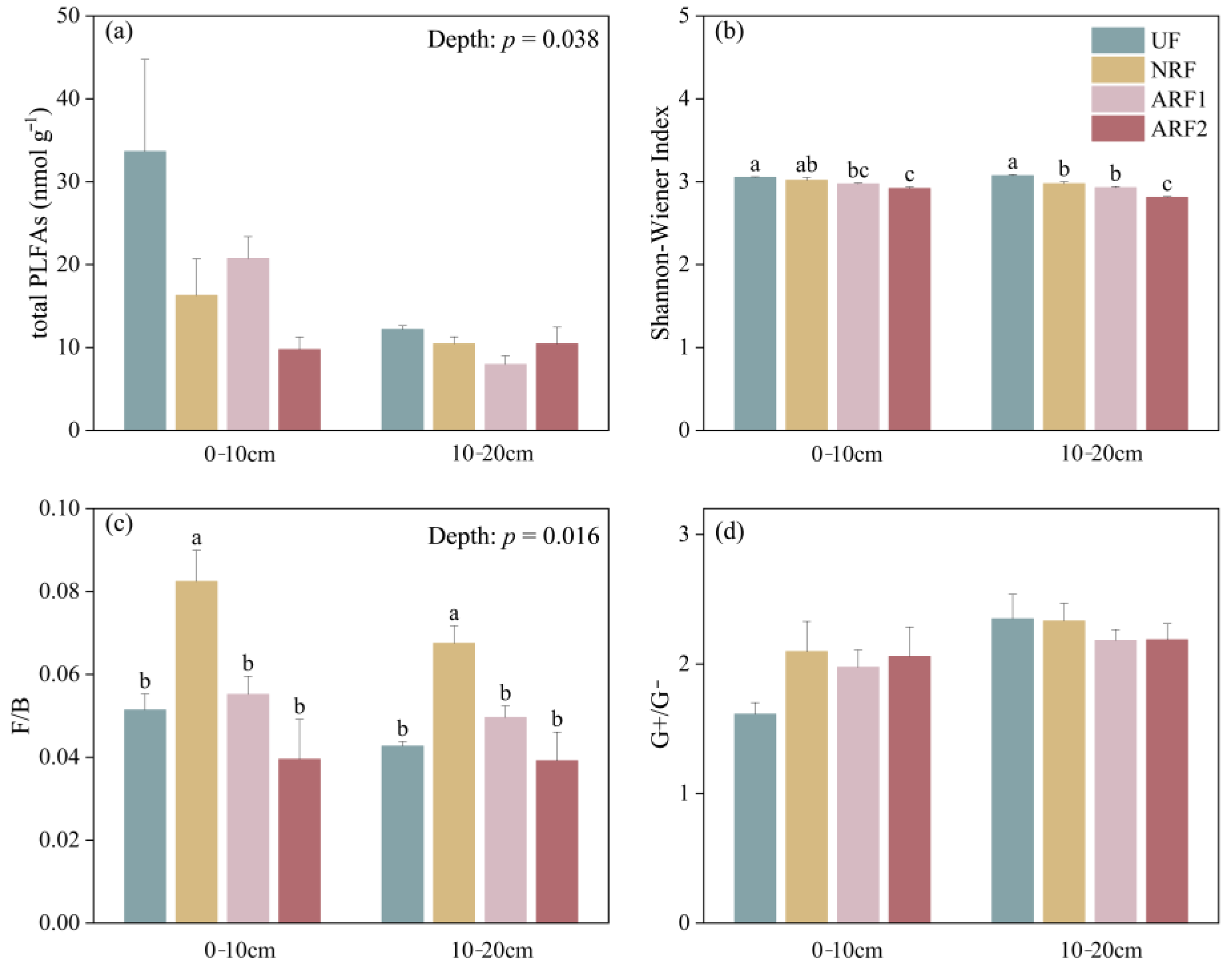
3.2. Impacts of Fire, Soil Depth, and Post-Fire Restoration Methods on Soil Microbial Characteristics
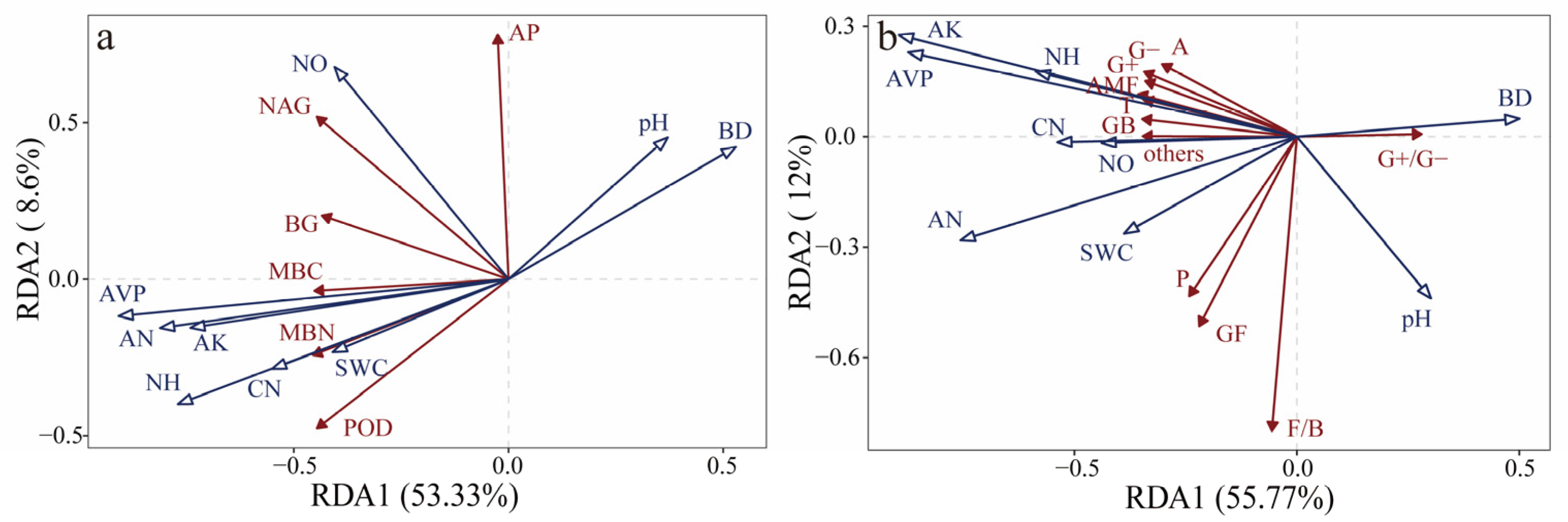
3.3. Correlations of Soil Enzyme Activity and Microbial Community Composition with Soil Physicochemical Properties
4. Discussion
4.1. Effects of Fire on Soil Physical and Chemical Properties
4.2. Effects of Fire on Soil Enzymatic Activities and the Structural of Microbial Communities
4.3. Implications for Depth
4.4. Implications for Post-Fire Management Strategies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taufik, M.; Torfs, P.J.J.F.; Uijlenhoet, R.; Jones, P.D.; Murdiyarso, D.; Van Lanen, H.A.J. Amplification of wildfire area burnt by hydrological drought in the humid tropics. Nat. Clim. Chang. 2017, 7, 428–431. [Google Scholar] [CrossRef]
- Gajendiran, K.; Kandasamy, S.; Narayanan, M. Influences of wildfire on the forest ecosystem and climate change: A comprehensive study. Environ. Res. 2024, 240, 117537. [Google Scholar] [CrossRef]
- Vieira, D.C.S.; Borrelli, P.; Jahanianfard, D.; Benali, A.; Scarpa, S.; Panagos, P. Wildfires in Europe: Burned soils require attention. Environ. Res. 2023, 217, 114936. [Google Scholar] [CrossRef]
- Santín, C.; Doerr, S.H. Fire effects on soils: The human dimension. Philos. Trans. R. Soc. B 2016, 371, 20150171. [Google Scholar] [CrossRef] [PubMed]
- Agbeshie, A.A.; Abugre, S.; Atta-Darkwa, T.; Awuah, R. A review of the effects of forest fire on soil properties. J. For. Res. 2022, 33, 1419–1441. [Google Scholar] [CrossRef]
- Roshan, A.; Biswas, A. Fire-induced geochemical changes in soil: Implication for the element cycling. Sci. Total Environ. 2023, 868, 161714. [Google Scholar] [CrossRef] [PubMed]
- Thomaz, E.L. Effects of fire on the aggregate stability of clayey soils: A meta-analysis. Earth-Sci. Rev. 2021, 221, 7. [Google Scholar] [CrossRef]
- Ibitoye, R.G.; Tijani, F.O.; Adeboye, O.B.; Akinde, B.P.; Oyedele, D.J. Prescribed fire and grass mulch impact on selected soil properties and amelioration potentials of amendments under an agricultural field in Ile-Ife, Nigeria. Soil Tillage Res. 2024, 244, 106249. [Google Scholar] [CrossRef]
- Arunrat, N.; Sereenonchai, S.; Hatano, R. Effects of fire on soil organic carbon, soil total nitrogen, and soil properties under rotational shifting cultivation in northern Thailand. J. Environ. Manag. 2022, 302, 113978. [Google Scholar] [CrossRef]
