Abstract
In coal mines, the mixture of coal dust and gas is more ignitable than gas alone, posing a high explosion risk to workers. Using the explosion tube, this study examines the explosion propagation characteristics and flame temperature of low-concentration gas and coal dust mixtures with various particle sizes. The CPD model and Chemkin-Pro 19.2 simulate the reaction kinetics of these explosions. Findings show that when the gas concentration is below its explosive limit, coal dust addition lowers the gas’s explosive threshold, potentially causing an explosion. Coal particle size significantly affects explosion propagation dynamics, with smaller particles producing faster flame velocities and higher temperatures. Due to their larger surface area, smaller particles absorb heat faster and undergo thermal decomposition, releasing combustible gases that intensify the explosion flame. The predicted yield of light gases from both coal types exceeds 40 wt% daf, raising combustible gas concentrations in the system. When accumulated reaction heat elevates the gas concentration to its explosive limit, an explosion occurs. These results are crucial for preventing gas and coal dust explosion accidents in coal mines.
1. Introduction
Gas, a flammable and explosive combustible gas, is prevalent throughout the coal mining process. In underground coal mine operations, a thorough mixture of coal dust and gas is more susceptible to ignition than gas alone [1], which can easily trigger explosion accidents and pose a serious threat to the life safety of underground workers [2,3]. Furthermore, the confined space of mine workings and the necessity for ventilation undoubtedly increase the risk of mixed gas–coal dust explosions. Consequently, investigating the explosion propagation characteristics and the mechanism of combustion flames in gas–coal dust mixtures is of major importance in reducing the occurrence of mixed gas–coal dust explosion incidents [4,5]. At this stage, researchers primarily study gas–coal dust mixed explosions through experimental approaches [6,7], numerical simulations [8,9,10], and theoretical analyses [11,12,13].
In the investigation of the characteristics and laws of gas–coal dust explosions, scholars have primarily focused on the variation patterns of mixed gas–coal dust explosions after altering the initial concentration ratio of gas to coal dust, coal dust parameters, and pipeline conditions. Studies have found that conditions such as the gas equivalent ratio [14,15], coal dust particle size and concentration [16], initial pressure [17], and the presence of obstacles [18,19] significantly affect the overpressure and flame propagation velocity of gas–coal dust explosions. Moreover, compared to single coal dust flames, the mixture of gas and coal dust substantially increases the flame propagation velocity and the flame temperature [20]. Cashdollar [21] found that the lower explosion limit of coal dust is influenced by factors such as coal dust particle size and composition, gas concentration, initial pressure, and initial temperature. In addition to experimental research, scholars have utilized numerical simulation methods to investigate the variation patterns of shock waves and maximum overpressure during gas–coal dust explosions [22,23], deeply analyzing the diffusion mechanism of coal dust and the interaction between gas explosion shock waves and coal dust layers [24,25]. They have also revealed the flame structure and the mechanism of explosion flame propagation in mixed gas–coal dust explosions [26]. Shimura and Matsuo [27] applied a CFD–DEM model to simulate the flame structure during the shock wave-induced combustion of stratified coal dust, thereby revealing the flame structure in the combustion of layered coal dust. Cloney et al. [28] classified the laminar flame structures of mixtures below the lower flammability limit of gases based on computational fluid dynamics, not only confirming that the flammability limit of gas and combustible dust mixtures is lower than that of gas alone but also exploring the flammability limits of gas–coal dust mixtures. Moreover, in theoretical research, investigators have primarily focused on the structure and thickness of combustion flames, the critical Peclet number [29], laminar burning velocities, and the Markstein length [30]. Krazinski et al. [31] analyzed the flame model of coal dust combustion and pointed out that the type of volatiles and the combustion process of coal dust depends on the acceleration effect of the flame. Based on radiative heat transfer and two-phase flow conservation equations, a laminar flame structure and flame propagation model for coal dust combustion were proposed. Cuervo et al. [32] studied the instability of flames by analyzing the Markstein length of dust mixtures, obtaining the unstretched laminar burning velocity.
Overall, current research on gas–coal dust mixed explosions primarily focuses on initial conditions to explore the fundamental laws of explosions, with particular attention to the maximum overpressure, flame propagation velocity, and explosion limits of mixed explosions. However, most researchers have deeply investigated gas–coal dust mixed explosions from a single perspective, lacking in-depth studies on the mechanism of coal dust explosions at a low concentration of gas. Therefore, this study investigates the variation patterns of flames during the deflagration of different coal samples with low-concentration gas in a Hartmann tube, thereby refining the dynamic propagation characteristics of coal dust explosions at a low concentration of gas. The focus is on the influence of coal dust particle size and type, as well as gas and coal dust concentrations, on the flame propagation velocity and temperature of mixed explosions. Reaction kinetics simulations are used to deeply analyze the dynamic mechanism of gas–coal dust mixed deflagration reactions. The research findings contribute to understanding the interaction patterns between coal dust particles and flames in mixed explosions at a low concentration of gas, providing necessary references for accident prevention and safety measures during coal mining processes.
2. Experimental
2.1. Experimental Setup and Materials
The experimental setup consists of a combustion pipe, a gas distribution and powder injection system, a high-voltage ignition system, a temperature measurement system, a data acquisition system, a high-speed photography system, and a timing control system. The combustion pipe is a semi-closed combustion pipe. The combustion pipe is 500 mm in length with a cross-sectional area of 73 mm × 73 mm. The high-voltage ignition system consists of a pair of tungsten wire electrodes with a diameter of 0.4 mm and a 15 kV transformer, which produces an arc providing an ignition energy of 737 mJ. Ignition delay time is 60 ms. Two R-type thermocouples composed of Pt-Pt/Rh 13% are used to measure the flame temperature. To ensure the accuracy and reliability of the data collected by the thermocouples, a correction process was implemented to mitigate biases resulting from thermal inertia of the sensors. Assuming that convective heat transfer played a more significant role than radiative and conductive heat transfer, a correction equation for the temperature measurements was subsequently developed:
where T is correct flame temperature, Td is measured flame temperature. The equation was used to correct the flame temperatures for all subsequent studies and analyzes.
A certain mass of dust is spread evenly over the hemispherical bottom surface. Upon opening the solenoid valve, the high-pressure gas flow, after passing through the conical cap of the mushroom-shaped launcher, carries the dust particles into the combustion pipe, forming a dust cloud. Each data set in this experiment was repeated five times under the same conditions to ensure the reproducibility of the explosion experiment results.
The coal dust utilized in the experiment originates from the Dafosi Coal Mine in Shaanxi, China, and the Dongtan Coal Mine in Shandong, China. The coal dust used has particle sizes of 50, 100, and 200 µm, with all sizes referring to the Sauter mean diameter (D[3,2]). The ultimate and proximate analysis results for the two types of coal are presented in Table 1.

Table 1.
Fuel properties.
2.2. Kinetic Model
The light gases in coal volatiles participate in the gas-phase combustion reactions. The chemical percolation devolatilization (CPD) model was applied to calculate the predicted yield of light gases in the volatiles of Dafosi coal and Dongtan coal. The CPD model is a theoretical framework used to describe the devolatilization phenomena of solid materials during heating, particularly applicable to the pyrolysis process of materials such as polymers and coal. The core concept of this model is that as solid materials are heated, the chemical bonds within them break, leading to the release of volatile components, such as gases and liquids. During the heating process, coal undergoes pyrolysis, decomposing into volatile substances, such as light gases (e.g., methane, hydrogen, carbon monoxide, etc.) and solid residues (char). The CPD model posits that the chemical constituents in coal undergo cracking reactions at high temperatures, forming light gases that escape through the coal’s pore network. The CPD model describes the interplay between temperature, chemical reactions, and gas diffusion through a set of differential equations, enabling the quantitative prediction of the release characteristics of light gases under different heating temperatures and times.
