Polyurethane Materials for Fire Retardancy: Synthesis, Structure, Properties, and Applications
Abstract
:1. Introduction
- Synthesis and structure: covering the synthesis of polyurethane bases and modification of additive compounds.
- Performance: studying physical properties and thermal degradation processes.
- Application: evaluating the commercial potential of polyurethane polymers.
- Flame retardancy: analyzing five established FR mechanisms.
2. Synthesis and Structure
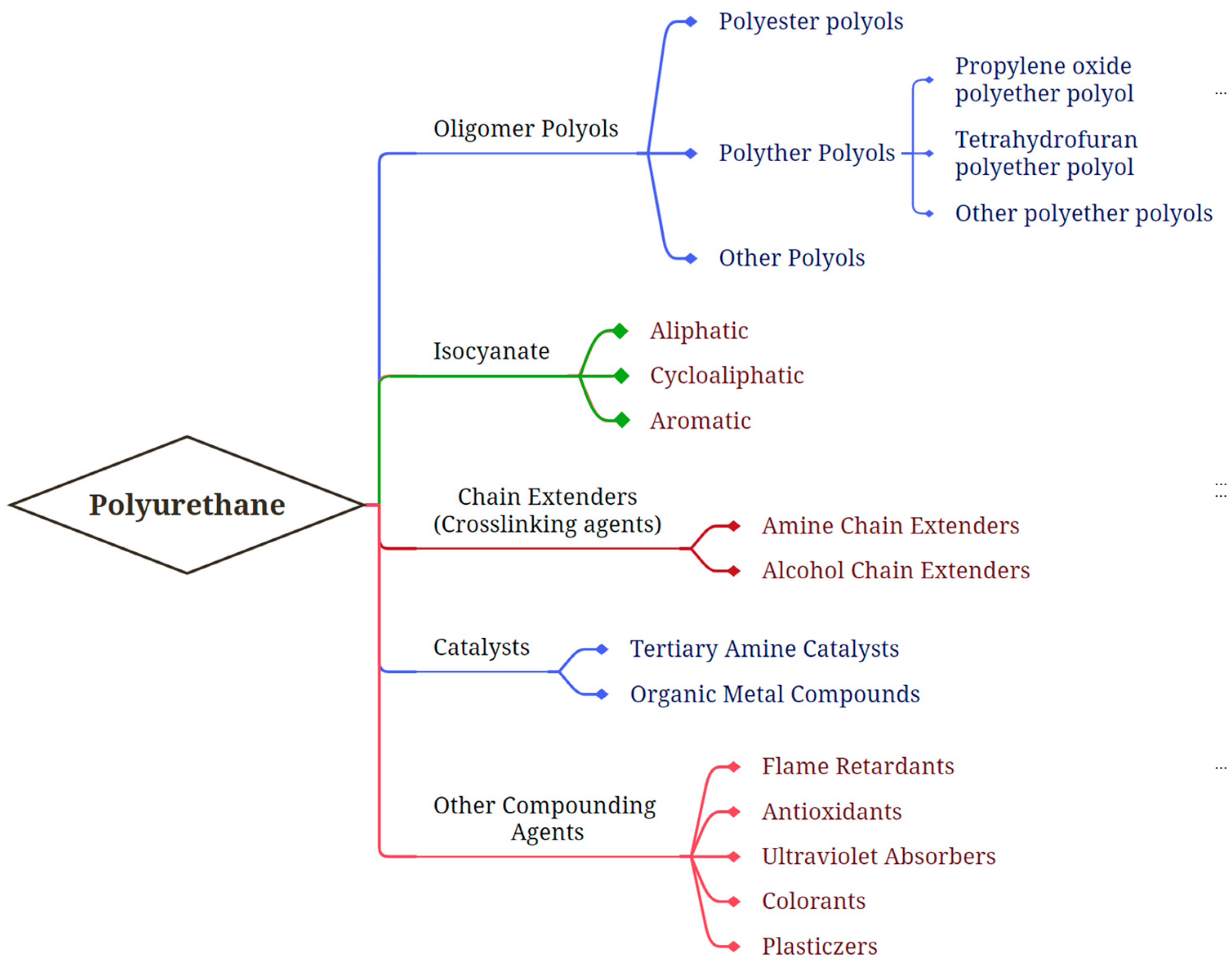
2.1. Chemical Components
2.1.1. Polyols
2.1.2. Isocyanates
2.1.3. Other Additives
2.2. Polymerization
2.2.1. Reaction with Active Hydrogen


2.2.2. Self-Polymerization


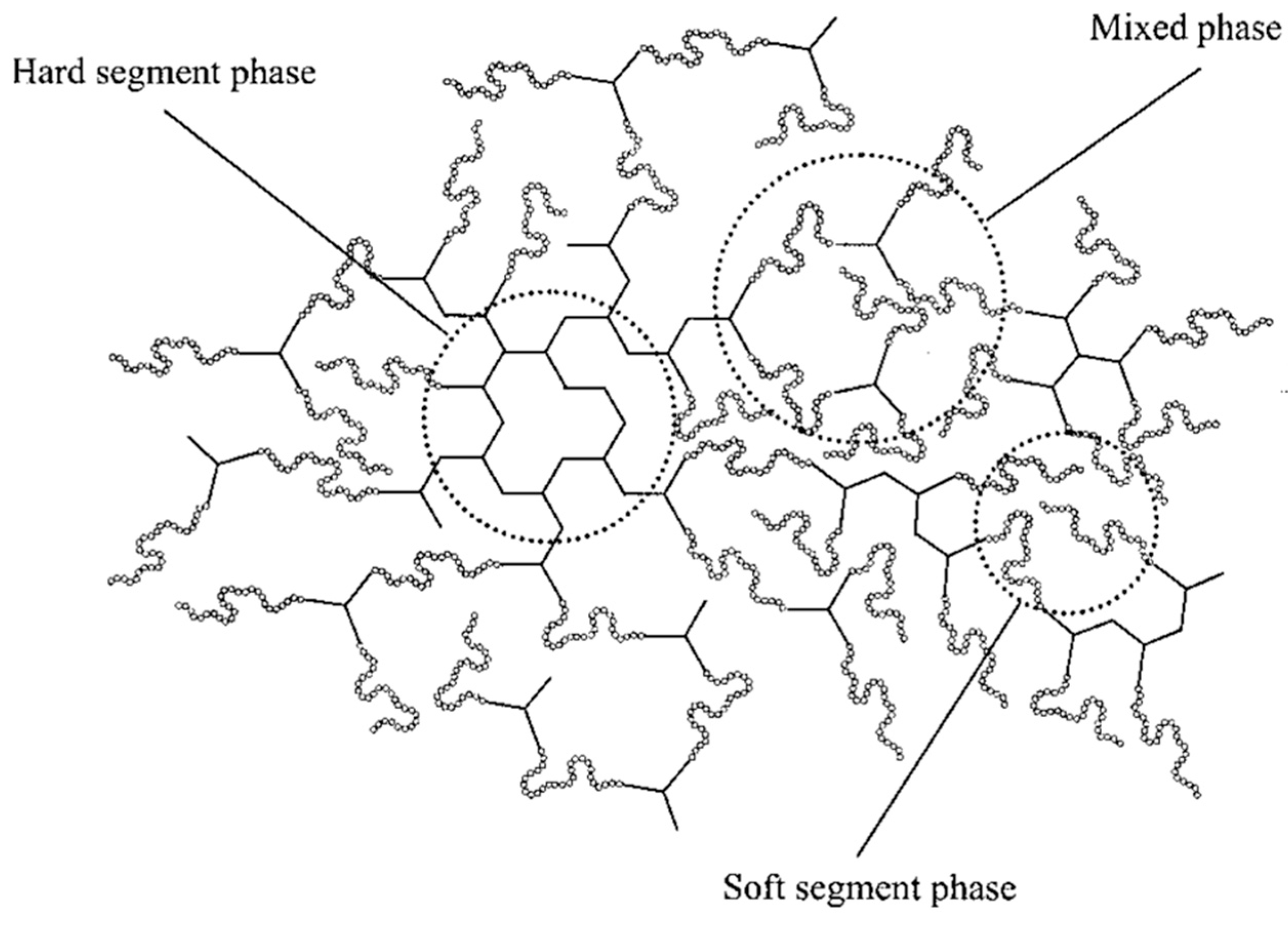
2.3. Phase Structures
2.3.1. Linear
2.3.2. Star-Shaped
2.3.3. Cross-Linked
2.4. Structural Modification
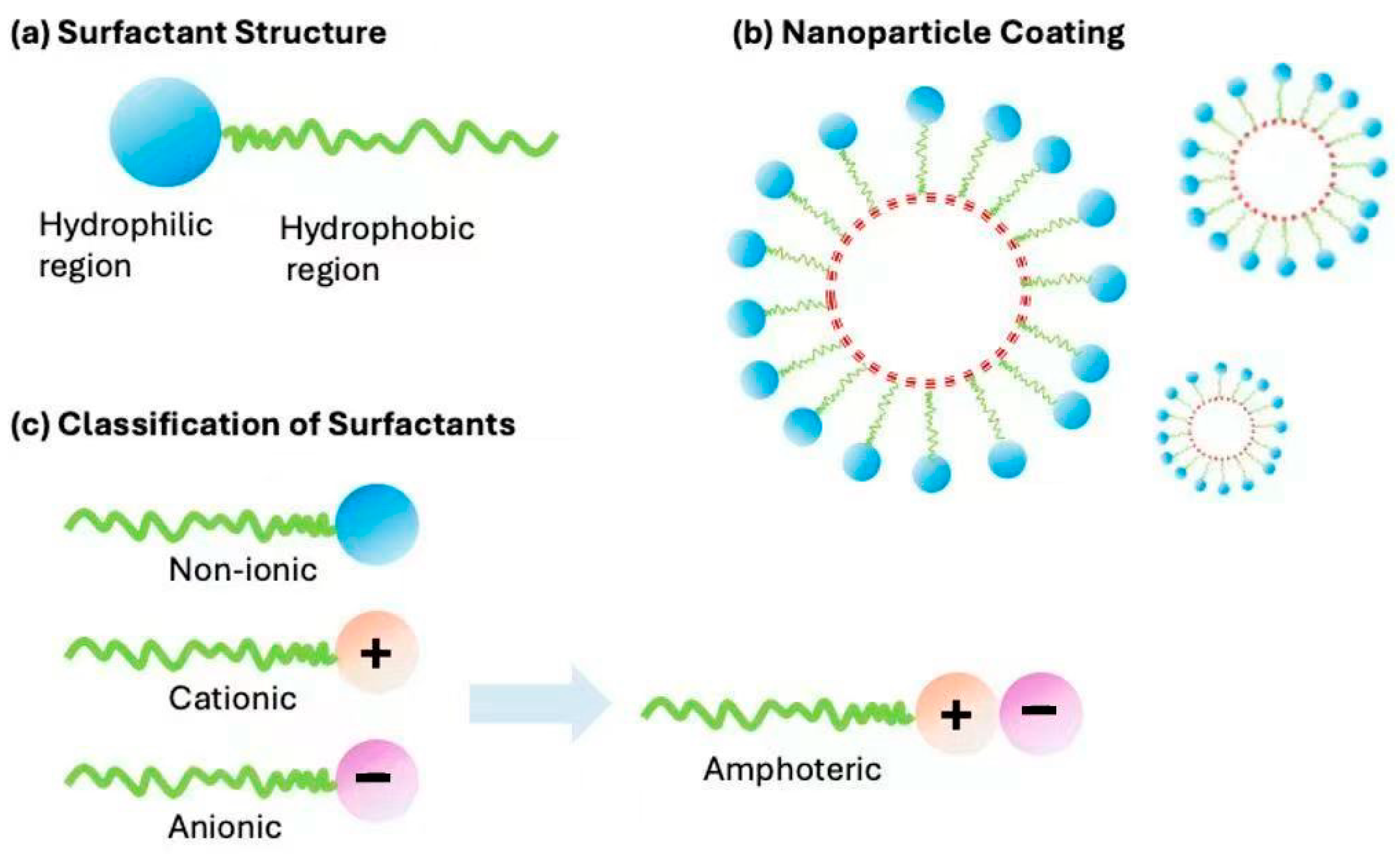
Surfactants
3. Properties
3.1. Molecular Weight in Polyols
3.2. Strength
3.3. Mechanism of Thermal Degradation
3.3.1. Initial Decomposition
3.3.2. Depolymerization and Fragmentation
3.3.3. Cross-Linking and Char Formation
3.3.4. Further Decomposition and Residue Formation
3.4. Biodegradation
3.4.1. Fungal Biodegradation
3.4.2. Bacterial Biodegradation
3.4.3. Degradation of PU by Polyurethanes Enzymes
4. Applications
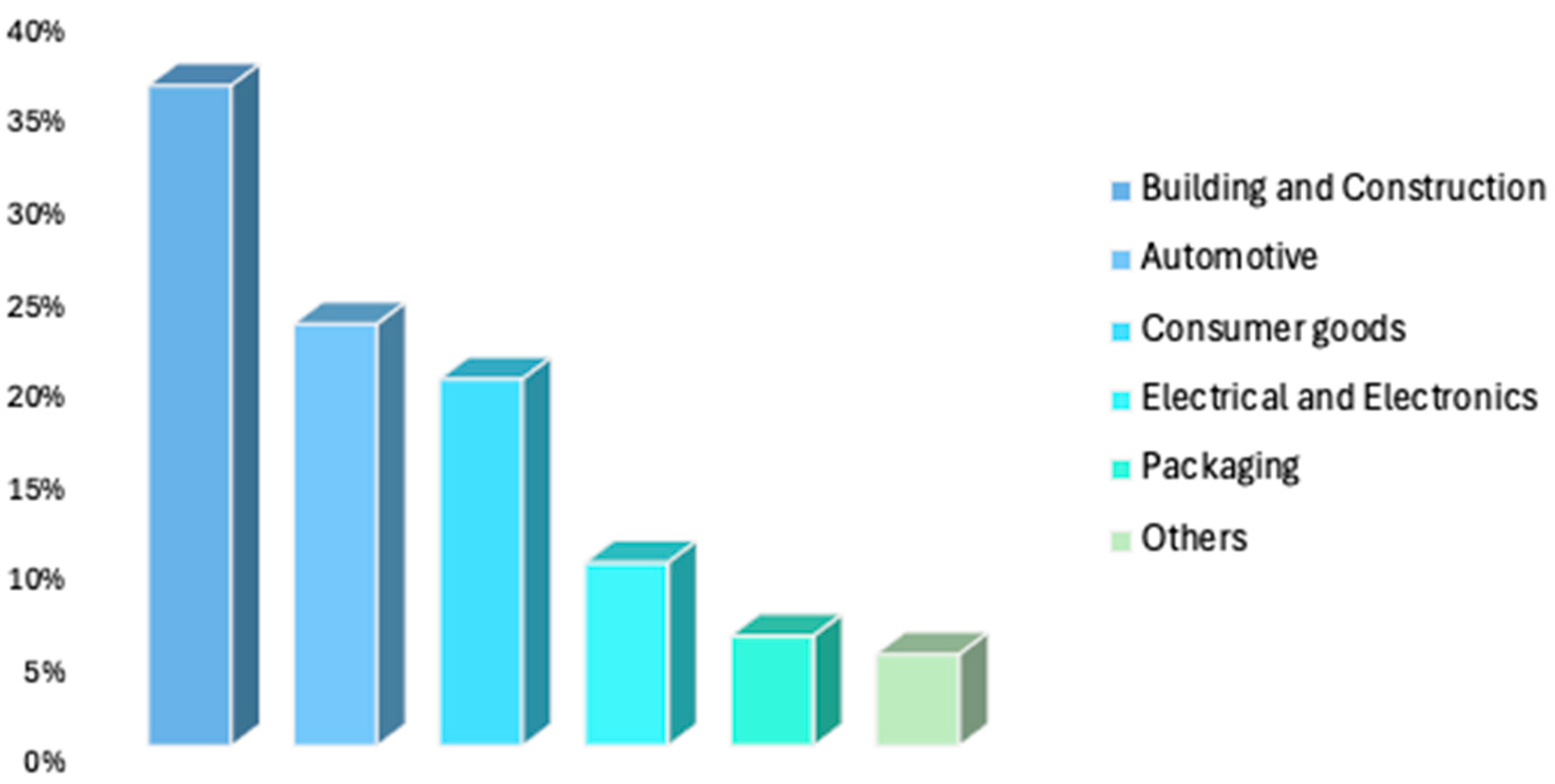
4.1. Global PU Market
4.2. Commercial Application
4.2.1. Construction Industry
4.2.2. Automotive Industry
4.2.3. Artificial Leather
4.2.4. Industrial Manufacture
4.2.5. Medical Industry
5. Fire Retardancy
5.1. Material Fabrication
5.2. Fire Retardants
5.2.1. Halogen-Free FR
5.2.2. Inorganic Metal Hydroxides
5.2.3. Nanoparticles
5.2.4. Synergistic Effect of FR
6. Green and Sustainable Development
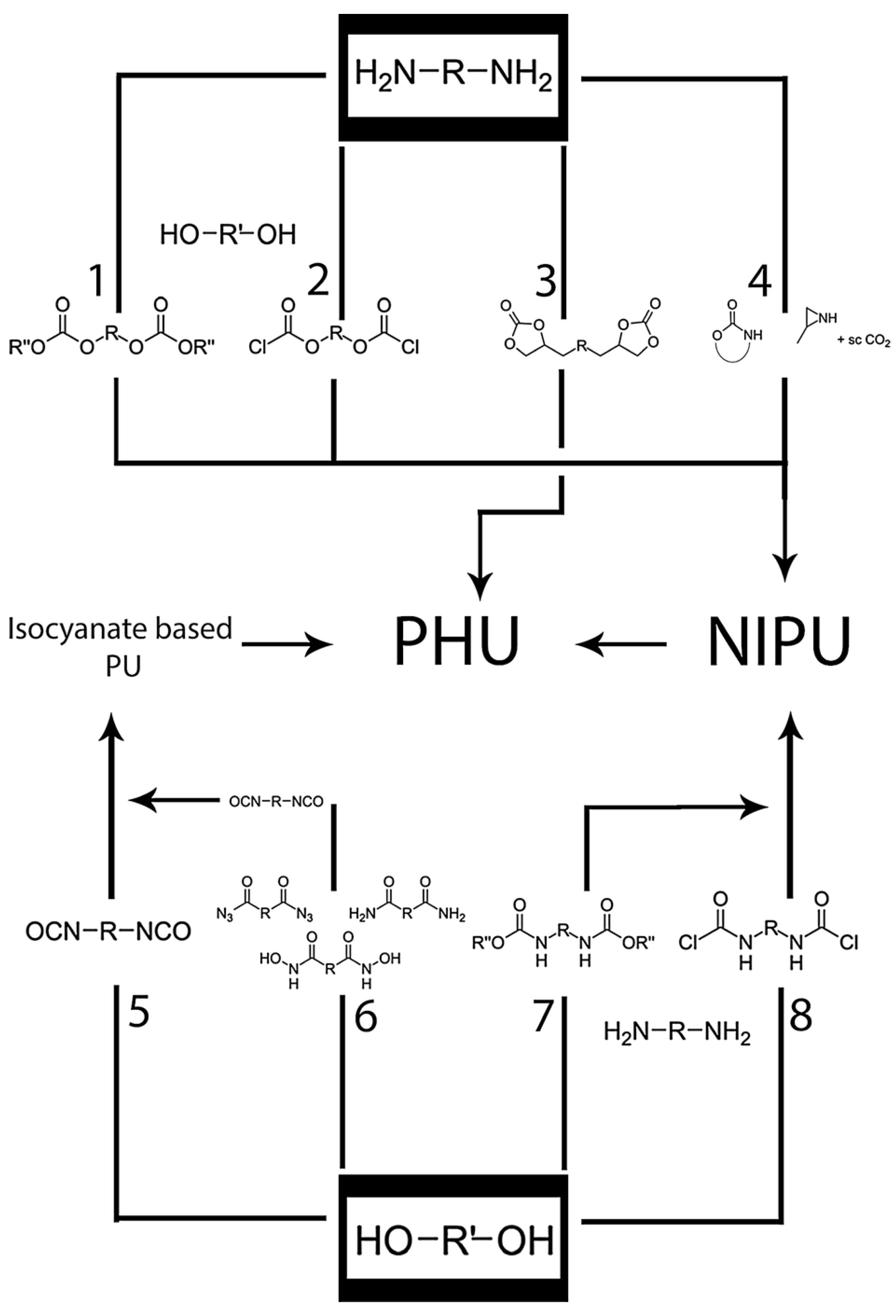
6.1. Non-Isocyanate PU
6.2. Foaming Process
7. Summary and Outlook
7.1. Summary
7.2. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| DOPO | 9,10-dihydro-9-oxa-10-phosphaphenanthrene 10-oxide |
| FR | Flame retardant |
| GWP | Global warming potential |
| HFC | Hydrofluorocarbon |
| MADP | Melamine-derived polyol |
| MDI | Diphenylmethane diisocyanate |
| MH | Magnesium hydroxide |
| MMT | Nano-montmorillonite |
| NIPU | Non-isocyanate PU |
| ODP | Ozone depletion potential |
| P-N | Phosphorus–nitrogen |
| PAPI | Polyaryl polymethylene isocyanate |
| PBDE | Pentabromodiphenyl ether |
| PP-PA | Polypropylene–polyamide |
| PPO | Polyphenylene ether |
| Sb2O3 | Antimony trioxide |
| SBD | Surface binding domain |
| SiO2 | Nano-silicon dioxide |
| SPCL | Star poly(ε-caprolactone) |
| SPU | Star polyurethane |
| TDI | Toluene diisocyanate |
| VOC | Volatile organic compound |
| WPU | Waterborne polyurethane |
| ZHS | Zinc hydroxystannate |
| ZS | Zinc stannate |
References
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane foams: Past, present, and future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- de Souza, F.M.; Kahol, P.K.; Gupta, R.K. Introduction to Polyurethane Chemistry. In Polyurethane Chemistry: Renewable Polyols and Isocyanates; American Chemical Society: Washington, DC, USA, 2021; Volume 1380, pp. 1–24. [Google Scholar]
- Mistry, M.; Prajapati, V.; Dholakiya, B.Z. Redefining Construction: An In-Depth Review of Sustainable Polyurethane Applications. J. Polym. Environ. 2024, 32, 3448–3489. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, T.; Zhang, Y.; Guo, B.; Xu, J. Reprocessable cross-linked polyurethane with dynamic and tunable phenol–carbamate network. ACS Sustain. Chem. Eng. 2019, 8, 1207–1218. [Google Scholar] [CrossRef]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-isocyanate polyurethanes: From chemistry to applications. RSC Adv. 2013, 3, 4110–4129. [Google Scholar] [CrossRef]
- Li, Y.; Luo, X.; Hu, S. Introduction to Bio-based Polyols and Polyurethanes. In Bio-Based Polyols and Polyurethanes; Li, Y., Luo, X., Hu, S., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–13. [Google Scholar] [CrossRef]
- Huang, H.; Pang, H.; Huang, J.; Yu, P.; Li, J.; Lu, M.; Liao, B. Influence of hard segment content and soft segment length on the microphase structure and mechanical performance of polyurethane-based polymer concrete. Constr. Build. Mater. 2021, 284, 122388. [Google Scholar] [CrossRef]
- Howard, G.T. Biodegradation of polyurethane: A review. Int. Biodeterior. Biodegrad. 2002, 49, 245–252. [Google Scholar] [CrossRef]
- Dukarski, W.; Rykowska, I.; Krzyżanowski, P.; Gonsior, M. Flame Retardant Additives Used for Polyurea-Based Elastomers—A Review. Fire 2024, 7, 50. [Google Scholar] [CrossRef]
- Kausar, A.; Rafique, I.; Anwar, Z.; Muhammad, B. Recent developments in different types of flame retardants and effect on fire retardancy of epoxy composite. Polym.-Plast. Technol. Eng. 2016, 55, 1512–1535. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Hou, L.; Chen, Y.; Xu, T. Effect of polyether/polyester polyol ratio on properties of waterborne two-component polyurethane coatings. Prog. Org. Coat. 2020, 141, 105545. [Google Scholar] [CrossRef]
- Suryawanshi, Y.; Sanap, P.; Wani, V. Advances in the synthesis of non-isocyanate polyurethanes. Polym. Bull. 2018, 76, 3233–3246. [Google Scholar] [CrossRef]
- Gaan, S.; Sun, G. Effect of phosphorus and nitrogen on flame retardant cellulose: A study of phosphorus compounds. J. Anal. Appl. Pyrolysis 2007, 78, 371–377. [Google Scholar] [CrossRef]