- Qin, Q.Q.; Liu, Y.H. Changes in microbial communities at different soil depths through the first rainy season following severe wildfire in North China artificial forest. J. Environ. Manag. 2021, 280, 111865. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Eisenhauer, N.; Pellegrini, A.F.A.; Wang, J.; Certini, G.; Guerra, C.A.; Lai, D.Y.F. Fire frequency and type regulate the response of soil carbon cycling and storage to fire across soil depths and ecosystems: A meta-analysis. Sci. Total Environ. 2022, 825, 153921. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.L.; Chen, H.; Marod, D.; Wu, J.P. Effect of fire on microbial necromass carbon content is regulated by soil depth, time since fire, and plant litter input in subtropical forests. Plant Soil. 2024. [Google Scholar] [CrossRef]
- Cui, J.; Holden, N.M. The relationship between soil microbial activity and microbial biomass, soil structure and grassland management. Soil Tillage Res. 2015, 146, 32–38. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Chen, Y.R.; Li, A.G.; Wang, C.; Hu, M.J.; Liu, W.X. Taxonomic turnover dominates changes in soil microbial communities and functions in response to wildfire in subtropical forest. Appl. Soil Ecol. 2024, 202, 105572. [Google Scholar] [CrossRef]
- Hu, M.J.; Wang, J.L.; Lu, L.L.; Shao, P.S.; Zhou, Z.X.; Wang, D.; Han, S.J.; Osborne, B.; Chen, J. Post-fire soil extracellular enzyme activities in subtropical-warm temperate climate transitional forests. Land Degrad. Dev. 2023, 34, 1973–1983. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Fu, S.L. Biological Indices for Soil Quality Evaluation: Perspectives and Limitations. Land Degrad. Dev. 2016, 27, 14–25. [Google Scholar] [CrossRef]
- Certini, G.; Moya, D.; Lucas-Borja, M.E.; Mastrolonardo, G. The impact of fire on soil-dwelling biota: A review. For. Ecol. Manag. 2021, 488, 118989. [Google Scholar] [CrossRef]
- Huerta, S.; Marcos, E.; Fernandez-Garcia, V.; Calvo, L. Short-term effects of burn severity on ecosystem multifunctionality in the northwest Iberian Peninsula. Sci. Total Environ. 2022, 844, 157193. [Google Scholar] [CrossRef]
- Metz, M.R.; Frangioso, K.M.; Meentemeyer, R.K.; Rizzo, D.M. Interacting disturbances: Wildfire severity affected by stage of forest disease invasion. Ecol. Appl. 2011, 21, 313–320. [Google Scholar] [CrossRef]