The gas-phase combustion reaction process of gas–coal dust was simulated using Chemkin-Pro 19.2. The simulator employed was the premixed laminar flame-speed calculation within the flame simulator. GRI-Mech 3.0 model is employed to simulate the combustion and explosion process of gas and coal dust [33,34]. Chemkin-Pro 19.2 is utilized to simulate the gas phase chemical reactions, in which the light gases from coal volatiles participate in the gas phase chemical reactions of gas–coal dust combustion. The light gas content of coal volatiles, as calculated by the CPD model, is employed in the Chemkin-Pro 19.2 simulations.
3. Results and Discussion
3.1. The Flame Propagation Characteristics of Gas and Coal Dust Explosion
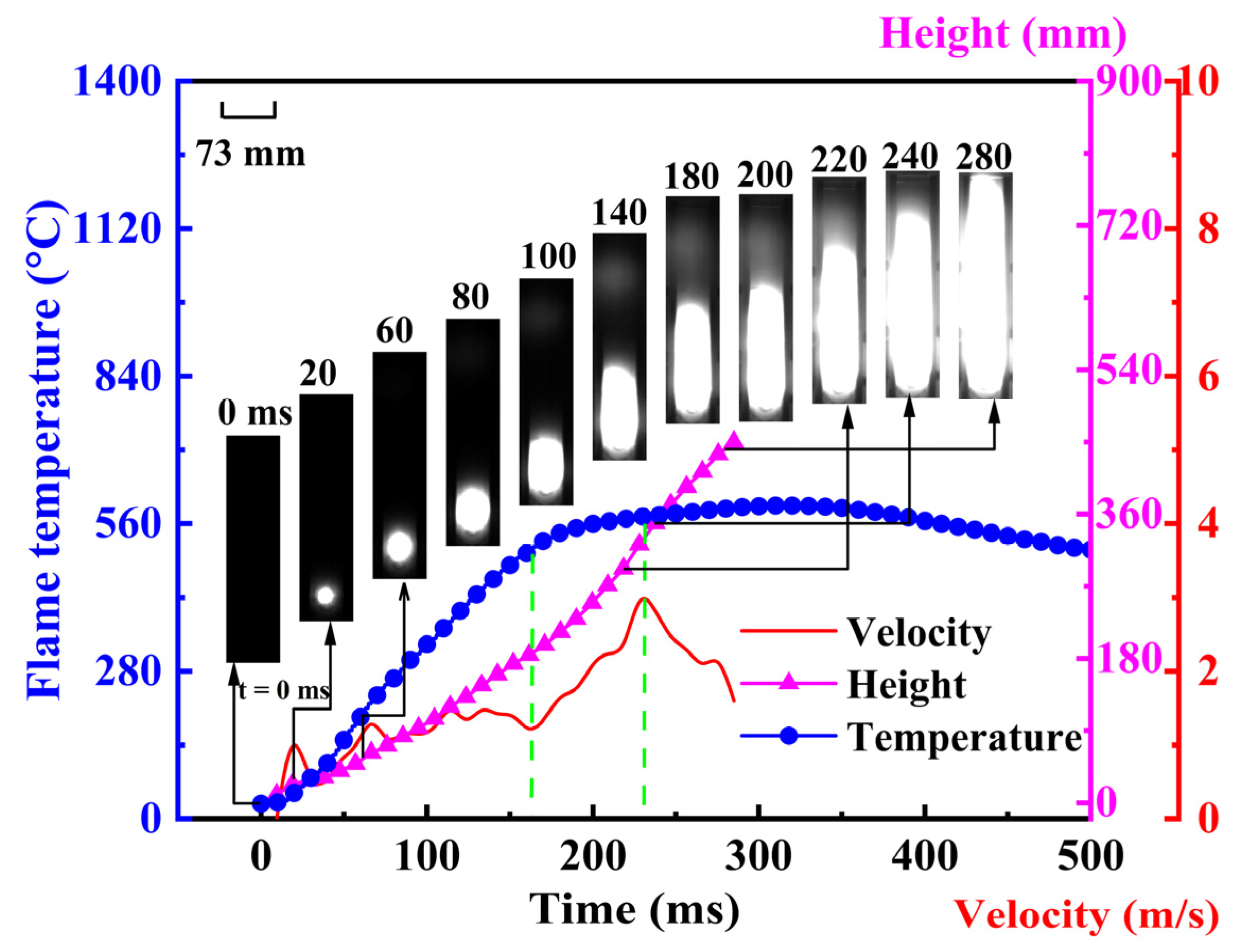
Figure 1 shows the variation of flame propagation velocity and temperature during the mixed explosion of gas and coal dust at a gas concentration of 2 vol%, with a particle size of 200 μm for Dongtan coal at a concentration of 560 g/m3. It can be observed that after ignition the mixed explosion flame transitions from a spherical shape to a tulip shape and finally develops into a planar flame, which is consistent with the pattern of flame changes in pipeline explosions. Moreover, at 280 ms, the mixed explosion flame reaches the top of the pipe, after which the flame fills the entire pipe, with the entire explosion lasting around 500 ms. From the perspective of the flame temperature’s change over time, the mixed explosion flame temperature reaches its peak at approximately 165 ms, with a peak temperature of around 512 K. At this point, the gas–coal dust mixed explosion flame has developed to the middle of the Hartmann pipe. This allows the entire explosion to be divided into two stages: the first stage with a rapid rise in flame temperature, and the second stage with a stable flame temperature. The heat exchange effect in the first stage of the mixed explosion is not strong, leading to an upward trend in flame temperature. Subsequently, in the second stage, as the flame gradually propagates towards the top, the flame temperature stabilizes at the peak temperature, indicating that after 165 ms, the heat transfer interaction between the combustion flame and the pipe intensifies, with an increasing amount of heat transferred to the external environment, preventing the flame temperature from rising further. Additionally, the slow decrease in flame temperature in the second stage may be due to the incomplete combustion of coal dust on the flame surface, with some coal dust continuing to burn behind the flame front. This is also evidenced by high-speed imaging, which shows a white luminous area behind the flame front, filling the entire pipe after 280 ms and resulting in a slow decrease in flame temperature.
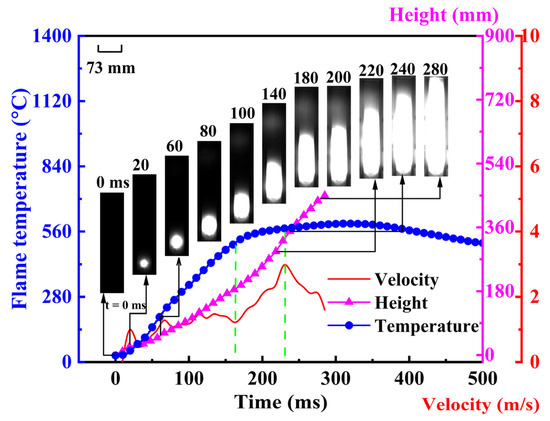
Figure 1.
Changes in flame propagation velocity and flame temperature in coal-dust mixture explosions at 2 vol% Gas (200 μm, 560 g/m3 Dongtan coal).
Furthermore, in the first stage, the flame propagation velocity of the mixed gas–coal dust explosion initially changes slightly, maintaining around 2 m/s, during which the flame transitions from a spherical shape to a tulip shape. When the explosion time reaches 220 ms, the mixed explosion flame develops to the upper region of the Hartmann tube, at which point both the flame rise height and flame propagation velocity are significant, with a peak velocity of 3.15 m/s. Thereafter, the flame transitions to a planar shape, and the increased contact area between the flame and the wall surface leads to a decrease in flame propagation velocity. Moreover, due to the low gas concentration, the unburned fuel in the flame front is primarily coal dust. In other words, the propagation of the mixed explosion flame is controlled by both gas and coal dust, but it is mainly influenced by the combustion and explosion of coal dust. Therefore, overall, the flame propagation velocity of the mixed gas–coal dust explosion remains at a relatively low level.