- Lenz, J.U.; Pospiech, D.; Komber, H.; Korwitz, A.; Kobsch, O.; Paven, M.; Albach, R.W.; Gunther, M.; Schartel, B. Effective Halogen-Free Flame-Retardant Additives for Crosslinked Rigid Polyisocyanurate Foams: Comparison of Chemical Structures. Materials 2022, 16, 172. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Ma, Z.; Liu, L.; Zhang, J.; Huo, S.; Song, P. Recent advances in fire-retardant rigid polyurethane foam. J. Mater. Sci. Technol. 2022, 112, 315–328. [Google Scholar] [CrossRef]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; iSmithers Rapra Publishing: Akron, OH, USA, 2005. [Google Scholar]
- Izarra, I.; Borreguero, A.; Garrido, I.; Rodríguez, J.; Carmona, M. Comparison of flexible polyurethane foams properties from different polymer polyether polyols. Polym. Test. 2021, 100, 107268. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, G.; Zhang, Y.; Gao, Y.; Zhao, Y.; Zhou, C.; Zhang, Q.; Wang, X. Synthesis, characterization, and properties of novel polyetherester polyols and developed polyurethanes. J. Appl. Polym. Sci. 2006, 103, 417–424. [Google Scholar] [CrossRef]
- Foy, E.; Farrell, J.B.; Higginbotham, C.L. Synthesis of linear aliphatic polycarbonate macroglycols using dimethylcarbonate. J. Appl. Polym. Sci. 2008, 111, 217–227. [Google Scholar] [CrossRef]
- Huang, S.; Xiao, J.; Zhu, Y.a.; Qu, J. Synthesis and properties of spray-applied high solid content two component polyurethane coatings based on polycaprolactone polyols. Prog. Org. Coat. 2017, 106, 60–68. [Google Scholar] [CrossRef]
- Ozaki, S. Recent advances in isocyanate chemistry. Chem. Rev. 1972, 72, 457–496. [Google Scholar] [CrossRef]
- Saunders, J.H. The Reactions of Isocyanates and Isocyanate Derivatives at Elevated Temperatures. Rubber Chem. Technol. 1959, 32, 337–345. [Google Scholar] [CrossRef]
- Oprea, S. The effect of chain extenders structure on properties of new polyurethane elastomers. Polym. Bull. 2010, 65, 753–766. [Google Scholar] [CrossRef]
- Mandrekar, K.S.; Kadam, H.K.; Tilve, A.; Tilve, S.G. Advances in the Synthesis of AmidesviaAlpha Oxygenation of Amines. Curr. Org. Chem. 2022, 26, 1185–1217. [Google Scholar] [CrossRef]
- Murugesan, K.; Senthamarai, T.; Chandrashekhar, V.G.; Natte, K.; Kamer, P.C.; Beller, M.; Jagadeesh, R.V. Catalytic reductive aminations using molecular hydrogen for synthesis of different kinds of amines. Chem. Soc. Rev. 2020, 49, 6273–6328. [Google Scholar] [CrossRef] [PubMed]
- Karpov, S.V.; Dzhalmukhanova, A.S.; Chernyayev, D.A.; Lodygina, V.P.; Firsova, A.I.; Badamshina, E.R. The investigation of triethylammonium carboxylates influence on the kinetics of urethane formation processing during waterborne polyurethane synthesis. Polym. Adv. Technol. 2021, 32, 2727–2734. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, D. Covalent organic frameworks for heterogeneous catalysis: Principle, current status, and challenges. ACS Cent. Sci. 2020, 6, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-C.; Kang, B.-H.; Chen, L.-S.; Lu, X. Enhancing toughness of poly (lactic acid)/Thermoplastic polyurethane blends via increasing interface compatibility by polyurethane elastomer prepolymer and its toughening mechanism. Polym. Test. 2020, 87, 106521. [Google Scholar] [CrossRef]
- Sivakumar, P.; Du, S.M.; Selter, M.; Daye, J.; Cho, J. Improved adhesion of polyurethane-based nanocomposite coatings to tin surface through silane coupling agents. Int. J. Adhes. Adhes. 2021, 110, 102948. [Google Scholar] [CrossRef]
- Yuan, Y.; Peng, C.; Chen, D.; Wu, Z.; Li, S.; Sun, T.; Liu, X. Synthesis of a coupling agent containing polyurethane chain and its influence on improving the dispersion of SiO2 nanoparticles in epoxy/amine thermoset. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106573. [Google Scholar] [CrossRef]
- Dhaliwal, G.S.; Anandan, S.; Bose, M.; Chandrashekhara, K.; Nam, P. Effects of surfactants on mechanical and thermal properties of soy-based polyurethane foams. J. Cell. Plast. 2020, 56, 611–629. [Google Scholar] [CrossRef]
- Kaminska, K.; Barczewski, M.; Kuranska, M.; Malewska, E.; Polaczek, K.; Prociak, A. The Effect of a Chemical Foaming Agent and the Isocyanate Index on the Properties of Open-Cell Polyurethane Foams. Materials 2022, 15, 6087. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, M.; Grignard, B.; Detrembleur, C. Water-induced self-blown non-isocyanate polyurethane foams. Angew. Chem. Int. Ed. 2022, 61, e202213422. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, X.; Zhang, X.; Ahmed, N.; Fan, H.; Wan, J.; Bittencourt, C.; Li, B.-G. Synthesis of CO2-derived, siloxane-functionalized poly (ether carbonate) s and waterborne polyurethanes. Ind. Eng. Chem. Res. 2020, 59, 3044–3051. [Google Scholar] [CrossRef]
- LeGrow, G.E.; Petroff, L.J. Silicone polyether copolymers: Synthetic methods and chemical compositions. In Silicone Surfactants; Routledge: London, UK, 2019; pp. 49–64. [Google Scholar]
- Lin, C.; Debeli, D.K.; Gan, L.; Deng, J.; Hu, L.; Shan, G. Polyether-modified siloxane stabilized dispersion system on the physical stability and control release of double (W/O/W) emulsions. Food Chem. 2020, 332, 127381. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and photostabilization of polymers, especially polystyrene. SpringerPlus 2013, 2, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Gijsman, P. Chapter 18—Polymer Stabilization. In Handbook of Environmental Degradation of Materials, 3rd ed.; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 369–395. [Google Scholar] [CrossRef]