- Ammitzboll, H.; Jordan, G.J.; Baker, S.C.; Freeman, J.; Bissett, A. Contrasting successional responses of soil bacteria and fungi to post-logging burn severity. For. Ecol. Manag. 2022, 508, 120059. [Google Scholar] [CrossRef]
- Weber, C.F.; Lockhart, J.S.; Charaska, E.; Aho, K.; Lohse, K.A. Bacterial composition of soils in ponderosa pine and mixed conifer forests exposed to different wildfire burn severity. Soil Biol. Biochem. 2014, 69, 242–250. [Google Scholar] [CrossRef]
- Pei, J.; Wan, J.; Wang, H.; Fang, C.; Nie, M.; Li, J. Changes in the activity of soil enzymes after fire. Geoderma 2023, 437, 116599. [Google Scholar] [CrossRef]
- Juan-Ovejero, R.; Molinas-González, C.R.; Leverkus, A.B.; Martín Peinado, F.J.; Castro, J. Decadal effect of post-fire management treatments on soil carbon and nutrient concentrations in a burnt Mediterranean forest. For. Ecol. Manag. 2021, 498, 119570. [Google Scholar] [CrossRef]
- Lewis, S.A.; Robichaud, P.R.; Archer, V.A.; Hudak, A.T.; Eitel, J.U.H.; Strand, E.K. Informing Sustainable Forest Management: Remote Sensing Strategies for Assessing Soil Disturbance after Wildfire and Salvage Logging. Forests 2023, 14, 2218. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, K.; Yao, Z.; Liu, X.; Zhao, D.; Wang, Y. Naturally Deposited Charcoal Enhances Water Retention Capacity of Subtropical Forest Soils. Forests 2024, 15, 1939. [Google Scholar] [CrossRef]
- Li, M.P.; Miao, N.; Liu, S.R. Effects of nitrogen-fixing tree species Alnus nepalensis on the degraded soils and understory restoration in the upper reaches of the Jinsha River, China. Acta Ecol. Sin. 2022, 42, 2321–2330. [Google Scholar] [CrossRef]
- Kelly, J.; Ibanez, T.S.; Santin, C.; Doerr, S.H.; Nilsson, M.C.; Holst, T.; Lindroth, A.; Kljun, N. Boreal forest soil carbon fluxes one year after a wildfire: Effects of burn severity and management. Glob. Chang. Biol. 2021, 27, 4181–4195. [Google Scholar] [CrossRef]
- Zhang, H.L.; Deng, Q.; Hui, D.F.; Wu, J.P.; Xiong, X.; Zhao, J.Q.; Zhao, M.D.; Chu, G.W.; Zhou, G.Y.; Zhang, D.Q. Recovery in soil carbon stock but reduction in carbon stabilization after 56-year forest restoration in degraded tropical lands. For. Ecol. Manag. 2019, 441, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.C.; Heathman, G.C.; Wang, Y.Q.; Huang, C.H. Fractal features of soil particle-size distribution as affected by plant communities in the forested region of Mountain Yimeng, China. Geoderma 2009, 154, 123–130. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-Total. In Methods of Soil Analysis; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1996; pp. 1085–1121. [Google Scholar]