3.2. The Influence of Coal Particle Size on Flame Propagation Velocity
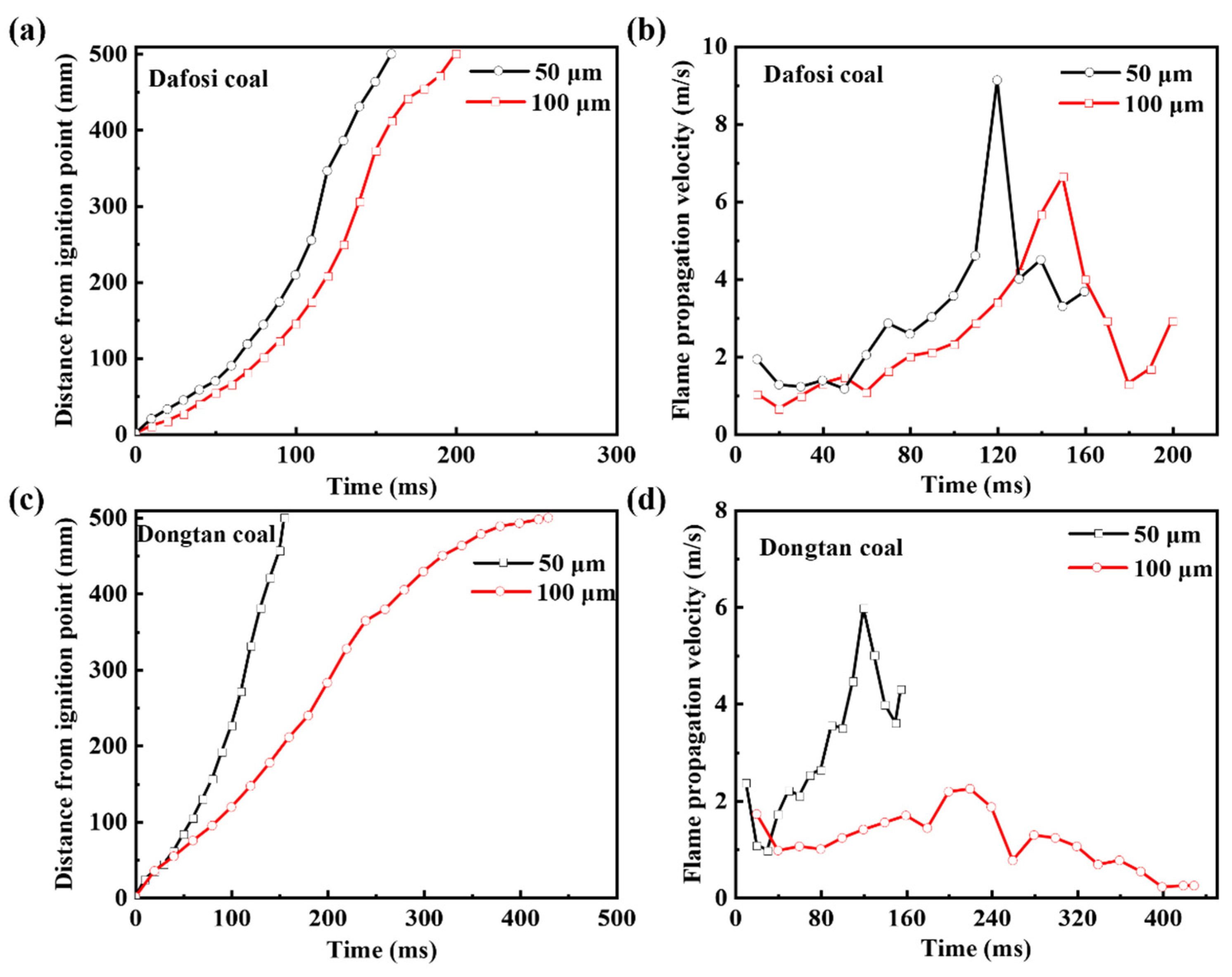
According to References [35,36,37], the calculated flame propagation velocity was statistically analyzed to obtain the mean and standard deviation to correct the flame propagation velocity. The flame propagation distance and velocity of the mixed explosion with 2 vol% gas and 560 g/m3 coal dust are shown in Figure 2. The development of the flame propagation can be divided into three stages. From 0 to 50 ms, the flame propagation distance and velocity increase relatively slowly. In this stage, the gas is ignited, releasing heat, and the coal particles absorb heat and dehydrate, resulting in slow flame development. From 50 to 150 ms, the flame propagation distance and velocity increase rapidly, reaching a peak and indicating that the combustion reaction has entered a more intense phase. The volatile matter of the coal particles decomposes to release combustible gases, leading to an increase in the concentration of combustible gases in the combustion system, promoting heat release, and increasing the combustion rate. After 150 ms, the flame propagation distance reaches a peak and the flame propagation velocity decreases. As shown in Figure 2, the flame propagation velocity of the mixed explosion with finer coal dust and gas is greater than that with coarser dust. Finer coal particles have a larger specific surface area, which allows for more sufficient contact with oxygen and combustible gases, making it easier for the coal particles to decompose upon heating, release combustible gases, and promote the combustion of gas and coal dust, thereby increasing the flame propagation velocity and steepening the slope of the flame propagation distance curve.
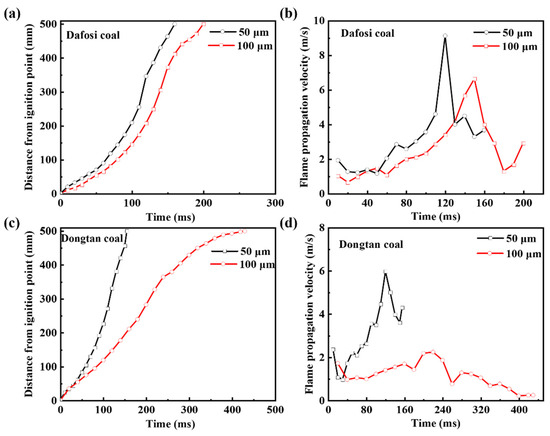
Figure 2.
(a,c) Flame propagation distance and (b,d) velocity for gas-coal dust combustion (2 vol% Gas and 560 g/m3 coal dust).
