Sorption Drying of Wheat Seeds Using Kieserite as a Solid Desiccant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Moisture Content Determination
2.3. Desiccant Characterization Methods
2.4. Sorption Drying Experiments
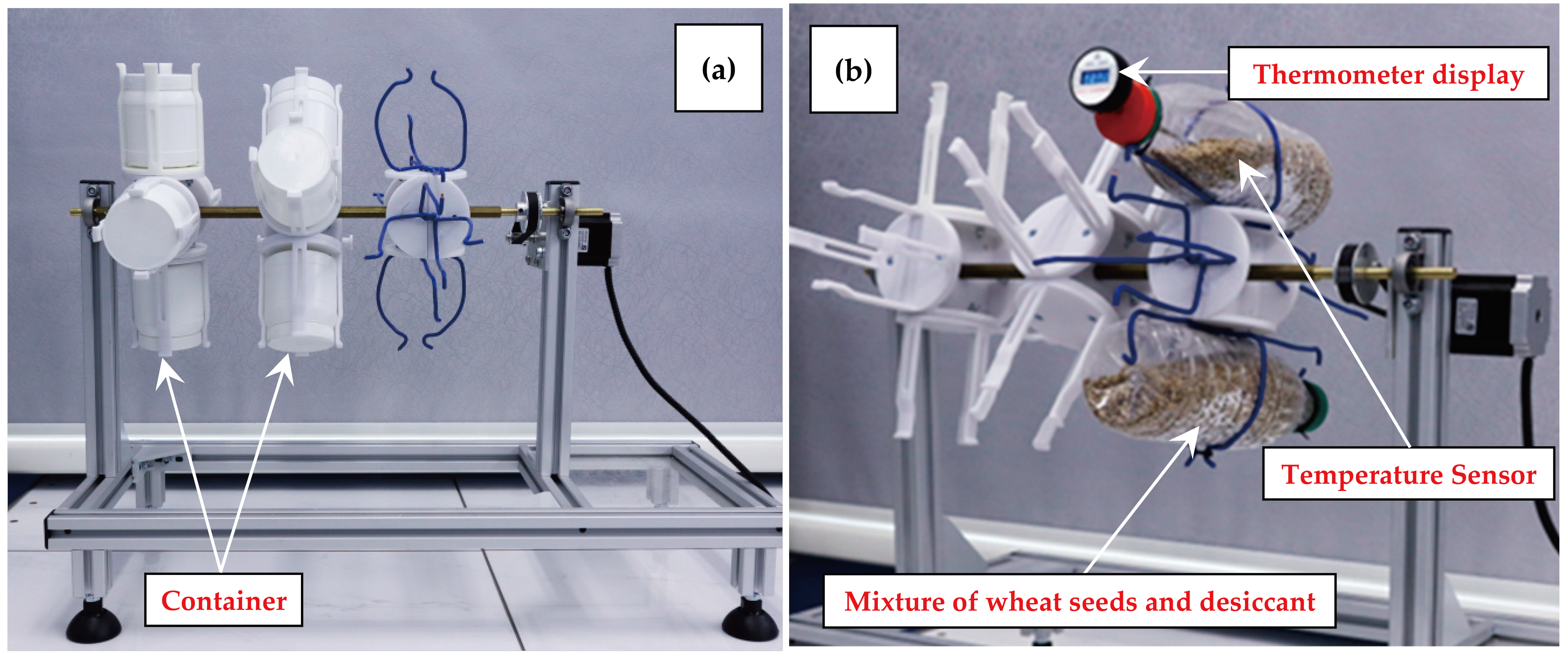
2.4.1. Drying Setup