- Pierzynski, G.M. Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters; North Carolina State University: Raleigh, NC, USA, 2000; pp. 45–49. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Lu, R.K. (Ed.) Methods for Agrochemical Analysis of Soil; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Bao, S. Soil Agrochemical Analysis. China Agricultural Press; Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Joergensen, R.G. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEC value. Soil Biol. Biochem. 1996, 28, 25–31. [Google Scholar] [CrossRef]
- Deng, S.; Popova, I. Carbohydrate Hydrolases. In Methods of Soil. Enzymology; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 185–209. [Google Scholar]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and Galactosidases in Soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Vestal, J.R.; White, D.C. Lipid analysis in microbial ecology: Quantitative approaches to the study of microbial communities. Bioscience 1989, 39, 535–541. [Google Scholar] [CrossRef]
- Bossio, D.A.; Fleck, J.A.; Scow, K.M.; Fujii, R. Alteration of soil microbial communities and water quality in restored wetlands. Soil Biol. Biochem. 2006, 38, 1223–1233. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.J.; Ren, T.S.; Tian, Z.C.; Wang, G.M.; He, X.Y.; Tian, C.J. Short-term effect of tillage and crop rotation on microbial community structure and enzyme activities of a clay loam soil. Biol. Fert. Soils 2014, 50, 1077–1085. [Google Scholar] [CrossRef]
- Cai, K.; Zhao, Y.; Kang, Z.; Ma, R.; Wright, A.L.; Jiang, X. Pyrolysis-assisted transesterification for accurate quantification of phospholipid fatty acids: Application to microbial community analysis in 1000-years paddy soil chronosequence. Geoderma 2022, 406, 115504. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W.; Wiener, N. The mathematical theory of communication. Phys. Today 1950, 3, 31–32. [Google Scholar] [CrossRef]
- Murphy, M. semEff: Automatic Calculation of Effects for Piecewise Structural Equation Models (Version R Package Version 0.6.0). Available online: https://murphymv.github.io/semEff/ (accessed on 6 June 2022).