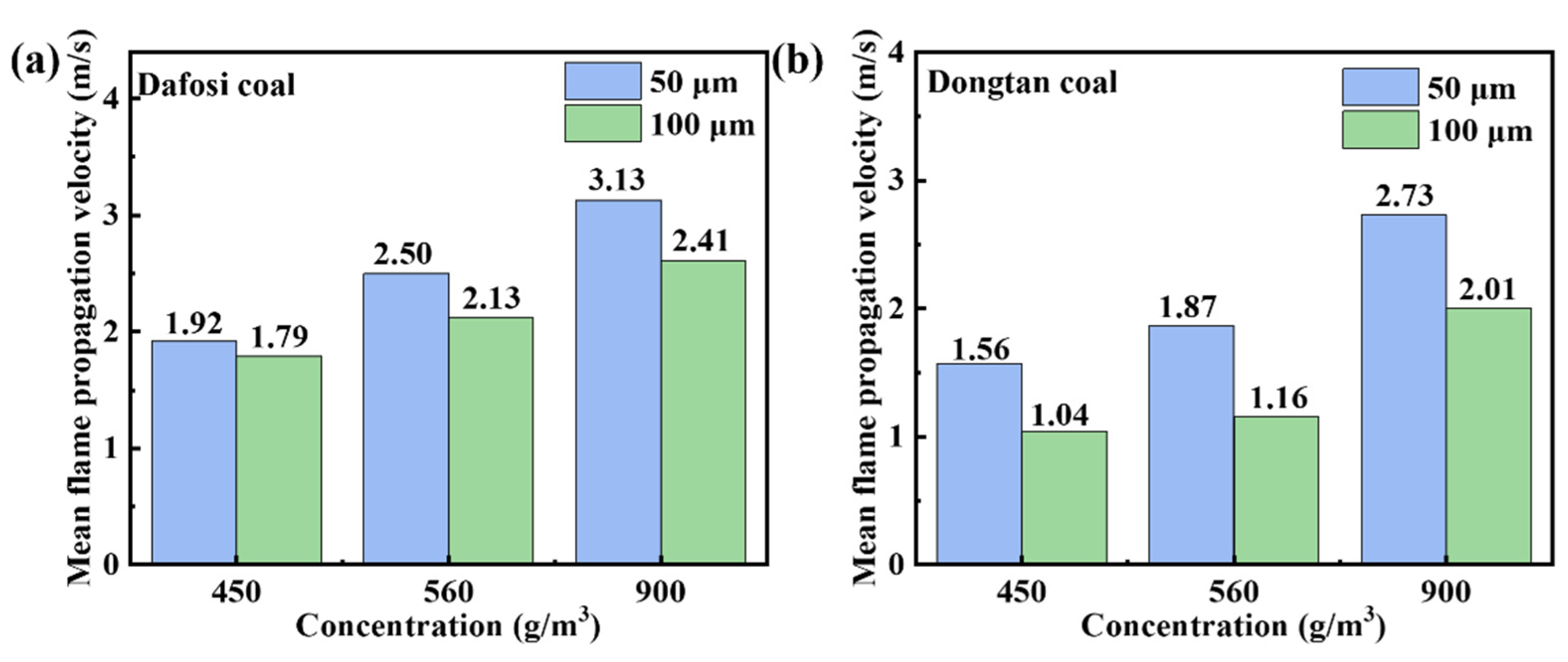
Figure 3 shows that when the gas concentration is below the lower explosion limit, the mean flame propagation velocity increases with the increase in coal dust concentration. The graph indicates that the mean flame propagation velocity is greater for particles with a diameter of 50 μm compared to those with a diameter of 100 μm. According to thermodynamic and combustion theories, the smaller the particle size of coal dust per unit mass, the larger the specific surface area and surface energy, which in turn enhances the explosiveness and increases the average flame propagation velocity, thereby affecting the dynamic characteristics of flame propagation.
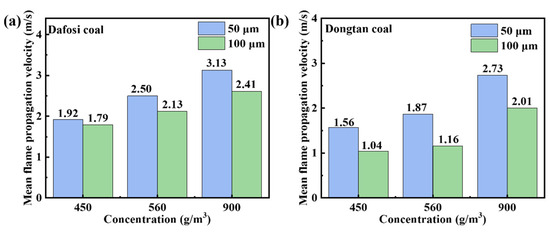
Figure 3.
Variation of mean flame propagation velocity for mixed combustion of 2 vol% (a) Dafosi coal (b) Dongtan coal.
3.3. The Effect of Coal Particle Size on Flame Temperature
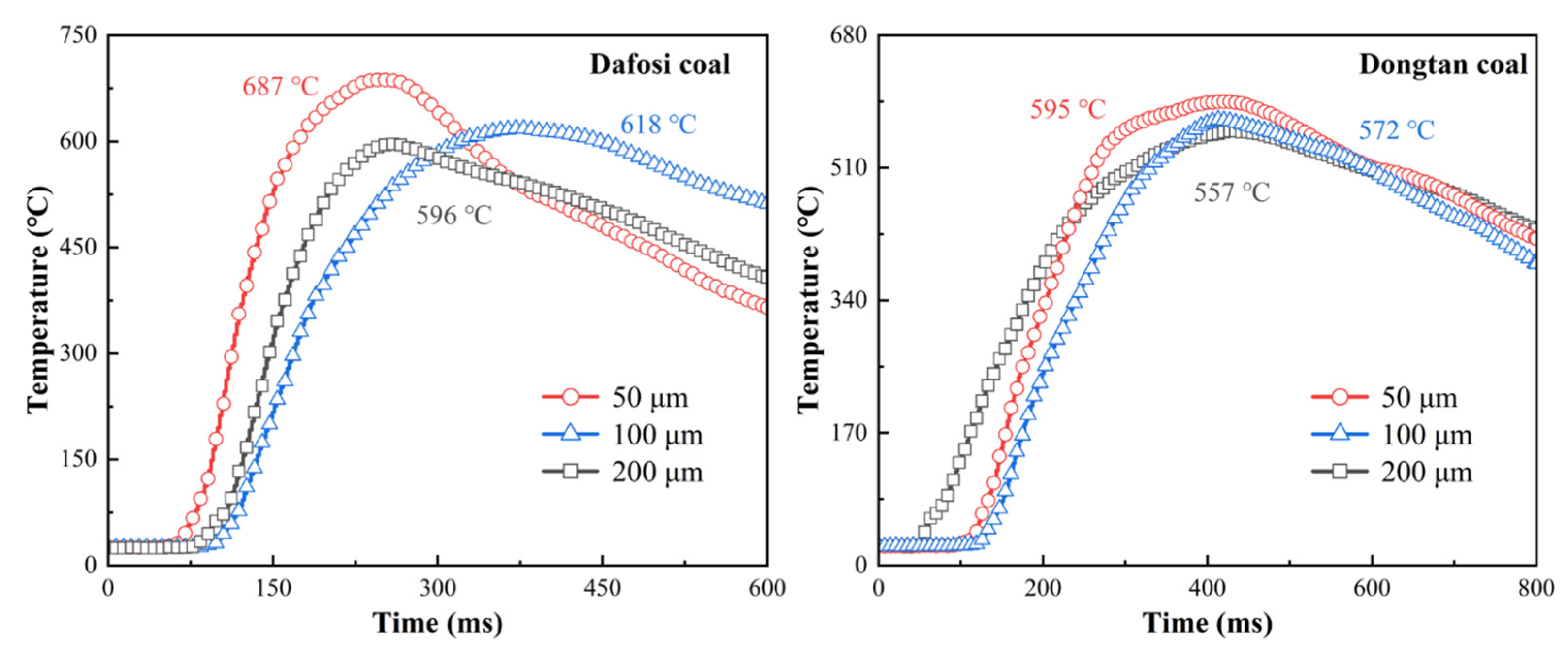
Figure 4 presents the temperature variations of the flame during the mixed explosion of coal dust at a concentration of 560 g/m3 and gas at 2 vol% with different coal particle sizes. The flame temperature curves exhibit two distinct phases: a rapid heating phase and a slow cooling phase. By comparing and analyzing the flame temperature curves of different coal samples, it can be observed that there are differences in the flame propagation velocity between the two coal samples. The flame of Dafosi coal spreads more rapidly, reaching the peak flame temperature at approximately 200 ms, whereas Dongtan coal requires 400 ms to reach the peak flame temperature. The results indicate that under the same conditions, the pyrolysis process of Dafosi coal occurs earlier than that of Dongtan coal. The combustible volatile components produced by this process burn rapidly in the abruptly heated environment, significantly promoting the combustion reaction process of the coal dust and gas, thereby reducing the time required to reach the peak temperature. The peak temperature of Dafosi coal is as high as 687 °C, which is higher than that of Dongtan coal (595 °C).
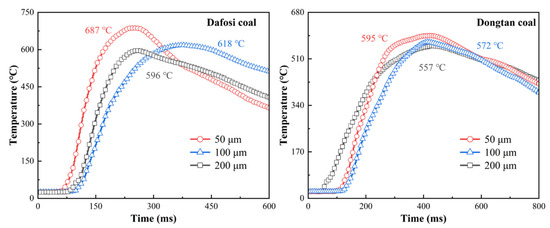
Figure 4.