- Gaponenko, V.; Poluboyarov, V.; Korotaeva, Z.; Zhdanok, A.; Gorbunov, F.; Berdnikova, L.; Zobov, K.; Bardakhanov, S. Enforcement of polyurethane composites with nano-sized particles. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2020. [Google Scholar]
- Delebecq, E.; Pascault, J.-P.; Boutevin, B.; Ganachaud, F. On the Versatility of Urethane/Urea Bonds: Reversibility, Blocked Isocyanate, and Non-isocyanate Polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Unland, M. Reaction of surface isocyanate groups with selected compounds. J. Phys. Chem. 1975, 79, 610–615. [Google Scholar] [CrossRef]
- Satchell, D.; Satchell, R. Acylation by ketens and isocyanates. A mechanistic comparison. Chem. Soc. Rev. 1975, 4, 231–250. [Google Scholar] [CrossRef]
- Saberov, V.S.; Rayenko, G.; Avksentiev, A.; Vakhitova, L.; Korotkikh, N. Catalytic Hydrodehalogenation of Haloarenes with Hydrogen and Hydrogen-Containing Compounds: A Review. Theor. Exp. Chem. 2023, 59, 151–177. [Google Scholar] [CrossRef]
- Lucio, B.; de la Fuente, J.L. Kinetic and thermodynamic analysis of the polymerization of polyurethanes by a rheological method. Thermochim. Acta 2016, 625, 28–35. [Google Scholar] [CrossRef]
- Devi, P.P.K.; Maznee, T.I.T.N.; Hoong, S.S.; Zailan, A.B.; Yeong, S.K.; Hazimah, A.H.; Schiffman, C.M.; Sendijarevic, A.; Sendijarevic, V. Urethane-forming reaction kinetics of natural oil polyols versus petroleum-based polyether polyol. React. Kinet. Mech. Catal. 2016, 119, 93–106. [Google Scholar] [CrossRef]
- Hanopolskyi, A.I.; Smaliak, V.A.; Novichkov, A.I.; Semenov, S.N. Autocatalysis: Kinetics, Mechanisms and Design. ChemSystemsChem 2020, 3, e2000026. [Google Scholar] [CrossRef]
- Goering, H.; Krüger, H.; Bauer, M. Multimodal polymer networks: Design and characterisation of nanoheterogeneous PU elastomers. Macromol. Mater. Eng. 2000, 278, 23–35. [Google Scholar] [CrossRef]
- Król, P. Linear Polyurethanes: Synthesis Methods, Chemical Structures, Properties and Applications; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Yang, X.; Wang, L.; Wang, W.; Chen, H.; Yang, G.; Zhou, S. Triple shape memory effect of star-shaped polyurethane. ACS Appl. Mater. Interfaces 2014, 6, 6545–6554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, H.; Chen, P.; Zhang, Z.; Hu, C. Preparation and properties of waterborne polyurethane with star-shaped hyperbranched structure. Polymer 2019, 180, 121690. [Google Scholar] [CrossRef]
- Versteegen, R.M.; Sijbesma, R.P.; Meijer, E.W. [n]-Polyurethanes: Synthesis and Characterization. Angew. Chem. Int. Ed. 1999, 38, 2917–2919. [Google Scholar] [CrossRef]
- Li, F.; Liang, Z.; Li, Y.; Wu, Z.; Yi, Z. Synthesis of waterborne polyurethane by inserting polydimethylsiloxane and constructing dual crosslinking for obtaining the superior performance of waterborne coatings. Compos. Part B Eng. 2022, 238, 109889. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, H. Design, Preparation and Properties of Polyurethane Dispersions via Prepolymer Method. Molecules 2023, 28, 625. [Google Scholar] [CrossRef] [PubMed]
- Shaban, S.M.; Kang, J.; Kim, D.-H. Surfactants: Recent advances and their applications. Compos. Commun. 2020, 22, 100537. [Google Scholar] [CrossRef]
- Cortes, H.; Hernandez-Parra, H.; Bernal-Chavez, S.A.; Prado-Audelo, M.L.D.; Caballero-Floran, I.H.; Borbolla-Jimenez, F.V.; Gonzalez-Torres, M.; Magana, J.J.; Leyva-Gomez, G. Non-Ionic Surfactants for Stabilization of Polymeric Nanoparticles for Biomedical Uses. Materials 2021, 14, 3197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, S.y.; Li, H.; Li, C.p.; Zhang, B.s.; Long, Y. Surfactant Regulated Chemical Modification of Rice Straw Powder for Biomass Composite Polyurethane Synthetic Leather. ChemistrySelect 2024, 9, e202402197. [Google Scholar] [CrossRef]
- Bossion, A.; Jones, G.O.; Taton, D.; Mecerreyes, D.; Hedrick, J.L.; Ong, Z.Y.; Yang, Y.Y.; Sardon, H. Non-Isocyanate Polyurethane Soft Nanoparticles Obtained by Surfactant-Assisted Interfacial Polymerization. Langmuir 2017, 33, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Cable, C. An Examination of the Effect of Surface Modifications on the Physicochemical and Biological Properties of Non-Ionic Surfactant Vesicles. Ph.D. Thesis, University of Strathclyde, Glasgow, Scotland, 1990. [Google Scholar]
- Ahmad, M.; Xu, B.; Purnawali, H.; Fu, Y.; Huang, W.; Miraftab, M.; Luo, J. High Performance Shape Memory Polyurethane Synthesized with High Molecular Weight Polyol as the Soft Segment. Appl. Sci. 2012, 2, 535–548. [Google Scholar] [CrossRef]
- El Eraki, M.; El Lawindy, A.; Hassan, H.; Mahmoud, W. The physical properties of pressure sensitive rubber composites. Polym. Degrad. Stab. 2006, 91, 1417–1423. [Google Scholar] [CrossRef]
- Schweitzer, P.A. Metallic Materials: Physical, Mechanical, and Corrosion Properties; CRC Press: Boca Raton, FL, USA, 2003; Volume 19. [Google Scholar]
- Kirchhof, G. Plastic properties. Encycl. Soil Sci. 2006, 2, 1311–1313. [Google Scholar]
- Simon, J.; Barla, F.; Kelemen-Haller, A.; Farkas, F.; Kraxner, M. Thermal stability of polyurethanes. Chromatographia 1988, 25, 99–106. [Google Scholar] [CrossRef]
- Deanin, R.D. Structure-property relations in polyurethanes. In High Performance Biomaterials; Routledge: London, UK, 2017; pp. 51–70. [Google Scholar]
- Herrera, M.; Matuschek, G.; Kettrup, A. Thermal degradation of thermoplastic polyurethane elastomers (TPU) based on MDI. Polym. Degrad. Stab. 2002, 78, 323–331. [Google Scholar] [CrossRef]