2.4.2. One-Stage Sorption Drying
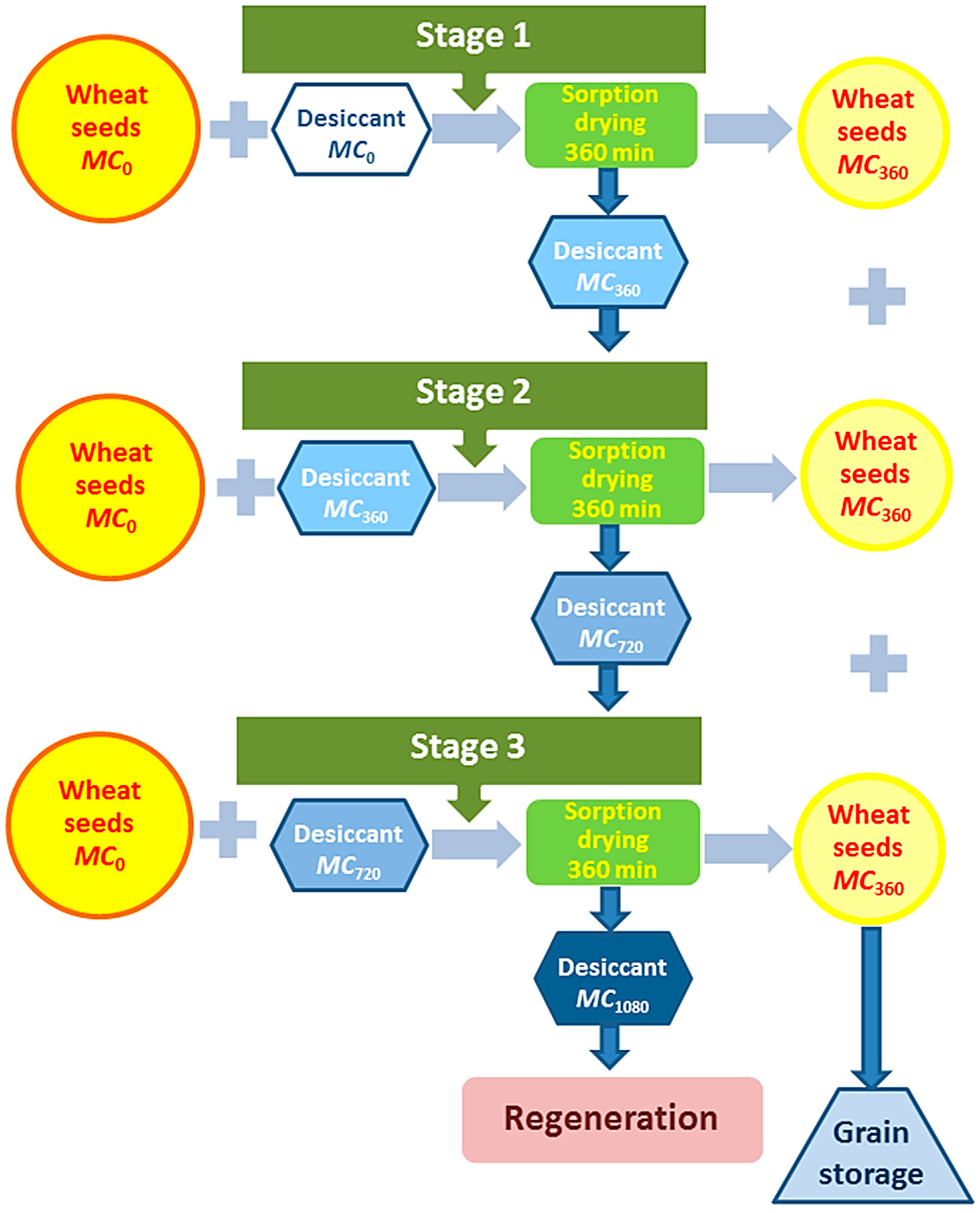
2.4.3. Three-Stage Sorption Drying
2.4.4. Drying Kinetics
2.5. Seed Germination Test
3. Results and Discussion
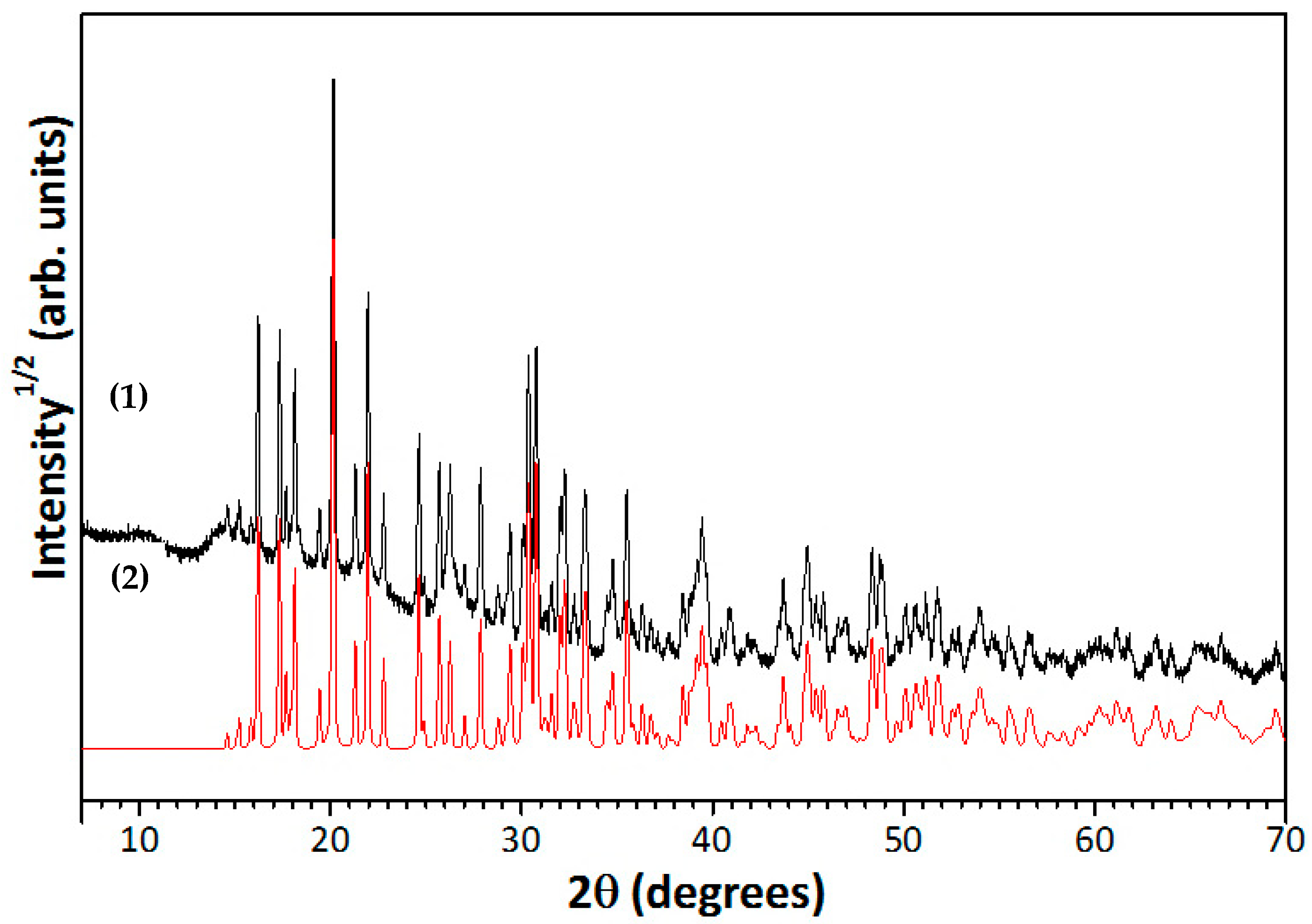
3.1. Desiccant Characterization
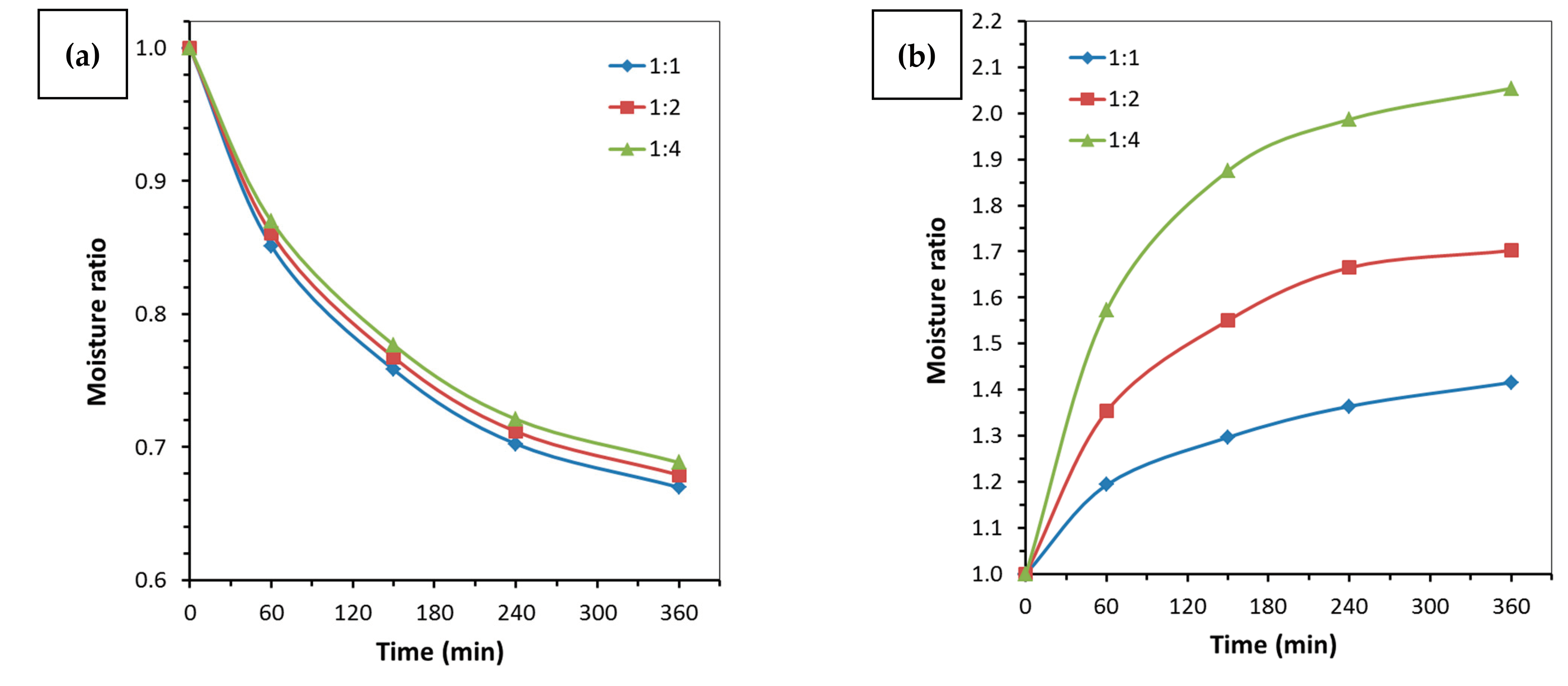
3.2. One-Stage Sorption Drying