- Heydari, M.; Rostamy, A.; Najafi, F.; Dey, D.C. Effect of fire severity on physical and biochemical soil properties in Zagros oak (Quercus brantii Lindl.) forests in Iran. J. For. Res. 2017, 28, 95–104. [Google Scholar] [CrossRef]
- Thomaz, E.L. Fire changes the larger aggregate size classes in slash-and-burn agricultural systems. Soil Tillage Res. 2017, 165, 210–217. [Google Scholar] [CrossRef]
- Rodríguez-Cardona, B.M.; Coble, A.A.; Wymore, A.S.; Kolosov, R.; Podgorski, D.C.; Zito, P.; Spencer, R.G.M.; Prokushkin, A.S.; McDowell, W.H. Wildfires lead to decreased carbon and increased nitrogen concentrations in upland arctic streams. Sci. Rep. 2020, 10, 8722. [Google Scholar] [CrossRef]
- Caon, L.; Vallejo, V.R.; Ritsema, C.J.; Geissen, V. Effects of wildfire on soil nutrients in Mediterranean ecosystems. Earth Sci. Rev. 2014, 139, 47–58. [Google Scholar] [CrossRef]
- Francos, M.; Vieira, A.; Bento-Gonçalves, A.; Úbeda, X.; Zema, D.A.; Lucas-Borja, M.E. Effects of wildfire, torrential rainfall and straw mulching on the physicochemical soil properties in a Mediterranean forest. Ecol. Eng. 2023, 192, 106987. [Google Scholar] [CrossRef]
- Pellegrini, A.F.A.; Harden, J.; Georgiou, K.; Hemes, K.S.; Malhotra, A.; Nolan, C.J.; Jackson, R.B. Fire effects on the persistence of soil organic matter and long-term carbon storage. Nat. Geosci. 2022, 15, 5–13. [Google Scholar] [CrossRef]
- Ji, S.Z.; Zhao, H.M.; Wang, G.P.; Cong, J.X.; Li, G.X.; Han, D.X.; Gao, C.Y. Effects of Fire Regime on Nitrogen Distribution in Marshlands of the Sanjiang Plain (NE China). Fire 2024, 7, 16. [Google Scholar] [CrossRef]
- Hoogmoed, M.; Cunningham, S.C.; Baker, P.; Beringer, J.; Cavagnaro, T.R. N-fixing trees in restoration plantings: Effects on nitrogen supply and soil microbial communities. Soil Biol. Biochem. 2014, 77, 203–212. [Google Scholar] [CrossRef]
- Ponder, F.; Tadros, M.; Loewenstein, E.F. Microbial properties and litter and soil nutrients after two prescribed fires in developing savannas in an upland Missouri Ozark Forest. For. Ecol. Manag. 2009, 257, 755–763. [Google Scholar] [CrossRef]
- Rong, S.; Jing-Chao, L.; Xin, Z.; Yao-Quan, Y.; Fa-Ping, Z.; Davide, F.; Xiao-Yan, Y.; Wen, X. Heat input determines the response and rapid recovery of post-fire soil microbial biomass. Int. J. Wildl. Fire 2024, 33, WF23095. [Google Scholar] [CrossRef]
- Arunrat, N.; Sansupa, C.; Sereenonchai, S.; Hatano, R.; Lal, R. Fire-Induced Changes in Soil Properties and Bacterial Communities in Rotational Shifting Cultivation Fields in Northern Thailand. Biology 2024, 13, 383. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Estiarte, M. Changes in soil enzymes related to C and N cycle and in soil C and N content under prolonged warming and drought in a Mediterranean shrubland. Appl. Soil Ecol. 2008, 39, 223–235. [Google Scholar] [CrossRef]