- Montaudo, G.; Puglisi, C.; Scamporrino, E.; Vitalini, D. Mechanism of thermal degradation of polyurethanes. Effect of ammonium polyphosphate. Macromolecules 1984, 17, 1605–1614. [Google Scholar] [CrossRef]
- Włodarczak, D. Studies of temperature and atmosphere composition influence on thermal degradation products of polyurethane foam. J. Appl. Polym. Sci. 1988, 36, 377–386. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Zulfiqar, M.; Kausar, T.; McNeill, I.C. Thermal degradation of phenyl methacrylate-methyl methacrylate copolymers. Polym. Degrad. Stab. 1987, 17, 327–339. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Kumagai, S.; Motokucho, S.; Kameda, T.; Saito, Y.; Watanabe, A.; Nakatani, H.; Yoshioka, T. Temperature-dependent pyrolysis behavior of polyurethane elastomers with different hard- and soft-segment compositions. J. Anal. Appl. Pyrolysis 2020, 145, 104754. [Google Scholar] [CrossRef]
- Coutinho, F.M.; Delpech, M.C. Degradation profile of films cast from aqueous polyurethane dispersions. Polym. Degrad. Stab. 2000, 70, 49–57. [Google Scholar] [CrossRef]
- Liaw, D.J. The relative physical and thermal properties of polyurethane elastomers: Effect of chain extenders of bisphenols, diisocyanate, and polyol structures. J. Appl. Polym. Sci. 1997, 66, 1251–1265. [Google Scholar] [CrossRef]
- Oprea, S. Effect of structure on the thermal stability of curable polyester urethane urea acrylates. Polym. Degrad. Stab. 2002, 75, 9–15. [Google Scholar] [CrossRef]
- Semsarzadeh, M.; Navarchian, A. Effects of NCO/OH ratio and catalyst concentration on structure, thermal stability, and crosslink density of poly (urethane-isocyanurate). J. Appl. Polym. Sci. 2003, 90, 963–972. [Google Scholar] [CrossRef]
- Zhang, Y.-D.; Shang, S.-B.; Zhang, X.-Y.; Wang, D.; Hourston, D. Influence of the composition of rosin-based rigid polyurethane foams on their thermal stability. J. Appl. Polym. Sci. 1996, 59, 1167–1171. [Google Scholar] [CrossRef]
- Pathirana, R.; Seal, K. Gliocladium roseum (Bainier), a potential biodeteriogen of polyester polyurethane elastomers. In Biodeterioration 5: Papers Presented at the 5th International Biodeterioration Symposium, Aberdeen, September, 1981; Oxley, T.A., Barry, S., Eds.; Wiley: Chichester, UK, 1983. [Google Scholar]
- Huang, S.J.; Roby, M.S. Biodegradable polymers poly (amide-urethanes)[1]. J. Bioact. Compat. Polym. 1986, 1, 61–71. [Google Scholar] [CrossRef]
- Kay, M.; Morton, L.; Prince, E. Bacterial degradation of polyester polyurethane. Int. Biodeterior. 1991, 27, 205–222. [Google Scholar] [CrossRef]
- Howard, G.; Hilliard, N. Use of Coomassie blue-polyurethane interaction inscreening of polyurethanase proteins andpolyurethanolytic bacteria. Int. Biodeterior. Biodegrad. 1999, 43, 23–30. [Google Scholar] [CrossRef]
- Christenson, E.; Anderson, J.; Hiltner, A. Biodegradation mechanisms of polyurethane elastomers. Corros. Eng. Sci. Technol. 2007, 42, 312–323. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Magnin, A.; Pollet, E.; Phalip, V.; Avérous, L. Evaluation of biological degradation of polyurethanes. Biotechnol. Adv. 2020, 39, 107457. [Google Scholar] [CrossRef]
- Akutsu, Y.; Nakajima-Kambe, T.; Nomura, N.; Nakahara, T. Purification and properties of a polyester polyurethane-degrading enzyme from Comamonas acidovorans TB-35. Appl. Environ. Microbiol. 1998, 64, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.T.; Ruiz, C.; Hilliard, N.P. Growth of Pseudomonas chlororaphis on apolyester–polyurethane and the purification andcharacterization of a polyurethanase–esterase enzyme. Int. Biodeterior. Biodegrad. 1999, 43, 7–12. [Google Scholar] [CrossRef]
- Allen, A.B.; Hilliard, N.P.; Howard, G.T. Purification and characterization of a solublepolyurethane degrading enzyme from Comamonasacidovorans. Int. Biodeterior. Biodegrad. 1999, 43, 37–41. [Google Scholar] [CrossRef]
- Haritz, S.; Mecerreyes, D.; Basterretxea, A.; Averous, L.; Jehanno, C. From lab to market: Current strategies for the production of biobased polyols. ACS Sustain. Chem. Eng. 2021, 9, 10664–10677. [Google Scholar]
- Jiang, R.; Zheng, X.; Zhu, S.; Li, W.; Zhang, H.; Liu, Z.; Zhou, X. Recent Advances in Functional Polyurethane Chemistry: From Structural Design to Applications. ChemistrySelect 2023, 8, e202204132. [Google Scholar] [CrossRef]
- Delavarde, A.; Savin, G.; Derkenne, P.; Boursier, M.; Morales-Cerrada, R.; Nottelet, B.; Pinaud, J.; Caillol, S. Sustainable polyurethanes: Toward new cutting-edge opportunities. Prog. Polym. Sci. 2024, 151, 101805. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.; Li, Y.; Feng, L.; Huang, S. Performance development of polyurethane elastomer composites in different construction and curing environments. Constr. Build. Mater. 2023, 365, 130047. [Google Scholar] [CrossRef]
- Alsuhaibani, A.M.; Refat, M.S.; Qaisrani, S.A.; Jamil, F.; Abbas, Z.; Zehra, A.; Baluch, K.; Kim, J.-G.; Mubeen, M. Green buildings model: Impact of rigid polyurethane foam on indoor environment and sustainable development in energy sector. Heliyon 2023, 9, e14451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Gong, K.; Wang, C.; Zhou, M.; Xiao, J. Construction of Ti(3)C(2) MXene based fire resistance nanocoating on flexible polyurethane foam for highly efficient photothermal conversion and solar water desalination. J. Colloid Interface Sci. 2023, 630, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Qi, Y.; Gong, R.; Hu, Y.; Yao, F.; Liu, Y.; Liu, B.; Zhao, Y.; Dai, Y.; Dong, X.; et al. Nanocellulose-reinforced polyurethane as flexible coating for cork floor. Prog. Org. Coat. 2023, 178, 107480. [Google Scholar] [CrossRef]