3.3. Three-Stage Sorption Drying
3.4. Wheat Seed Germination
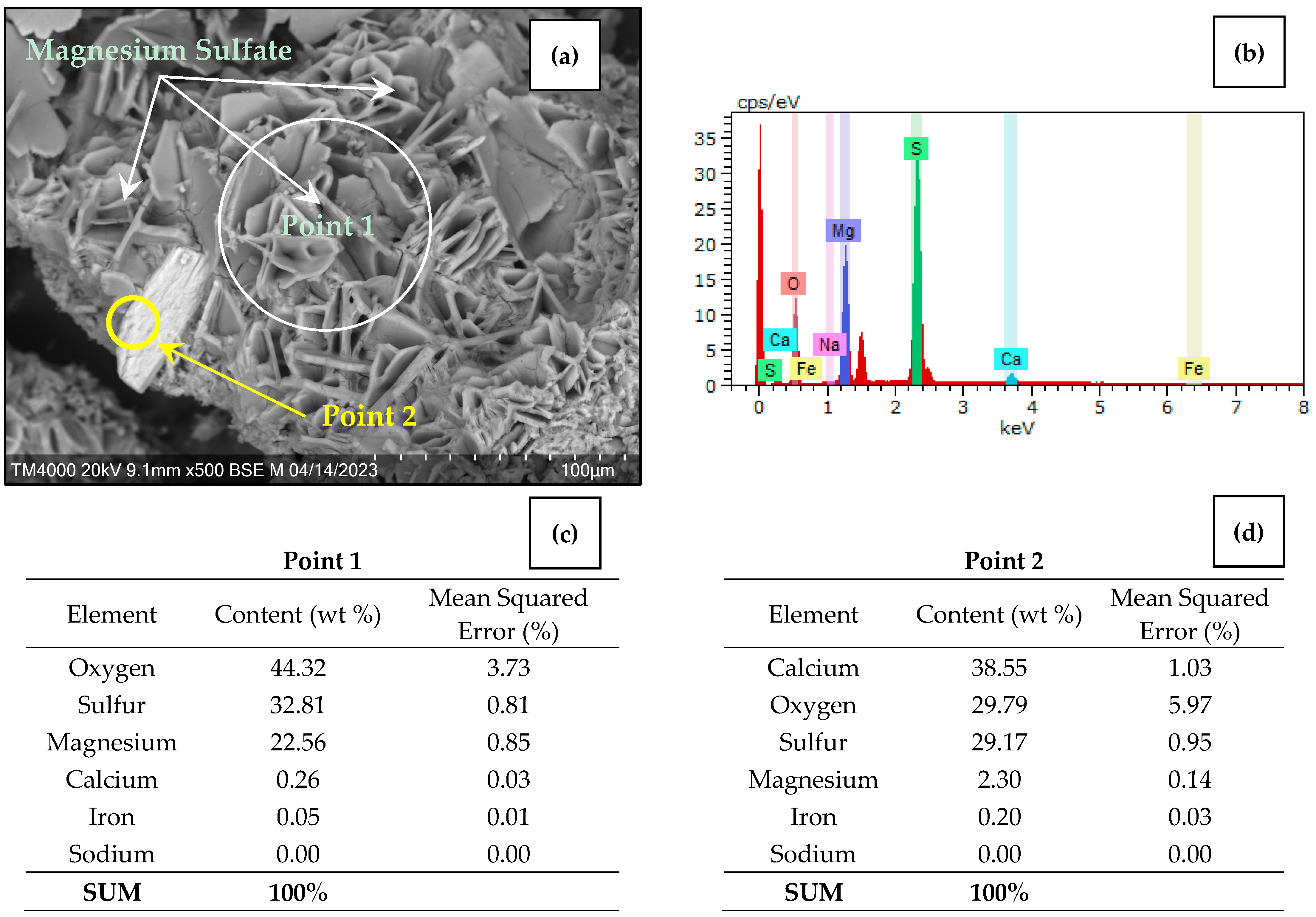
3.5. Characterization of Desiccant after Sorption Drying
3.6. Cyclicity Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Sousa, T.; Ribeiro, M.; Sabença, C.; Igrejas, G. The 10,000-Year Success Story of Wheat! Foods 2021, 10, 2124. [Google Scholar] [CrossRef] [PubMed]
- FAO; IPAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2022, Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable. Rome. FAOSTAT. 2022. Available online: https://www.fao.org/ (accessed on 28 April 2024).