Variation of flame temperature with coal particle size in gas and coal dust explosions (2 vol% gas and 560 g/m3 coal dust).
The variation in coal dust particle size has a certain impact on the flame temperature of gas–coal dust mixtures. As the particle size increases, the peak flame temperature gradually decreases, which may be attributed to differences in the specific surface area, pyrolysis rate, and volatile release characteristics of coal dust particles at different sizes. Coal dust particles with smaller sizes have a larger specific surface area, allowing them to absorb heat more rapidly and undergo pyrolysis, thereby promoting the combustion reaction. However, as the particle size increases, heat conduction within the particles is limited, the pyrolysis rate slows down, and the release of combustible volatiles decreases, thereby affecting the peak flame temperature. The results indicate that when Dafosi coal with a particle size of 50 μm is mixed with 2 vol% gas, the resulting flame has the highest temperature, indicating the most intense development of the combustion reaction and the highest flame intensity. Additionally, observations of the flame temperature characteristics of the Dongtan coal–gas mixture show that the overall trend of the flame temperature curves is relatively consistent across the three different particle sizes, suggesting that the combustion characteristics of Dongtan coal are less sensitive to changes in particle size.
3.4. Mechanism of Dust and Low-Concentration Gas Explosions
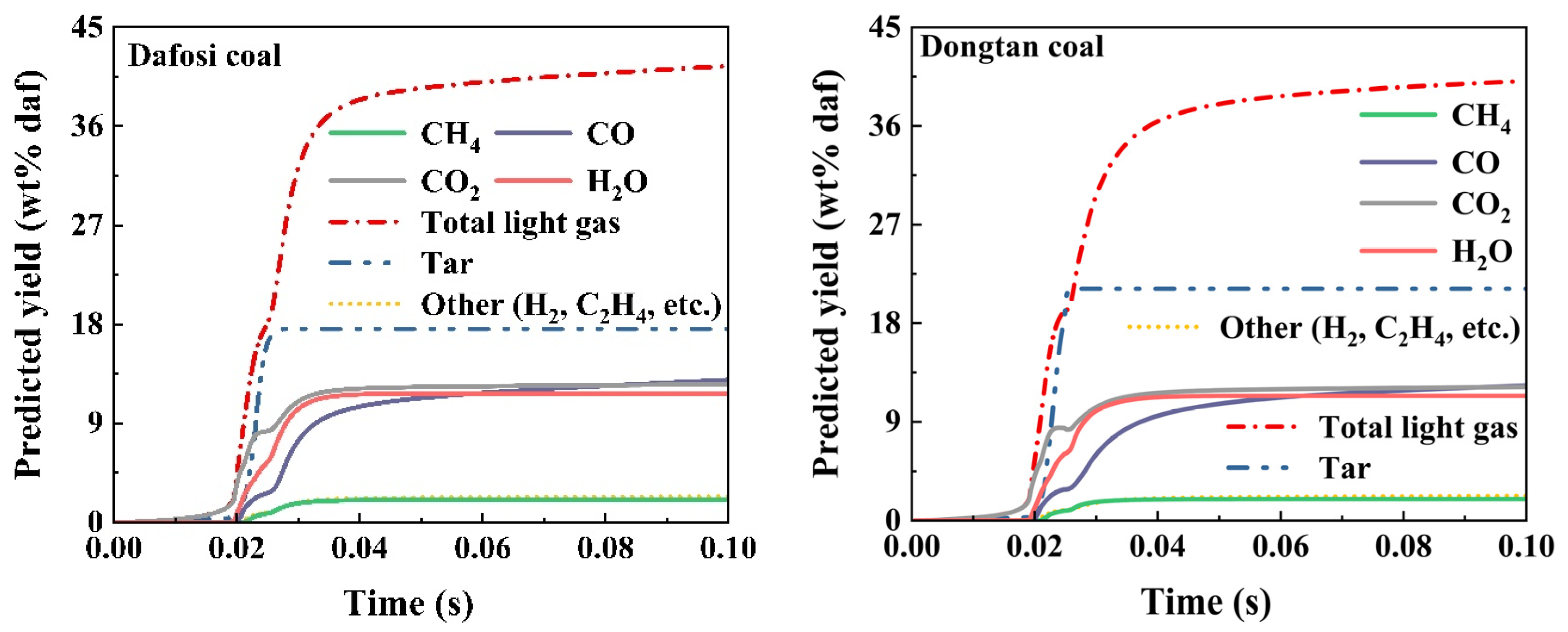
The light gases in coal volatiles participate in the gas phase combustion reactions. The chemical percolation devolatilization (CPD) model was applied to calculate the predicted yield of light gases in the volatiles of Dafosi coal and Dongtan coal. The variation trends of the predicted yield of light gases and tar for both coals are shown in Figure 5. According to the industrial analysis results, the volatile content of Dafosi coal and Dongtan coal is 28.85% and 28.63%, respectively, indicating a similar degree of coalification for both types. The total predicted yields of light gases for the two coals are 42.781 wt% daf and 41.909 wt% daf, respectively. Dafosi coal has a higher volatile content and a higher degree of coalification, resulting in a higher total predicted yield of light gases compared to Dongtan coal.
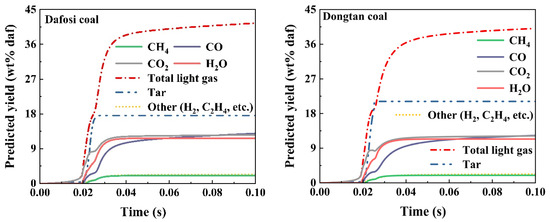
Figure 5.
Predicted yield of light gases in coal volatiles by CPD model calculated.