- Xavier, J.R. Investigation of anticorrosion, flame retardant and mechanical properties of polyurethane/GO nanocomposites coated AJ62 Mg alloy for aerospace/automobile components. Diam. Relat. Mater. 2023, 136, 110025. [Google Scholar] [CrossRef]
- Xavier, J.; Vinodhini, S.; Ganesan, R. Innovative nanocomposite coating for aluminum alloy: Superior corrosion resistance, flame retardancy, and mechanical strength for aerospace applications. J. Mater. Sci. 2024, 59, 1–32. [Google Scholar] [CrossRef]
- Liu, J.; Recupido, F.; Lama, G.C.; Oliviero, M.; Verdolotti, L.; Lavorgna, M. Recent advances concerning polyurethane in leather applications: An overview of conventional and greener solutions. Collagen Leather 2023, 5, 8. [Google Scholar] [CrossRef]
- Xing, W.; Xi, J.; Cai, W.; Zhang, W.; Wang, B.; Chen, L.; Hu, Y. Preparation and properties of multifunctional polyurethane synthetic leather nanocomposites. Compos. Part A Appl. Sci. Manuf. 2023, 169, 107534. [Google Scholar] [CrossRef]
- Xu, W.; Liu, D. High solid content waterborne polyurethane with high foaming rate to artificial leather: Synthesis and characterization. Prog. Org. Coat. 2024, 189, 108332. [Google Scholar] [CrossRef]
- Sur, S.-H.; Choi, P.-J.; Ko, J.-W.; Lee, J.-Y.; Lee, Y.-H.; Kim, H.-D. Preparation and Properties of DMF-Based Polyurethanes for Wet-Type Polyurethane Artificial Leather. Int. J. Polym. Sci. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Shi, M.; Wang, X.; Yang, J. Development of lignin-based waterborne polyurethane materials for flame retardant leather application. Polym. Bull. 2023, 80, 5553–5571. [Google Scholar] [CrossRef]
- Mhaske, S.; Chugh, K.W.; Mahajan, U.R.; Mohanty, J. Polymers for Adhesives and Sealants. In Specialty Polymers; CRC Press: Boca Raton, FL, USA, 2023; pp. 279–293. [Google Scholar]
- Dang, G.-P.; Gu, J.-T.; Wan, Q.-Q.; Niu, L.-N. Polyurethanes for Sealants. In Polyurethanes: Preparation, Properties, and Applications Volume 2: Advanced Applications; American Chemical Society: Washington, DC, USA, 2023; Volume 1453, pp. 153–168. [Google Scholar]
- Choffat, F.; Corsaro, A.; Di Fratta, C.; Kelch, S. 3—Advances in polyurethane structural adhesives. In Advances in Structural Adhesive Bonding, 2nd ed.; Dillard, D.A., Ed.; Woodhead Publishing: Cambridge, UK, 2023; pp. 103–136. [Google Scholar] [CrossRef]
- Du, J.; Wang, H.; Huang, Z.; Liu, X.; Yin, X.; Wu, J.; Lin, W.; Lin, X.; Yi, G. Construction and mechanism study of lignin-based polyurethane with high strength and high self-healing properties. Int. J. Biol. Macromol. 2023, 248, 125925. [Google Scholar] [CrossRef]
- Wienen, D.; Gries, T.; Cooper, S.L.; Heath, D.E. An overview of polyurethane biomaterials and their use in drug delivery. J. Control. Release 2023, 363, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Jiang, Y.; Li, X.; Yuan, X.; Zhang, J.; He, Q.; Ye, F.; Luo, G.; Guo, S.; Zhang, Y.; et al. A Flexible and Stretchable MXene/Waterborne Polyurethane Composite-Coated Fiber Strain Sensor for Wearable Motion and Healthcare Monitoring. Sensors 2024, 24, 271. [Google Scholar] [CrossRef]
- Kemona, A.; Piotrowska, M. Polyurethane Recycling and Disposal: Methods and Prospects. Polymers 2020, 12, 1752. [Google Scholar] [CrossRef]
- Qian, X.; Song, L.; Bihe, Y.; Yu, B.; Shi, Y.; Hu, Y.; Yuen, R.K.K. Organic/inorganic flame retardants containing phosphorus, nitrogen and silicon: Preparation and their performance on the flame retardancy of epoxy resins as a novel intumescent flame retardant system. Mater. Chem. Phys. 2014, 143, 1243–1252. [Google Scholar] [CrossRef]
- Xu, Z.-Z.; Huang, J.-Q.; Chen, M.-J.; Tan, Y.; Wang, Y.-Z. Flame retardant mechanism of an efficient flame-retardant polymeric synergist with ammonium polyphosphate for polypropylene. Polym. Degrad. Stab. 2013, 98, 2011–2020. [Google Scholar] [CrossRef]
- Papa, A.J. Reactive flame retardants for polyurethane foams. Ind. Eng. Chem. Prod. Res. Dev. 1970, 9, 478–496. [Google Scholar] [CrossRef]
- Xiao, W.-D.; Kibble, K.A. Comparison of Aluminium Hydroxide and Magnesium Hydroxide as Flame Retardants in Sebs-Based Composites. Polym. Polym. Compos. 2008, 16, 415–422. [Google Scholar] [CrossRef]
- Zhou, R.; Ming, Z.; He, J.; Ding, Y.; Jiang, J. Effect of Magnesium Hydroxide and Aluminum Hydroxide on the Thermal Stability, Latent Heat and Flammability Properties of Paraffin/HDPE Phase Change Blends. Polymers 2020, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-S.; Yu, Z.-Z.; Liu, W.; Zhang, S. Synergistic effect of decabromodiphenyl ethane and montmorillonite on flame retardancy of polypropylene. Polym. Degrad. Stab. 2009, 94, 1520–1525. [Google Scholar] [CrossRef]
- Scionti, G.; Piperopoulos, E.; Atria, M.; Calabrese, L.; Proverbio, E. Effect of Magnesium Hydroxide and Aluminum Hydroxide as Thermal Barriers on the Flame-Retardant Behavior of Acrylic-Based Coating. Coatings 2023, 13, 1517. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, D.W.; Hwang, K.H.; Yoon, B.S.; Wu, J.P.; Park, J.W.; Hahm, H.S.; Im, W.B. Preparation and characterization of polyurethane flame-retardant coatings using pyrophosphoric lactone-modified polyesters/isophorone diisocyanate–isocyanurate. J. Appl. Polym. Sci. 2001, 80, 2316–2327. [Google Scholar] [CrossRef]