- Yan, D.; Liu, X.; Hao, X.-P.; Li, J. Tracing environmental impacts of grain losses along the supply chain in the North China Plain: An integrated framework. Resour. Conserv. Recycl. 2023, 189, 106771. [Google Scholar] [CrossRef]
- Hashim, N.; Onwude, D.I.; Maringgal, B. Technological advances in postharvest management of food grains. Res. Technol. Adv. Food Sci. Book 2022, 15, 371–406. [Google Scholar] [CrossRef]
- Kirov, G.; Petrova, N.; Staminirova, T. Matching of the water states of products and zeolite during contact adsorption drying. Dry. Technol. 2017, 35, 2015–2020. [Google Scholar] [CrossRef]
- Afzal, I.; Khalid, E.; Basra, S.M.A.; Afzal, A.; Mahmood, K. Maintaining seed quality of maize and wheat through dry chain technology in Pakistan. Int. J. Agric. Biol. 2019, 22, 1363–1368. [Google Scholar]
- Hanif, S.; Sultan, M.; Miyazaki, T.; Koyama, S. Investigation of energy-efficient solid desiccant system for wheat drying. Int. J. Agric. Biol. Eng. 2019, 12, 221–228. [Google Scholar] [CrossRef]
- Hanif, S.; Sultan, M.; Miyazaki, T.; Koyama, S. Evaluation of Solid Desiccant based Drying System for Energy-Efficient Drying of Agricultural Products. Trans. Jpn. Soc. Refrig. Air Cond. Eng. 2018, 35, 347–352. [Google Scholar] [CrossRef]
- Jani, D.B. Progressive Review on Use of Desiccant Drying in Agricultural Applications. J. Agric. Sci. Eng. 2019, 1, 24–31. [Google Scholar]
- Sultana, R.; Kunusoth, K.; Amineni, L.; Dahal, P.; Bradford, K.J. Desiccant drying prior to hermetic storage extends viability and reduces bruchid (Callosobruchus chinensis L.) infestation of mung bean (Vigna radiate (L.) R. Wilczek) seeds. J. Stored Prod. Res. 2021, 94, 101888. [Google Scholar] [CrossRef]
- Sundareswaran, S.; Ray Choudhury, P.; Vanitha, C.; Yadava, D.K. Seed Quality: Variety Development to Planting—An Overview. In Seed Science and Technology; Dadlani, M., Yadava, D.K., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Wang, R.; Liu, L.; Guo, Y.; Hea, X.; Lu, Q. Effects of deterioration and mildewing on the quality of wheat seeds with different moisture contents during storage. RSC Adv. 2020, 10, 14581. [Google Scholar] [CrossRef]
- Corbineau, F. The Effects of Storage Conditions on Seed Deterioration and Ageing: How to Improve Seed Longevity. Seeds 2024, 3, 56–75. [Google Scholar] [CrossRef]
- Nadarajan, J.; Walters, C.; Pritchard, H.W.; Ballesteros, D.; Colville, L. Seed Longevity—The Evolution of Knowledge and a Conceptual Framework. Plants 2023, 12, 471. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.H. Predicting the storage life of seeds. Seed Sci. Technol. 1973, 1, 499–514. [Google Scholar]
- Ellis, R.H. Invited Review: Seed ageing, survival and the improved seed viability equation; forty years on. Seed Sci. Technol. 2022, 50 (Suppl. S1), 1–20. [Google Scholar] [CrossRef]
- Jimoh, K.A.; Hashim, N.; Shamsudin, R.; Che Man, H.; Jahari, M.; Onwude, D.I. Recent Advances in the Drying Process of Grains. Food Eng. Rev. 2023, 15, 548–576. [Google Scholar] [CrossRef]
- Codex Alimentarius. Wheat and Durum Wheat Codex Standard 199-1995. Codex Alimentarius. 1995. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 13 February 2024).
- GOST 52325-2005; Seeds of Agricultural Plants. Variety and Sowing Qualities. General Technical Conditions. Standardinform: Moscow, Russia, 2005; 21p. (In Russian)
- Barrozo, M.A.S.; Mujumdar, A.; Freire, J.T. Air-Drying of Seeds: A Review. Dry. Technol. 2014, 32, 1127–1141. [Google Scholar] [CrossRef]
- Kudra, T.; Mujumdar, A.S. Advanced Drying Technologies, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Motevali, A.; Chayjan, R.A. Effect of Various Drying Bed on Thermodynamic Characteristics. Case Stud. Therm. Eng. 2017, 10, 399–406. [Google Scholar] [CrossRef]
- Henderson, S. Moisture transfer between mixed wet and dry barley grains. J. Agric. Eng. Res. 1987, 37, 163–170. [Google Scholar] [CrossRef]
- Li, Z.; Kobayashi, N.; Watanabe, F.; Hasatani, M. Sorption drying of soybean seeds with silica gel. I. Hydrodynamics of a fluidized bed dryer. Dry. Technol. 2002, 20, 223–233. [Google Scholar] [CrossRef]
- Danziger, M.T.; Steinberg, M.P.; Nelson, A.I. Drying of Field Corn with Silica Gel. Trans. ASAE 1972, 15, 1071–1074. [Google Scholar] [CrossRef]
- Chamurliysky, P.; Tsenov, N.; Stoyanova, S. Drying of seeds from common wheat (Triticum aestivum L.) by using Silica Gel for ex situ storage. Agric. Sci. Technol. 2013, 3, 252–256. [Google Scholar]
- Dhaliwal, S.S.; Singh, S.; Singh, P.P. Desiccant seed dryer. Int. J. Ambient Energy 2006, 27, 52–56. [Google Scholar] [CrossRef]
- Watts, K.C.; Bilanski, W.K.; Menzies, D.R. Simulation of adsorption drying of corn, wheat, barley and oats using bentonite. Can. Agric. Eng. 1987, 29, 173–178. [Google Scholar]
- Falabella, M.C.; Suarez, K.; Viollaz, P.E. Drying Kinetics of Corn in Contact with a Solid Adsorbent. J. Food Eng. 1991, 13, 273–283. [Google Scholar] [CrossRef]
- Graham, V.A.; Bilanski, W.K.; Menzies, D.R. Adsorption Grain Drying Using Bentonite. Trans. ASAE 1983, 26, 1512–1515. [Google Scholar] [CrossRef]
- Alikhani, Z.; Raghavan, G.S.V.; Mujumbar, A.S. Adsorption drying of corn in zeolite granules using a rotary drum. Dry. Technol. 1992, 10, 783–797. [Google Scholar] [CrossRef]
- Gordon, A.J.; Ford, R.A. The Chemist’s Companion: A Handbook of Practical Data, Techniques, and References; Wiley: New York, NY, USA, 1972; 560p. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands; Academic Press: London, UK, 1995; 899p. [Google Scholar]
- Grzebisz, W.; Zielewicz, W.; Przygocka-Cyna, K. Deficiencies of Secondary Nutrients in Crop Plants—A Real Challenge to Improve Nitrogen Management. Agronomy 2023, 13, 66. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Surin, N.A.; Akimochkina, G.V.; Rabchevskiy, E.V.; Rogovenko, E.S.; Butkovskaya, L.K.; Kozulina, N.S.; Lipshin, A.G.; Spedt, A.A.; et al. Method of Contact Drying of Grain. Russian Patent RU2788857C1, 25 January 2023. Available online: https://patents.google.com/patent/RU2788857C1/en (accessed on 28 April 2024).