- Rodríguez, J.; González-Pérez, J.A.; Turmero, A.; Hernández, M.; Ball, A.S.; González-Vila, F.J.; Arias, M.E. Physico-chemical and microbial perturbations of Andalusian pine forest soils following a wildfire. Sci. Total Environ. 2018, 634, 650–660. [Google Scholar] [CrossRef]
- Luo, M.; Moorhead, D.L.; Ochoa-Hueso, R.; Mueller, C.W.; Ying, S.C.; Chen, J. Nitrogen loading enhances phosphorus limitation in terrestrial ecosystems with implications for soil carbon cycling. Funct. Ecol. 2022, 36, 2845–2858. [Google Scholar] [CrossRef]
- Wu, Q.Q. Season-dependent effect of snow depth on soil microbial biomass and enzyme activity in a temperate forest in Northeast China. Catena 2020, 195, 104760. [Google Scholar] [CrossRef]
- Moya, D.; Gonzalez-De Vega, S.; Lozano, E.; Garcia-Orenes, F.; Mataix-Solera, J.; Lucas-Borja, M.E.; de Las Heras, J. The burn severity and plant recovery relationship affect the biological and chemical soil properties of Pinus halepensis Mill. stands in the short and mid-terms after wildfire. J. Environ. Manag. 2019, 235, 250–256. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Treseder, K.K. A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol. 2004, 164, 347–355. [Google Scholar] [CrossRef]
- Fox, S.; Sikes, B.A.; Brown, S.P.; Cripps, C.L.; Glassman, S.I.; Hughes, K.W.; Semenova-Nelsen, T.A.; Jumpponen, A.J.M. Fire as a driver of fungal diversity—A synthesis of current knowledge. Mycologia 2022, 114, 215–241. [Google Scholar] [CrossRef]
- Wang, C.; Lu, X.K.; Mori, T.; Mao, Q.G.; Zhou, K.J.; Zhou, G.Y.; Nie, Y.X.; Mo, J.M. Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol. Biochem. 2018, 121, 103–112. [Google Scholar] [CrossRef]
- Bárcenas-Moreno, G.; Rousk, J.; Bååth, E. Fungal and bacterial recolonisation of acid and alkaline forest soils following artificial heat treatments. Soil Biol. Biochem. 2011, 43, 1023–1033. [Google Scholar] [CrossRef]
- MacKenzie, M.D.; Quideau, S.A. Microbial community structure and nutrient availability in oil sands reclaimed boreal soils. Appl. Soil Ecol. 2010, 44, 32–41. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; de Menezes, A.B.; Macdonald, L.M.; Toscas, P.; Bissett, A.; Baker, G.; Farrell, M.; Richardson, A.E.; Wark, T.; Thrall, P.H. Wildfire impact: Natural experiment reveals differential short-term changes in soil microbial communities. Soil Biol. Biochem. 2017, 109, 1–13. [Google Scholar] [CrossRef]
- Zhang, A.; Chen, S.; Chen, J.; Cui, H.; Jiang, X.; Xiao, S.; Wang, J.; Gao, H.; An, L.; Cardoso, P. Shrub and precipitation interactions shape functional diversity of nematode communities on the Qinghai–Tibet Plateau. Glob. Change Biol. 2023, 29, 2746–2758. [Google Scholar] [CrossRef]