- Wang, Z.; Han, E.; Ke, W. Effect of nanoparticles on the improvement in fire-resistant and anti-ageing properties of flame-retardant coating. Surf. Coat. Technol. 2006, 200, 5706–5716. [Google Scholar] [CrossRef]
- Lu, H.; Song, L.; Hu, Y. A review on flame retardant technology in China. Part II: Flame retardant polymeric nanocomposites and coatings. Polym. Adv. Technol. 2011, 22, 379–394. [Google Scholar] [CrossRef]
- Wu, K.; Kandola, B.K.; Kandare, E.; Hu, Y. Flame retardant effect of polyhedral oligomeric silsesquioxane and triglycidyl isocyanurate on glass fibre-reinforced epoxy composites. Polym. Compos. 2010, 32, 378–389. [Google Scholar] [CrossRef]
- Sharma, V.; Agarwal, S.; Mathur, A.; Singhal, S.; Wadhwa, S. Advancements in nanomaterial based flame-retardants for polymers: A comprehensive overview. J. Ind. Eng. Chem. 2024, 133, 38–52. [Google Scholar] [CrossRef]
- Araby, S.; Philips, B.; Meng, Q.; Ma, J.; Laoui, T.; Wang, C.H. Recent advances in carbon-based nanomaterials for flame retardant polymers and composites. Compos. Part B Eng. 2021, 212, 108675. [Google Scholar] [CrossRef]
- Jiao, C.M.; Wang, Z.Z.; Ye, Z.; Hu, Y.; Fan, W.C. Flame Retardation of Ethylene-Vinyl Acetate Copolymer Using Nano Magnesium Hydroxide and Nano Hydrotalcite. J. Fire Sci. 2006, 24, 47–64. [Google Scholar] [CrossRef]
- Shen, C.; Shao, R.; Wang, W.; Wu, X.; Zhou, B.; Zhao, L.; Siddique, A.; Xu, Z. Progress of flame retardant research on flexible polyurethane foam. Eur. Polym. J. 2024, 220, 113478. [Google Scholar] [CrossRef]
- Wang, S.; Du, X.; Jiang, Y.; Xu, J.; Zhou, M.; Wang, H.; Cheng, X.; Du, Z. Synergetic enhancement of mechanical and fire-resistance performance of waterborne polyurethane by introducing two kinds of phosphorus-nitrogen flame retardant. J. Colloid Interface Sci. 2019, 537, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-S.; Zhang, J.; Wang, H.; He, M.; Ding, L.; Zhao, W.-W. Simultaneously improving the fracture toughness and flame retardancy of soybean oil-based waterborne polyurethane coatings by phosphorus-nitrogen chain extender. Ind. Crops Prod. 2021, 163, 113328. [Google Scholar] [CrossRef]
- Mahajan, M.S.; Gite, V.V. Self-healing polyurethane coatings of eugenol-based polyol incorporated with linseed oil encapsulated cardanol-formaldehyde microcapsules: A sustainable approach. Prog. Org. Coat. 2022, 162, 106534. [Google Scholar] [CrossRef]
- Farshchi, N.; Gedan-Smolka, M. Polyurethane powder coatings: A review of composition and characterization. Ind. Eng. Chem. Res. 2020, 59, 15121–15132. [Google Scholar] [CrossRef]
- Paraskar, P.M.; Prabhudesai, M.S.; Hatkar, V.M.; Kulkarni, R.D. Vegetable oil based polyurethane coatings–A sustainable approach: A review. Prog. Org. Coat. 2021, 156, 106267. [Google Scholar] [CrossRef]
- Wan, L.; Deng, C.; Chen, H.; Zhao, Z.-Y.; Huang, S.-C.; Wei, W.-C.; Yang, A.-H.; Zhao, H.-B.; Wang, Y.-Z. Flame-retarded thermoplastic polyurethane elastomer: From organic materials to nanocomposites and new prospects. Chem. Eng. J. 2021, 417, 129314. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liu, B.; Zhao, Q.; Qi, Y.; Wang, Y.; Sun, Z.; Liu, B.; Zhang, N.; Hu, W.; et al. A novel phosphorus-containing lignin-based flame retardant and its application in polyurethane. Compos. Commun. 2020, 21, 100382. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Cai, W.; Wang, J.; Song, L.; Hu, Y. Recent advances in construction of hybrid nano-structures for flame retardant polymers application. Appl. Mater. Today 2020, 20, 100762. [Google Scholar] [CrossRef]
- Nguyen-Ha, T.M.; Nguyen, T.B.; Nguyen, T.A.; Pham, L.H.; Nguyen, D.H.; Nguyen, D.M.; Hoang, D.; Oh, E.; Suhr, J. Novel high-performance sustainable polyurethane nanocomposite foams: Fire resistance, thermal stability, thermal conductivity, and mechanical properties. Chem. Eng. J. 2023, 474, 145585. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, T.; Peng, H.; Ma, Z.; Zhang, M.; Lynch, M.; Dinh, T.; Zhou, Z.; Zhou, Y.; Song, P. Fire-retardant, anti-dripping, biodegradable and biobased polyurethane elastomers enabled by hydrogen-bonding with cellulose nanocrystals. Nano Res. 2024, 17, 2186–2194. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. A review of recent progress in phosphorus-based flame retardants. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorus-based flame retardancy mechanisms—Old hat or a starting point for future development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huo, S.; Ye, G.; Zhang, Q.; Cao, C.-F.; Lynch, M.; Wang, H.; Song, P.; Liu, Z. Strong self-healing close-loop recyclable vitrimers via complementary dynamic covalent/non-covalent bonding. Chem. Eng. J. 2024, 500, 157418. [Google Scholar] [CrossRef]
- Wang, C.; Huo, S.; Ye, G.; Song, P.; Wang, H.; Liu, Z. A P/Si-containing polyethylenimine curing agent towards transparent, durable fire-safe, mechanically-robust and tough epoxy resins. Chem. Eng. J. 2023, 451, 138768. [Google Scholar] [CrossRef]
- Arunkumar, T.; Anand, G.; Venkatachalam, P.; Anish, M.; Jayaprabakar, J.; Sajin, J.B. Effect of plural spray coating process parameters on bonding strength of polyurea with steel and aluminum for liquid storage applications. J. Test. Eval. 2021, 49, 3319–3332. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S. An overview of some recent advances in DOPO-derivatives: Chemistry and flame retardant applications. Polym. Degrad. Stab. 2015, 113, 119–134. [Google Scholar] [CrossRef]