- Fomenko, E.V.; Anshits, N.N.; Akimochkina, G.V.; Rabchevsky, E.V.; Rogovenko, E.S.; Shabanov, V.F.; Anshits, A.G. Method of Obtaining Microspherical Composite Desiccant for Bulk Materials. Russian Patent RU2789376C1, 2 February 2023. Available online: https://patents.google.com/patent/RU2789376C1/en (accessed on 28 April 2024).
- Fomenko, E.V.; Anshits, N.N.; Solovyov, L.A.; Shabanov, V.F.; Anshits, A.G. The Preparation and Contact Drying Performance of Encapsulated Microspherical Composite Sorbents Based on Fly Ash Cenospheres. Molecules 2024, 29, 2391. [Google Scholar] [CrossRef] [PubMed]
- Trausel, F.; de Jong, A.-J.; Cuypers, R. A Review on the properties of salt hydrates for thermochemical storage. Energy Procedia 2014, 48, 447–452. [Google Scholar] [CrossRef]
- Donkers, P.A.J.; Sögütoglu, L.C.; Huinink, H.P.; Fischer, H.R.; Adan, O.C.G. A review of salt hydrates for seasonal heat storage in domestic applications. Appl. Energy 2017, 199, 45–68. [Google Scholar] [CrossRef]
- Steiger, M.; Linnow, K.; Ehrhardt, D.; Rohde, M. Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4–H2O and Na+–Mg2+–Cl−–SO42−–H2O systems with implications for Mars. Geochim. Cosmochim. Acta 2011, 75, 3600–3626. [Google Scholar] [CrossRef]
- Scheidema, M.N.; Taskinen, P. Decomposition thermodynamics of magnesium sulfate. Ind. Eng. Chem. Res. 2011, 50, 9550–9556. [Google Scholar] [CrossRef]
- van Essen, V.M.; Zondag, H.A.; Cot Gores, J.; Bleijendaal, L.P.J.; Bakker, M.; Schuitema, R.; van Helden, W.G.J.; He, Z.; Rindt, C.C.M. Characterization of MgSO4 hydrate for thermochemical seasonal heat storage. J. Sol. Energy Eng. 2009, 131, 041014. [Google Scholar] [CrossRef]
- Hamad, S.E.D. An experimental study of salt hydrate MgSO4∙7H2O. Thermochim. Acta 1975, 13, 409. [Google Scholar] [CrossRef]
- Emons, H.H.; Ziegenbalg, G.; Naumann, R.; Paulik, F. Thermal decomposition of the magnesium sulphate hydrates under quasi-isothermal and quasi-isobaric conditions. J. Therm. Anal. 1990, 36, 1265–1279. [Google Scholar] [CrossRef]
- Donkers, P.A.J.; Beckert, S.; Pel, L.; Stallmach, F.; Steiger, M.; Adan, O.C.G. Water Transport in MgSO4·7H2O During Dehydration in View of Thermal Storage. J. Phys. Chem. C 2015, 119, 28711–28720. [Google Scholar] [CrossRef]
- Grevel, K.-D.; Majzlan, J. Internally consistent thermodynamic data for magnesium sulfate hydrates. Geochim. Cosmochim. Acta 2009, 73, 6805–6815. [Google Scholar] [CrossRef]
- TU 20.13.41-001-23877036-2017; Agrochemical Magnesium Sulfate Grades: Fine-Crystalline Epsomite, Granulated Epsomite, Fine-Crystalline Kieserite, Granulated Kieserite. South Ural Magnesium Compounds Plant: Kuvandyk, Russia, 2017; 22p. (In Russian)
- Ministry of Agriculture of Russia. State Catalog of Pesticides and Agrochemicals Approved for Use in the Russian Federation, as of July 18, 2022; Ministry of Agriculture of Russia: Moscow, Russia, 2022. [Google Scholar]
- GOST 13586.5-2015; Grain. Moisture Determination Method. Standartinform: Moscow, Russia, 2019; 12p. (In Russian)
- ISO 712:2009; Cereals and Cereal Products Determination of Moisture Content Reference Method. ISO: Geneva, Switzerland, 2009.