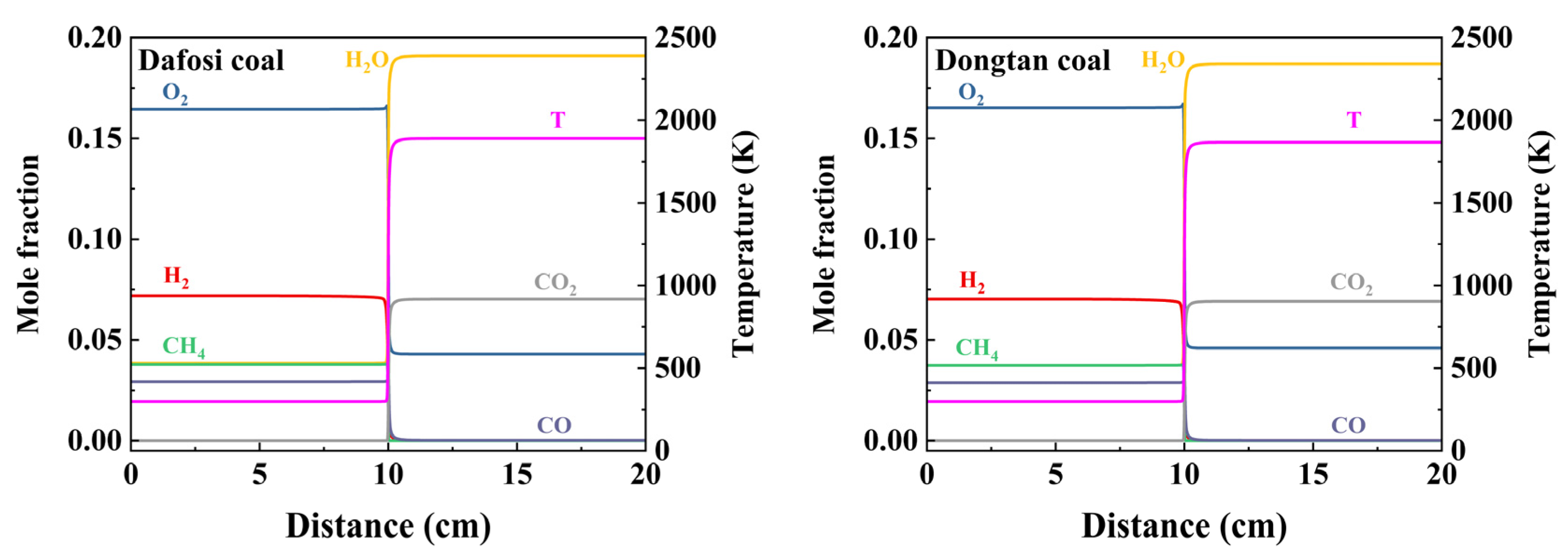
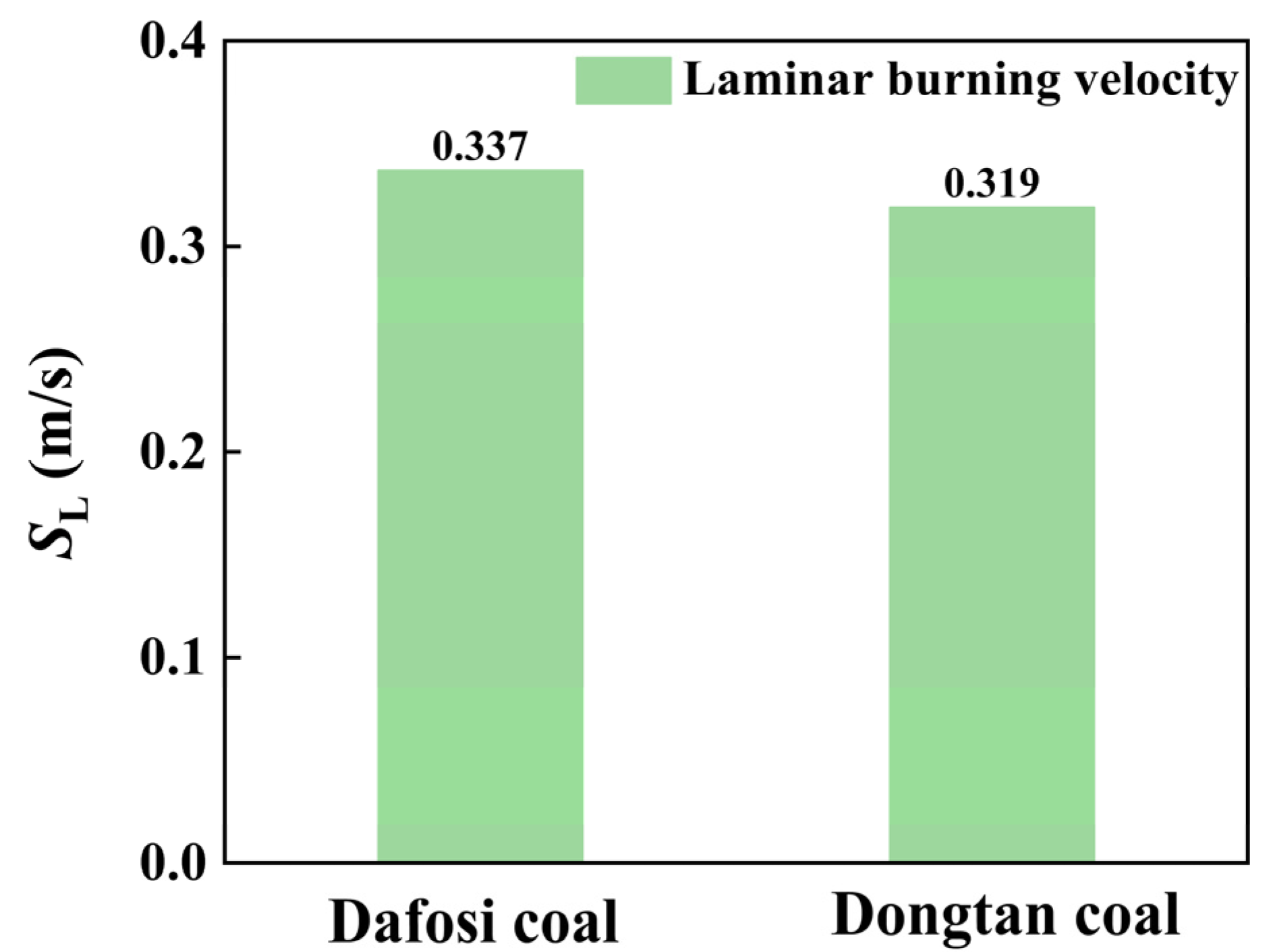
At a gas concentration of 2 vol% and a coal dust concentration of 560 g/m3, the variation trends of key species and flame temperature with flame propagation distance for the gas–coal dust combustion are shown in Figure 6, while the maximum value of the laminar burning velocity is presented in Figure 7. The adiabatic flame temperature and laminar burning velocity for the combustion of Dafosi coal with gas are 1890.186 K and 0.337 m/s, respectively. For the combustion of Dongtan coal with gas, the adiabatic flame temperature and laminar burning velocity are 1867.078 K and 0.319 m/s, respectively. Dafosi coal has a higher volatile content, which results in a greater predicted yield of light gases and more combustible materials. Under conditions of sufficient oxygen, the adiabatic flame temperature and laminar burning velocity are higher.
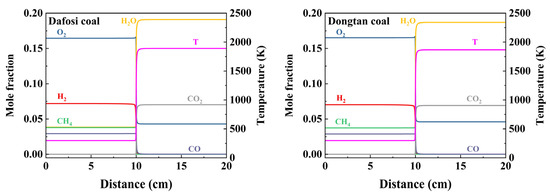
Figure 6.
Evolution of key species and flame temperature with flame propagation distance in gas and coal dust combustion.
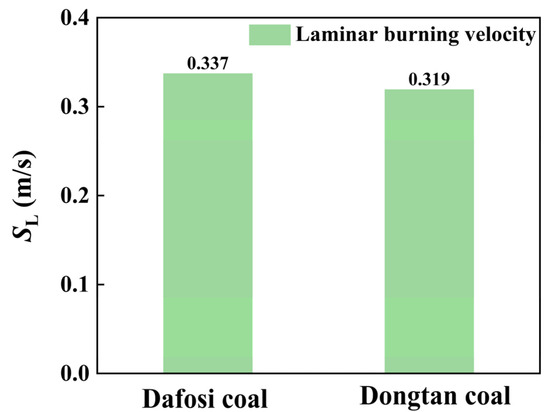
Figure 7.
Laminar combustion velocity for coal combustion at the coal dust concentration of 560 g/m3 and the gas concentration of 2 vol%.