- Wang, C.; Huo, S.; Ye, G.; Wang, B.; Guo, Z.; Zhang, Q.; Song, P.; Wang, H.; Liu, Z. Construction of an epoxidized, phosphorus-based poly(styrene butadiene styrene) and its application in high-performance epoxy resin. Compos. Part B Eng. 2024, 268, 111075. [Google Scholar] [CrossRef]
- Brown, S.C. Flame retardants: Inorganic oxide and hydroxide systems. In Plastics Additives: An A-Z Reference; Pritchard, G., Ed.; Springer: Dordrecht, The Netherlands, 1998; pp. 287–296. [Google Scholar] [CrossRef]
- Bourbigot, S.; Duquesne, S. Fire retardant polymers: Recent developments and opportunities. J. Mater. Chem. 2007, 17, 2283–2300. [Google Scholar] [CrossRef]
- Casetta, M.; Michaux, G.; Ohl, B.; Duquesne, S.; Bourbigot, S. Key role of magnesium hydroxide surface treatment in the flame retardancy of glass fiber reinforced polyamide 6. Polym. Degrad. Stab. 2018, 148, 95–103. [Google Scholar] [CrossRef]
- Choe, C.-G.; Jang, Y.-M.; Jo, C.-H.; Yu, C.-J. Preparation and flame retardance of polyethylene composites with microencapsulated resorcinol bis(diphenyl phosphate), red phosphorus and magnesium hydroxide. Polym. Bull. 2023, 80, 9727–9744. [Google Scholar] [CrossRef]
- Rothon, R.; Hornsby, P. Fire retardant fillers for polymers. Polym. Green Flame Retard. 2014, 9, 289–321. [Google Scholar] [CrossRef]
- Kind, D.J.; Hull, T.R. A review of candidate fire retardants for polyisoprene. Polym. Degrad. Stab. 2012, 97, 201–213. [Google Scholar] [CrossRef]
- Hou, Y.; Xu, Z.; Chu, F.; Gui, Z.; Song, L.; Hu, Y.; Hu, W. A review on metal-organic hybrids as flame retardants for enhancing fire safety of polymer composites. Compos. Part B Eng. 2021, 221, 109014. [Google Scholar] [CrossRef]
- Olejnik, M. Polymer nano-composites with montmorillonyte-obtaining, assesment methods, properties and applications. Tech. Wyr. Włókiennicze 2008, 16, 67–74. [Google Scholar]
- Feng, F.; Qian, L. The flame retardant behaviors and synergistic effect of expandable graphite and dimethyl methylphosphonate in rigid polyurethane foams. Polym. Compos. 2014, 35, 301–309. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, H.; Yu, B.; Shi, Y.; Wang, W.; Song, L.; Hu, Y.; Zhang, Y. Phosphorus and nitrogen-containing polyols: Synergistic effect on the thermal property and flame retardancy of rigid polyurethane foam composites. Ind. Eng. Chem. Res. 2016, 55, 10813–10822. [Google Scholar] [CrossRef]
- Zhang, P.; Fan, H.; Tian, S.; Chen, Y.; Yan, J. Synergistic effect of phosphorus–nitrogen and silicon-containing chain extenders on the mechanical properties, flame retardancy and thermal degradation behavior of waterborne polyurethane. RSC Adv. 2016, 6, 72409–72422. [Google Scholar] [CrossRef]
- Mouren, A.; Avérous, L. Sustainable cycloaliphatic polyurethanes: From synthesis to applications. Chem. Soc. Rev. 2023, 52, 277–317. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, H.; Iqbal, S.; Irfan, M.; Darda, A.; Rawat, N.K. A review on the production, properties and applications of non-isocyanate polyurethane: A greener perspective. Prog. Org. Coat. 2021, 154, 106124. [Google Scholar] [CrossRef]
- Parcheta, P.; Głowińska, E.; Datta, J. Effect of bio-based components on the chemical structure, thermal stability and mechanical properties of green thermoplastic polyurethane elastomers. Eur. Polym. J. 2020, 123, 109422. [Google Scholar] [CrossRef]
- Phung Hai, T.A.; Tessman, M.; Neelakantan, N.; Samoylov, A.A.; Ito, Y.; Rajput, B.S.; Pourahmady, N.; Burkart, M.D. Renewable Polyurethanes from Sustainable Biological Precursors. Biomacromolecules 2021, 22, 1770–1794. [Google Scholar] [CrossRef]
- Stemmler, K.; Folini, D.; Ubl, S.; Vollmer, M.K.; Reimann, S.; O'Doherty, S.; Greally, B.R.; Simmonds, P.G.; Manning, A.J. European emissions of HFC-365mfc, a chlorine-free substitute for the foam blowing agents HCFC-141b and CFC-11. Environ. Sci. Technol. 2007, 41, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Sayad, R.S.; Williams, K.W. Methylene Chloride Urethane Grade As a Viable Auxiliary Blowing Agent In Flexible Slabstock Foam. J. Cell. Plast. 1979, 15, 32–38. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, B.; Lee, L.J. Extrusion foaming of polystyrene/carbon particles using carbon dioxide and water as co-blowing agents. Polymer 2011, 52, 1847–1855. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Poly (lactic acid) foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Western, L.M.; Redington, A.L.; Manning, A.J.; Trudinger, C.M.; Hu, L.; Henne, S.; Fang, X.; Kuijpers, L.J.; Theodoridi, C.; Godwin, D.S. A renewed rise in global HCFC-141b emissions between 2017–2021. Atmos. Chem. Phys. 2022, 22, 9601–9616. [Google Scholar] [CrossRef]
- Li, P.; Mühle, J.; Montzka, S.A.; Oram, D.E.; Miller, B.R.; Weiss, R.F.; Fraser, P.J.; Tanhua, T. Atmospheric histories, growth rates and solubilities in seawater and other natural waters of the potential transient tracers HCFC-22, HCFC-141b, HCFC-142b, HFC-134a, HFC-125, HFC-23, PFC-14 and PFC-116. Ocean. Sci. 2019, 15, 33–60. [Google Scholar] [CrossRef]
- Schilling, S. Appliance rigid foams blown with cyclopentane and cyclopentane/isopentane blends. J. Cell. Plast. 2000, 36, 190–206. [Google Scholar] [CrossRef]
- Zipfel, L.; Barthtlemy, P.; Dournel, P. The next generation blowing agents: From one single product to a product range. J. Cell. Plast. 1999, 35, 345–364. [Google Scholar] [CrossRef]