- Li, S.; Tang, S.; Chen, H.; Jin, K. Soil nitrogen availability drives the response of soil microbial biomass to warming. Sci. Total Environ. 2024, 917, 170505. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Li, C.; Liu, Y. The Negative Effects of Tourist Trampling on the Soil Physical Properties and Microbial Community Composition in a Natural Oak Forest. Forests 2024, 15, 1419. [Google Scholar] [CrossRef]
- Bruns, T.D.; Chung, J.A.; Carver, A.A.; Glassman, S.I. A simple pyrocosm for studying soil microbial response to fire reveals a rapid, massive response by Pyronema species. PLoS ONE 2020, 15, e0222691. [Google Scholar] [CrossRef]
- Zhang, W.; You, Y.; Su, X.; Yan, J.; Gao, G.; Ming, A.; Shen, W.; Huang, X. Introducing N2-fixing tree species into Eucalyptus plantations promotes soil organic carbon sequestration in aggregates by increasing microbial carbon use efficiency. Catena 2023, 231, 107321. [Google Scholar] [CrossRef]
- García-Carmona, M.; García-Orenes, F.; Mataix-Solera, J.; Roldán, A.; Pereg, L.; Caravaca, F. Salvage logging alters microbial community structure and functioning after a wildfire in a Mediterranean forest. Appl. Soil Ecol. 2021, 168, 104130. [Google Scholar] [CrossRef]
- Pereira, P.; Francos, M.; Brevik, E.C.; Ubeda, X.; Bogunovic, I. Post-fire soil management. Curr. Opin. Environ. Sci. Health 2018, 5, 26–32. [Google Scholar] [CrossRef]
- Lingua, E.; Marques, G.; Marchi, N.; Garbarino, M.; Marangon, D.; Taccaliti, F.; Marzano, R. Post-Fire Restoration and Deadwood Management: Microsite Dynamics and Their Impact on Natural Regeneration. Forests 2023, 14, 1820. [Google Scholar] [CrossRef]



| Depth (cm) | Sites | p | ||||
|---|---|---|---|---|---|---|
| UF | NRF | ARF1 | ARF2 | Depth | ||
| SWC (%) | 0–10 | 16.85 ± 0.32 a | 15.67 ± 0.21 a | 10.87 ± 1.17 b | 12.01 ± 1.26 b | |
| 10–20 | 15.91 ± 0.56 a | 15.98 ± 0.13 a | 11.41 ± 0.82 b | 13.8 ± 0.7 ab | 0.700 | |
| FWC (%) | 0–10 | 20.04 ± 1.35 a | 17.53 ± 0.72 a | 11.42 ± 1.04 b | 12.24 ± 0.67 b | |
| 10–20 | 17.74 ± 0.75 a | 16.15 ± 0.35 a | 12.34 ± 0.09 b | 11.94 ± 1.55 b | 0.611 | |
| CP (%) | 0–10 | 39.95 ± 0.53 a | 39.38 ± 0.73 ab | 31.34 ± 1.15 c | 35.17 ± 1.51 bc | |
| 10–20 | 37.47 ± 1.42 a | 37.43 ± 1.01 a | 31.99 ± 1.29 ab | 30.88 ± 1.55 b | 0.234 | |
| BD (g cm−3) | 0–10 | 1.19 ± 0.01 | 1.31 ± 0.07 | 1.41 ± 0.05 | 1.48 ± 0.03 | |
| 10–20 | 1.18 ± 0.01 | 1.47 ± 0.04 | 1.37 ± 0.07 | 1.44 ± 0.01 | 0.787 |
| Depth (cm) | Sites | p | ||||
|---|---|---|---|---|---|---|
| UF | NRF | ARF1 | ARF2 | Depth | ||
| pH | 0–10 | 4.26 ± 0.03 | 4.37 ± 0.05 | 4.4 ± 0.01 | 4.36 ± 0.04 | |
| 10–20 | 4.26 ± 0.03 | 4.34 ± 0.05 | 4.41 ± 0.01 | 4.35 ± 0.04 | 0.947 | |
| SOC | 0–10 | 25.96 ± 3.44 a | 19.1 ± 0.77 ab | 11.18 ± 3.31 b | 9.61 ± 0.9 b | |
| (g kg−1) | 10–20 | 15.16 ± 2.82 a | 11.83 ± 0.9 a | 7.05 ± 0.37 a | 7.06 ± 0.44 a | 0.031 |