- Powder Diffraction File Database. Available online: https://www.icdd.com/pdf-2 (accessed on 13 February 2024).
- Solovyov, L.A. Full-profile refinement by derivative difference minimization. J. Appl. Crystallogr. 2004, 37, 743–749. [Google Scholar] [CrossRef]
- DIN 51007:1994-06; Thermal Analysis; Differential Thermal Analysis; Principles. Deutsche Institut für Normung e.V. (DIN): Berlin, Germany, 1994.
- GOST 12038-84; Seeds of Agricultural Crops. Germination Methods. Publishing of Standards: Moscow, Russia, 2004; 29p. (In Russian)
- ISTA. International Rules for Seed Testing (Bassersdorf: International Seed Testing Association). 2005. Available online: https://www.seedtest.org/en/publications/international-rules-seed-testing.html (accessed on 13 February 2024).
- A Minerals Database. Available online: https://www.mindat.org/ (accessed on 13 February 2024).
- Kovba, L.M. Radiography in Inorganic Chemistry; Moscow State University: Moscow, Russia, 1991; 256p. [Google Scholar]
- Pilyaeva, O.V. Problems and Prospects of Post-Harvest Grain Processing; Krasnoyarsk State Agrarian University, Achinsk Branch: Achinsk, Russia, 2017; 74p. (In Russian) [Google Scholar]
- Jittanit, W.; Srzednicki, G.; Driscoll, R. Corn, Rice, and Wheat Seed Drying by Two-Stage Concept. Dry. Technol. 2010, 28, 807–815. [Google Scholar] [CrossRef]





| Parameter | Temperature Range (°C) | |||||||
|---|---|---|---|---|---|---|---|---|
| 40–100 | 40–150 | 40–200 | 40–250 | 40–300 | 40–350 | 40–400 | 40–450 | |
| ∆m (wt%) | 0.32 | 1.96 | 2.79 | 3.43 | 5.69 | 14.30 | 14.56 | 14.44 |
| Water capacity (mg/g) * | 4 | 23 | 33 | 40 | 67 | 167 | 170 | 169 |
| Desiccant/Grain Mass Ratio | Time (min) | ||||
|---|---|---|---|---|---|
| 0 | 60 | 150 | 240 | 360 | |
| Moisture content of wheat seeds (% wb), ±0.1 (p = 0.95) | |||||
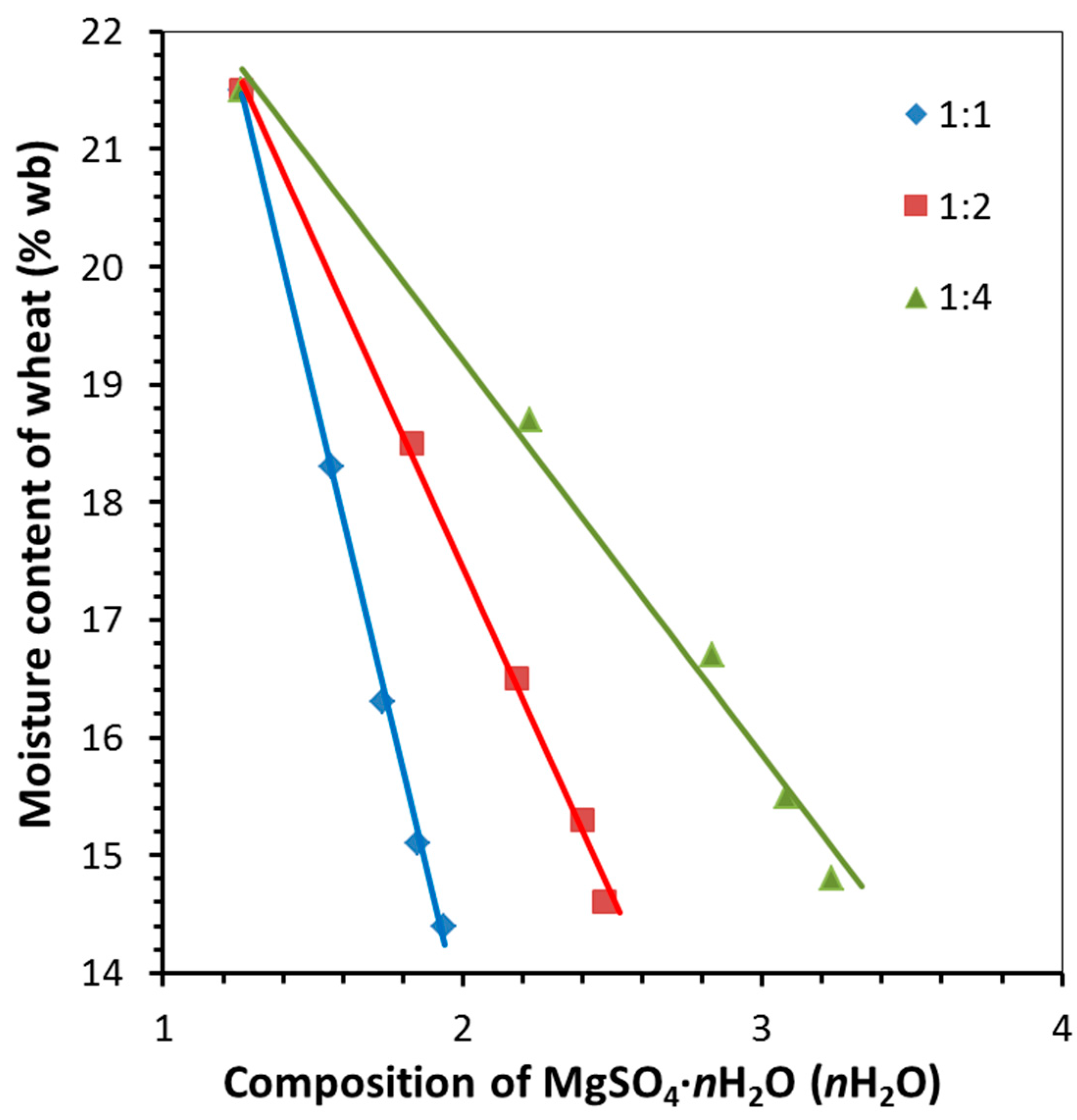
| 1:1 | 21.5 | 18.3 | 16.3 | 15.1 | 14.4 |
| 1:2 | 21.5 | 18.5 | 16.5 | 15.3 | 14.6 |
| 1:4 | 21.5 | 18.7 | 16.7 | 15.5 | 14.8 |
| Moisture content of desiccant (wt%), ±0.02 (p = 0.95) | |||||