- Zhou, T.; Shang, B.; Dong, H.; Tao, X.; Liu, T.; Wang, Y. Emission characteristics of volatile organic compounds during pilot swine manure composting. Trans. Chin. Soc. Agric. Eng. 2017, 33, 192–198. [Google Scholar]
- Tsai, W.-T. An overview of environmental hazards and exposure risk of hydrofluorocarbons (HFCs). Chemosphere 2005, 61, 1539–1547. [Google Scholar] [CrossRef]
- Fishback, T.; Reichel, C. Hydrofluorocarbons and hydrofluorocarbon ethers as blowing agents for rigid insulating urethane foams. J. Cell. Plast. 1994, 30, 84–89. [Google Scholar] [CrossRef]
- Zipfel, L.; Krucke, W.; Borner, K.; Barthtlemy, P.; Dournel, P. HFC-365mfc and HFC-245fa progress in application of new HFC blowing agents. J. Cell. Plast. 1998, 34, 511–525. [Google Scholar] [CrossRef]
- Vollmer, M.K.; Mühle, J.; Trudinger, C.M.; Rigby, M.; Montzka, S.A.; Harth, C.M.; Miller, B.R.; Henne, S.; Krummel, P.B.; Hall, B.D.; et al. Atmospheric histories and global emissions of halons H-1211 (CBrClF2), H-1301 (CBrF3), and H-2402 (CBrF2CBrF2). J. Geophys. Res. Atmos. 2016, 121, 3663–3686. [Google Scholar] [CrossRef]


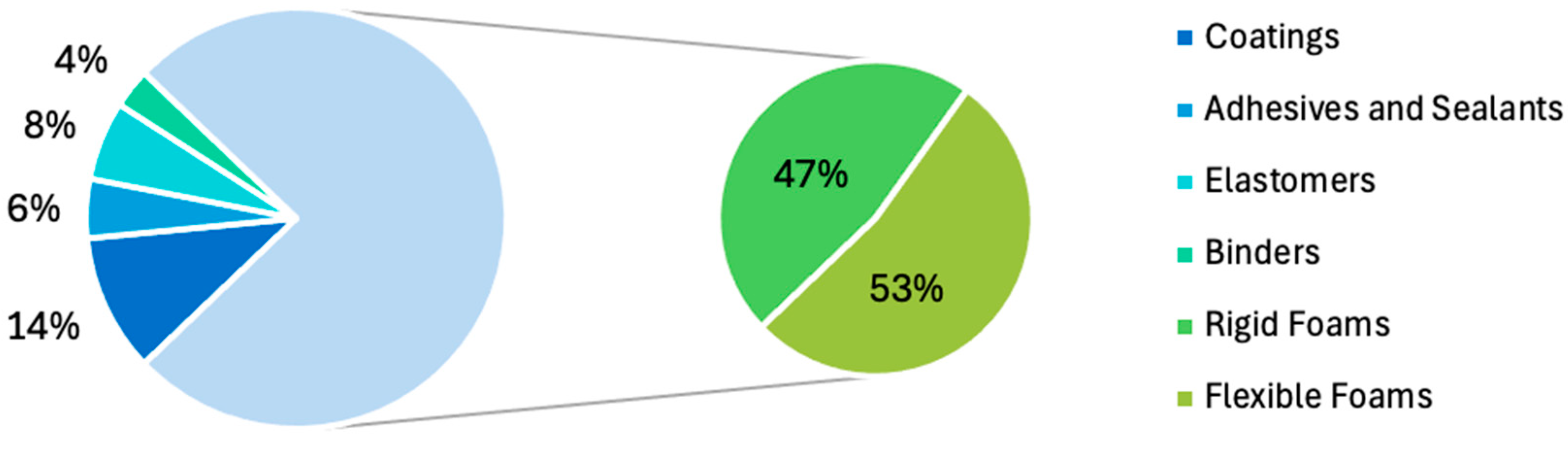
| Additives | Common Selections | Ref. |
|---|---|---|
| Chain extenders | Glycerol, Trimethylolpropane, Pentaerythritol | [24] |
| Catalyst | Amines: triethylenediamine, N-alkyl morphine | [25,26,27] |
| Organotin: dibutyltin diosilicate | [28] | |
| Interface agent | Coupling agents, surfactants | [29,30,31,32] |
| Foaming agent | Water, liquid carbon dioxide, chlorofluorocarbons, hydrochlorofluorocarbons, hydrofluorocarbons, pentane, cyclopentane | [33,34] |
| Foam stabilizer | Water-soluble polyether siloxane | [35,36,37] |
| Stabilizers | Antioxidants, UV absorbers, and polystyrene peroxide | [38,39] |
| Enforced fillers | Cellulose, glass fiber, carbon black, silica | [40] |
| Reactants | Reaction Formulas |
|---|---|
| Alcohol | 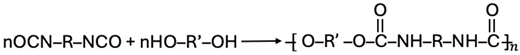 |
| Amines | 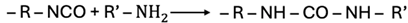 |
| Water | 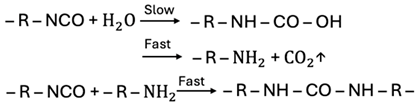 |
| Phenol | 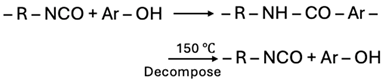 |
| Amide |  |
| Carboxylic acid | 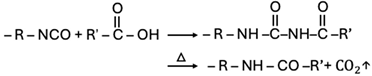 |
| Urea | 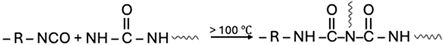 |
| Carbamate | 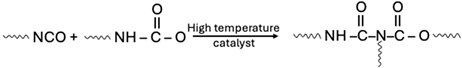 |
| Features | One-Step | Prepolymer |
|---|---|---|
| Efficiency | High | Moderate |
| Control Over Properties | Limited (less precise phase control) | High (allow tailored properties) |
| Morphology | Less defined | Better-defined phase separation |
| Application Suitability | Simple applications | High-performance materials |
| Property | Vs. Rubber [62] | Vs. Metal [63] | Vs. Plastic [64] |
|---|---|---|---|
| Abrasion Resistance | High | High | High |
| Impact Resistance | High | High | |
| Cut and Tear Resistance | High | - | - |
| Load Bearing Capacity | Superior | ||
| Elastic Memory | Present | ||
| Noise Reduction | High | High | |
| Corrosion Resistance | High | ||
| Resilience | High | High | |
| Flexibility | High | ||
| Surface Coating Durability | Frictions control | ||
| Temperature Resistance | Low | ||
| Radiation Resistance | High | High | |
| Ozone Resistance | High |
| Classifications | Representative Materials | Protection Mechanism | Ref. |
|---|---|---|---|
| Reactive FR | Phosphorus and nitrogen FR | Covalently bonded to the substrate to form a FR structure. | [14,15,16] |
| Ammonium polyphosphate (APP) Pentaerythritol phosphate | Introduction of phosphorus/nitrogen monomers during polymerization. | [108,109,110] | |
| Non-Reactive FR | Aluminum hydroxide (ATH) Magnesium hydroxide (MH) | The FR is physically mixed into the PU system and decomposes to produce water vapor when heated. | [111,112] |
| Pentabromodiphenyl ether (PBDE) Antimony trioxide (Sb2O3) | Halogenated compounds decompose endothermally, releasing harmful gases to suppress flames. | [113] | |
| Encapsulation and Coating | Phosphate coating Siloxane coating | Coating insulation and oxygen insulation. | [114,115] |
| Nano-silicon dioxide (SiO2) Nano-montmorillonite (MMT) | Nanomaterials improve surface thermal stability. | [116,117] | |
| FR Structural Units | Aromatic polyester Epoxy resin | Non-flammable structural units affect the thermal decomposition path of materials. | [118] |
| Nanomaterial Modification | Nanographene Nano-clay Carbon nanotubes | Nanomaterials in matrix improve heat resistance and oxygen isolation. | [119,120] |
| APP/nano-silicon dioxide composite material | The additional synergistic effects brought by nanoparticles produce multiple flame-retardant mechanisms. | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yeoh, G.H.; Kabir, I.I. Polyurethane Materials for Fire Retardancy: Synthesis, Structure, Properties, and Applications. Fire 2025, 8, 64. https://doi.org/10.3390/fire8020064
Zhang J, Yeoh GH, Kabir II. Polyurethane Materials for Fire Retardancy: Synthesis, Structure, Properties, and Applications. Fire. 2025; 8(2):64. https://doi.org/10.3390/fire8020064
Chicago/Turabian StyleZhang, Jiemin, Guan Heng Yeoh, and Imrana I. Kabir. 2025. "Polyurethane Materials for Fire Retardancy: Synthesis, Structure, Properties, and Applications" Fire 8, no. 2: 64. https://doi.org/10.3390/fire8020064
APA StyleZhang, J., Yeoh, G. H., & Kabir, I. I. (2025). Polyurethane Materials for Fire Retardancy: Synthesis, Structure, Properties, and Applications. Fire, 8(2), 64. https://doi.org/10.3390/fire8020064