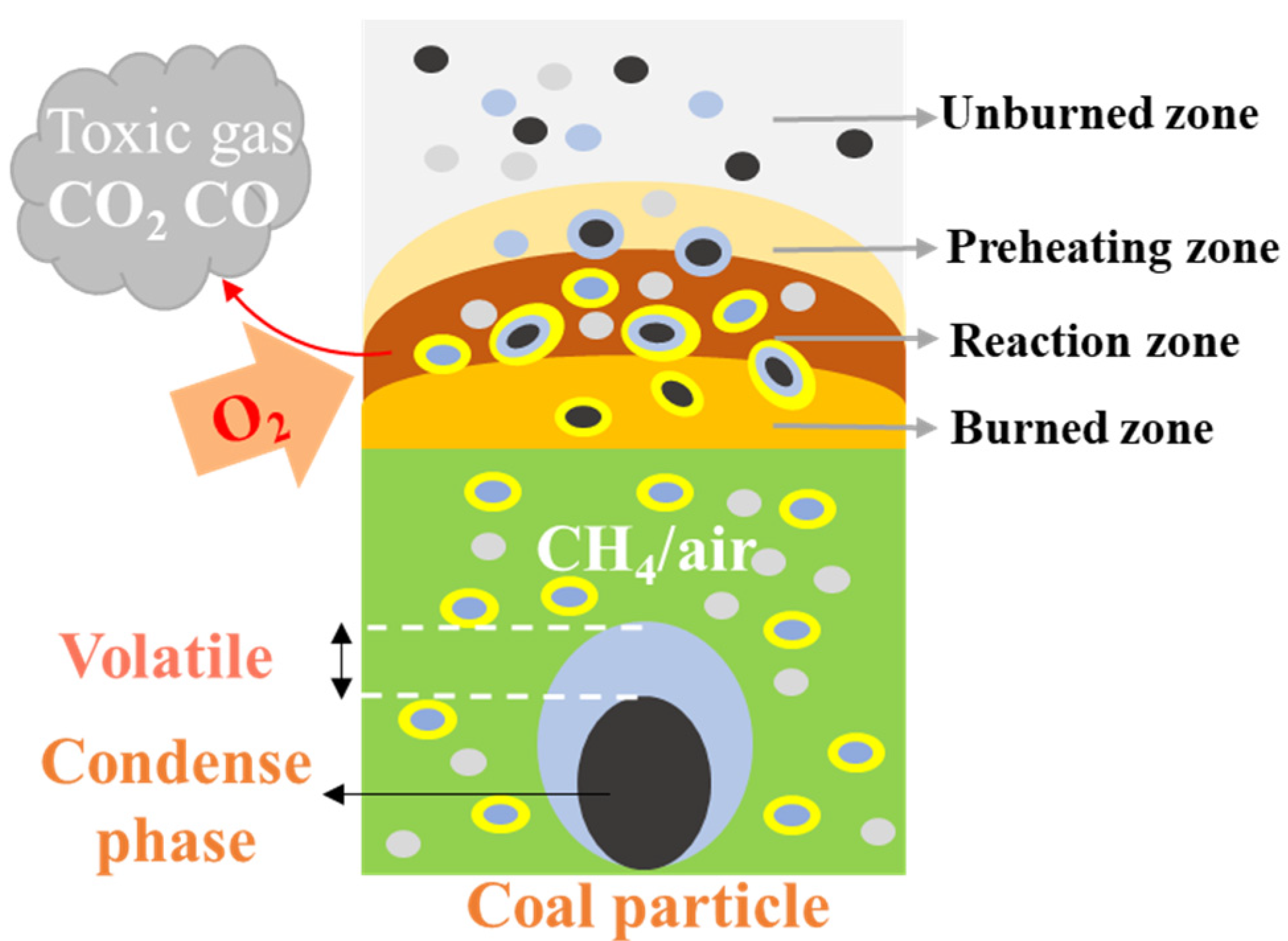
In an atmosphere of low concentration gas that has not reached the lower explosion limit, the gas is adsorbed by the pores on the surface of coal particles. The ignition energy of the gas is much lower than that of the coal dust; therefore, the gas is ignited first. The combustion of the gas produces heat, which in turn heats the coal particles and the surrounding gas. The heated coal particles undergo thermal decomposition, releasing volatiles, and generating light gases such as CH4, CO, and H2, which increase the concentration of combustible gases within the system. Once the concentration reaches the lower explosion limit, an explosion occurs. After ignition, the combustible gases produce a large number of active radicals such as H, OH, and HO2. These active radicals undergo vigorous collisions with combustible gas molecules and oxygen, causing the combustible gases to decompose and oxidize rapidly. This process generates more free radicals, releases more heat, promotes the oxidative decomposition of coal particles, and continuously releases volatiles from the coal. The fixed carbon continues to burn and the combustion process of the gas and coal dust cycles maintains flame development. The number of free radicals grows exponentially and the heat released by the reaction accumulates. Under certain critical conditions, this transformation leads to an explosion. The mechanism of coal dust explosion under low concentration gas conditions is shown in Figure 8.
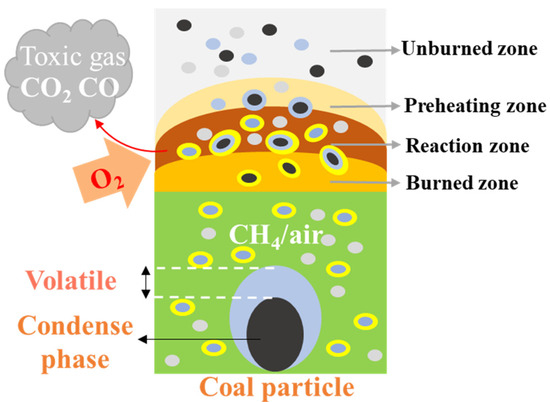
Figure 8.
Mechanism of coal dust explosion under low concentration gas conditions.
4. Conclusions
The Hartmann tube was used to study the explosion propagation characteristics of low concentration gas and coal dust mixtures with varying particle sizes. The CPD model and Chemkin-Pro 19.2 were applied to simulate the reaction kinetics of gas and coal dust explosions. The main conclusions are as follows:
- Under low concentration gas conditions, the gas–coal dust explosion flame evolves from a spherical flame to a fingertip-shaped flame, finally forming a planar flame, with the entire explosion lasting about 500 ms. The forward propagation of the flame is controlled by both gas and coal dust, with coal dust having a more significant impact.
- The flame propagation velocity during low concentration gas–coal dust explosions develop in three stages: relatively slow growth in the flame distance and velocity, followed by rapid increase, and finally a decrease in flame velocity. Smaller coal dust particles produce greater flame propagation velocity, with a steeper slope in the flame distance curve.
- At low gas concentrations, combustible gases released by coal decomposition accelerate the gas–coal dust explosion reaction. The peak temperature for Dafosi coal reaches 687 °C, higher than that of Dongtan coal (595 °C). Smaller coal dust particles with gas explosions result in higher flame temperatures.
- According to the CPD model calculations, the total predicted yields of light gases for both coals exceed 40 wt% daf. Kinetic simulations show that heat generated by gas ignition heats coal particles and surrounding gas, leading to coal decomposition and the release of light combustible gases, raising combustible gas concentrations in the system. Explosions occur when the concentration reaches the lower explosive limit.