| 1:1 | 15.88 | 18.95 | 20.59 | 21.66 | 22.48 |
| 1:2 | 15.88 | 21.49 | 24.62 | 26.44 | 27.03 |
| 1:4 | 15.88 | 24.98 | 29.78 | 31.55 | 32.62 |
| Stage | Time (min) | MR360 | ||||
|---|---|---|---|---|---|---|
| 0 | 60 | 150 | 240 | 360 | ||
| Moisture Content of Wheat Seeds (% wb), ±0.1 (p = 0.95)Desiccant/Grain Mass Ratio = 1:1 | ||||||
| 1 | 21.5 | 18.3 | 16.5 | 15.3 | 14.4 | 0.67 |
| 2 | 21.5 | – | – | – | 14.6 | 0.68 |
| 3 | 21.5 | – | – | – | 15.5 | 0.72 |
| Moisture Content of Wheat Seeds(% wb), ±0.1 (p = 0.95)Desiccant/Grain Mass Ratio = 1:2 | ||||||
| 1 | 21.5 | 18.5 | 16.5 | 15.4 | 14.6 | 0.68 |
| 2 | 21.5 | – | – | – | 14.8 | 0.69 |
| 3 | 21.5 | – | – | – | 15.9 | 0.74 |
| Stage | Moisture Content (wt%), ±0.02 (p = 0.95) | Composition |
|---|---|---|
| Desiccant/grain mass ratio = 1:1 | ||
| 1 | 22.48 | MgSO4·1.94H2O |
| 2 | 28.10 | MgSO4·2.61H2O |
| 3 | 32.30 | MgSO4·3.19H2O |
| Desiccant/grain mass ratio = 1:2 | ||
| 1 | 27.03 | MgSO4·2.48H2O |
| 2 | 35.50 | MgSO4·3.68H2O |
| 3 | 42.83 | MgSO4·5.00H2O |
| Parameter | Temperature Range (°C) | |||||||
|---|---|---|---|---|---|---|---|---|
| 40–100 | 40–150 | 40–200 | 40–250 | 40–300 | 40–350 | 40–400 | 40–450 | |
| ∆m (wt%) | 23.43 | 29.45 | 33.01 | 34.43 | 36.43 | 37.83 | 37.71 | 37.58 |
| Water capacity (mg/g) * | 377 | 474 | 531 | 554 | 586 | 608 | 607 | 604 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fomenko, E.V.; Anshits, N.N.; Shabanov, V.F.; Anshits, A.G. Sorption Drying of Wheat Seeds Using Kieserite as a Solid Desiccant. AgriEngineering 2024, 6, 2023-2042. https://doi.org/10.3390/agriengineering6030118
Fomenko EV, Anshits NN, Shabanov VF, Anshits AG. Sorption Drying of Wheat Seeds Using Kieserite as a Solid Desiccant. AgriEngineering. 2024; 6(3):2023-2042. https://doi.org/10.3390/agriengineering6030118
Chicago/Turabian StyleFomenko, Elena V., Natalia N. Anshits, Vasily F. Shabanov, and Alexander G. Anshits. 2024. "Sorption Drying of Wheat Seeds Using Kieserite as a Solid Desiccant" AgriEngineering 6, no. 3: 2023-2042. https://doi.org/10.3390/agriengineering6030118