| TN | 0–10 | 2.51 ± 0.27 ab | 2.24 ± 0.08 a | 1.8 ± 0.15 ab | 1.47 ± 0.06 b | |
| (g kg−1) | 10–20 | 1.55 ± 0.28 a | 1.66 ± 0.03 a | 1.2 ± 0.07 a | 1.38 ± 0.08 a | 0.004 |
| TP | 0–10 | 0.6 ± 0.02 a | 0.28 ± 0.02 b | 0.18 ± 0.01 c | 0.15 ± 0.01 c | |
| (g kg−1) | 10–20 | 0.53 ± 0.02 a | 0.25 ± 0.02 b | 0.16 ± 0.01 c | 0.15 ± 0.01 c | 0.694 |
| C/N | 0–10 | 10.25 ± 0.31 | 8.54 ± 0.28 | 6.05 ± 1.51 | 6.52 ± 0.5 | |
| 10–20 | 9.79 ± 0.67 | 7.16 ± 0.65 | 5.89 ± 0.10 | 5.13 ± 0.18 | 0.349 | |
| N/P | 0–10 | 4.22 ± 0.53 b | 8.13 ± 0.74 a | 9.98 ± 0.37 a | 10.11 ± 0.83 a | |
| 10–20 | 3 ± 0.64 c | 6.71 ± 0.53 b | 7.36 ± 0.38 b | 9.42 ± 0.21 a | 0.185 | |
| NH4+-N | 0–10 | 5.11 ± 0.59 | 2.52 ± 0.22 | 2.74 ± 0.25 | 2.39 ± 0.08 | |
| (mg kg−1) | 10–20 | 3.06 ± 0.23 | 2.14 ± 0.07 | 2.38 ± 0.23 | 2.48 ± 0.03 | 0.117 |
| NO3−-N | 0–10 | 1 ± 0.08 a | 0.86 ± 0.07 a | 1.78 ± 0.29 a | 0.86 ± 0.04 a | |
| (mg kg−1) | 10–20 | 0.62 ± 0.04 b | 0.68 ± 0.02 b | 1.06 ± 0.13 a | 0.51 ± 0.06 b | 0.019 |
| AN | 0–10 | 155.9 ± 8.29 ab | 124.42 ± 3.06 a | 79.76 ± 9.24 b | 67.25 ± 5.6 b | |
| (mg kg−1) | 10–20 | 104.84 ± 10.35 ab | 98.99 ± 2.94 a | 50.47 ± 2.08 b | 42.85 ± 2.94 b | 0.034 |
| AVP | 0–10 | 22.11 ± 2.25 a | 9.37 ± 1.02 b | 11.9 ± 1.55 b | 8.47 ± 0.32 b | |
| (mg kg−1) | 10–20 | 12.14 ± 1.24 a | 6.57 ± 0.7 b | 8.35 ± 0.71 b | 4.49 ± 0.96 b | 0.024 |
| AK | 0–10 | 5.56 ± 0.41 a | 3.31 ± 0.32 b | 3.88 ± 0.19 b | 3.71 ± 0.34 b | |
| (mg kg−1) | 10–20 | 4.14 ± 0.44 a | 3.21 ± 0.39 a | 2.92 ± 0.11 a | 3.35 ± 0.25 a | 0.074 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Yang, M.; Luo, X.; Yang, Z.; Wang, L.; Liu, S.; Zhang, Q.; Luo, M.; Ou, J.; Xiong, S.; et al. Short-Term Impacts of Fire and Post-Fire Restoration Methods on Soil Properties and Microbial Characteristics in Southern China. Fire 2024, 7, 474. https://doi.org/10.3390/fire7120474
Zhou H, Yang M, Luo X, Yang Z, Wang L, Liu S, Zhang Q, Luo M, Ou J, Xiong S, et al. Short-Term Impacts of Fire and Post-Fire Restoration Methods on Soil Properties and Microbial Characteristics in Southern China. Fire. 2024; 7(12):474. https://doi.org/10.3390/fire7120474
Chicago/Turabian StyleZhou, Hongen, Mengmeng Yang, Xuan Luo, Zefang Yang, Lanqing Wang, Shizhong Liu, Qianmei Zhang, Mingdao Luo, Jinwei Ou, Shiyang Xiong, and et al. 2024. "Short-Term Impacts of Fire and Post-Fire Restoration Methods on Soil Properties and Microbial Characteristics in Southern China" Fire 7, no. 12: 474. https://doi.org/10.3390/fire7120474
APA StyleZhou, H., Yang, M., Luo, X., Yang, Z., Wang, L., Liu, S., Zhang, Q., Luo, M., Ou, J., Xiong, S., Qin, Y., & Li, Y. (2024). Short-Term Impacts of Fire and Post-Fire Restoration Methods on Soil Properties and Microbial Characteristics in Southern China. Fire, 7(12), 474. https://doi.org/10.3390/fire7120474