Author Contributions
Conceptualization, L.L.; methodology, L.L. and X.M.; software, X.M. and Y.J.; validation, Y.T.; formal analysis, X.M. and L.S.; investigation, Y.T.; resources, L.S.; data curation, L.L. and X.M.; writing—original draft preparation, L.L., X.M., Y.J. and Y.T.; writing—review and editing, L.L., X.M. and Y.J.; funding acquisition, L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Project of Yulin, grant number (No. CXY-2020-030).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amyotte, P.R. Solid inertants and their use in dust explosion prevention and mitigation. J. Loss Prev. Process Ind. 2006, 19, 161–173. [Google Scholar] [CrossRef]
- Han, W.; Wang, C.; Law, C.K. Role of transversal concentration gradient in detonation propagation. J. Fluid Mech. 2019, 865, 602–649. [Google Scholar] [CrossRef]
- Liu, T.; Liu, S. The impacts of coal dust on miners’ health: A review. Environ. Res. 2020, 190, 109849. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Qin, B.; Zhang, Y.; Ma, D.; Xu, J. Experimental investigation of OH* emission spectrum characteristics and transient ignition dynamics in methane and coal dust mixtures explosions. Process Saf. Environ. Prot. 2024, 192, 669–679. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, J.; Huang, C.; Zhang, H.; Li, Y.; Chen, X.; Dai, H. Characteristics of coal dust deflagration under the atmosphere of methane and their inhibition by coal ash. Fuel 2021, 291, 120121. [Google Scholar] [CrossRef]
- Li, Q.; Lin, B.; Dai, H.; Zhao, S. Explosion characteristics of H2/CH4/air and CH4/coal dust/air mixtures. Powder Technol. 2012, 229, 222–228. [Google Scholar] [CrossRef]
- Rockwell, S.R.; Rangwala, A.S. Influence of coal dust on premixed turbulent methane–air flames. Combust. Flame 2013, 160, 635–640. [Google Scholar] [CrossRef]
- Houim, R.W.; Oran, E.S. Structure and flame speed of dilute and dense layered coal-dust explosions. J. Loss Prev. Process Ind. 2015, 36, 214–222. [Google Scholar] [CrossRef]
- Collecutt, G.; Humphreys, D.; Proud, D. CFD simulation of underground coal dust explosions and active explosion barriers. In Proceedings of the 7th International Conference on CFD in the Minerals and Process Industries, Melbourne, Australia, 9–11 December 2009; pp. 1–6. [Google Scholar]
- Li, G.; Gao, W.; Jiang, H.; Liu, J.; Zhao, F.; Jin, S.; Lu, Z. Ignition and combustion of AlH3-nanoparticles: A molecular dynamics study. Combust. Flame 2024, 269, 113667. [Google Scholar] [CrossRef]
- Kundu, S.K.; Zanganeh, J.; Eschebach, D.; Moghtaderi, B. Explosion severity of methane–coal dust hybrid mixtures in a ducted spherical vessel. Powder Technol. 2018, 323, 95–102. [Google Scholar] [CrossRef]
- Huéscar Medina, C.; Phylaktou, H.N.; Andrews, G.E.; Gibbs, B.M. Explosion characteristics of pulverised torrefied and raw Norway spruce (Picea abies) and Southern pine (Pinus palustris) in comparison to bituminous coal. Biomass Bioenergy 2015, 79, 116–127. [Google Scholar] [CrossRef]
- Jin, S.; Gao, W.; Li, G.; Geng, X.; Bi, M.; Jiang, H. Atomistic insights into p-nitrotoluene combustion via the ReaxFF molecular dynamics and density functional theory study. Process Saf. Environ. Prot. 2024, 192, 484–494. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, L.; Wang, H.; Zhang, Y.; Li, Y.; Qiu, D.; Chen, X. Effects of methane concentration on flame propagation mechanisms and dynamic characteristics of methane/coal dust explosions. Powder Technol. 2024, 439, 119744. [Google Scholar] [CrossRef]
- Collins, P.K.; Schroeder, A.R.; Buckius, R.O.; Krier, H.; Peters, J.E. Flammability Characteristics of Treated Coals. J. Energy Resour. Technol. 1992, 114, 65–69. [Google Scholar] [CrossRef]
- Yan, Z.X.; Zhang, T.; Yan, D.Z.; Yang, H.P.; Gong, A.; Liao, Q.; Chen, Q. A Comparison Study of Hybrid Flame of Methane/Coal Dust Mixture in Vented Explosions. Adv. Mater. Res. 2014, 864–867, 801–808. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Wang, X. Experimental Study on the Influence of Initial Pressure on Explosion of Methane-coal Dust Mixtures. Procedia Eng. 2013, 62, 980–984. [Google Scholar] [CrossRef]
- Dong, C.; Bi, M.; Zhou, Y. Effects of obstacles and deposited coal dust on characteristics of premixed methane–air explosions in a long closed pipe. Saf. Sci. 2012, 50, 1786–1791. [Google Scholar] [CrossRef]
- Oh, K.H.; Kim, H.; Kim, J.B.; Lee, S.E. A study on the obstacle-induced variation of the gas explosion characteristics. J. Loss Prev. Process Ind. 2001, 14, 597–602. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.; Chen, D. Flame propagation in hybrid mixture of coal dust and methane. J. Loss Prev. Process Ind. 2007, 20, 691–697. [Google Scholar] [CrossRef]
- Cashdollar, K.L. Overview of dust explosibility characteristics. J. Loss Prev. Process Ind. 2000, 13, 183–199. [Google Scholar] [CrossRef]
- Song, B.; Li, Y. Study on propagation characteristics of the secondary explosion of coal dust. Int. J. Low-Carbon Technol. 2019, 15, 89–96. [Google Scholar] [CrossRef]
- Song, B.; Li, Y. Numerical simulation on flame propagation characteristics of coal dust explosion in diagonal structure network. Chem. Eng. Trans. 2018, 66, 277–282. [Google Scholar]
- Houim, R.W.; Oran, E.S. Numerical simulation of dilute and dense layered coal-dust explosions. Proc. Combust. Inst. 2015, 35, 2083–2090. [Google Scholar] [CrossRef]
- Salamonowicz, Z.; Kotowski, M.; Półka, M.; Barnat, W. Numerical simulation of dust explosion in the spherical 20l vessel. Bull. Pol. Acad. Sci. Tech. Sci. 2015, 63, 289–293. [Google Scholar] [CrossRef]
- Semenov, I.; Utkin, P.; Markov, V. Numerical modelling of dust-layered detonation structure in a narrow tube. J. Loss Prev. Process Ind. 2013, 26, 380–386. [Google Scholar] [CrossRef]
- Shimura, K.; Matsuo, A. Using an extended CFD–DEM for the two-dimensional simulation of shock-induced layered coal-dust combustion in a narrow channel. Proc. Combust. Inst. 2019, 37, 3677–3684. [Google Scholar] [CrossRef]
- Cloney, C.T.; Ripley, R.C.; Pegg, M.J.; Amyotte, P.R. Laminar combustion regimes for hybrid mixtures of coal dust with methane gas below the gas lower flammability limit. Combust. Flame 2018, 198, 14–23. [Google Scholar] [CrossRef]
- Kim, W.; Imamura, T.; Mogi, T.; Dobashi, R. Experimental investigation on the onset of cellular instabilities and acceleration of expanding spherical flames. Int. J. Hydrogen Energy 2017, 42, 14821–14828. [Google Scholar] [CrossRef]
- Shu, T.; Xue, Y.; Liang, W.; Ren, Z. Extrapolations of laminar flame speeds from expanding spherical flames based on the finite-structure stretched flames. Combust. Flame 2021, 226, 445–454. [Google Scholar] [CrossRef]
- Krazinski, J.L.; Buckius, R.O.; Krier, H. Coal dust flames: A review and development of a model for flame propagation. Prog. Energy Combust. Sci. 1979, 5, 31–71. [Google Scholar] [CrossRef]
- Cuervo, N.; Dufaud, O.; Perrin, L. Determination of the burning velocity of gas/dust hybrid mixtures. Process Saf. Environ. Prot. 2017, 109, 704–715. [Google Scholar] [CrossRef]
- Jin, S.; Gao, W.; Huang, Z.; Bi, M.; Jiang, H.; Si, R.; Wen, G. Suppression characteristics of methane/coal dust explosions by active explosion suppression system in the large mining tunnel. Fire Saf. J. 2024, 150, 104251. [Google Scholar] [CrossRef]
- Huang, Z.; Si, R.; Wen, G.; Jin, S.; Xue, S. Experimental Study on the Isolation Effect of an Active Flame-Proof Device on a Gas Explosion in an Underground Coal Mine. Fire 2023, 6, 468. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Vanierschot, M.; Norman, F.; Verplaetsen, F.; Berghmans, J. Flame propagation and flow field measurements in a Hartmann dust explosion tube. Powder Technol. 2018, 323, 346–356. [Google Scholar] [CrossRef]
- Andrews, G.E.; Bradley, D. Determination of burning velocities: A critical review. Combust. Flame 1972, 18, 133–153. [Google Scholar] [CrossRef]
- Cuervo, N. Influences of Turbulence and Combustion Regimes on Explosions of Gas-Dust Hydrid Mixtures. Ph.D. Thesis, Universite de Lorraine, Lorraine, France, 2015. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).